Physical Properties and Hydration Characteristics of Low-Heat Portland Cement at High-Altitude
Abstract
:1. Introduction
2. Materials and Experimental Methods
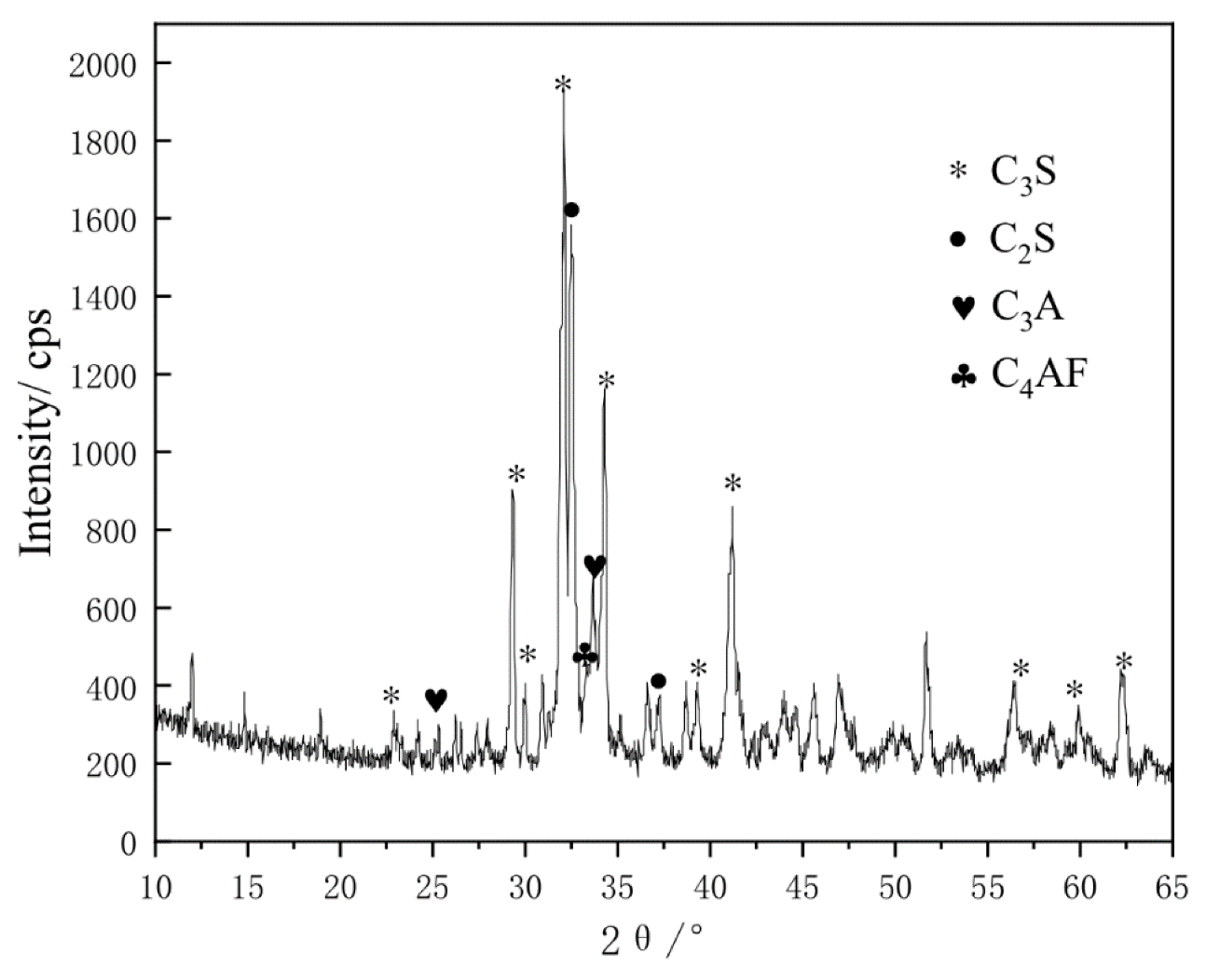
2.1. Raw Materials
2.2. Sample Preparation and Maintenance
2.2.1. Sample Preparation
2.2.2. Curing Regimes
2.3. Test Method
2.3.1. XRF
2.3.2. Mechanical Strength
2.3.3. Dry Shrinkage
2.3.4. XRD-Rietveld Analysis
2.3.5. Thermal Analysis
2.3.6. SEM and EDS
2.3.7. Pore Structure
3. Result and Discussion
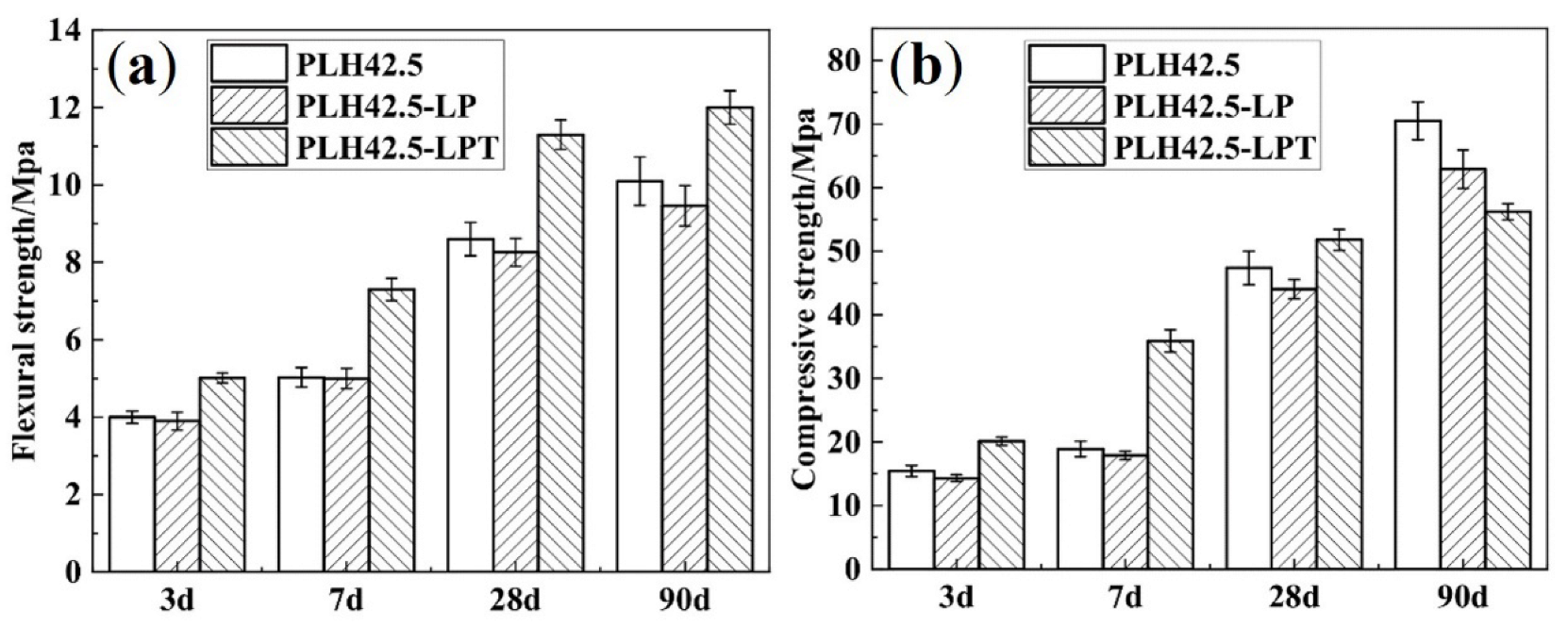
3.1. Mechanical Strength
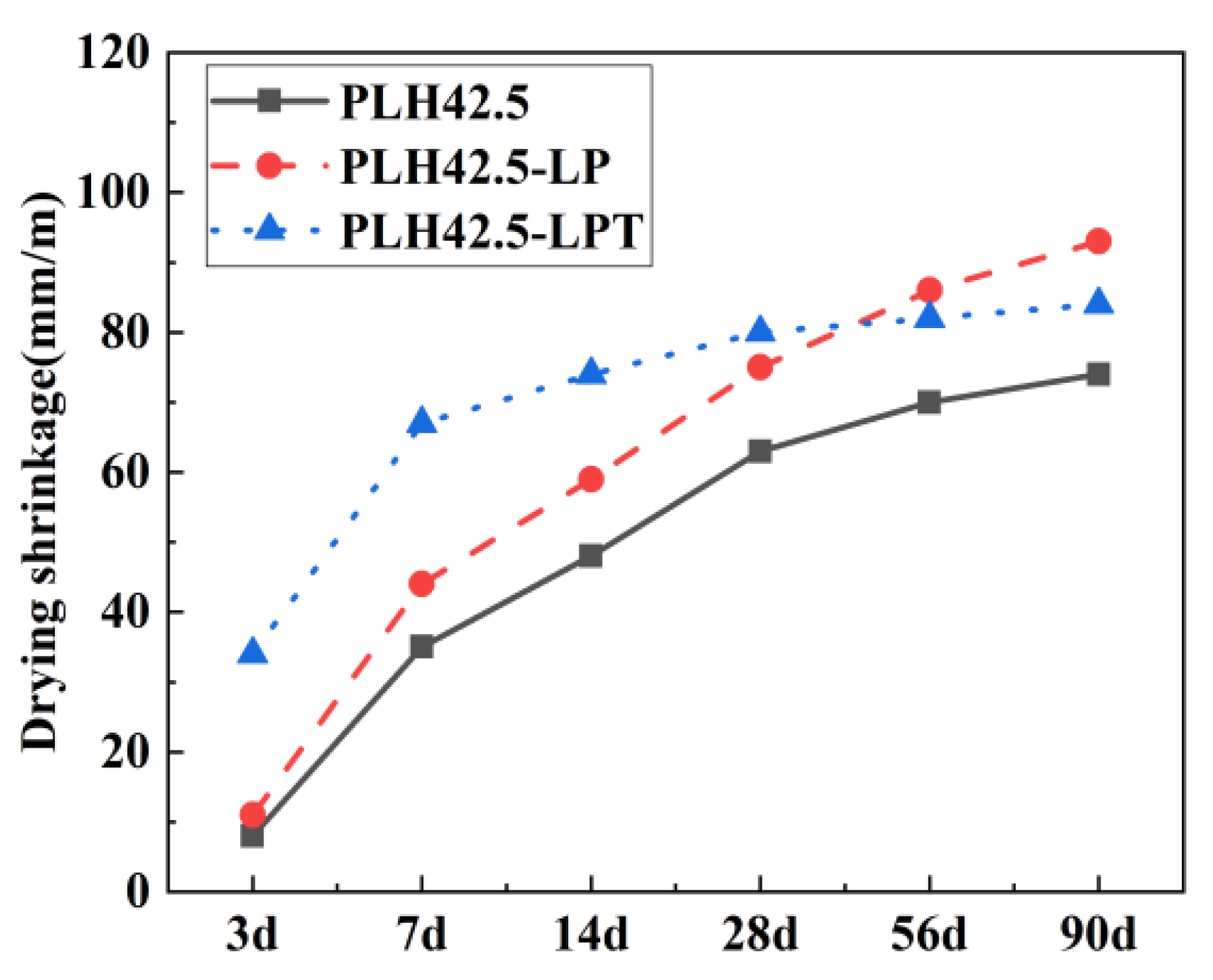
3.2. Drying Shrinkage
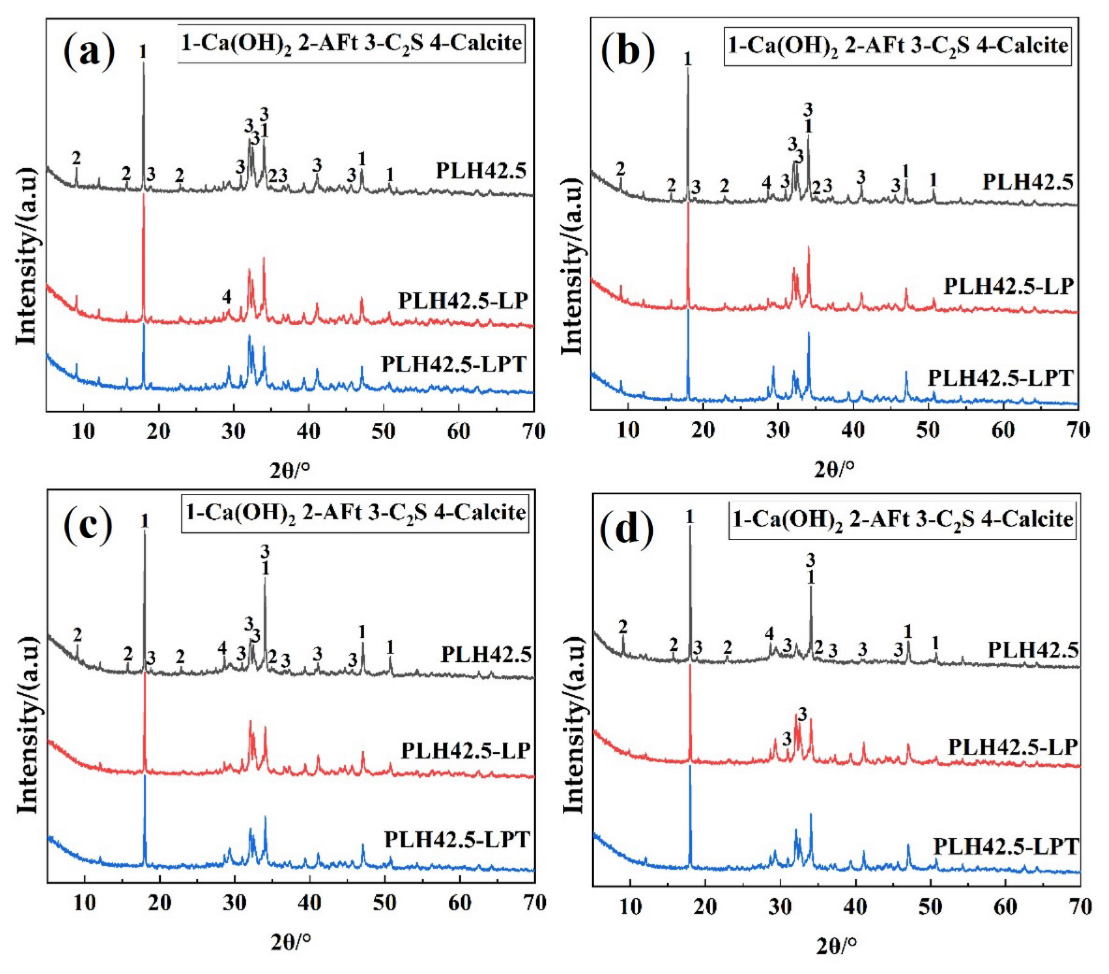
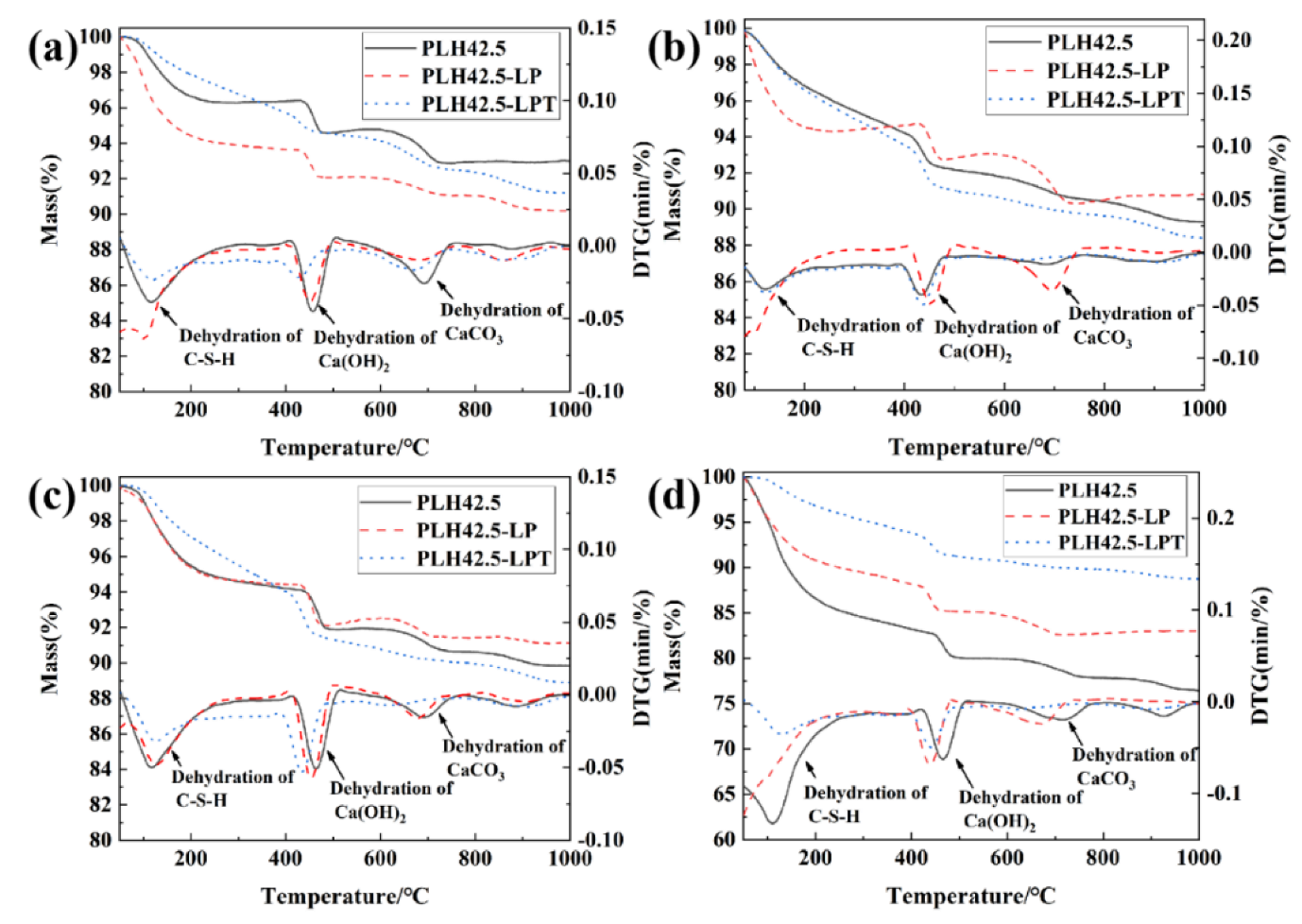
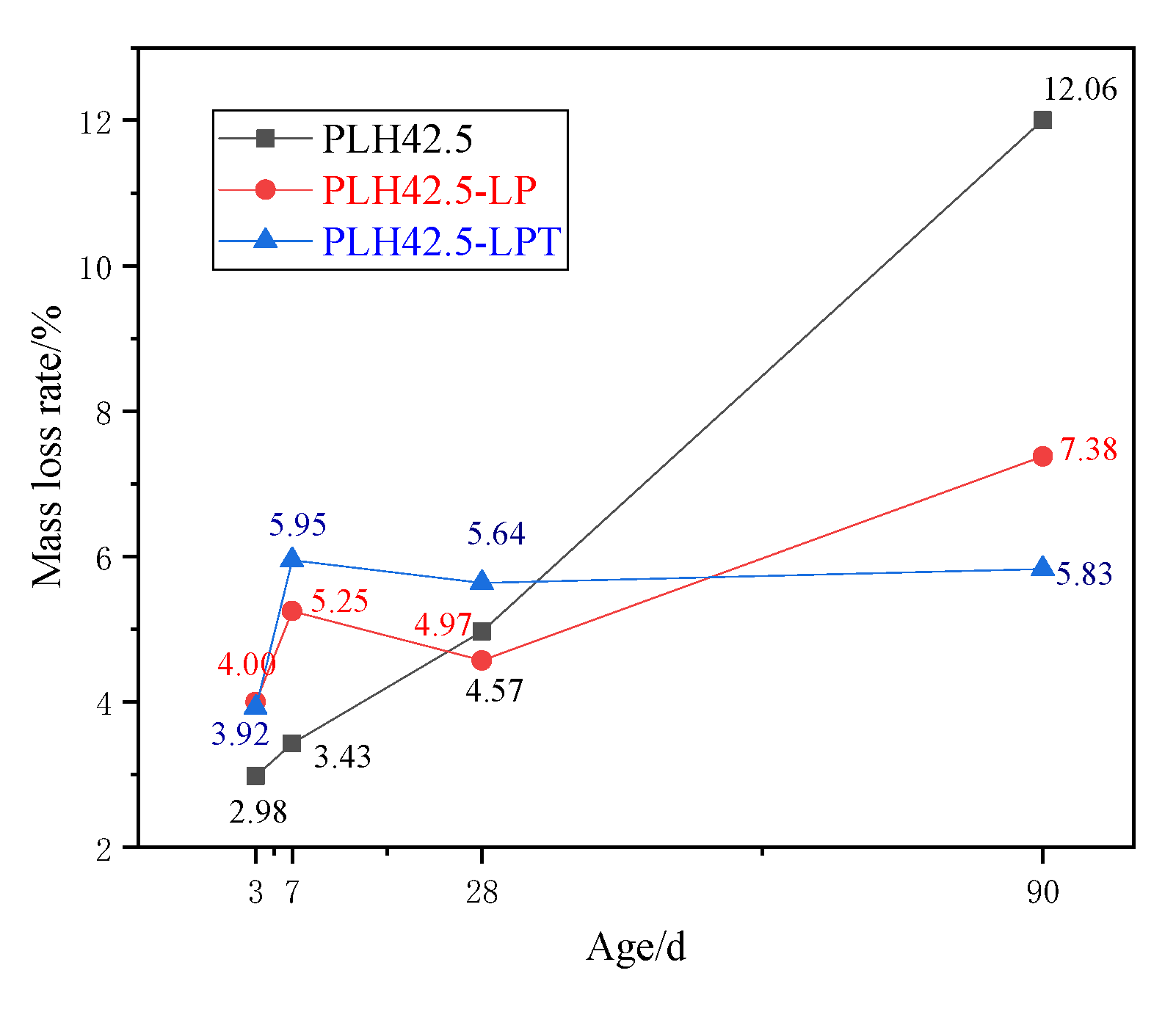
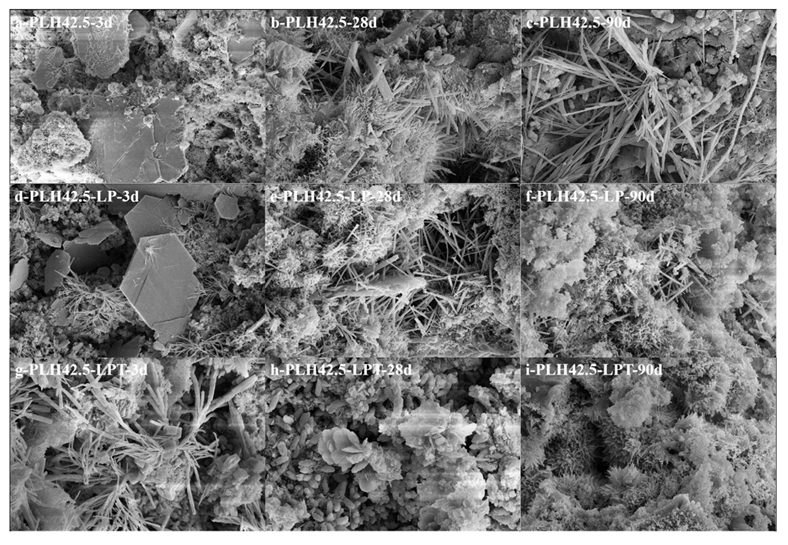
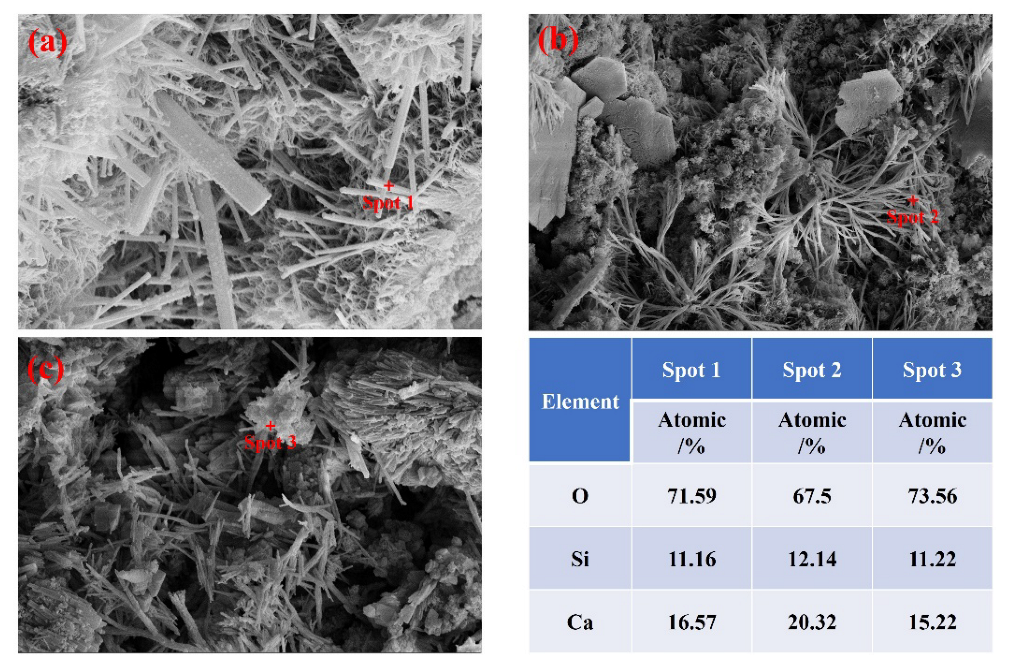
3.3. Hydration Characteristics
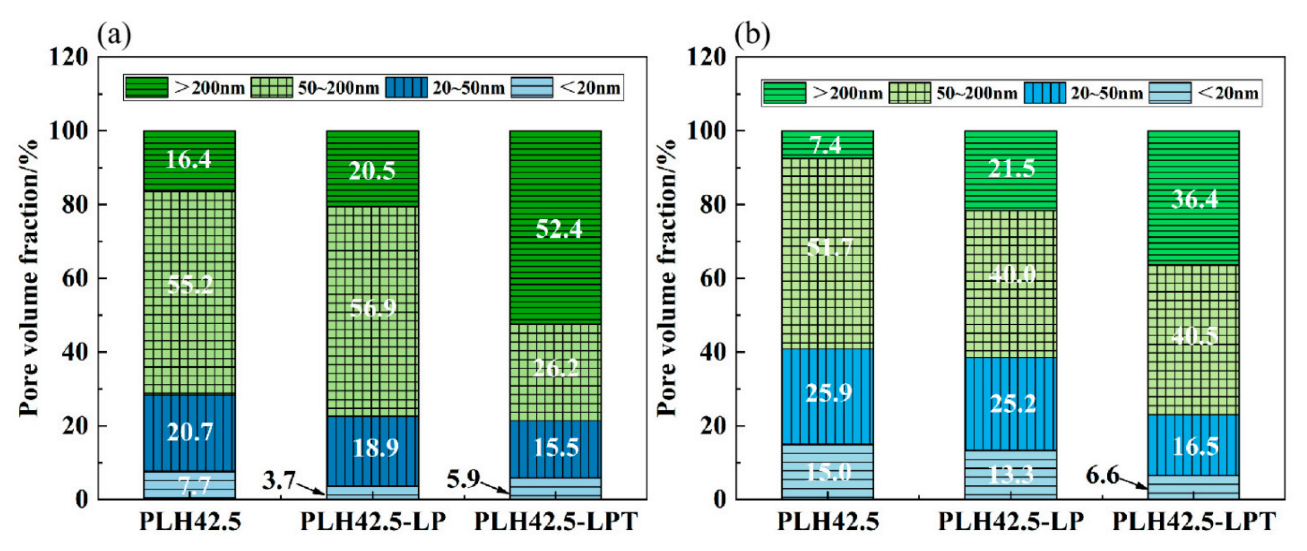
3.4. Pore Size Distribution
3.5. Ca/Si Ratio of C-S-H at an Early Curing Stage
4. Conclusions
- (1)
- The compressive strength of the PLH mortar cured under the LPT conditions was higher than that of the specimens cured under standard conditions at an early curing stage (3, 7, or 28 d). This was because hydration products formed faster upon curing under the LPT conditions at an early hydration stage but slower than those formed upon curing under standard conditions at a later curing stage (90 d), owing to the dehydration of the hydration products. The flexural strength was higher in the PLH mortar cured under the LPT conditions than that of the PLH cured under the standard conditions after curing for 3, 7, 28, or 90 d.
- (2)
- A low air pressure accelerated the moisture evaporation and drying shrinkage of the PLH mortar. In addition, drying shrinkage under LPT conditions developed rapidly at an early stage but slowly at a later stage. Owing to the temperature variation (5–60 °C), the moisture losses of the PLH-LPT mortars in the first 7 d were consistently higher than those of the standard-cured and LP mortars. Moreover, when the mortar was cured at various temperatures, the hydration products C-S-H and Ca(OH)2 formed rapidly, which reduced the pore connectivity and water migration pathways within the PLH mortar.
- (3)
- Hydration products were formed faster than curing products under the LPT conditions at an early hydration stage. Under the LPT conditions, the characteristic peaks of AFt were not observed in the XRD pattern after curing for 28 d, and AFt transformed into AFm.
- (4)
- The pore size distribution characteristics of the specimens cured under the LPT conditions deteriorated, owing to water evaporation and micro-crack formation at low air pressures.
- (5)
- Low pressure led to water transport and evaporation, hindering the reaction between belite and water and contributing to significant changes in the C-S-H Ca/Si ratios in the early curing stages in different curing environments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krausmann, F.; Lauk, C.; Haas, W.; Wiedenhofer, D. From resource extraction to outflows of wastes and emissions: The socioeconomic metabolism of the global economy, 1900–2015. Glob. Environ. Change 2018, 52, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.K.; Singh, S. Embodied energy analysis of higher education buildings using an input-output-based hybrid method. Energy Build. 2018, 161, 41–54. [Google Scholar] [CrossRef]
- Heravi, G.; Nafisi, T.; Mousavi, R. Evaluation of energy consumption during production and construction of concrete and steel frames of residential buildings. Energy Build. 2016, 130, 244–252. [Google Scholar] [CrossRef]
- Tan, C.; Yu, X.; Guan, Y. A technology-driven pathway to net-zero carbon emissions for China’s cement industry. Appl. Energy 2022, 325, 119804. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; De Wolf, C.; Chin, S.; Ochsendorf, J.; Hajiah, A.E.; Al-Mumin, A.; Büyüköztürk, O. Impact of Embodied Energy on materials/buildings with partial replacement of ordinary Portland Cement (OPC) by natural Pozzolanic Volcanic Ash. J. Clean. Prod. 2018, 177, 547–554. [Google Scholar] [CrossRef]
- Monahan, J.; Powell, J.C. An embodied carbon and energy analysis of modern methods of construction in housing: A case study using a lifecycle assessment framework. Energy Build. 2011, 43, 179–188. [Google Scholar] [CrossRef]
- Cuesta, A.; Ayuela, A.; Aranda, M.A.G. Belite cements and their activation. Cem. Concr. Res. 2021, 140, 106319. [Google Scholar] [CrossRef]
- Jiang, C.; Yu, L.; Tang, X.; Chu, H.; Jiang, L. Deterioration process of high belite cement paste exposed to sulfate attack, calcium leaching and the dual actions. J. Mater. Res. Technol. 2021, 15, 2982–2992. [Google Scholar] [CrossRef]
- Sinyoung, S.; Kunchariyakun, K.; Asavapisit, S.; MacKenzie, K.J.D. Synthesis of belite cement from nano-silica extracted from two rice husk ashes. J. Environ. Manag. 2017, 190, 53–60. [Google Scholar] [CrossRef]
- Kurdowski, W.; Duszak, S.; Trybalska, B. Belite produced by means of low-temperature synthesis. Cem. Concr. Res. 1997, 27, 51–62. [Google Scholar] [CrossRef]
- Shen, W.; Liu, Y.; Yan, B.; Wang, J.; He, P.; Zhou, C.; Huo, X.; Zhang, W.; Xu, G.; Ding, Q. Cement industry of China: Driving force, environment impact and sustainable development. Renew. Sustain. Energy Rev. 2017, 75, 618–628. [Google Scholar] [CrossRef]
- García-Díaz, I.; Palomo, J.G.; Puertas, F. Belite cements obtained from ceramic wastes and the mineral pair CaF2/CaSO4. Cem. Concr. Compos. 2011, 33, 1063–1070. [Google Scholar] [CrossRef]
- Staněk, T.; Sulovský, P. Active low-energy belite cement. Cem. Concr. Res. 2015, 68, 203–210. [Google Scholar] [CrossRef]
- Zentar, R.; Wang, H.; Wang, D. Comparative study of stabilization/solidification of dredged sediments with ordinary Portland cement and calcium sulfo-aluminate cement in the framework of valorization in road construction material. Constr. Build. Mater. 2021, 279, 122447. [Google Scholar] [CrossRef]
- Natkunarajah, K.; Masilamani, K.; Maheswaran, S.; Lothenbach, B.; Amarasinghe, D.A.S.; Attygalle, D. Analysis of the trend of pH changes of concrete pore solution during the hydration by various analytical methods. Cem. Concr. Res. 2022, 156, 106780. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Qing, Y.; Huxing, C.; Yuqing, W.; Shangxian, W.; Zonghan, L. Effect of MgO and gypsum content on long-term expansion of low heat Portland slag cement with slight expansion. Cem. Concr. Compos. 2004, 26, 331–337. [Google Scholar] [CrossRef]
- Jiang, C.; Jiang, L.; Tang, X.; Gong, J.; Chu, H. Impact of calcium leaching on mechanical and physical behaviors of high belite cement pastes. Constr. Build. Mater. 2021, 286, 122983. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Zhou, S.H.; Chen, E.; Tang, S.W. Energy saving benefit; mechanical performance; volume stabilities, hydration properties and products of low heat cement-based materials. Energy Build. 2018, 170, 157–169. [Google Scholar] [CrossRef]
- Wang, N.; Luo, K.; Peng, K.; Li, J.; Lu, Z.; Xia, Y.; Lin, Y.; Zhong, W. Thermal deformation and microstructure characteristics of low-heat Portland cement-based concrete in a high plateau environment. J. Build. Eng. 2022, 58, 105025. [Google Scholar] [CrossRef]
- Maruyama, I.; Lura, P. Properties of early-age concrete relevant to cracking in massive concrete. Cem. Concr. Res. 2019, 123, 105770. [Google Scholar] [CrossRef]
- Hooton, R.D. Future directions for design, specification, testing, and construction of durable concrete structures. Cem. Concr. Res. 2019, 124, 105827. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, W.; Ge, Y.; Liu, P. Effect of nanomaterials on the mechanical properties and microstructure of cement mortar under low air pressure curing. Constr. Build. Mater. 2020, 249, 118787. [Google Scholar] [CrossRef]
- Chang, H.; Wang, X.; Wang, Y.; Li, S.; Wang, J.; Liu, J.; Feng, P. Influence of low vacuum condition on mechanical performance and microstructure of hardened cement paste at early age. Constr. Build. Mater. 2022, 346, 128358. [Google Scholar] [CrossRef]
- Huo, J.; Wang, Z.; Zhang, T.; Ji, X.; Zhang, H.; He, R. The evolution of early-age cracking of cement paste cured in low air pressure environment. J. Build. Eng. 2022, 52, 104489. [Google Scholar] [CrossRef]
- Pang, X.; Sun, L.; Sun, F.; Zhang, G.; Guo, S.; Bu, Y. Cement hydration kinetics study in the temperature range from 15 °C to 95 °C. Cem. Concr. Res. 2021, 148, 106552. [Google Scholar] [CrossRef]
- JC/T 603-2004; Standard Test Method for Drying Shinkage of Mortar. National Standardization Technical Committee Cement: Beijing, China, 2004.
- ISO 679-2009; Cement-Test Methods-Determination of Strength. ISO: London, UK, 2009.
- ASTM C596-2009; Standard Test Method for Drying Shrinkage of Mortar Containing Hydraulic Cement. ASTM: West Conshohocken, PA, USA, 2009.
- Thom, A.S.; Oliver, H.R. On Penman’s equation for estimating regional evaporation. Q. J. R. Meteorol. Soc. 1977, 103, 345–357. [Google Scholar] [CrossRef]
- Ge, X.; Ge, Y.; Li, Q.; Cai, X.; Yang, W.; Du, Y. Effect of low air pressure on the durability of concrete. Constr. Build. Mater. 2018, 187, 830–838. [Google Scholar] [CrossRef]
- Hu, X.; Shi, Z.; Shi, C.; Wu, Z.; Tong, B.; Ou, Z.; de Schutter, G. Drying shrinkage and cracking resistance of concrete made with ternary cementitious components. Constr. Build. Mater. 2017, 149, 406–415. [Google Scholar] [CrossRef]
- Kristiawan, S.A.; Aditya, M.T.M. Effect of High Volume Fly Ash on Shrinkage of Self-compacting Concrete. Procedia Eng. 2015, 125, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Shirani, S.; Cuesta, A.; Morales-Cantero, A.; De la Torre, A.G.; Olbinado, M.P.; Aranda, M.A.G. Influence of curing temperature on belite cement hydration: A comparative study with Portland cement. Cem. Concr. Res. 2021, 147, 106499. [Google Scholar] [CrossRef]
- Gallucci, E.; Zhang, X.; Scrivener, K.L. Effect of temperature on the microstructure of calcium silicate hydrate (C-S-H). Cem. Concr. Res. 2013, 53, 185–195. [Google Scholar] [CrossRef]
- Huang, H.; Qian, C.; Zhao, F.; Qu, J.; Guo, J.; Danzinger, M. Improvement on microstructure of concrete by polycarboxylate superplasticizer (PCE) and its influence on durability of concrete. Constr. Build. Mater. 2016, 110, 293–299. [Google Scholar] [CrossRef]
- Matos, P.R.D.; Prudêncio, L.R.; Oliveira, A.L.D.; Pelisser, F.; Gleize, P.J.P. Use of porcelain polishing residue as a supplementary cimentitious material in self-compacting concrete. Constr. Build. Mater. 2018, 193, 623–630. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Lu, Z.; Li, X.; Jiang, J.; Niu, Y.; Xiang, Y. Silicomanganese slag: Hydration mechanism and leaching behavior of heavy metal ions. Constr. Build. Mater. 2022, 326, 126857. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Jin, M.; Liu, J.; Zhang, J.; Huang, J.; Lu, C.; Zeng, H.; Wang, J.; Zhao, H.; et al. Influences of leaching on the composition, structure and morphology of calcium silicate hydrate (C–S–H) with different Ca/Si ratios. J. Build. Eng. 2022, 58, 105017. [Google Scholar] [CrossRef]
- Luo, K.; Li, J.; Han, Q.; Lu, Z.; Deng, X.; Hou, L.; Niu, Y.; Jiang, J.; Xu, X.; Cai, P. Influence of nano-SiO2 and carbonation on the performance of natural hydraulic lime mortars. Constr. Build. Mater. 2020, 235, 117411. [Google Scholar] [CrossRef]
| SiO2 | Al2O3 | CaO | MgO | SO3 | Fe2O3 | K2O | Na2O | Other Components | Loss |
|---|---|---|---|---|---|---|---|---|---|
| 23.57 | 3.98 | 61.33 | 2.55 | 2.34 | 4.75 | 0.37 | 0.22 | 0.26 | 0.63 |
| C3A | C3S | C2S | C4AF | Other Minerals |
|---|---|---|---|---|
| 2.53 | 29.97 | 44.96 | 14.44 | 8.10 |
| Experiment | Specimen Size (mm) | Number of Specimens | Evaluation Age (d) |
|---|---|---|---|
| Mechanical strength | 40 × 40 × 160 | 36 | 3, 7, 28, and 90 |
| Drying shrinkage | 25 × 25 × 280 | 9 | 3, 7, 28, 56, and 90 |
| XRD, SEM, EDS, TG, and MIP | 20 × 20 × 20 | 36 | 3, 7, 28, and 90 |
| Specimen Size (mm) | Curing Condition | Environmental Parameters | Notation |
|---|---|---|---|
| 40 × 40 × 160 and 20 × 20 × 20 | Standard condition | 20 ± 1 °C, 90% RH, 101 kPa | PLH42.5 |
| Low air pressure condition (LP) | 1 d: 20 ± 1 °C, 90% RH, 101 kPa 2–7 d: 20 ± 1 °C, 90% RH, 50 kPa 8–90 d: 20 ± 1 °C, 10% RH, 50 kPa | PLH42.5-LP | |
| Low air pressure and variable temperature condition (LPT) | 1 d: 20 ± 1 °C 90% RH, 101 kPa 2–7 d: 5–60 °C, 90% RH, 50 kPa 8–90 d: 5–60 °C, 10% RH, 50 kPa | PLH42.5-LPT | |
| 25 × 25 × 280 | Standard condition | 20 ± 1 °C, 50% RH, 101 kPa | PLH42.5 |
| Low air pressure condition (LP) | 20 ± 1 °C, 10% RH, 50 kPa | PLH42.5-LP | |
| Low air pressure and variable temperature condition (LPT) | 5–60 °C, 10% RH, 50 kPa | PLH42.5-LPT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Liu, Q.; Xia, Y.; Li, J.; Lu, Z.; Xu, Y.; Zhong, W.; Lin, Y. Physical Properties and Hydration Characteristics of Low-Heat Portland Cement at High-Altitude. Materials 2023, 16, 3110. https://doi.org/10.3390/ma16083110
Wang N, Liu Q, Xia Y, Li J, Lu Z, Xu Y, Zhong W, Lin Y. Physical Properties and Hydration Characteristics of Low-Heat Portland Cement at High-Altitude. Materials. 2023; 16(8):3110. https://doi.org/10.3390/ma16083110
Chicago/Turabian StyleWang, Ning, Qiang Liu, Yanqing Xia, Jun Li, Zhongyuan Lu, Yigang Xu, Wen Zhong, and Yan Lin. 2023. "Physical Properties and Hydration Characteristics of Low-Heat Portland Cement at High-Altitude" Materials 16, no. 8: 3110. https://doi.org/10.3390/ma16083110
APA StyleWang, N., Liu, Q., Xia, Y., Li, J., Lu, Z., Xu, Y., Zhong, W., & Lin, Y. (2023). Physical Properties and Hydration Characteristics of Low-Heat Portland Cement at High-Altitude. Materials, 16(8), 3110. https://doi.org/10.3390/ma16083110





