Dynamic Molecular Simulation of Polyethylene/Organoclay Nanocomposites for Their Physical Properties and Foam Morphology
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Foaming Process
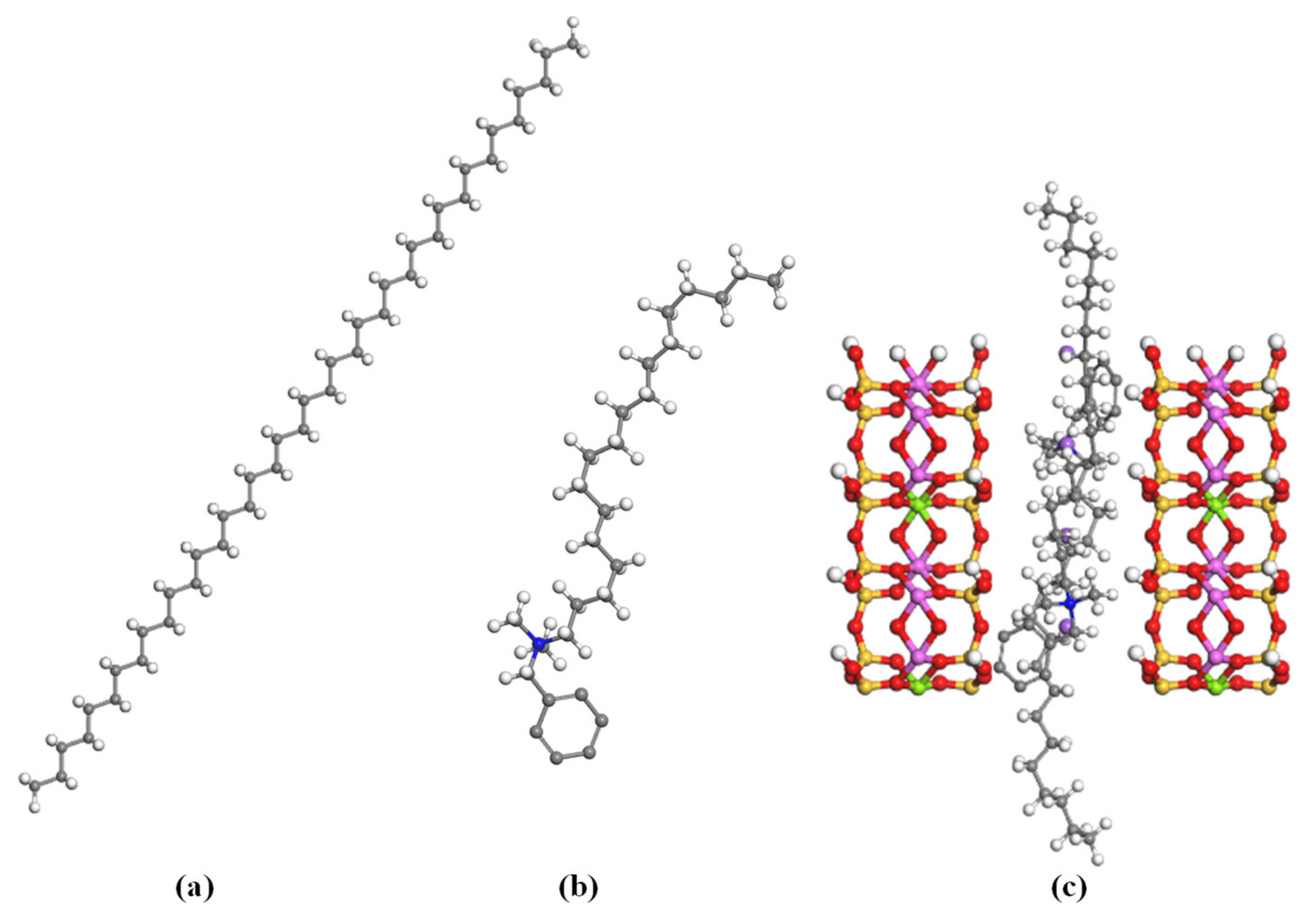
2.2. Molecular Modelling Methods
2.3. Analysis of Data
3. Results and Discussions
3.1. Thermal Characterization of PE/Organoclay Nanocomposites
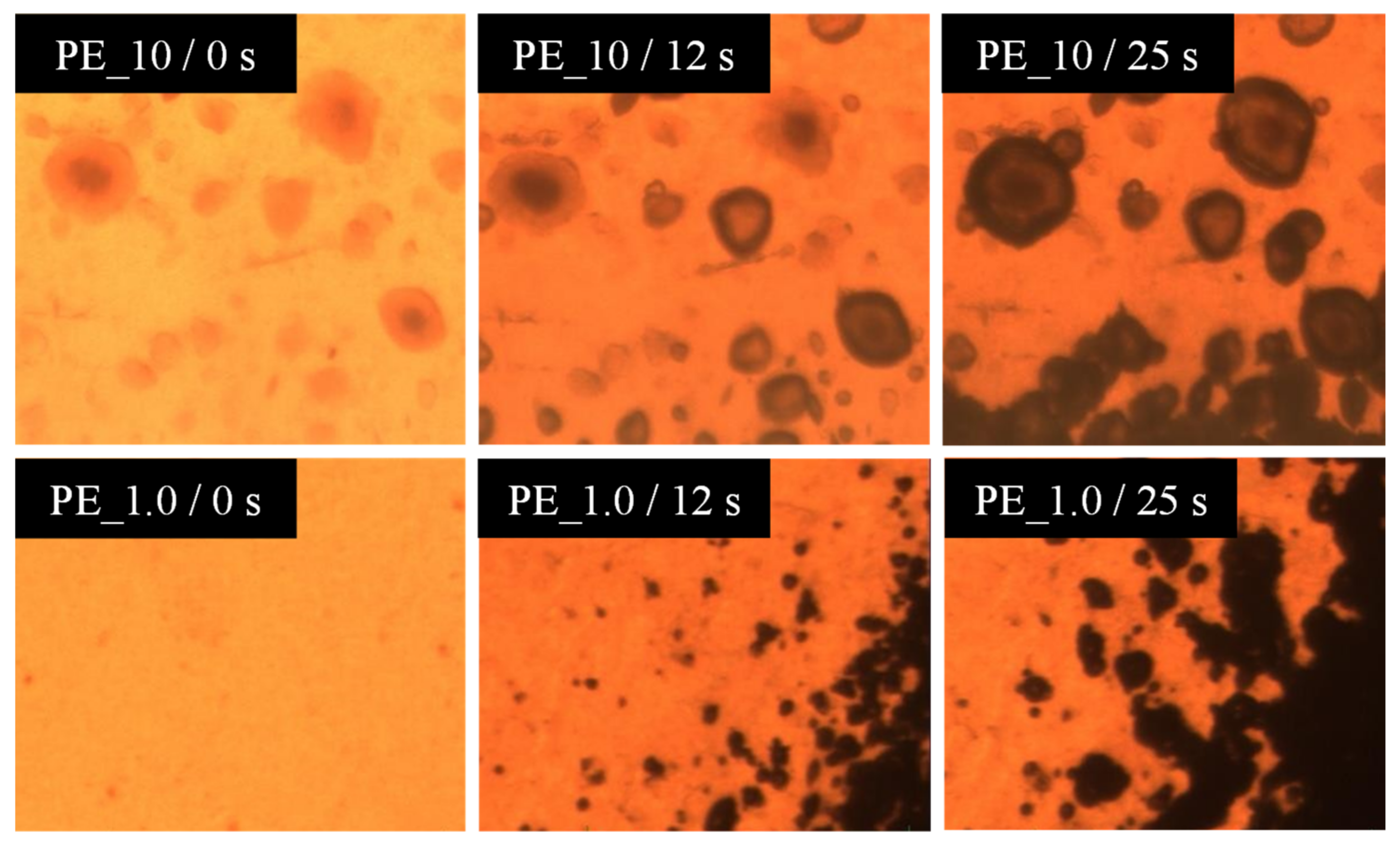
3.2. Foaming Behaviour of PE/Organoclay Nanocomposites
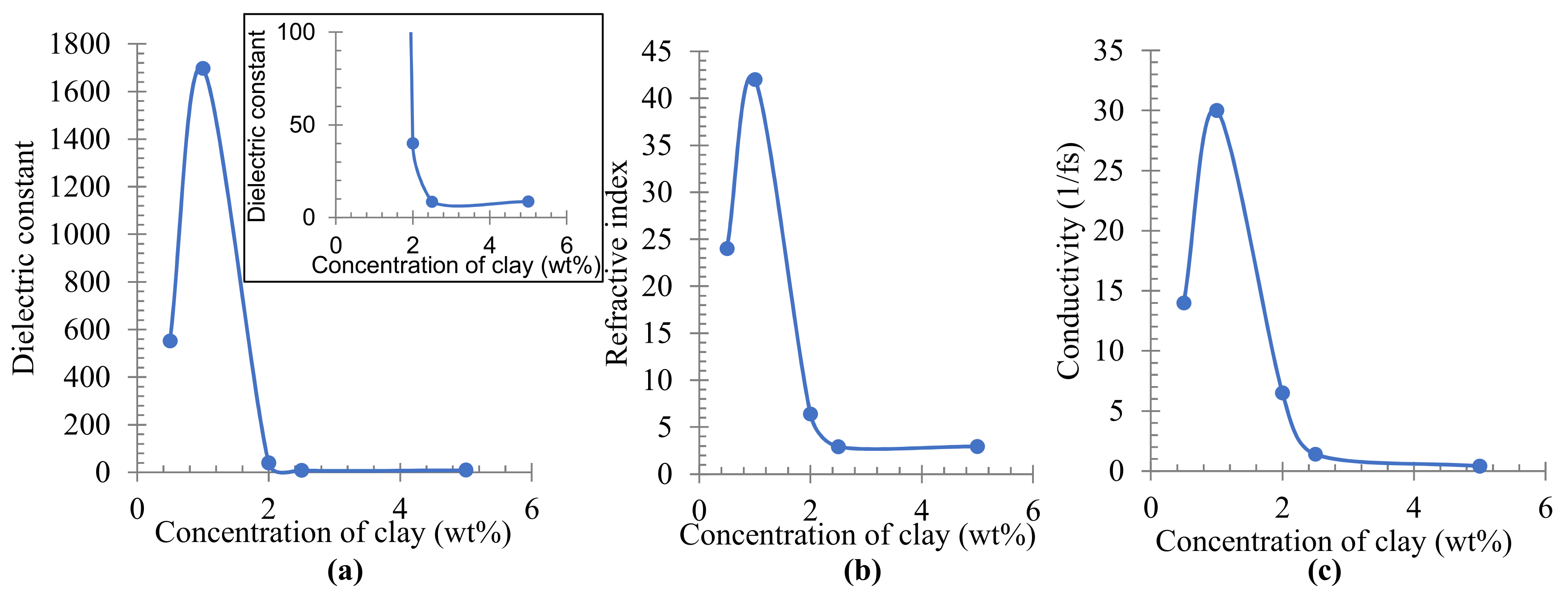
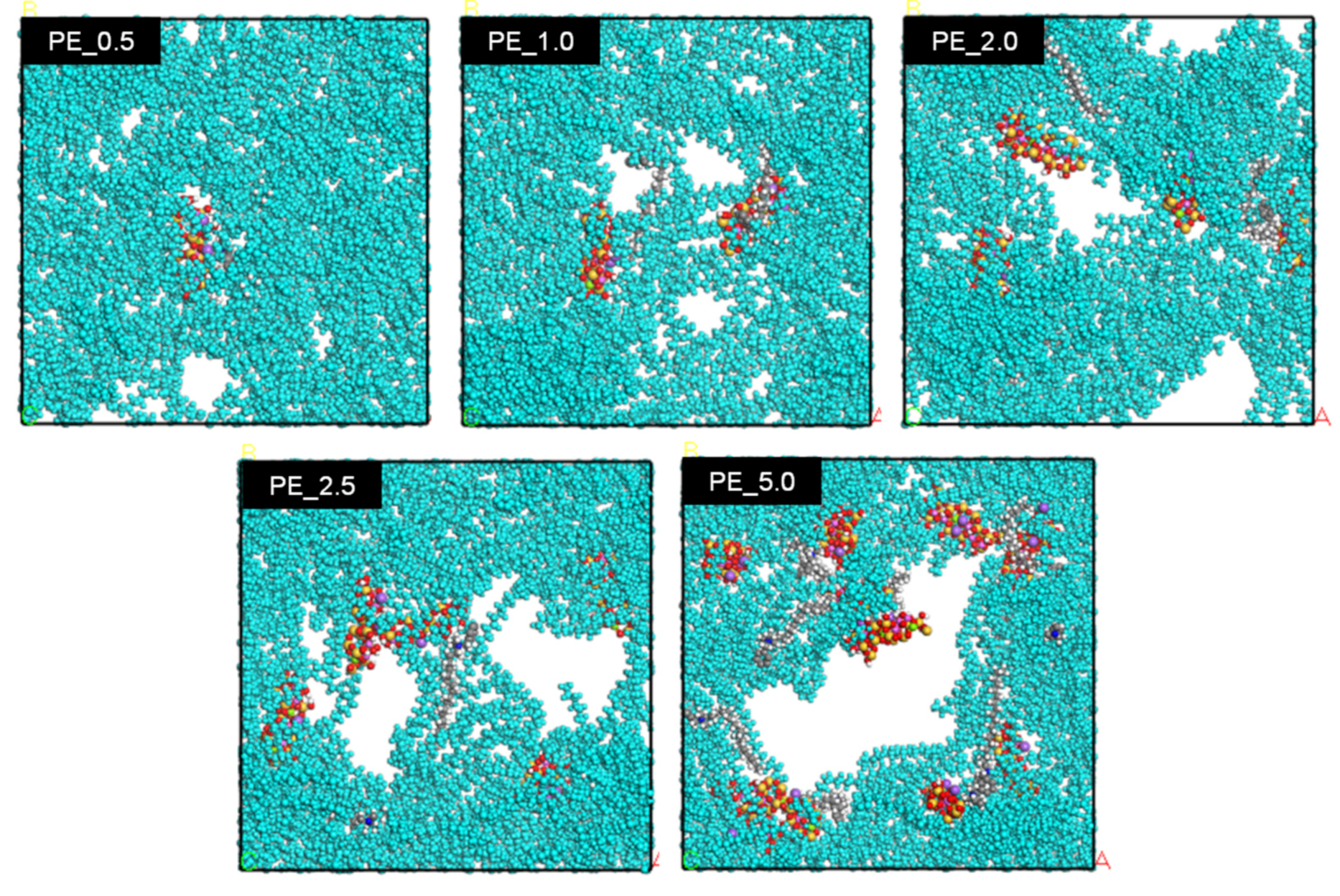
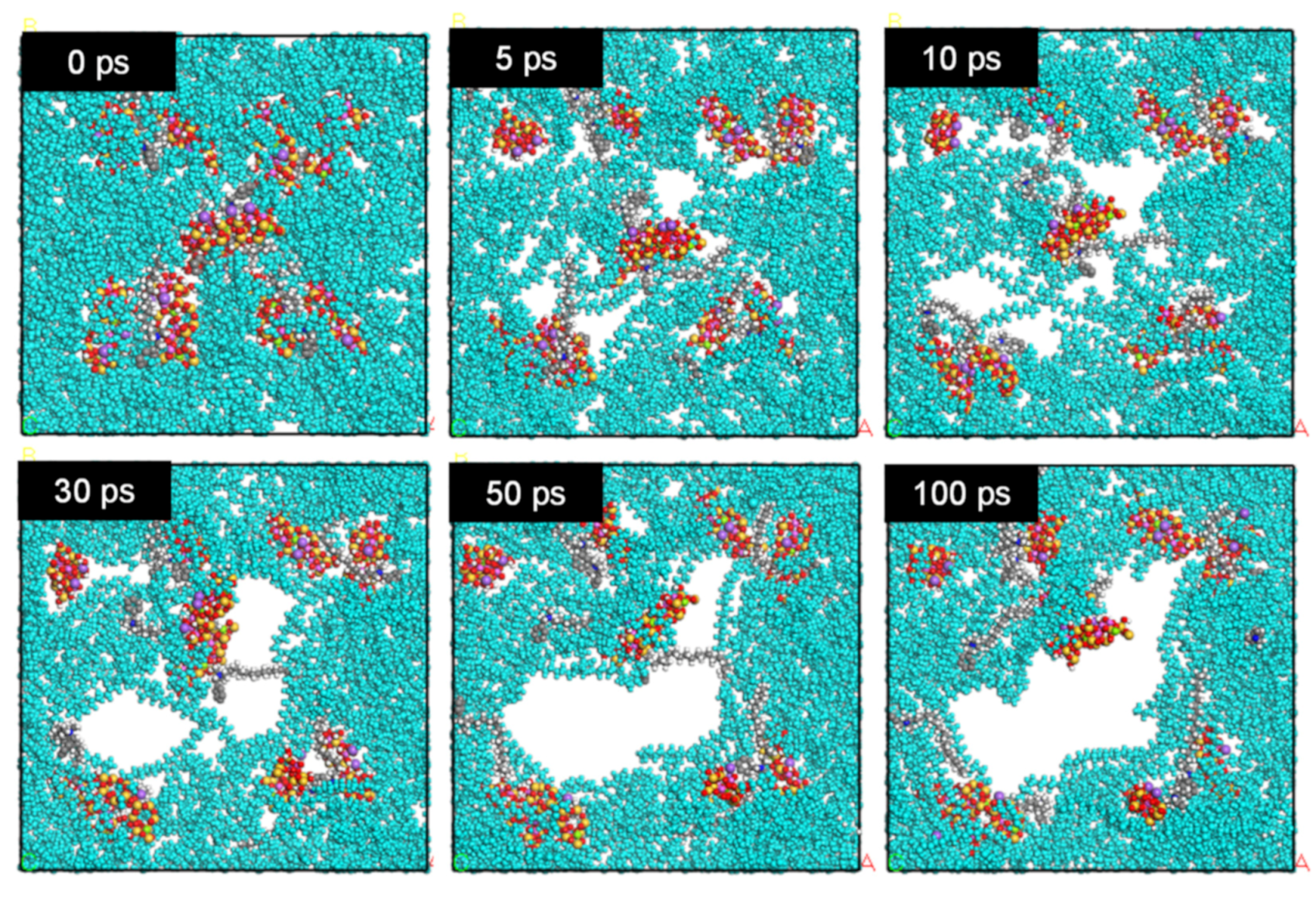
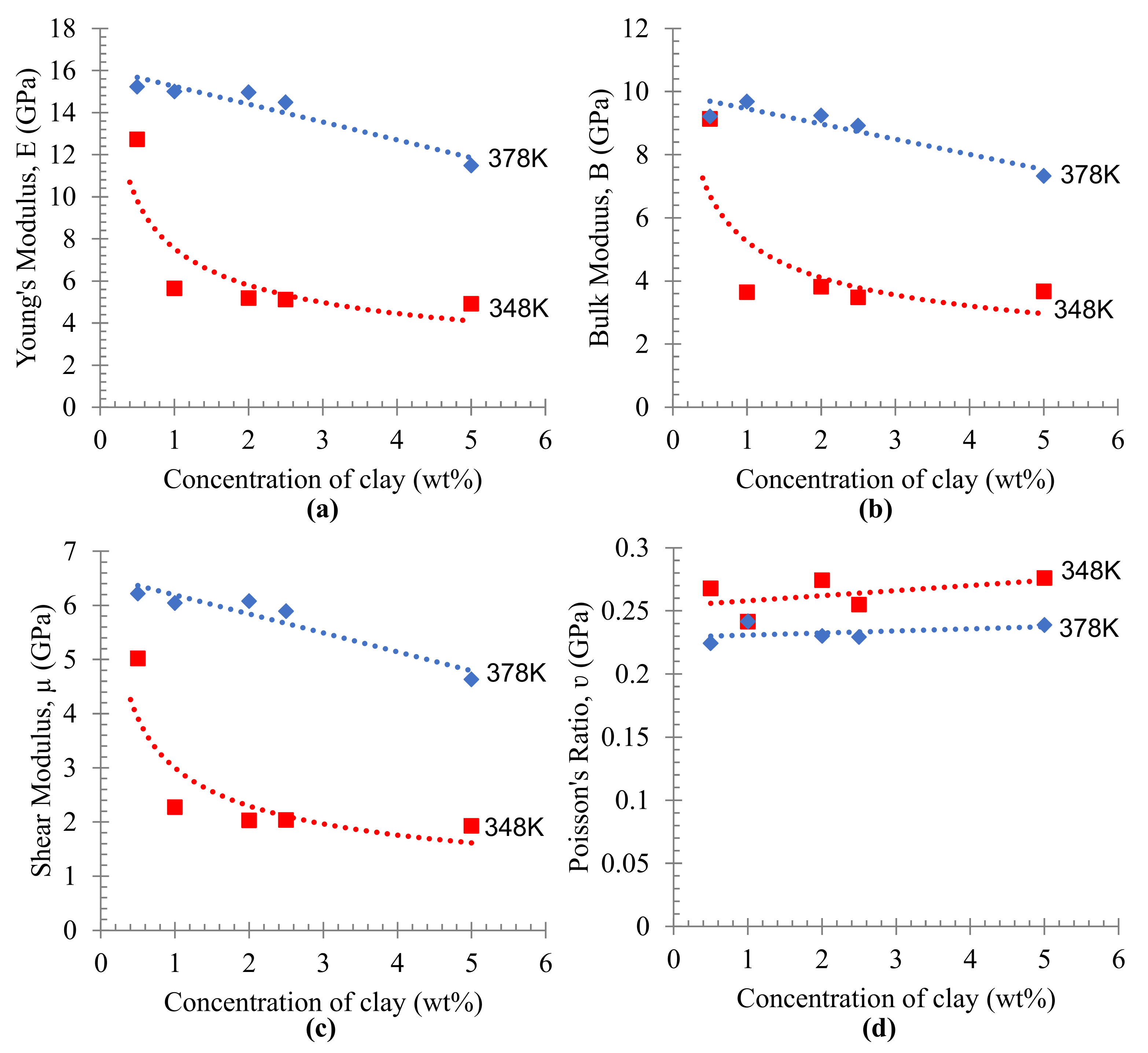
3.3. Simulation Calculation of PE/Organoclay Nanocomposite’s Properties
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panwar, V.; Sachdev, V.K.; Mehra, R.M. Insulator conductor transition in low-density polyethylene-graphite composites. Eur. Polym. J. 2007, 43, 573–585. [Google Scholar] [CrossRef]
- Plesa, I.; Notingher, P.V.; Stancu, C.; Wiesbrock, F.; Schlogl, S. Polyethylene nanocomposites for power cable insulations. Polymers 2019, 11, 24. [Google Scholar] [CrossRef]
- Wan Akmal, I.; Yanuar, Z.A.; Zuraimy, A.; Mohd, S. Partial Discharge characteristics of polymer nanocomposite materials in electrical insulation: A review of sample preparation techniques, analysis methods, potential applications, and future trends. Sci. World J. 2014, 2014, 735070. [Google Scholar] [CrossRef]
- Coppola, B.; Scarfato, P.; Incarnato, L.; Di Maio, L. Morphology development and mechanical properties variation during cold-drawing of polyethylene-clay nanocomposite fibers. Polymers 2017, 9, 235. [Google Scholar] [CrossRef]
- Wagner, J.R. Multilayer Flexible Packaging, 2nd ed.; William Andrew Publishing: Norwich, NY, USA, 2016. [Google Scholar]
- Zhou, Y.; Peng, S.; Hu, J.; He, J. Polymeric insulations materials for HVDC cables: Development, challenges and future perspective. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1308–1318. [Google Scholar] [CrossRef]
- Barber, K.; Alexander, G. Insulation of electrical cables over the past 50 years. IEEE Electr. Insul. Mag. 2013, 29, 27–32. [Google Scholar] [CrossRef]
- Andritsch, T.; Vaughan, A.S.; Stevens, G.C. Novel insulation materials for high voltage cable systems. IEEE Electr. Insul. Mag. 2017, 33, 27–33. [Google Scholar] [CrossRef]
- Zhong, W.H.; Li, B. Polymer Nanocomposites for Dielectrics; Pan Stanford Publishing: Singapore, 2017. [Google Scholar]
- Oliver-Ortega, H.; Tresserras, J.; Julian, F. Nanocomposites materials of PLA reinforces with nanoclays using a masterbatch and its sustainability. Polymers 2021, 13, 2133. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.W. Functionalization of nanocomposite dielectrics. In Proceedings of the IEEE International Symposium on Electrical Insulation (ISEI), San Diego, CA, USA, 6–9 June 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Plesa, I.; Notingher, P.V.; Scholgl, S.; Sumereder, C.; Muhr, M. Properties of polymer composites used in high-voltage applications. Polymers 2016, 8, 173. [Google Scholar] [CrossRef]
- Nalini, R.; Nagarajan, S.; Reddy, B.S.R. Polypropylene-blended organoclay nanocomposites-preparation, characterisation and properties. J. Exp. Nanosci. 2013, 8, 480–492. [Google Scholar] [CrossRef]
- Yang, S.; Qu, J. Computing thermomechanical properties of crosslinked epoxy by molecular dynamic simulations. Polymer 2012, 53, 4806–4817. [Google Scholar] [CrossRef]
- Schlick, T. Molecular Modelling and Simulations: An Interdisciplinary Guide, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Myerson, A.S. Molecular Modeling Applications in Crystallization; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar] [CrossRef]
- Ammala, A.; Pa, S.J.; Lawrence, K.A.; Stark, R.; Webb, R.I.; Hill, A.J. Poly(m-xylne adipamide)-montmorillonite nanocomposites: Effect of organo-modifier structure on free volume and oxygen barrier properties. J. Mater. Chem. 2008, 18, 911–916. [Google Scholar] [CrossRef]
- Scocchi, G.; Posocco, P.; Handgraaf, J.-W.; Fraaije, J.G.E.M.; Fermeglia, M.; Pricl, S. A complete modelling approach for polymer-clay nanocomposites. Chem. A Eur. J. 2009, 15, 7586–7592. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.C.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model. 2016, 22, 47. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Shi, J.; Sun, X.; Shen, Y.; Yan, Y.; Zhang, J.; Liu, B. Supercritical CO2 selective extraction inducing wettability alteration of oil reservoir. J. Supercrit. Fluids 2016, 113, 10–15. [Google Scholar] [CrossRef]
- Abdul Manap, A.H.; Shamsuddin, L.; Mohamed, K. The study of polydimethylsiloxane nanocone distortion in the demolding process using molecular dynamics method. AIP Adv. 2022, 12, 045011. [Google Scholar] [CrossRef]
- Chen, F.; Ren, Y.; He, L.; An, C.; Wen, S.; Shen, F. Molecular dynamics simulation of the interface interaction and mechanical properties of PYX and polymer binder. AIP Adv. 2022, 12, 025307. [Google Scholar] [CrossRef]
- Hong, H.; Song, S.A.; Kim, S.S. Phase transformation of poly (vinylidene fluoride)/TiO2 nanocomposite film prepared by microwave-assisted solvent evaporation: An experimental and molecular dynamics study. Compos. Sci. Technol. 2020, 199, 108375. [Google Scholar] [CrossRef]
- Sun, F.C.; Dongare, A.M.; Asandei, A.D.; Alpay, S.P.; Nakhmanson, S. Temperature dependent structural, elastic, and polar properties of ferroelectric polyvinylidene fluoride (PVDF) and trifluoroethylene (TrFE) copolymers. J. Mater. Chem. 2015, 32, 8389–8396. [Google Scholar] [CrossRef]
- Bohlén, M.; Bolton, K. Molecular dynamics studies of the influence of single wall carbon nanotubes on the mechanical properties of Poly (vinylidene fluoride). Comput. Mater. Sci. 2013, 68, 73–80. [Google Scholar] [CrossRef]
- Erdtman, E.; Satyanarayana, K.C.; Bolton, K. Simulation of α-and β-PVDF melting mechanisms. Polymer 2012, 53, 2919–2926. [Google Scholar] [CrossRef]
- Gee, R.H.; Fried, L.E.; Cook, R.C. Structure of chlorotrifluoroethylene/Vinylidene fluoride random copolymers and homopolymers by molecular dynamics simulations. Macromolecules 2001, 34, 3050–3059. [Google Scholar] [CrossRef]
- Makimura, D.; Kunieda, M.; Liang, Y.; Matsuoka, T.; Takahashi, S.; Okabe, H. Application of molecular simulations to CO2-enhanced oil recovery: Phase equilibria and interfacial phenomena. SPE J. 2013, 18, 319–330. [Google Scholar] [CrossRef]
- Liu, B.; Shi, J.; Wang, M.; Zhang, J.; Sun, B.; Shen, Y.; Sun, X. Reduction on interfacial tension of water oil interface by supercritical CO2 in enhanced oil recovery process studied with molecular dynamics simulation. J. Supercrit. Fluid 2016, 111, 171–178. [Google Scholar] [CrossRef]
- Md Azmi, N.S.; Rosli, N.R.; Tengku Mohd, T.A.; Tan, H.L.; Abu Bakar, N.F. Diffusion coefficient and interfacial tension with addition of silica nanoparticles in CO2-surfactant-water-hexane for enhanced oil recovery (EOR) using molecular dynamics simulation. Key Eng. Mater. 2019, 797, 375–384. [Google Scholar] [CrossRef]
- Hua, X.; Wang, L.; Yang, S. Molecular dynamics simulation of improving the physical properties of polytetrafluoroethylene cable insulation materials by boron nitride nanoparticle under moisture-temperature-electric fields conditions. Polymers 2019, 11, 971. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wen, H.; Wu, Y.J. Computational thermomechanical properties of silica-epoxy nanocomposites by molecular modelling simulation. Polymers 2017, 9, 430. [Google Scholar] [CrossRef]
- Doroudiani, S.; Park, C.B.; Kortschot, M.T. Effect of the crystallinity and morphology on the microcellular foam structure of semicrystalline polymers. Polym. Eng. Sci. 2013, 36, 2645–2662. [Google Scholar] [CrossRef]
- Tariq, Z.; Butt, F.K.; Rehman, S.U.; Ul Haq, B.; Aleem, F.; Li, C. First principles study of electronic and optical properties of sulfur doped tin monoxide: A potential applicant for optoelectronic devices. Ceram. Int. 2019, 45, 7495–7503. [Google Scholar] [CrossRef]
- Raabe, G.; Sadus, R.J. Molecular dynamics simulation of the dielectric constant of water: The effect of bond flexibility. J. Chem. Phys. 2011, 134, 234501. [Google Scholar] [CrossRef]
- Ahmad, Z. Polymeric dielectric materials. In Dielectric Material; Intech Open: London, UK, 2012; pp. 3–26. [Google Scholar] [CrossRef]
- Thakur, V.K. Recycled Polymers: Properties and Applications; Smithers Rapra Technology: Shrewsbury, UK, 2015; Volume 2. [Google Scholar]
- Yildirim, E.; Yurtsever, M.; Eriman, B.; Uyanik, N. Experimental and MD simulation study on the physical and mechanical properties of organically modified montmorillonite clate and compatibilized linear low density polyethylene nanocomposites. J. Appl. Polym. Sci. 2017, 135, 5817. [Google Scholar] [CrossRef]
- Firdaus, M.Y.; Octaviani, H.; Herlini, H.; Fatimah, N.; Mulyaningsih, T.; Fairuuz, Z.; Nandiyanto, A.B.D. Review: The comparison of clay modifier (cloisite types) in various epoxy-clay nanocomposite synthesis methods. Mediterr. J. Chem. 2021, 11, 54–74. [Google Scholar] [CrossRef]
- Shi, H.; Xu, C.; Hu, X.; Gan, W.; Wu, K.; Wang, X. Improving the Young’s modulus of Mg via alloying and compositing—A short review. J. Magnes. Alloy. 2022, 10, 2009–2024. [Google Scholar] [CrossRef]
- Jones, D.R.H.; Ashby, M.F. Elastic moduli. Eng. Mater. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Hong, J.S.; Kim, Y.K.; Ahn, K.H.; Lee, S.J.; Kim, C.Y. Interfacial tension reduction in PBT/PE/Clay nanocomposites. Rheol. Acta 2007, 46, 469–478. [Google Scholar] [CrossRef]




| Sample | Organoclay (wt%) |
|---|---|
| PE_0 | 0.0 |
| PE_0.5 | 0.5 |
| PE_1.0 | 1.0 |
| PE_2.0 | 2.0 |
| PE_2.5 | 2.5 |
| PE_5.0 | 5.0 |
| PE_10 | 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharudin, R.W.; Md Azmi, N.S.; Hanizan, A.; Akhbar, S.; Ahmad, Z.; Ohshima, M. Dynamic Molecular Simulation of Polyethylene/Organoclay Nanocomposites for Their Physical Properties and Foam Morphology. Materials 2023, 16, 3122. https://doi.org/10.3390/ma16083122
Sharudin RW, Md Azmi NS, Hanizan A, Akhbar S, Ahmad Z, Ohshima M. Dynamic Molecular Simulation of Polyethylene/Organoclay Nanocomposites for Their Physical Properties and Foam Morphology. Materials. 2023; 16(8):3122. https://doi.org/10.3390/ma16083122
Chicago/Turabian StyleSharudin, Rahida Wati, Nik Salwani Md Azmi, Anuaruddin Hanizan, Suffiyana Akhbar, Zakiah Ahmad, and Masahiro Ohshima. 2023. "Dynamic Molecular Simulation of Polyethylene/Organoclay Nanocomposites for Their Physical Properties and Foam Morphology" Materials 16, no. 8: 3122. https://doi.org/10.3390/ma16083122
APA StyleSharudin, R. W., Md Azmi, N. S., Hanizan, A., Akhbar, S., Ahmad, Z., & Ohshima, M. (2023). Dynamic Molecular Simulation of Polyethylene/Organoclay Nanocomposites for Their Physical Properties and Foam Morphology. Materials, 16(8), 3122. https://doi.org/10.3390/ma16083122







