The Effect of Dopants on Structure Formation and Properties of Cast SHS Alloys Based on Nickel Monoaluminide
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- Cast alloys based on NiAl-Cr-Co (base+) with complex dopants added (base+2.5Mo-0.5Re-0.5Ta, base+2.5Mo-1.5Re-1.5Ta, base+2.5Mo-1.5Ta-1.5La-0.5Ru, base+2.5Mo-1.5Re-1.5Ta-0.2Ti, and base+2.5Mo-1.5Re-1.5Ta-0.2Zr) were fabricated by centrifugal SHS metallurgy.
- The chemical composition was found to be consistent with the calculated one. The total content of impurity elements was 0.15 ± 0.02 wt.% and lay within the acceptance region. Due to binding of dissolved oxygen and nitrogen to form oxides and nitrides, doping with Ti and Ru reduced the negative role of gas impurities and enhanced high-temperature oxidation resistance of the alloy.
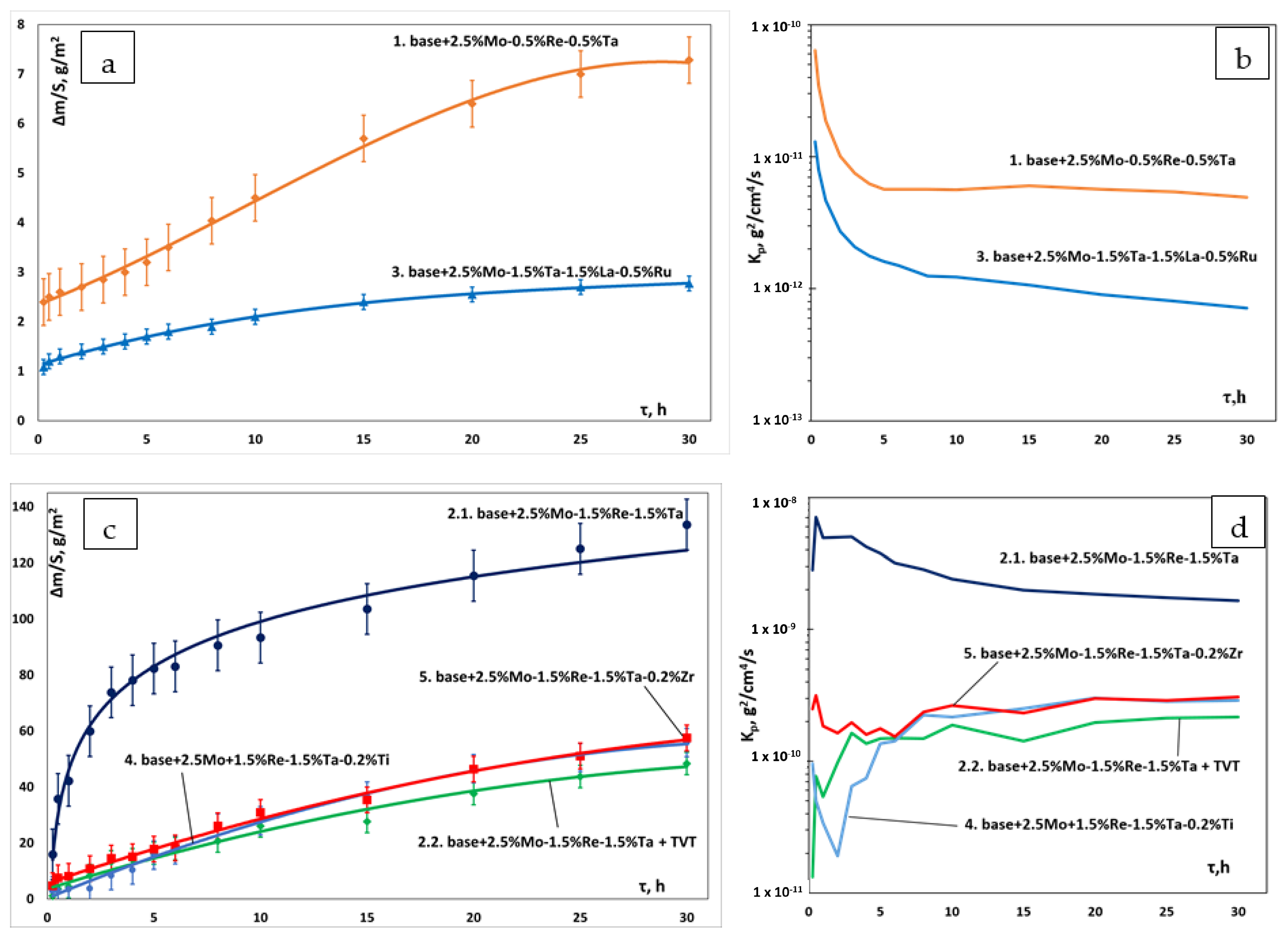
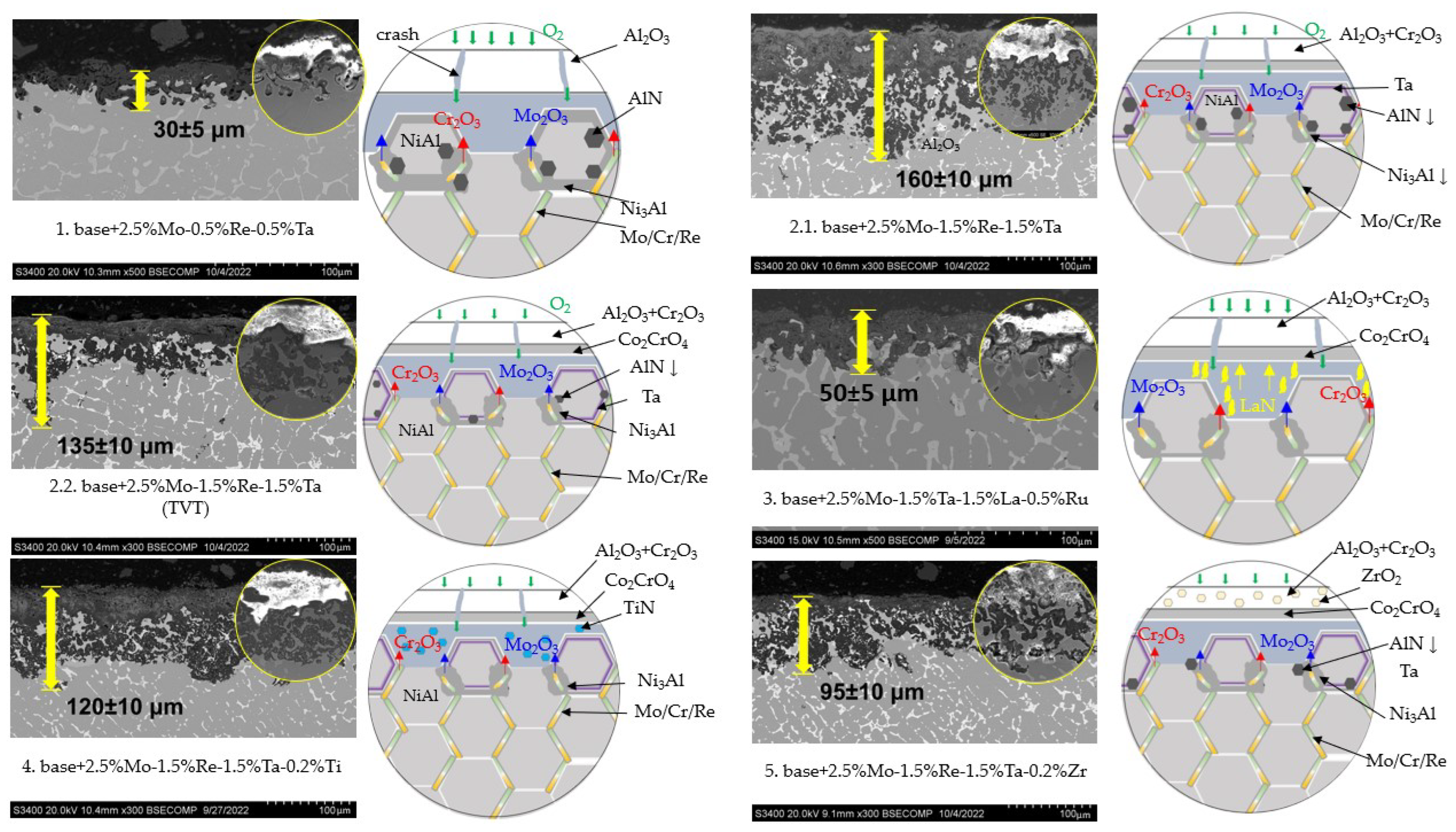
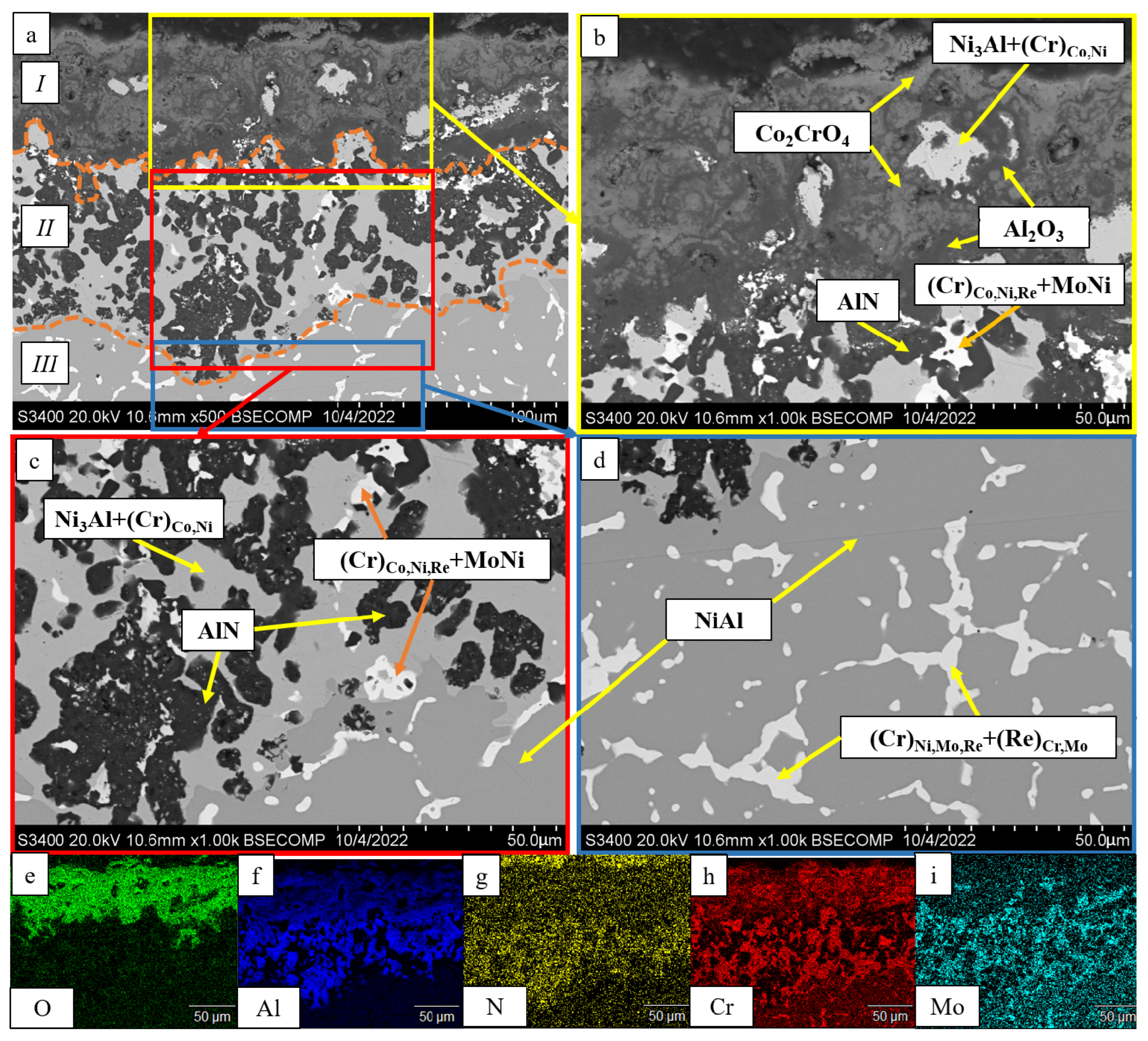
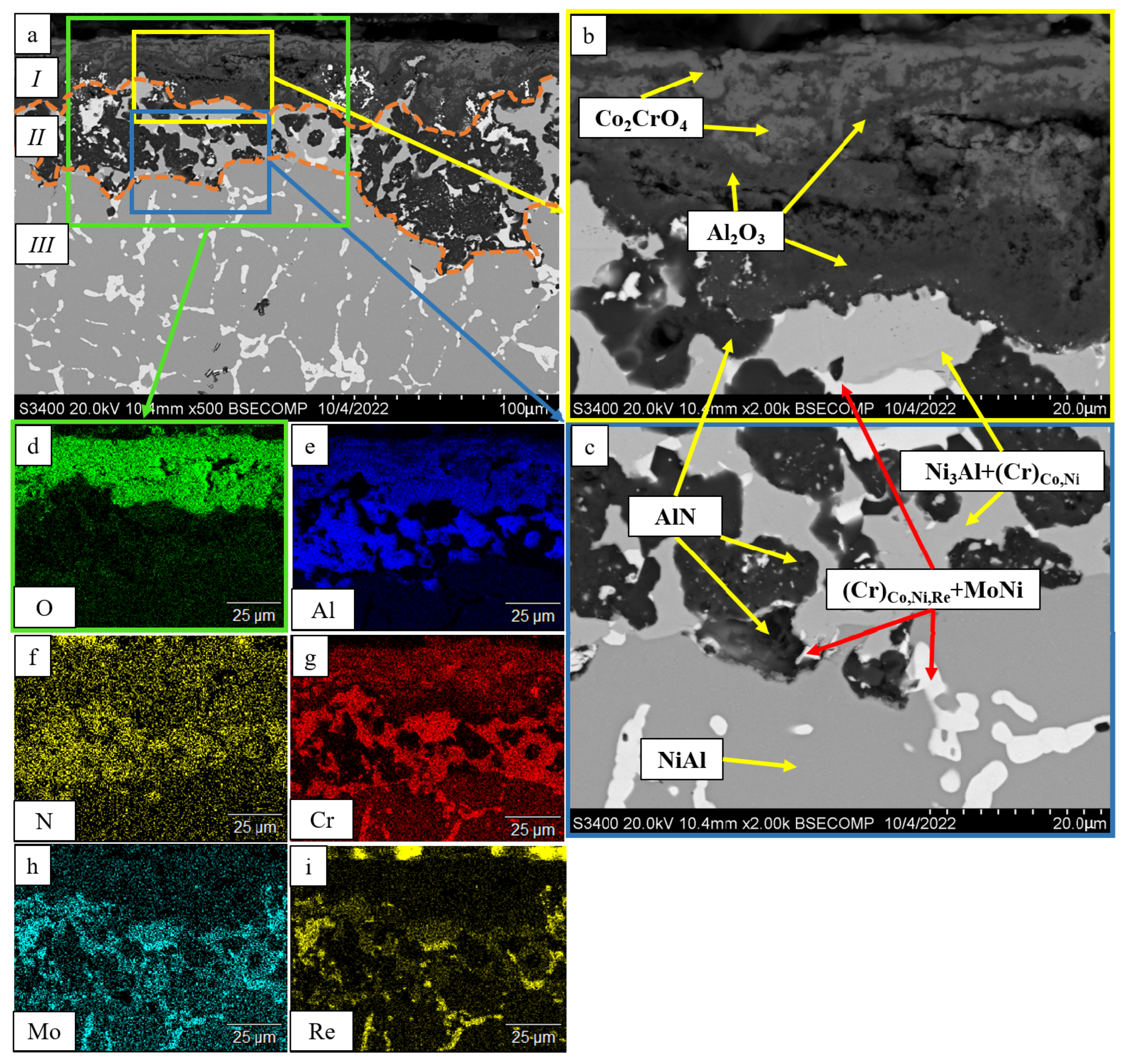
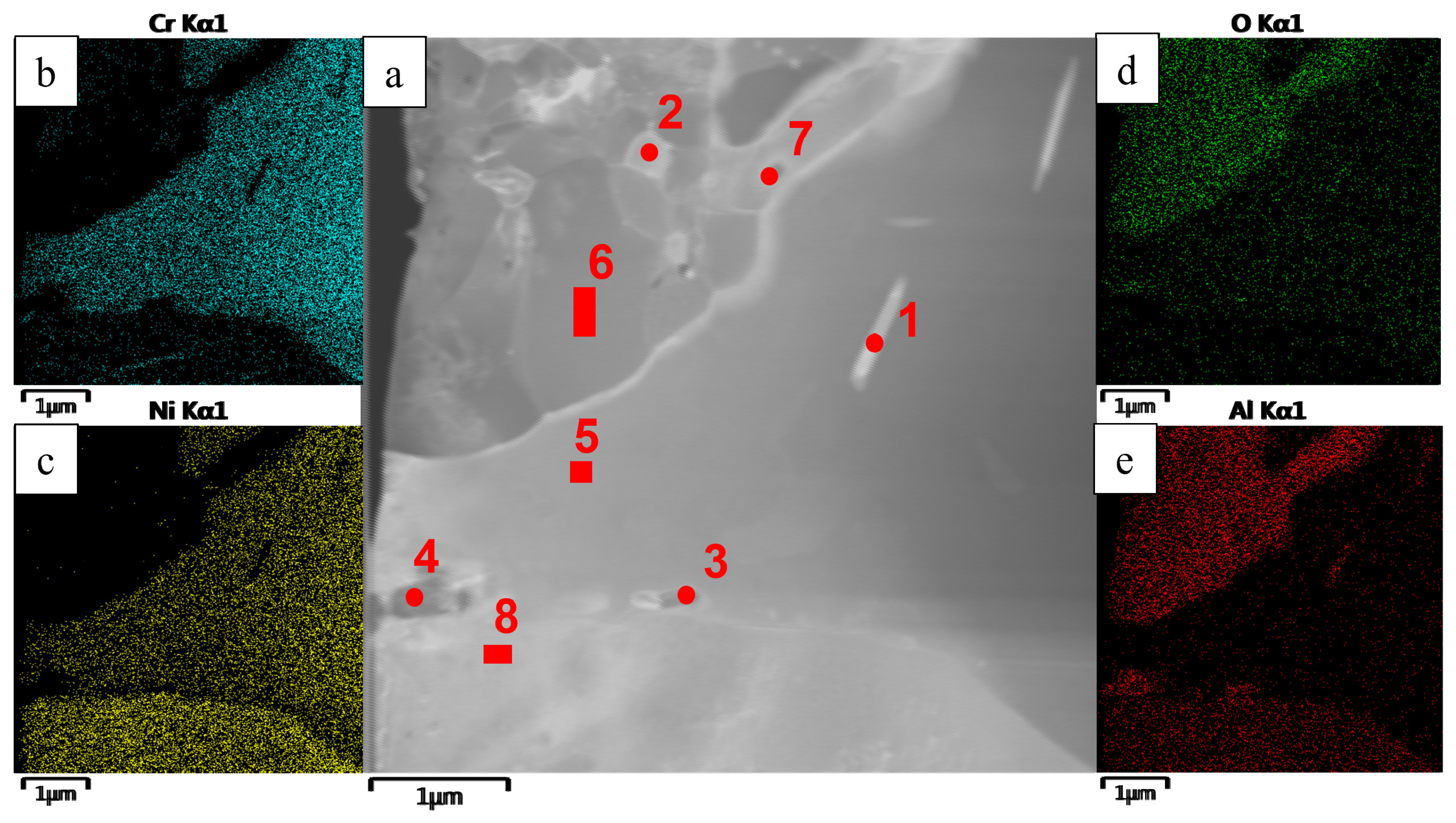
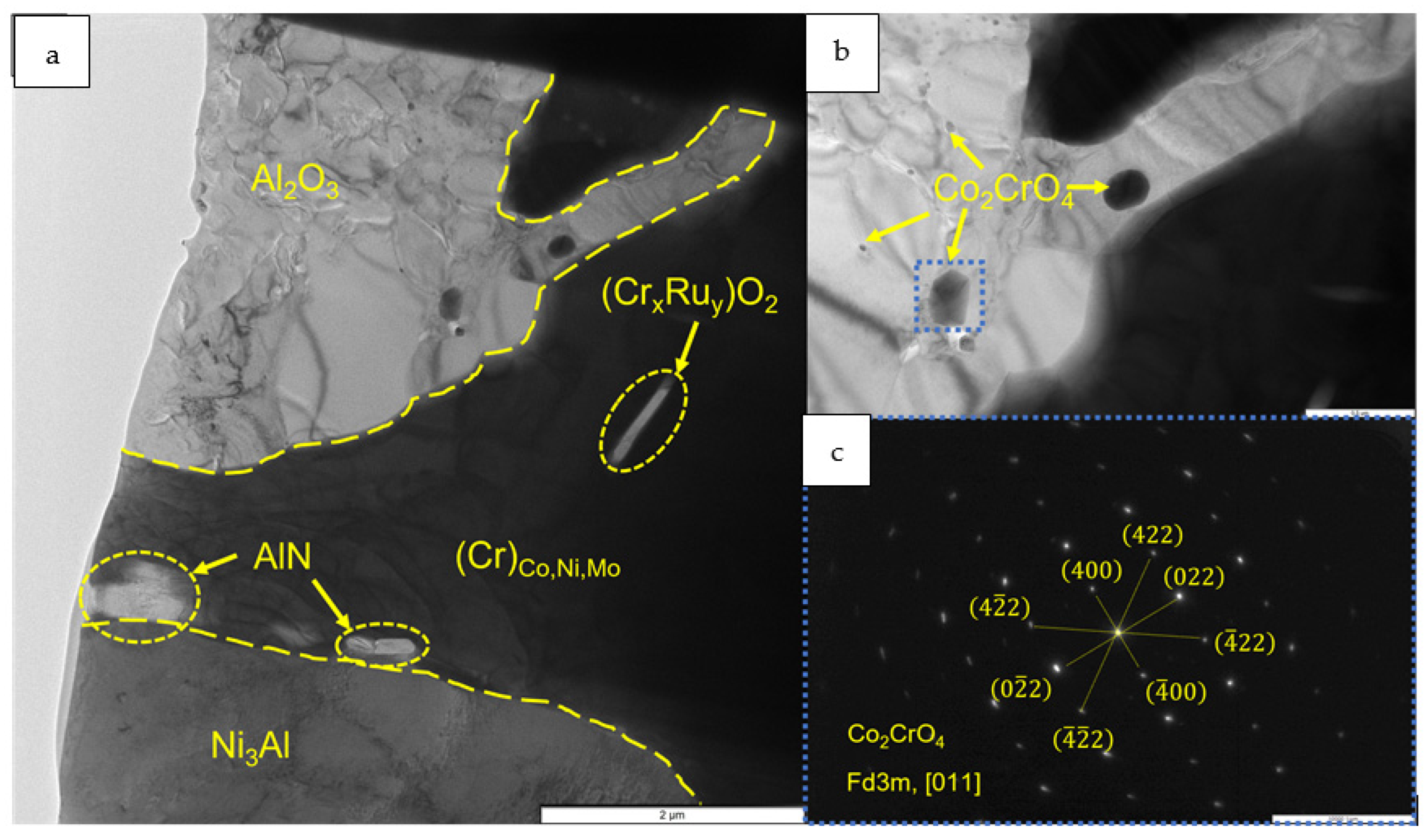
- The kinetics and the mechanism of oxidation of alloys at T = 1150 °C were studied; the kinetic regression equations describing the oxidation law were plotted. Al2O3 and Co2CrO4 are the major phases in the oxidized layer. Three layers were found to be formed: I—the continuous Al2O3 layer with Co2CrO4 inclusions; II—the transitional MeN-Me layer with AlN inclusions; and III—the metallic layer with local AlN inclusions.
- The positive effect of vacuum pre-annealing of ingots on their high-temperature oxidation resistance was observed for the base+2.5Mo-1.5Re-1.5Ta+(TVT) alloy as an example. The total weight gain of the annealed samples after the tests decreased threefold: from 120 ± 5 g/m2 to 40 ± 5 g/m2.
- Phases containing ruthenium and titanium, which reduce the content of gas impurities in the base+ 2.5Mo-1.5Ta-1.5La-0.5Ru alloy to the value ∑O,N = 0.0145 wt.% and the base+2.5Mo-1.5Re-1.5Ta-0.2Ti alloy to the value ∑O,N = 0.0223 wt.%, were identified by TEM.
- The NiAl-12Cr-6Co-2.5Mo-1.5Re-1.5Ta-0.2Ti alloy was found to have the optimal composition in this experimental series; it was characterized by strength properties σucs = 1644 ± 30 MPa, σys = 1518 ± 25 MPa and the total weight gain after oxidation of 52 g/m2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Logunov, A.V. Heat-Resistant Nickel-Based Alloys for Blades and Discs of Gas Turbines; Gas Turbine Technologies: Rybinsk, Russia, 2017; p. 854. [Google Scholar]
- Wang, Y.; Guo, H.B.; Peng, H.; Peng, L.Q.; Gong, S.K. Diffusion barrier behaviors of (Ru,Ni)Al/NiAl coatings on Ni-based superalloy substrate. Intermetallics 2011, 19, 191–195. [Google Scholar] [CrossRef]
- Wang, D.; Liang, Y.; Ning, H.; Wang, B. Effects of Zr and Co on the microstructure and mechanical properties of NiAl-based alloys. J. Alloys Compd. 2021, 883, 160815. [Google Scholar] [CrossRef]
- Sui, X.; Lu, J.; Wei, D.; Zhang, L.; Wang, R.; Zhao, W.; Zhang, W. Unveiling the influence of TiN on the microstructure and high-temperature oxidation behavior of Ti-Al-Cr composite coating. Corros. Sci. 2022, 206, 110539. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, G.; Hu, W.; Gottstein, G.; Bogner, S.; Bührig-Polaczek, A. Tensile creep of directionally solidified NiAl–9Mo in situ composites. Acta Mater. 2013, 61, 7155–7165. [Google Scholar] [CrossRef]
- Seemüller, C.; Heilmaier, M.; Haenschke, T.; Bei, H.; Dlouhy, A.; George, E.P. Influence of fiber alignment on creep in directionally solidified NiAl–10Mo in-situ composites. Intermetallics 2013, 35, 110–115. [Google Scholar] [CrossRef]
- Bei, H.; George, E.P. Microstructures and mechanical properties of a directionally solidified NiAl–Mo eutectic alloy. Acta Mater. 2005, 53, 69–77. [Google Scholar] [CrossRef]
- Shang, Z.; Shen, J.; Wang, L.; Du, Y.; Xiong, Y.; Fu, H. Investigations on the microstructure and room temperature fracture toughness of directionally solidified NiAl-Cr(Mo) eutectic alloy. Intermetallics 2015, 57, 25–33. [Google Scholar] [CrossRef]
- Walter, J.L.; Cline, H.E. The effect of solidification rate on structure and high-temperature strength of the eutectic NiAl-Cr Metall. Mater. Tran. B 1970, 1, 1221–1229. [Google Scholar] [CrossRef]
- Cui, C.Y.; Chen, Y.X.; Guo, J.T.; Li, D.X.; Ye, H.Q. Preliminary investigation of directionally solidified NiAl–28Cr–5.5Mo–0.5Hf composite. Mater. Lett. 2000, 43, 303–308. [Google Scholar] [CrossRef]
- Voitovich, R.F.; Golovko, E.I. High-Temperature Oxidation of Metals and Alloys; Naukova Dumka: Kyiv, Ukraine, 1980; p. 296. [Google Scholar]
- Klumpes, R.; Maree, C.H.M.; Schramm, E.; de Wit, J.H.W. The influence of chromium on the oxidation of ß-NiAl at 1000 °C. Mater. Corros. 1996, 47, 619–624. [Google Scholar] [CrossRef]
- Johnson, D.R.; Chen, X.F.; Oliver, B.F. Processing and mechanical properties of in-situ composites from the NiAlCr and the NiAl(Cr,Mo) eutectic systems. Intermetallics 1995, 3, 99–113. [Google Scholar] [CrossRef]
- Yang, J.C.; Schumann, E.; Levin, I.; Rühle, M. Transient oxidation of NiAl. Acta Mater. 1998, 46, 2195–2201. [Google Scholar] [CrossRef]
- Grabke, H. Oxidation of NiAl and FeAl. Intermetallics 1999, 7, 1153–1158. [Google Scholar] [CrossRef]
- Gao, W.; Li, Z.; Wu, Z.; Li, S.; He, Y. Oxidation behavior of Ni3Al and FeAl intermetallics under low oxygen partial pressures. Intermetallics 2002, 10, 263–270. [Google Scholar] [CrossRef]
- Bo, L.; Fei, L.; Cong, L.; Yimin, G.; Congmin, F.; Xiaohu, H. Effect of Cr element on the microstructure and oxidation resistance of novel NiAl-based high temperature lubricating composites. Corros. Sci. 2021, 188, 109554. [Google Scholar] [CrossRef]
- Ghoussoub, J.N.; Utada, S.; Pedraza, F.; Dick-Cleland, W.J.B.; Tang, Y.T.; Reed, R.C. Alloy Design for Additive Manufacturing: Early-Stage Oxidation of Nickel-Based Superalloys. Met. Mater. Trans. A. 2023, 54, 1721–1729. [Google Scholar] [CrossRef]
- Taylor, C.D.; Tossey, B.M. High temperature oxidation of corrosion resistant alloys from machine learning. NPJ Mater. Degrad. 2021, 5, 38. [Google Scholar] [CrossRef]
- Kurbatkina, V.V. Nickel Aluminides, in: Concise Encycl. In Self-Propagating High-Temperature Synthesis; Elsevier: Amsterdam, The Netherlands, 2017; pp. 212–213. [Google Scholar] [CrossRef]
- Kurbatkina, V.V.; Patsera, E.I.; Levashov, E.A.; Kaplanskii, Y.Y.; Samokhin, A.V. Fabrication of Narrow-Fraction Micropowders of NiAl-Based Refractory Alloy CompoNiAl-M5-3. Int. J. Self-Propagating High-Temp. Synth. 2018, 27, 236–244. [Google Scholar] [CrossRef]
- Tsvetkov, Y.V.; Samokhin, A.V.; Alekseev, N.V.; Fadeev, A.A.; Sinaiskii, M.A.; Levashov, E.A.; Kaplanskii, Y.Y. Plasma Spheroidization of Micropowders of a Heat-Resistant Alloy Based on Nickel Monoaluminide. Dokl. Chem. 2018, 483, 312–317. [Google Scholar] [CrossRef]
- Kaplanscky, Y.Y.; Levashov, E.A.; Korotitskiy, A.V.; Loginov, P.A.; Sentyurina, Z.A.; Mazalov, A.B. Influence of aging and HIP treatment on the structure and properties of NiAl-based turbine blades manufactured by laser powder bed fusion. Addit. Manuf. 2020, 31, 100999. [Google Scholar] [CrossRef]
- Sanin, V.; Andreev, D.; Ikornikov, D.; Yukhvid, V. Cast Intermetallic Alloys and Composites Based on Them by Combined Centrifugal Casting—SHS Process. Open J. Metal. 2013, 3, 12–24. [Google Scholar] [CrossRef]
- Sanin, V.V.; Filonov, M.R.; Yukhvid, V.I.; Anikin, Y.A.; Mikhailov, A.M. Investigation into the influence of the remelting temperature on the structural heredity of alloys fabricated by centrifugal SHS metallurgy. Russ. J. Non-Ferr. Met. 2016, 57, 124–130. [Google Scholar] [CrossRef]
- Zaitsev, A.A.; Sentyurina, Z.A.; Levashov, E.A.; Pogozhev, Y.S.; Sanin, V.N.; Loginov, P.A.; Petrzhik, M.I. Structure and properties of NiAl-Cr(Co,Hf) alloys prepared by centrifugal SHS casting. Part 1—Room temperature investigations. Mater. Sci. Eng. A 2017, 690, 463–472. [Google Scholar] [CrossRef]
- Zaitsev, A.A.; Sentyurina, Z.A.; Levashov, E.A.; Pogozhev, Y.S.; Sanin, V.N.; Sidorenko, D.A. Structure and properties of NiAl-Cr(Co,Hf) alloys prepared by centrifugal SHS casting followed by vacuum induction remelting. Part 2—Evolution of the structure and mechanical behavior at high temperature. Mater. Sci. Eng. A 2017, 690, 473–481. [Google Scholar] [CrossRef]
- Kaplanskii, Y.Y.; Zaitsev, A.A.; Sentyurina, Z.A.; Levashov, E.A.; Pogozhev, Y.S.; Loginov, P.A.; Logacheva, A.I. The Structure and Properties of Pre-Alloyed NiAl-Cr(Co,Hf) Spherical Powders Produced by Plasma Rotating Electrode Processing for Additive Manufacturing. J. Mater. Res. Technol. 2018, 7, 461–468. [Google Scholar] [CrossRef]
- Kaplanskii, Y.Y.; Zaitsev, A.A.; Levashov, E.A.; Loginov, P.A.; Sentyurina, Z.A. NiAl based alloy produced by HIP and SLM of pre-alloyed spherical powders. Evolution of the structure and mechanical behavior at high temperatures. Mater. Sci. Eng. A 2018, 717, 48–59. [Google Scholar] [CrossRef]
- Kaplanskii, Y.Y.; Levashov, E.A.; Bashkirov, E.A.; Korotitskiy, A.V. Effect of molybdenum on structural evolution and thermomechanical behavior of a heat-resistant nickel aluminide-based alloy. J. Alloys Compd. 2021, 892, 162247. [Google Scholar] [CrossRef]
- Sanin, V.V.; Kaplansky, Y.Y.; Aheiev, M.I.; Levashov, E.A.; Petrzhik, M.I.; Bychkova, M.Y.; Samokhin, A.V.; Fadeev, A.A.; Sanin, V.N. Structure and Properties of Heat-Resistant Alloys NiAl–Cr–Co–X (X = La, Mo, Zr, Ta, Re) and Fabrication of Powders for Additive Manufacturing. Materials 2021, 14, 3144. [Google Scholar] [CrossRef]
- Aheiev, M.I.; Sanin, V.V.; Shvindina, N.V.; Kaplanskii, Y.Y.; Levashov, E.A. Oxidation kinetics and mechanism of nickel alloys. Izv. Vuzov. Poroshkovaya Metall. I Funktsional’nye Pokrytiya (Powder Metall. Funct. Coat.) 2022, 16, 4–23. (In Russian) [Google Scholar] [CrossRef]
- Peng, X.; Li, W.; Fu, L.B.; Li, Y.T.; Jiang, S.M.; Gong, J.; Sun, C. Role of Re in NiAl bond coating on isothermal oxidation behavior of a thermal barrier coating system at 1100 °C. Corros. Sci. 2023, 218, 111151. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, X.; Jia, P.; Cai, C.; Huang, J.; Zhou, G. One-dimensional γ-Al2O3 growth from the oxidation of NiAl. Corros. Sci. 2023, 216, 11109. [Google Scholar] [CrossRef]
- Zhang, W.L.; Li, S.M.; Fu, L.B.; Li, W.; Sun, J.; Wang, T.G.; Jiang, S.M.; Gong, J.; Sun, C. Preparation and cyclic oxidation resistance of Hf-doped NiAl coating. Corros. Sci. 2022, 195, 110014. [Google Scholar] [CrossRef]
- Kovalev, A.I.; Wainstein, D.L.; Rashkovskiy, A.Y. Influence of Al grain boundaries segregations and La-doping on embrittlement of intermetallic NiAl. Appl. Surf. Sci. 2015, 254, 323–327. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, Y.; Zou, Z.; Luo, L.; Zhao, X.; Guo, F.; Xiao, P. Effect of alloyed Lu, Hf and Cr on the oxidation and spallation behavior of NiAl. Corros. Sci. 2017, 126, 334–343. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, Z.; Li, X.; Shen, S.; Ye, F. Effects of vacuum pre-oxidation process on thermally-grown oxides layer of CoCrAlY high temperature corrosion resistance coating. Trans. Nonferrous Met. Soc. China 2015, 25, 3305–3314. [Google Scholar] [CrossRef]
- Levashov, E.A.; Mukasyan, A.S.; Rogachev, A.S.; Shtansky, D.V. Self-Propagating High-Temperature Synthesis of Advanced Materials and Coatings. Int. Mater. Rev. 2017, 62, 203–239. [Google Scholar] [CrossRef]
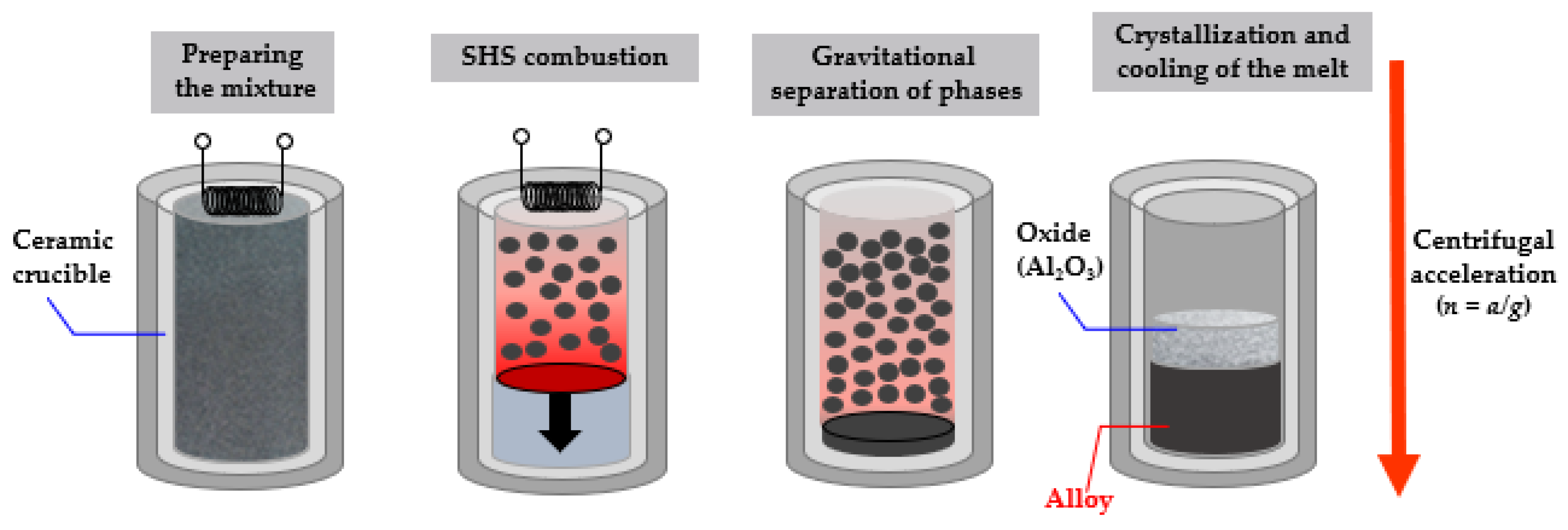
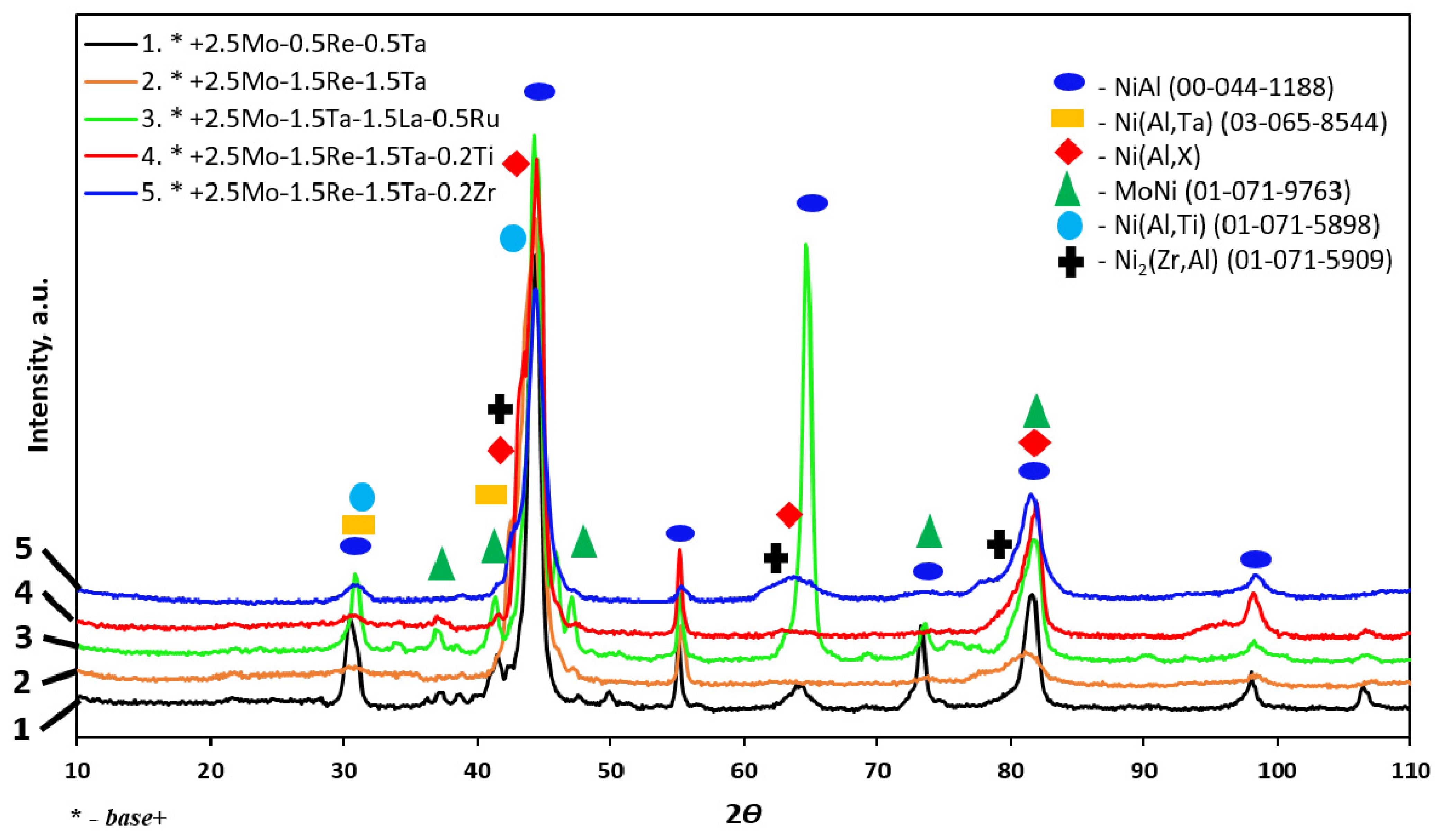
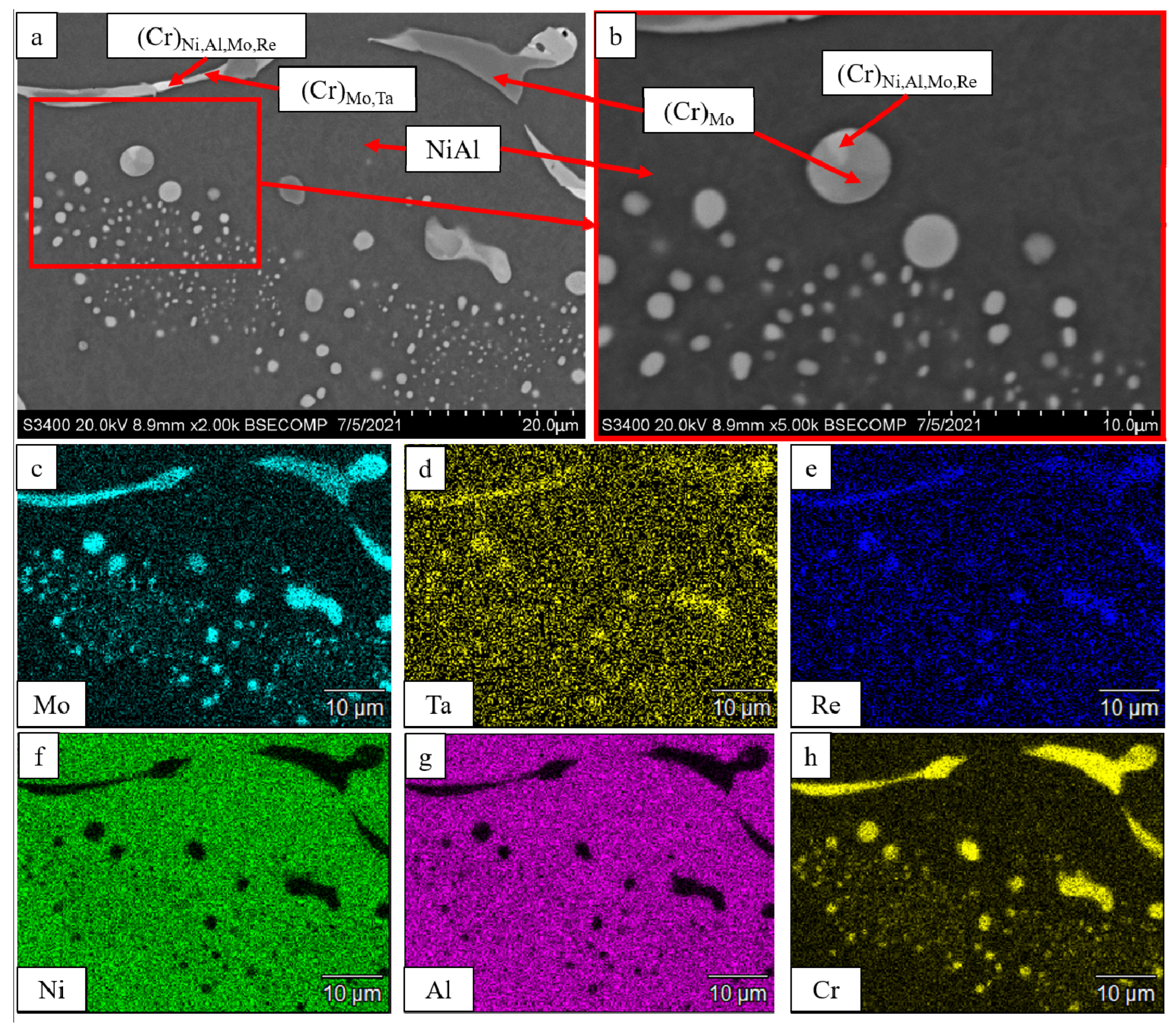
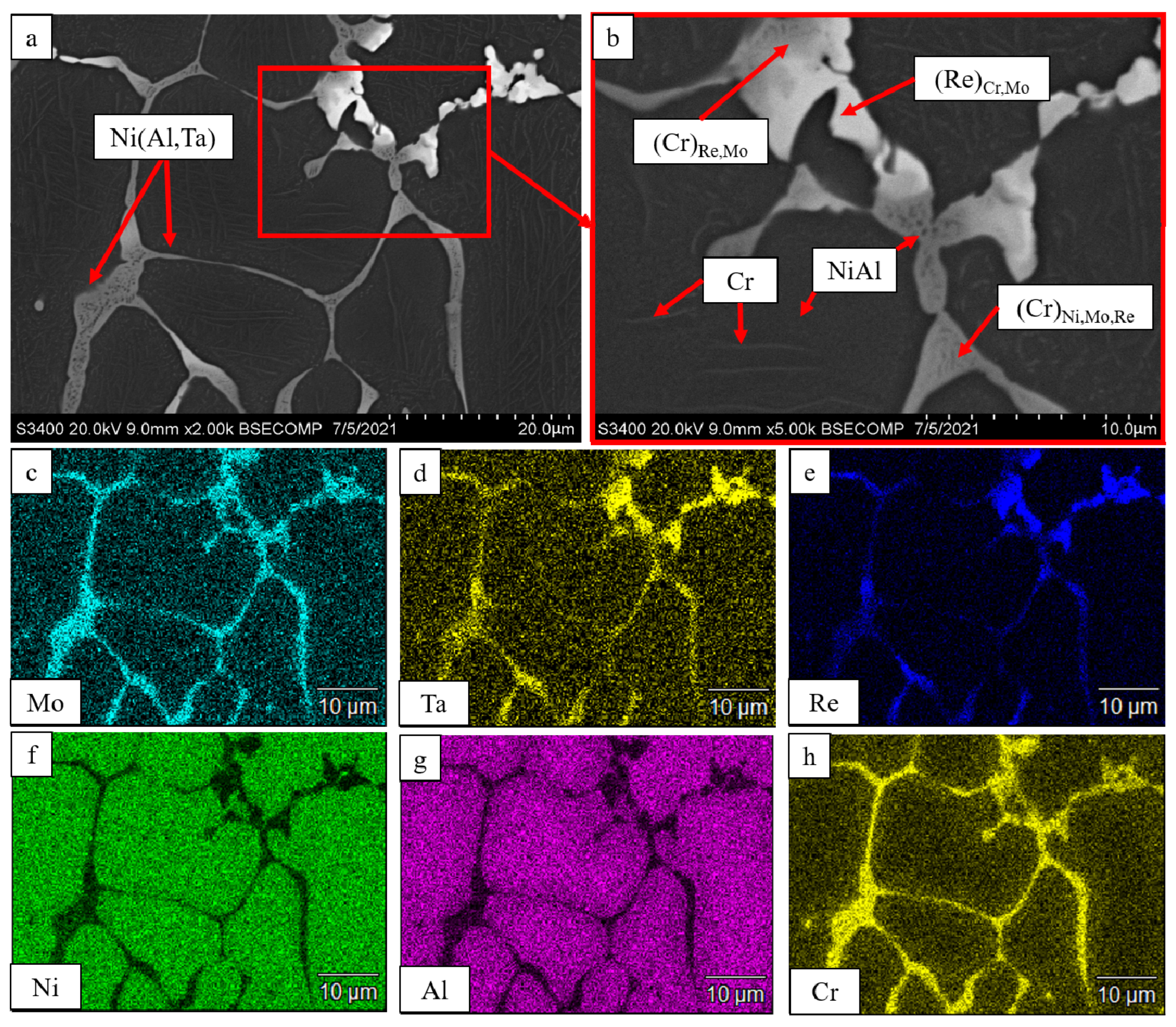
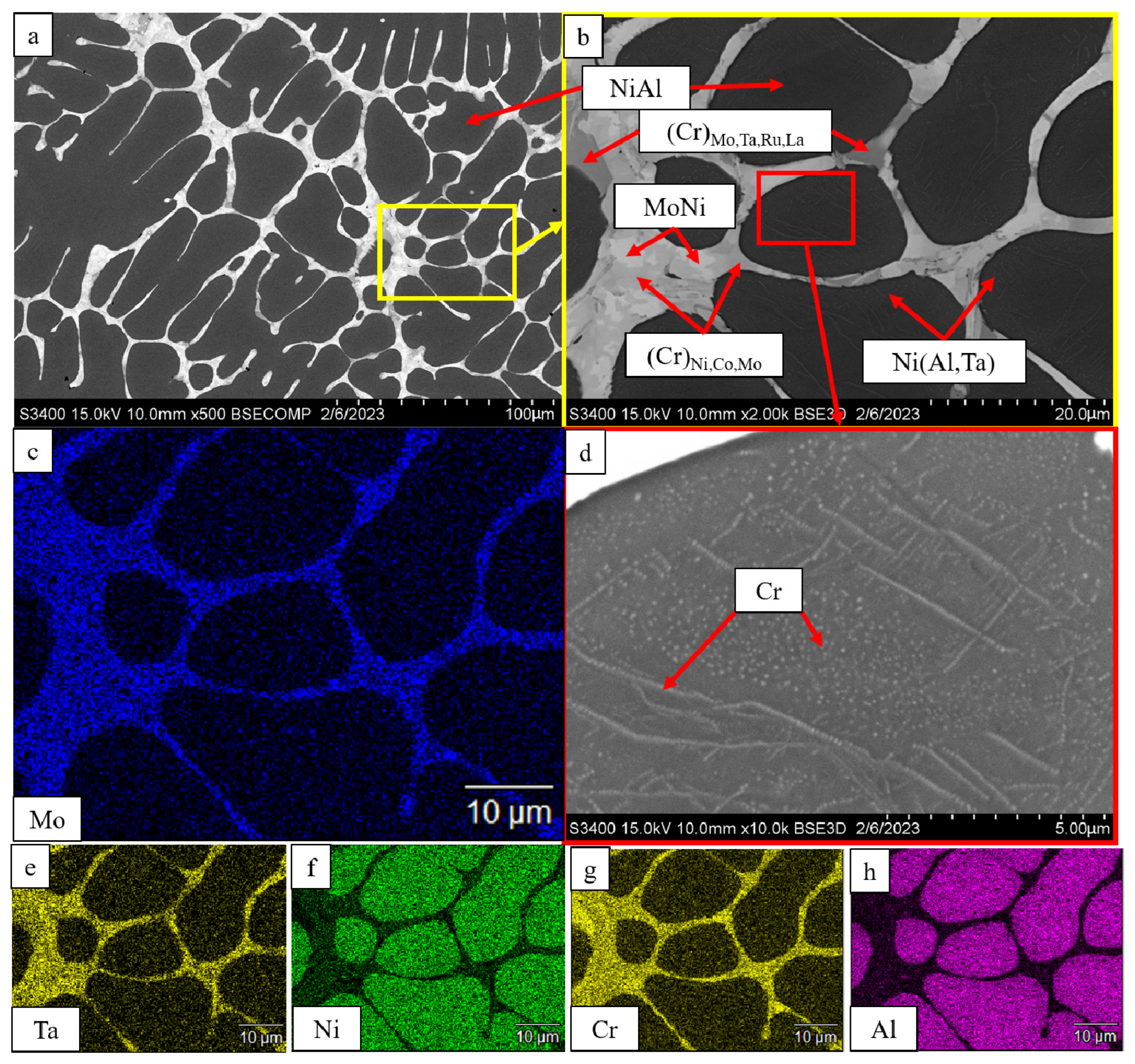
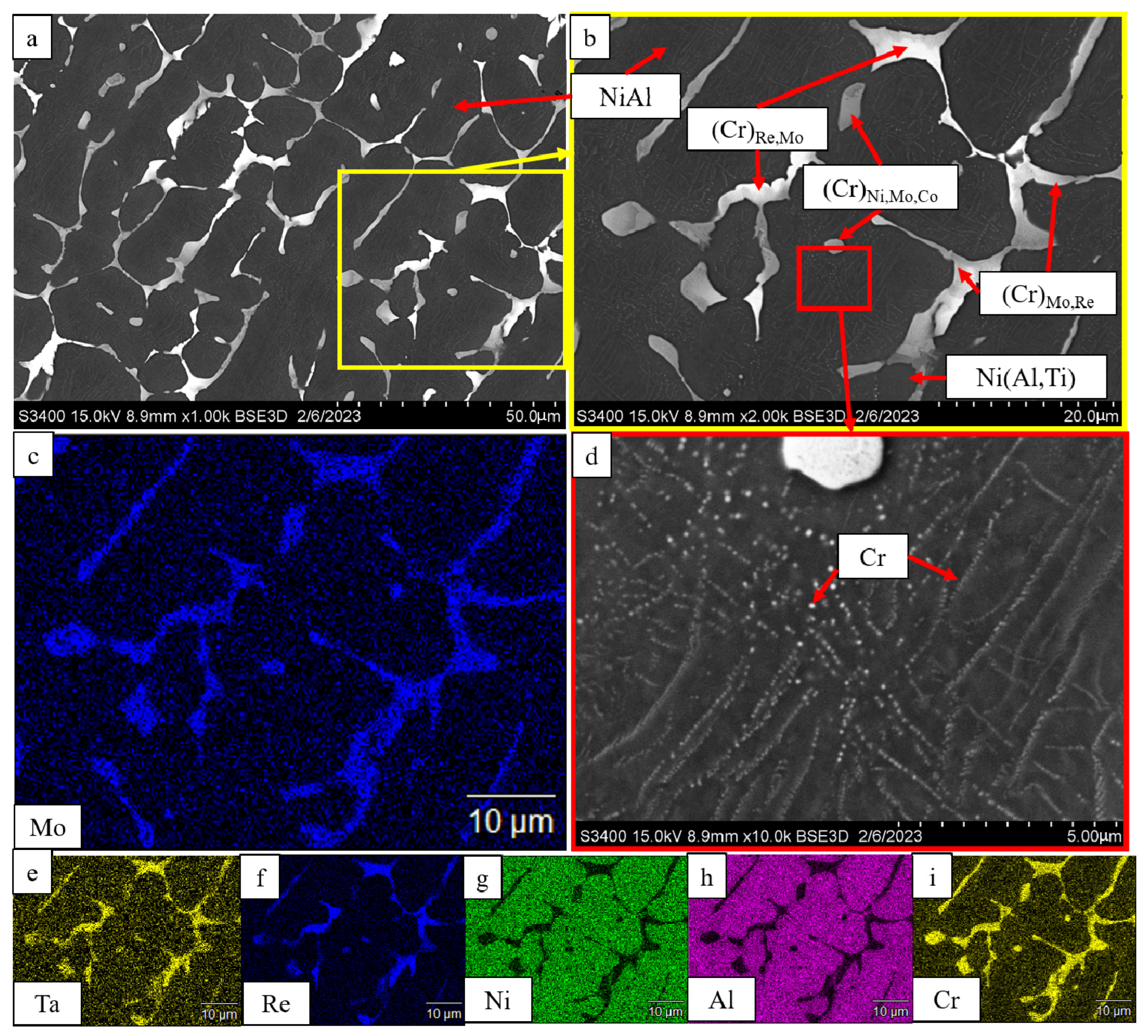
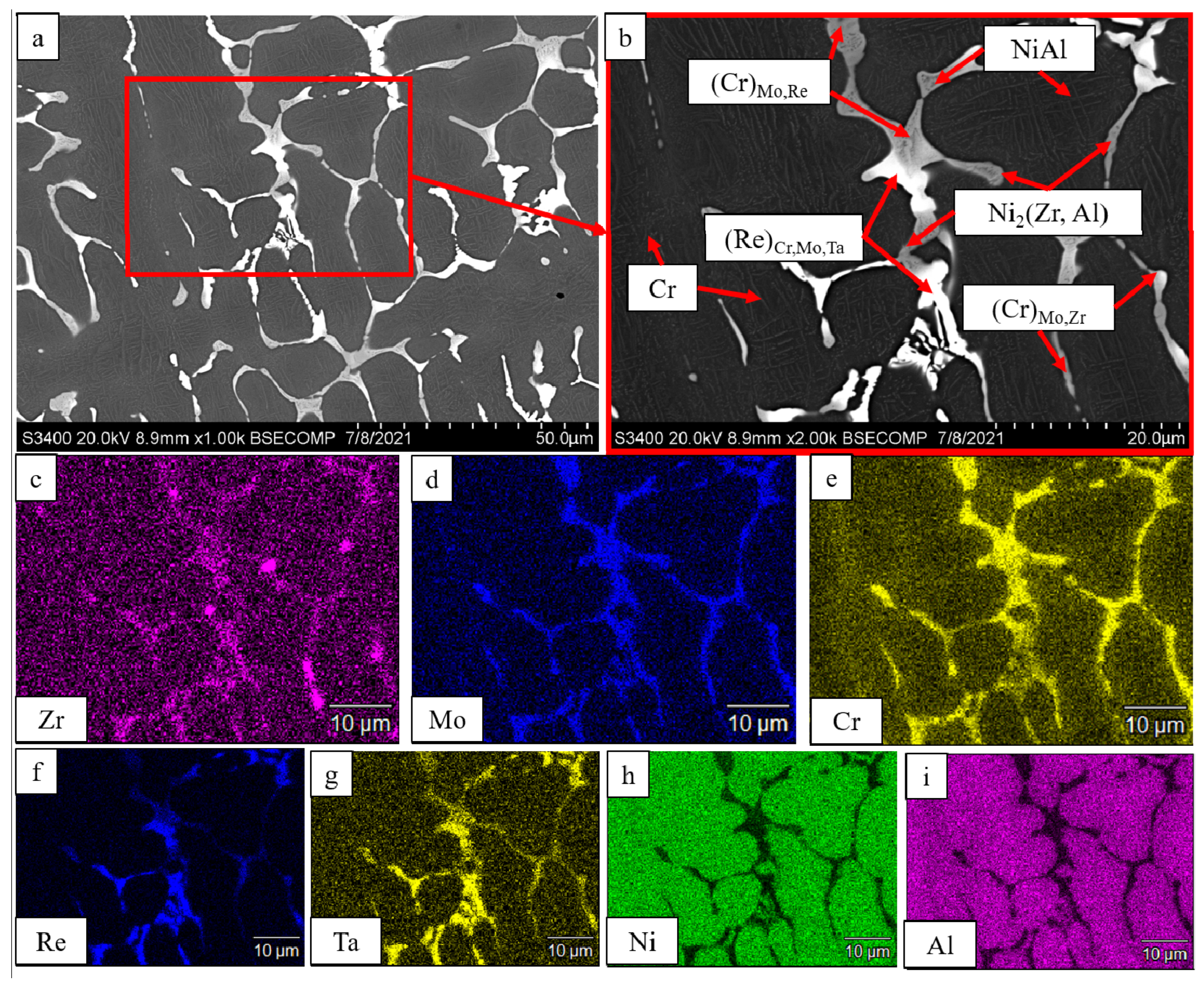
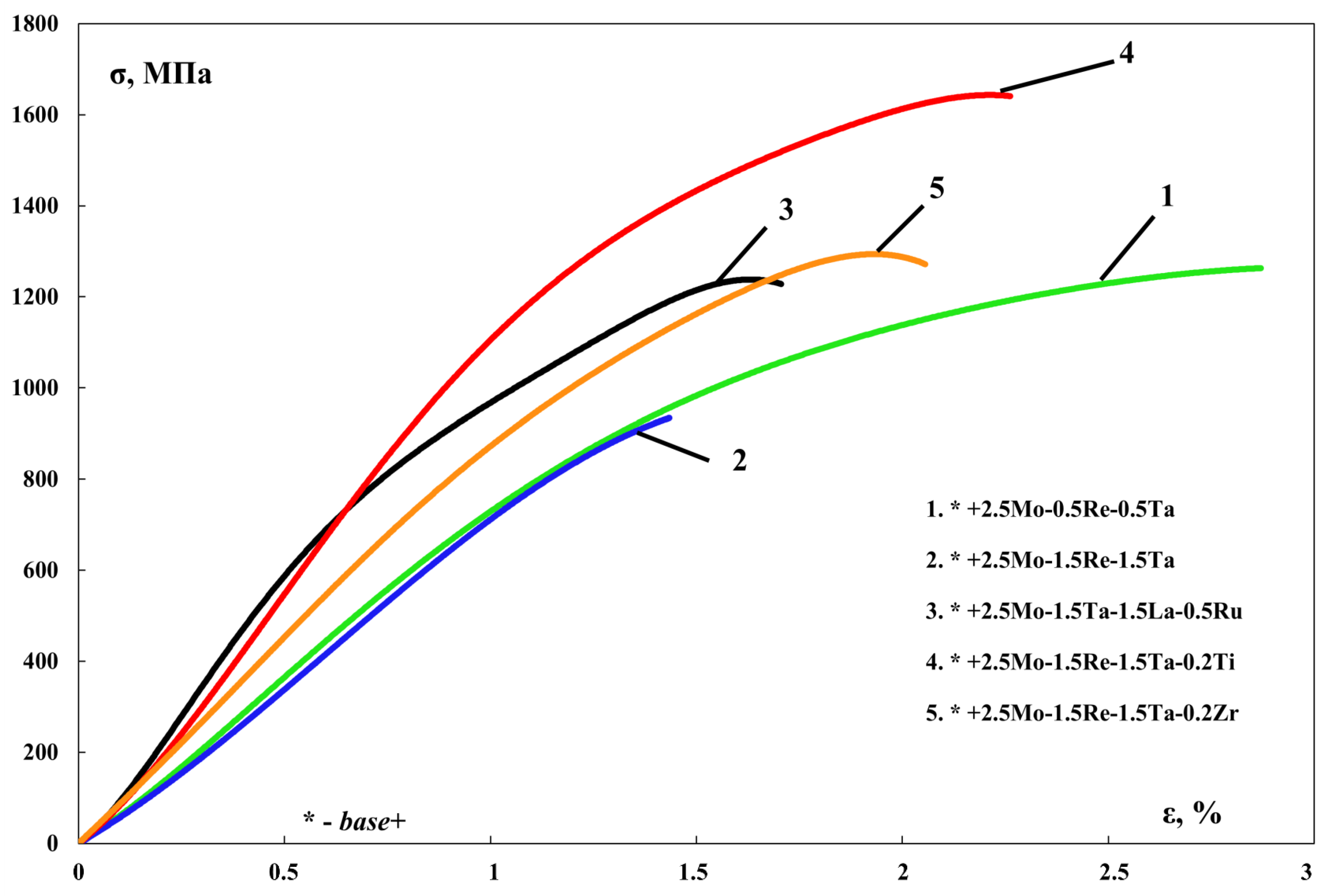


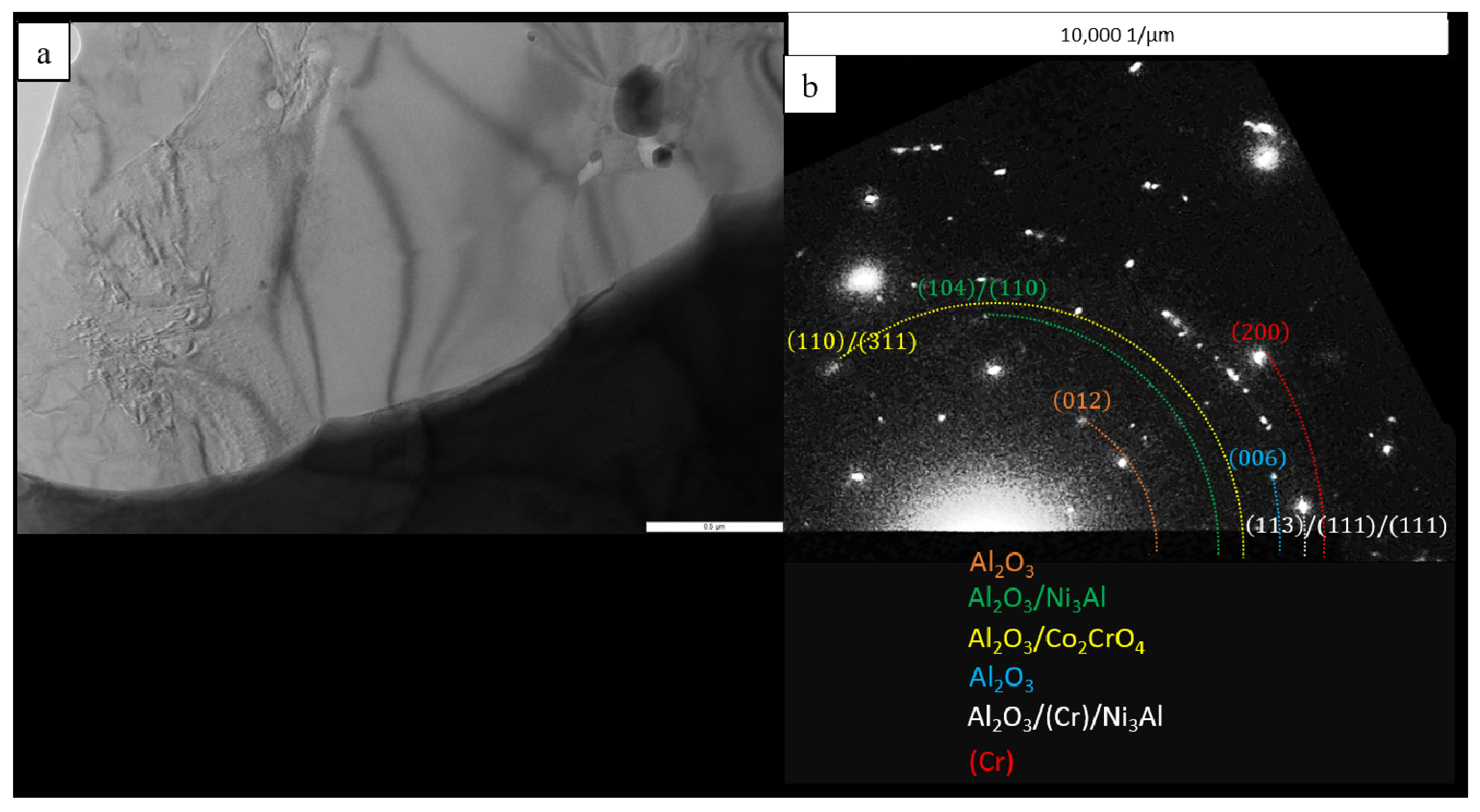
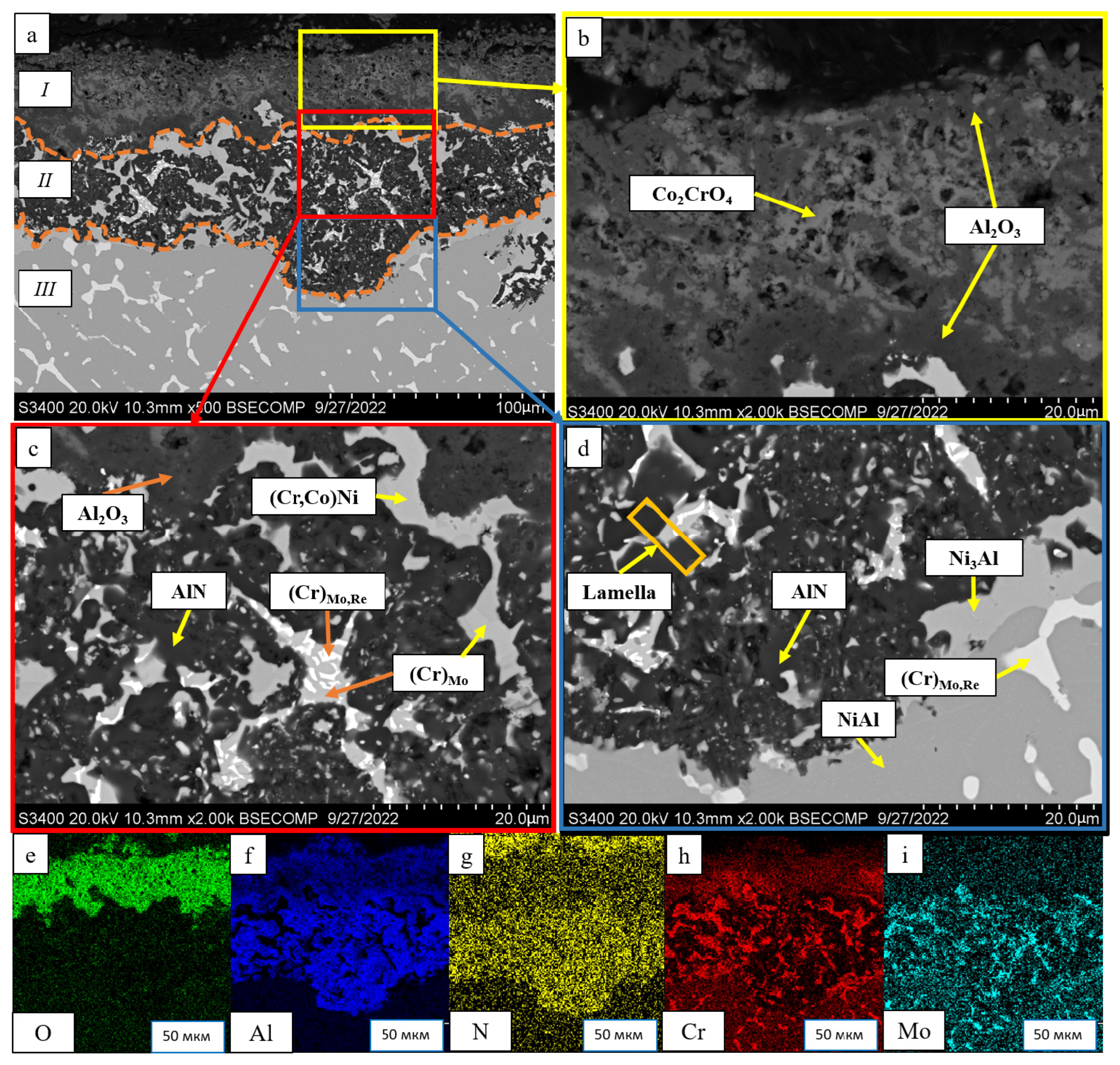

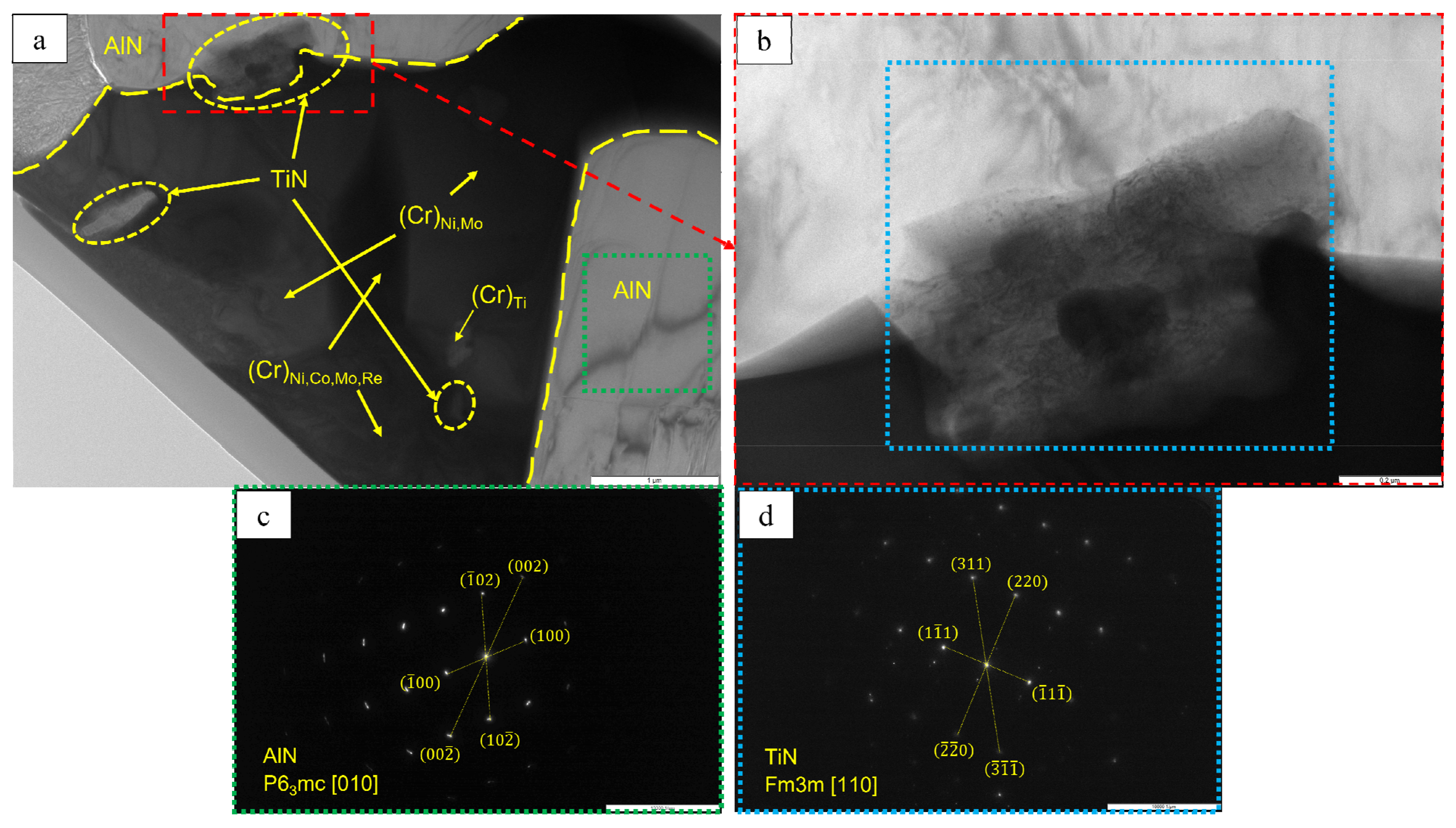

| No. | Composition of the Base | Composition of the Dopant (X), wt.% |
|---|---|---|
| 1 | NiAl-12Cr-6Co (base) | +2.5Mo-0.5Re-0.5Ta |
| 2 | +2.5Mo-1.5Re-1.5Ta | |
| 3 | +2.5Mo-1.5Ta-1.5La-0.5Ru | |
| 4 | +2.5Mo-1.5Re-1.5Ta-0.2Ti | |
| 5 | +2.5Mo-1.5Re-1.5Ta-0.2Zr |
| Compound | CAS No or Grade | Standard: GOST/TU/ISO/ASTM | Particle Size, µm | Chemical Composition, % |
|---|---|---|---|---|
| Major components | ||||
| NiO | 1313-99-1 | GOST 17607-72/ISO 12169 | <40 | 99.00 |
| Cr2O3 | 1308-38-9 | TU 6-09-4272-84/ISO 4621 | <20 | 99.00 |
| Co3O4 | 1307-96-6 | GOST 18671-73 | <30 | 99.00 |
| Al | P3 | GOST 6058-73/ASTM B221-21 & B595-2 | <140 | 98.00 |
| Al | Al300 | TU 48-5-226-87/ASTM B221-21 | <50 | 99.70 |
| Modifying additives (MA) | ||||
| MoO3 | 1313-27-5 | TU 6-09-4471-77/ASTM A146-04 | <50 | 99.00 |
| Zr | 702 (shaving) | TU 95.166-83/ASTM B551 | ≤600 | 99.80 |
| Ta | EB | TU 48-19-72-92/ASTM B708-12 | <20 | 98.00 |
| Re | 7440-15-5 | TU 48-4-195-87/ASTM E696-07 | <150 | 99.99 |
| Ru | 7440-18-8 | GOST 12343-79/ASTM-B717 | ≤100 | 99.95 |
| Ti | TF-0 | TU 14-22-57-92/ASTM B367-00 | ≤30 | 99.80 |
| La | F01 | TU 1-92-200-2000 | ≤100 | 99.97 |
| Element | Concentration, wt.% | ||||
|---|---|---|---|---|---|
| *+2.5Mo-0.5Re-0.5Ta | *+2.5Mo-1.5Re-1.5Ta | *+2.5Mo-1.5Ta-1.5La-0.5Ru | *+2.5Mo-1.5Re-1.5Ta-0.2Ti | *+2.5Mo-1.5Re-1.5Ta-0.2Zr | |
| O | 0.0261 | 0.0281 | 0.0136 | 0.0212 | 0.0321 |
| N | 0.0023 | 0.0029 | 0.0009 | 0.0013 | 0.0026 |
| ∑gas impurities | 0.0283 | 0.0310 | 0.0145 | 0.0223 | 0.0346 |
| ∑impurity elements ** | 0.1449 | 0.1135 | 0.1395 | 0.1478 | 0.1613 |
| No. | Modifying Additive X | Phase | Mass Fraction, % | Lattice Parameters, Å | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| 1 | 2.5%Mo-0.5%Re-0.5%Ta | NiAl | 100 | 2.867 | - | - |
| 2 | 2.5%Mo-1.5%Re-1.5%Ta | NiAl | 80 | 2.879 | - | - |
| Ni(Al,X) * | 14 | 2.931 | - | - | ||
| Ni(Al,Ta) | 6 | 2.997 | - | - | ||
| 3 | 2.5%Mo-1.5%Ta-1.5%La-0.5%Ru | NiAl | 74 | 2.882 | - | - |
| MoNi | 18 | 9.089 | 9.084 | 8.834 | ||
| Ni(Al,Ta) | 8 | 2.918 | - | - | ||
| 4 | 2.5%Mo-1.5%Re-1.5%Ta-0.2%Ti | NiAl | 54 | 2.884 | - | - |
| Ni(Al,Ti) | 46 | 2.927 | - | - | ||
| 5 | 2.5%Mo-1.5%Re-1.5%Ta-0.2%Zr | NiAl | 86 | 2.896 | - | - |
| Ni2(Zr,Al) | 14 | 2.983 | - | - | ||
| No. | base+X Alloy | Hardness, HV | σucs, MPa | σys, MPa | εpd, % |
|---|---|---|---|---|---|
| 1 | +2.5Mo-0.5%Re-0.5%Ta | 5.15 | 1266 | 1117 | <1 |
| 2 | +2.5Mo-1.5%Re-1.5%Ta | 4.94 | 924 | - | <1 * |
| 3 | +2.5Mo-1.5%Ta-1.5%La-0.5%Ru | 5.51 | 1241 | - | <1 * |
| 4 | +2.5Mo-1.5%Re-1.5%Ta-0.2%Ti | 5.56 | 1644 | 1518 | <1 |
| 5 | +2.5Mo-1.5%Re-1.5%Ta-0.2%Zr | 5.12 | 1304 | - | <1 * |
| No. | base+X alloy | Fitting Equation | Weight Gain, g/m2 |
|---|---|---|---|
| 1 | +2.5Mo-0.5%Re-0.5%Ta | y = 0.0002x3 − 0.0076x2 + 0.231x + 2.2869 | 7.282 |
| 2.1 | +2.5Mo-1.5%Re-1.5%Ta | y = 23.084ln(x) + 45.958 | 133.624 |
| 2.2 | +2.5Mo-1.5%Re-1.5%Ta (TVT *) | y = − 0.0295x2 + 2.3405x + 3.6274 | 48.372 |
| 3 | +2.5Mo-1.5%Ta-1.5%La-0.5%Ru | y = 0.0002x3 − 0.0094x2 + 0.165x + 1.096 | 2.772 |
| 4 | +2.5Mo-1.5%Re-1.5%Ta-0.2%Ti | y = −0.044x2 + 3.1537x + 0.3412 | 55.886 |
| 5 | +2.5Mo-1.5%Re-1.5%Ta-0.2%Zr | y = −0.0295x2 + 2.593x + 5.6503 | 57.546 |
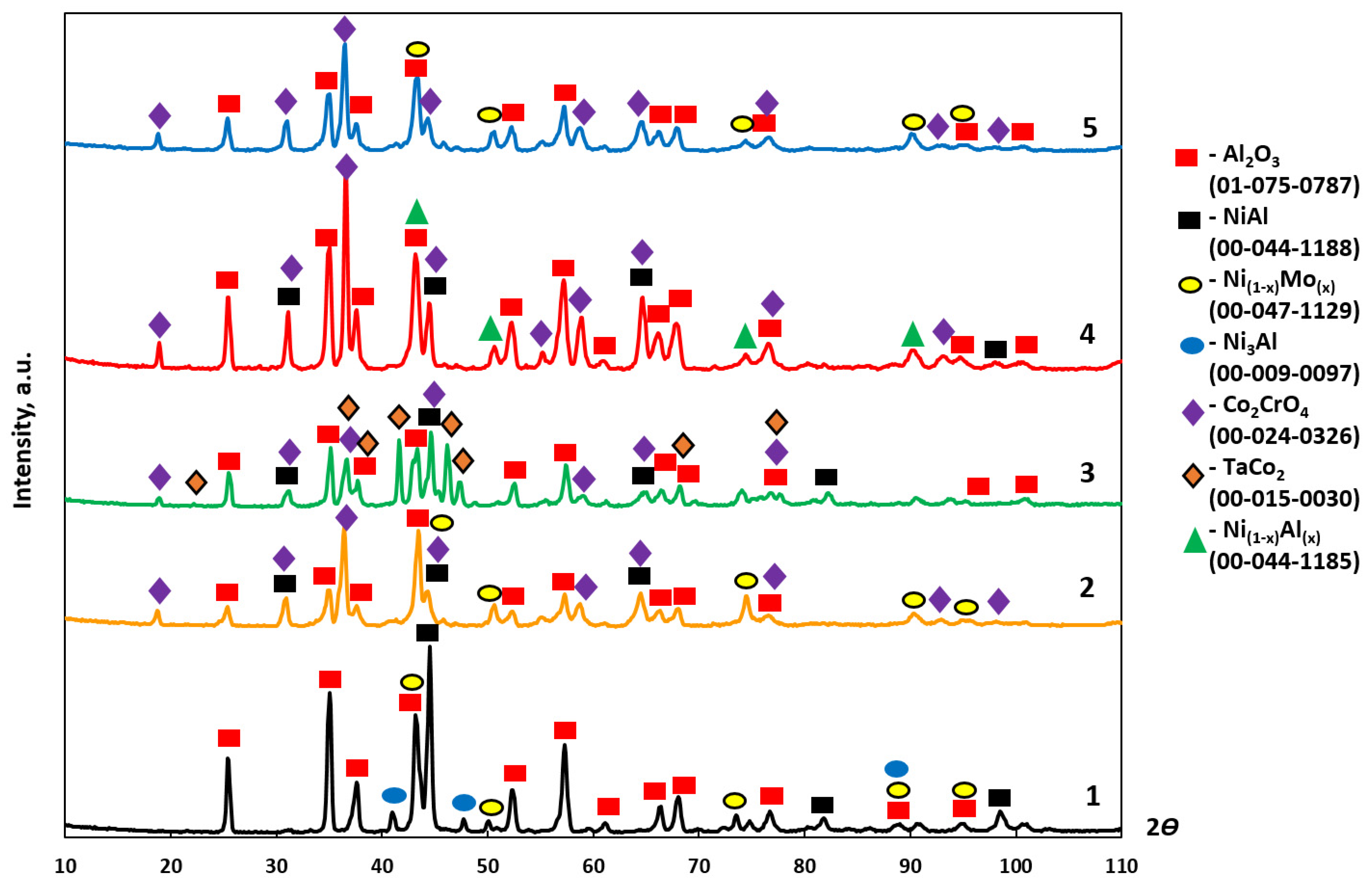
| No. | Base+X Alloy | Phase | wt.% | Lattice Parameters, Å | |
|---|---|---|---|---|---|
| a | c | ||||
| 1 | +2.5%Mo-0.5%Re-0.5%Ta | Al2O3 | 84 | 4.772 | 13.044 |
| NiAl | 11 | 2.878 | - | ||
| MoNi | 4 | 3.644 | - | ||
| Ni3AlN | 1 | 3.808 | - | ||
| 2 | +2.5%Mo-1.5%Re-1.5%Ta | Al2O3 | 42 | 4.770 | 13.054 |
| Co2CrO4 | 40 | 8.168 | - | ||
| MoNi | 13 | 3.598 | - | ||
| NiAl | 5 | 2.887 | - | ||
| 3 | +2.5%Mo-1.5%Ta-1.5%La-0.5%Ru | Al2O3 | 66 | 4.767 | - |
| Co2CrO4 | 13 | 8.135 | - | ||
| NiAl | 13 | 2.868 | - | ||
| TaCo2 | 8 | 4.765 | 15.287 | ||
| 4 | +2.5%Mo-1.5%Re-1.5%Ta-0.2%Ti | Al2O3 | 69 | 4.792 | 13.094 |
| Co2CrO4 | 20 | 8.163 | - | ||
| NiAl | 6 | 2.884 | - | ||
| Ni(1−x)Al(x) | 5 | 3.605 | - | ||
| 5 | +2.5%Mo-1.5%Re-1.5%Ta-0.2%Zr | Al2O3 | 63 | 4.781 | 13.066 |
| Co2CrO4 | 30 | 8.163 | - | ||
| MoNi | 7 | 3.605 | - | ||
| Spectra | O | N | Al | Cr | Co | Ni | Mo | Ru |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.32 | - | 8.18 | 41.52 | 11.91 | 16.07 | 14.16 | 1.83 |
| 2 | 49.55 | - | 49.06 | 1.39 | - | - | - | - |
| 3 | 14.36 | 7.54 | 50.32 | 1.37 | 2.06 | 24.34 | - | - |
| 4 | - | 32.57 | 67.43 | - | - | - | - | - |
| 5 | - | - | 0.64 | 50.95 | 14.52 | 19.46 | 14.42 | - |
| 6 | 50.65 | - | 49.35 | - | - | - | - | - |
| 7 | 20.58 | - | 22.52 | 19.71 | 10.36 | 10.80 | 16.04 | - |
| 8 | - | - | 15.67 | 12.89 | 7.24 | 64.19 | - | - |
| Spectra | N | Al | Ti | Cr | Co | Ni | Mo | Re |
|---|---|---|---|---|---|---|---|---|
| 1 | - | - | 18.93 | 45.84 | 6.16 | 19.54 | 9.55 | - |
| 2 | 1.91 | 0.34 | 4.42 | 39.57 | 9.56 | 16.15 | 13.57 | 14.48 |
| 3 | 24.58 | 78.06 | - | 0.36 | - | - | - | - |
| 4 | - | - | - | 37.41 | 10.60 | 17.47 | 15.16 | 19.36 |
| 5 | 1.87 | - | - | 51.61 | 7.96 | 25.89 | 12.68 | - |
| 6 | - | - | - | 35.57 | 10.44 | 17.34 | 14.69 | 21.97 |
| 7 | 23.39 | 76.61 | - | - | - | - | - | - |
| 8 | 23.16 | 79.84 | - | - | - | - | - | - |
| 9 | 24.37 | - | 63.48 | 9.64 | 0.73 | 1.80 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanin, V.V.; Aheiev, M.I.; Kaplanskii, Y.Y.; Loginov, P.A.; Bychkova, M.Y.; Levashov, E.A. The Effect of Dopants on Structure Formation and Properties of Cast SHS Alloys Based on Nickel Monoaluminide. Materials 2023, 16, 3299. https://doi.org/10.3390/ma16093299
Sanin VV, Aheiev MI, Kaplanskii YY, Loginov PA, Bychkova MY, Levashov EA. The Effect of Dopants on Structure Formation and Properties of Cast SHS Alloys Based on Nickel Monoaluminide. Materials. 2023; 16(9):3299. https://doi.org/10.3390/ma16093299
Chicago/Turabian StyleSanin, Vitalii V., Maksym I. Aheiev, Yury Yu. Kaplanskii, Pavel A. Loginov, Marina Ya. Bychkova, and Evgeny A. Levashov. 2023. "The Effect of Dopants on Structure Formation and Properties of Cast SHS Alloys Based on Nickel Monoaluminide" Materials 16, no. 9: 3299. https://doi.org/10.3390/ma16093299
APA StyleSanin, V. V., Aheiev, M. I., Kaplanskii, Y. Y., Loginov, P. A., Bychkova, M. Y., & Levashov, E. A. (2023). The Effect of Dopants on Structure Formation and Properties of Cast SHS Alloys Based on Nickel Monoaluminide. Materials, 16(9), 3299. https://doi.org/10.3390/ma16093299







