Enhanced Heterogeneous Peroxymonosulfate Activation by MOF-Derived Magnetic Carbonaceous Nanocomposite for Phenol Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cu-CuxO@C
2.3. Characterization of the Synthesized Activators
2.4. Catalytic Experiments
3. Discussion
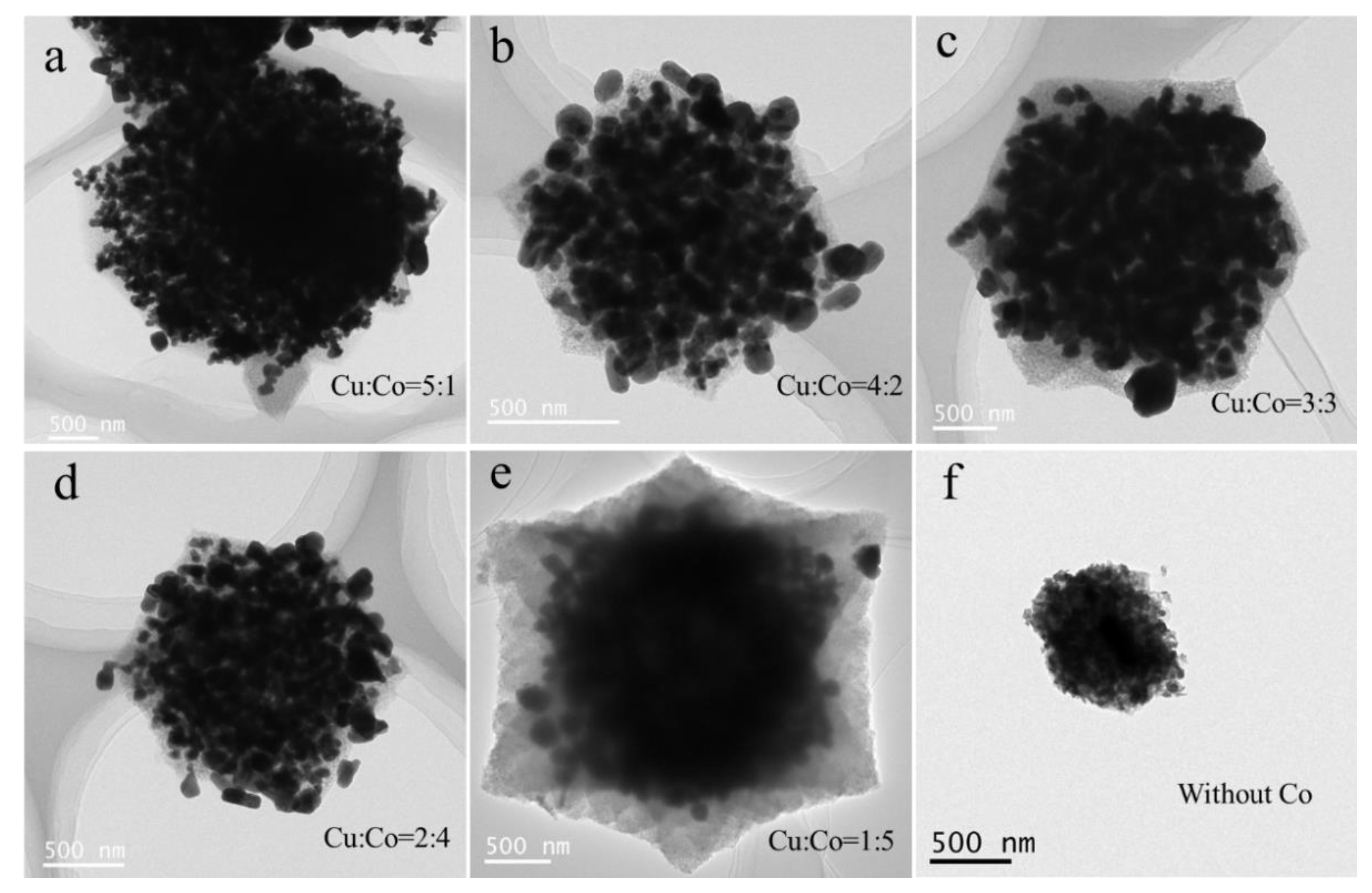
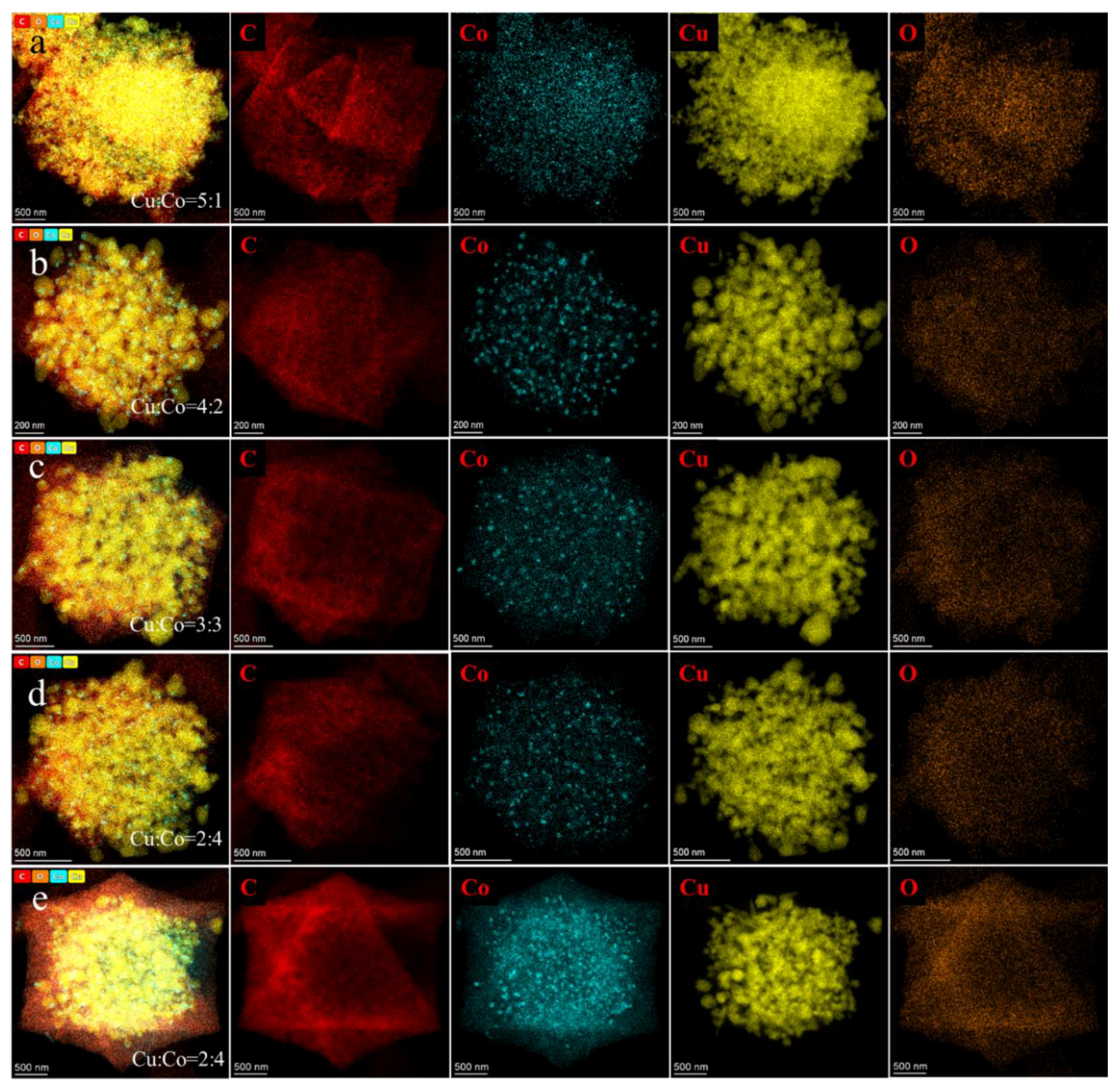
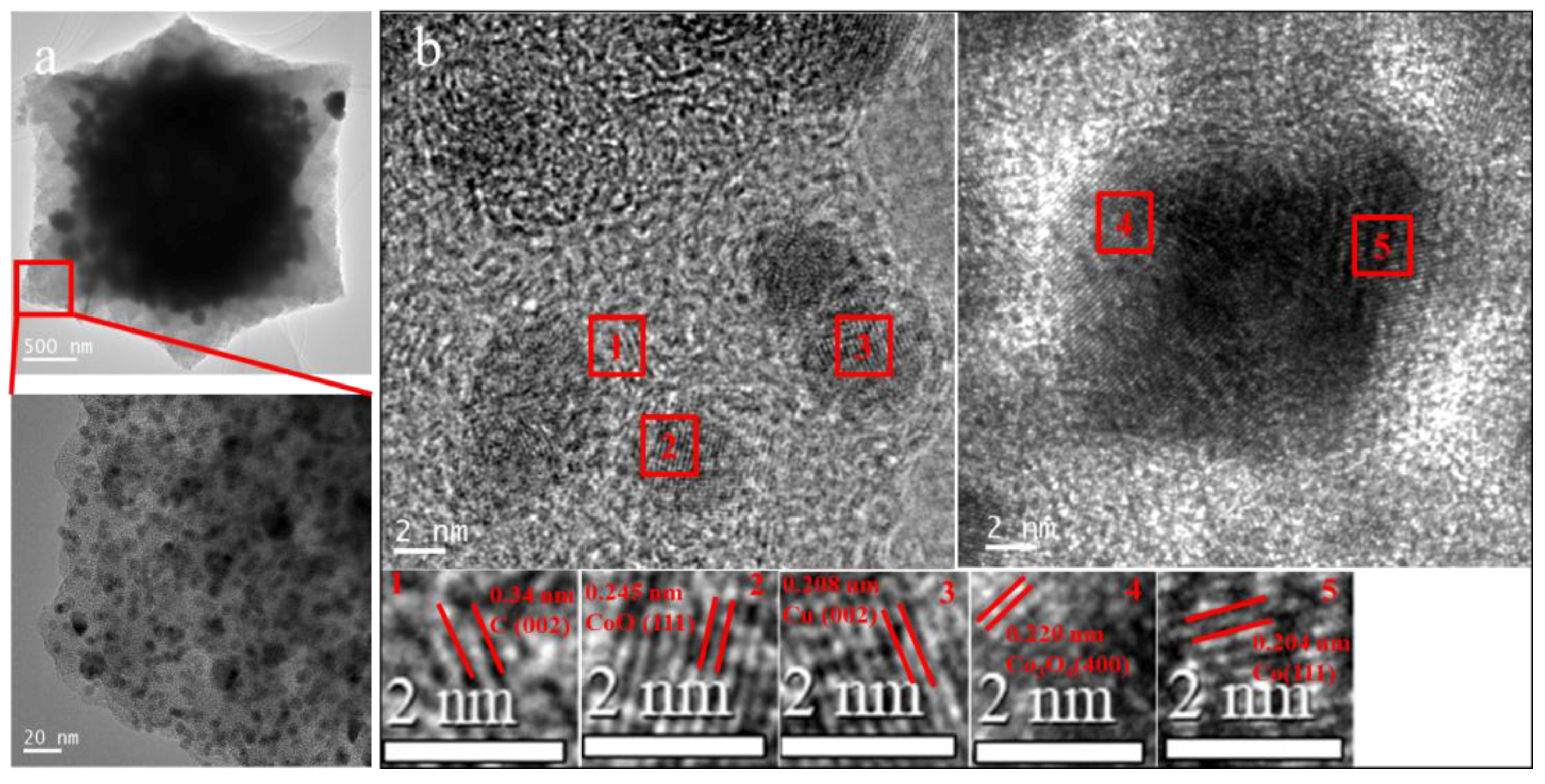
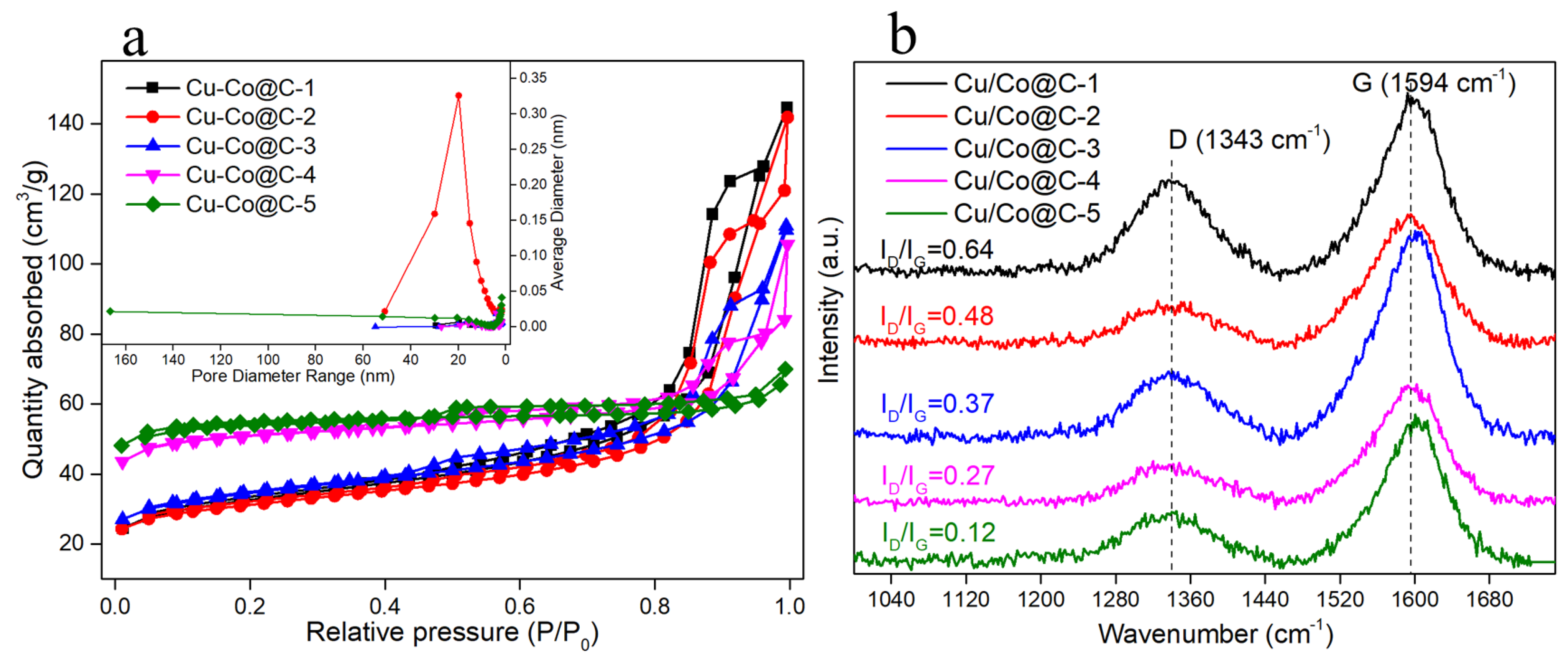
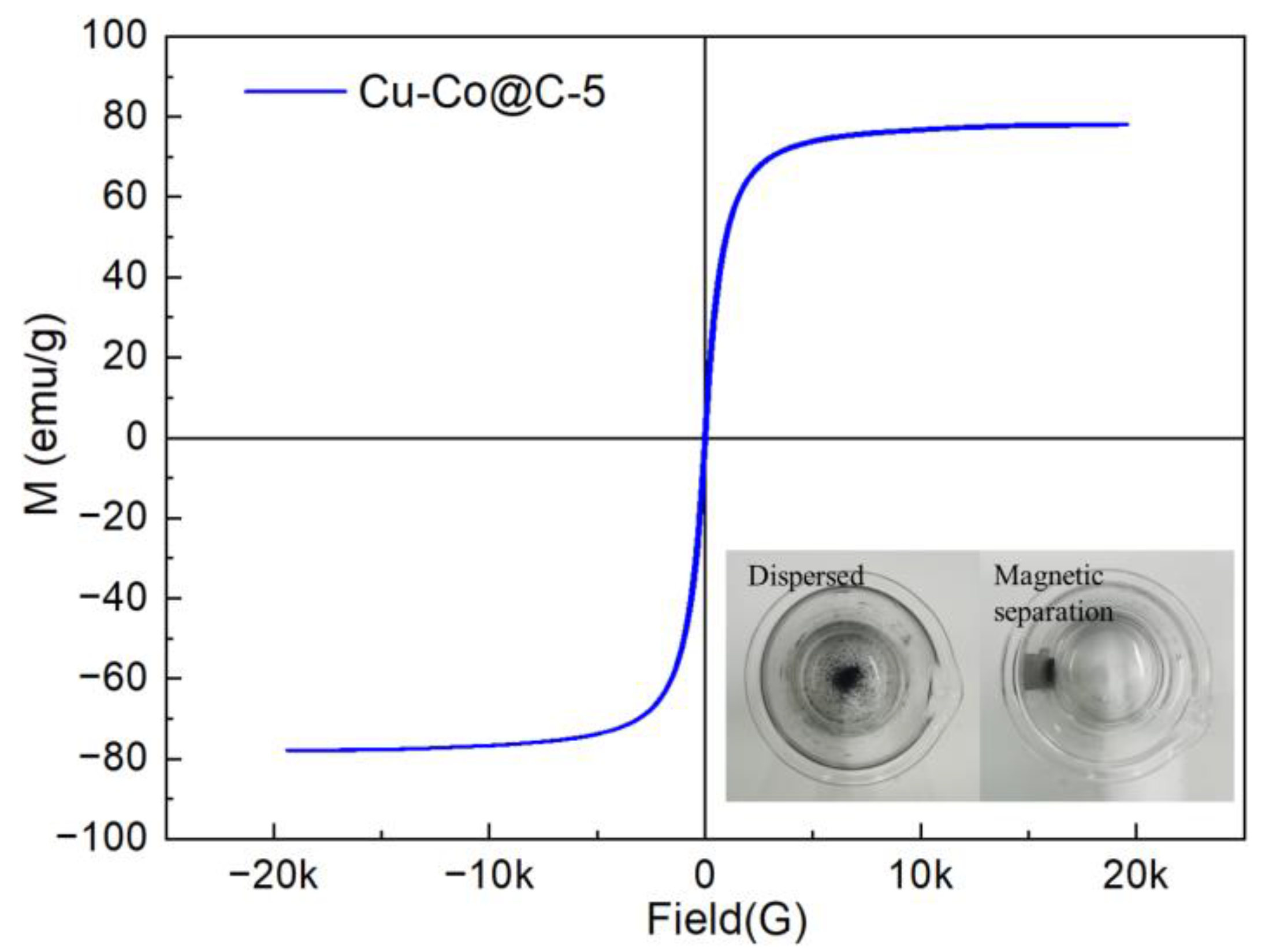
3.1. Structure and Morphology Characterization
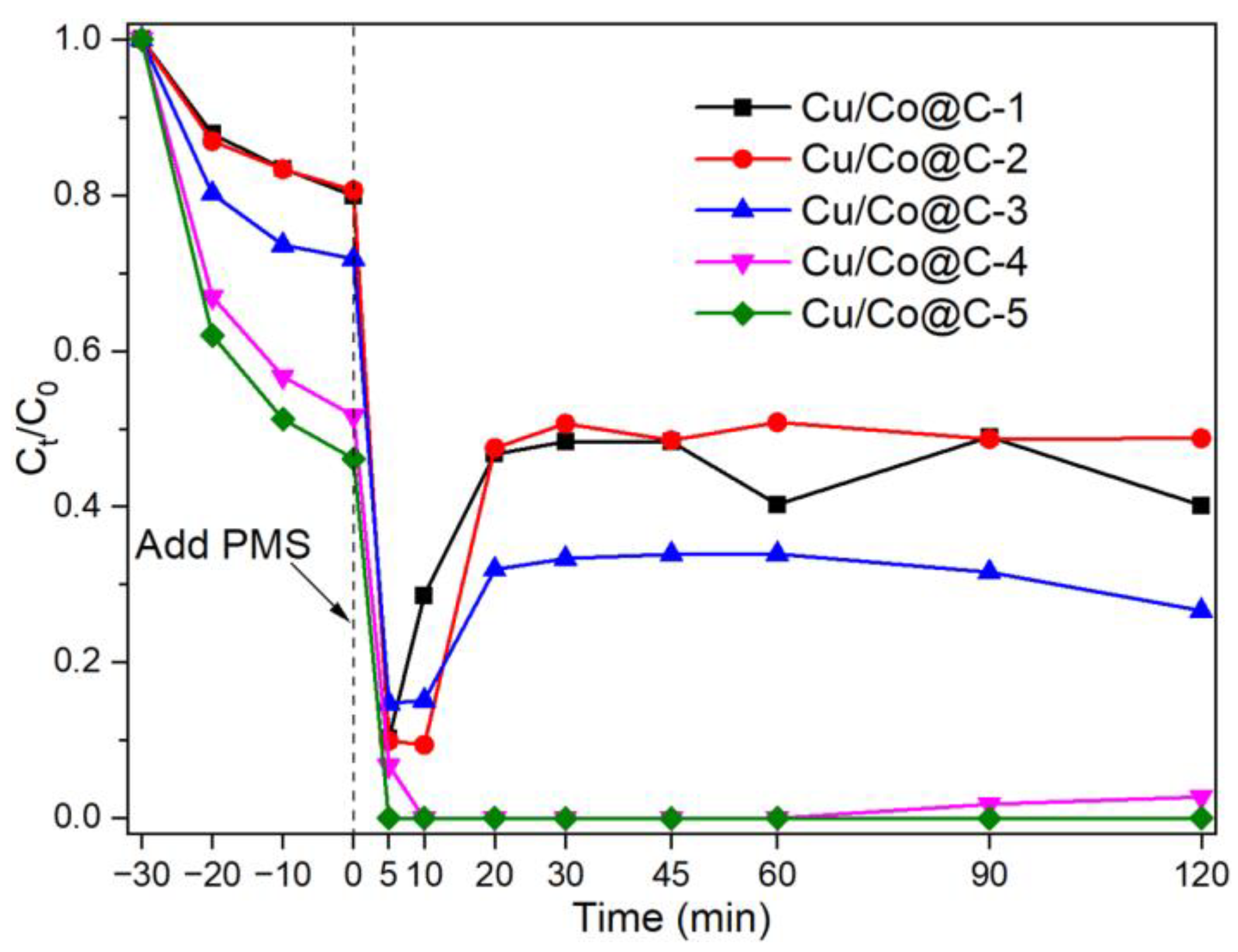
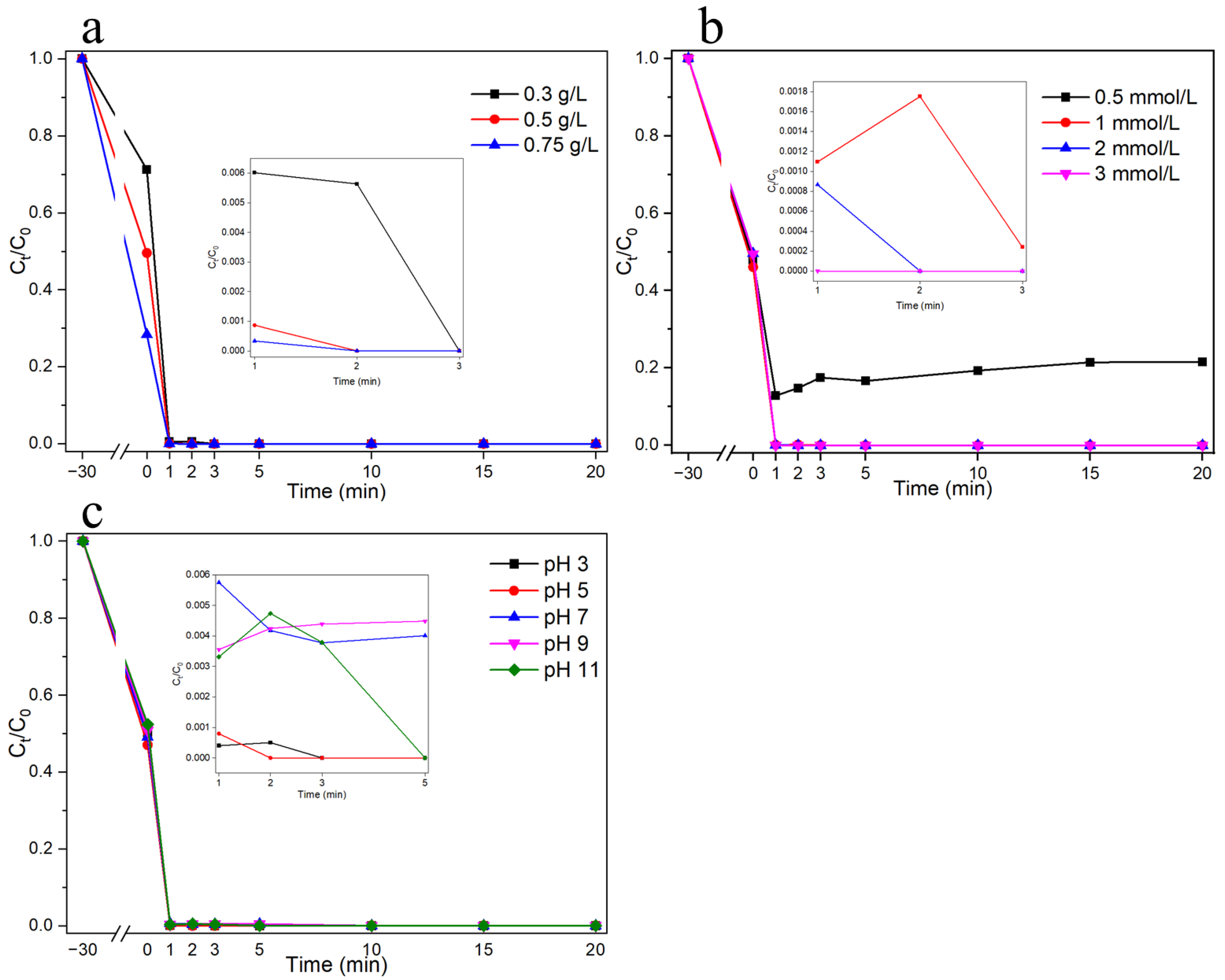
3.2. Catalytic Performances
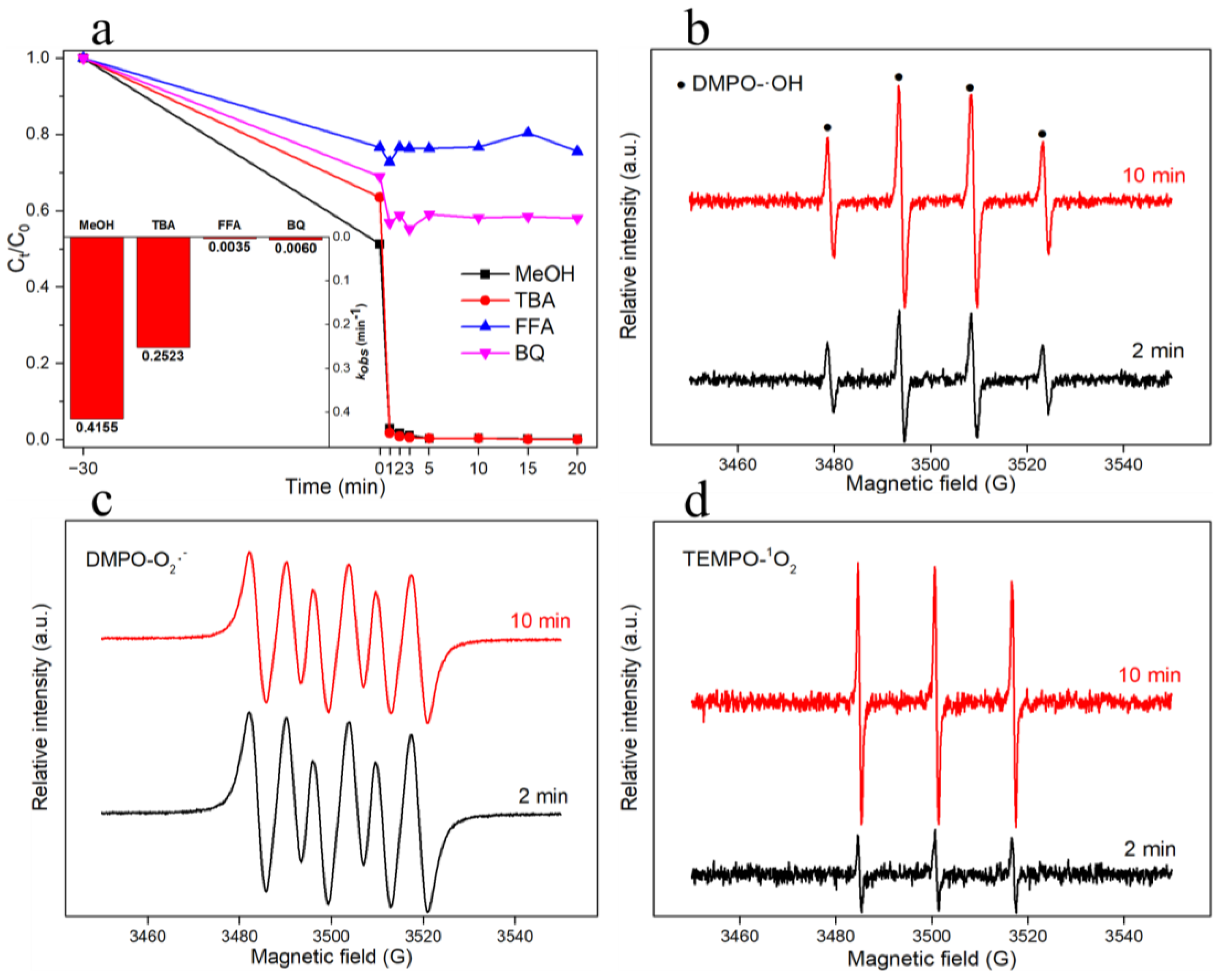
3.3. Identification of Radicals
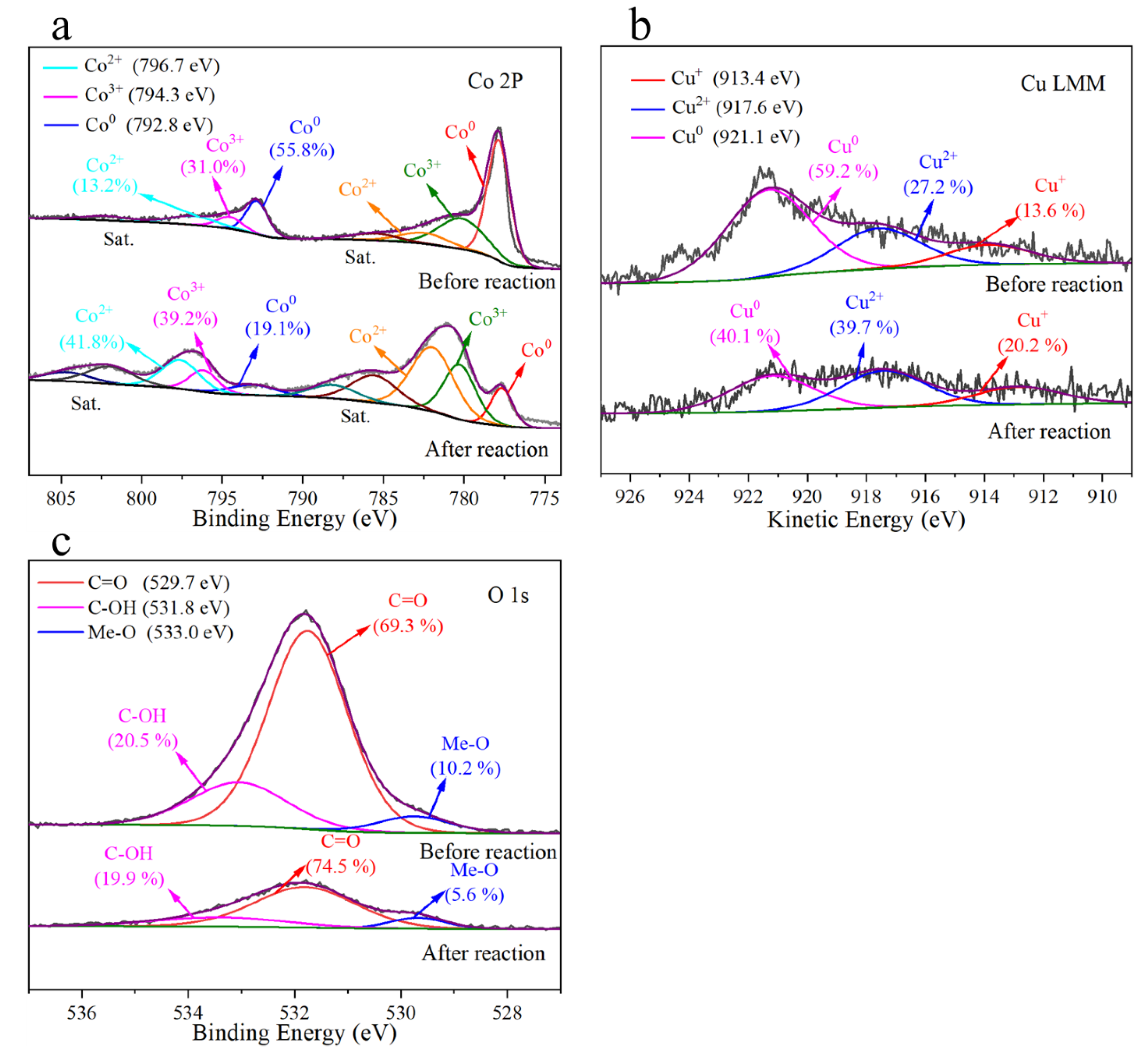
3.4. Cu-Co@C-5 Activated PMS and Phenol-Degradation Mechanisms
3.4.1. Reaction Pathway of Cu-Co@C-5
3.4.2. Electron-Transfer Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Duan, X.; Yang, S.; Waclawek, S.; Fang, G.; Xiao, R.; Dionysiou, D.D. Limitations and prospects of sulfate-radical based advanced oxidation processes. J. Environ. Chem. Eng. 2020, 8, 103849. [Google Scholar] [CrossRef]
- Hou, C.; Fu, L.; Wang, Y.; Chen, W.; Chen, F.; Zhang, S.; Wang, J. Co-MOF-74 based Co3O4/cellulose derivative membrane as dual-functional catalyst for colorimetric detection and degradation of phenol. Carbohydr. Polym. 2021, 273, 118548. [Google Scholar] [CrossRef]
- Liotta, L.F.; Gruttadauria, M.; Di Carlo, G.; Perrini, G.; Librando, V. Heterogeneous catalytic degradation of phenolic substrates: Catalysts activity. J. Hazard. Mater. 2009, 162, 588–606. [Google Scholar] [CrossRef]
- Domingues, E.; Silva, M.J.; Vaz, T.; Gomes, J.; Martins, R.C. Sulfate radical based advanced oxidation processes for agro-industrial effluents treatment: A comparative review with Fenton’s peroxidation. Sci. Total Environ. 2022, 832, 155029. [Google Scholar] [CrossRef]
- Li, J.; Liang, Y.; Jin, P.; Zhao, B.; Zhang, Z.; He, X.; Tan, Z.; Wang, L.; Cheng, X. Heterogeneous Metal-Activated Persulfate and Electrochemically Activated Persulfate: A Review. Catalysts 2022, 12, 1024. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.; Wu, S.; Yin, R.; Zhu, M. Surface dual redox cycles of Mn(III)/Mn(IV) and Cu(I)/Cu(II) for heterogeneous peroxymonosulfate activation to degrade diclofenac: Performance, mechanism and toxicity assessment. J. Hazard. Mater. 2021, 410, 124623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Chen, H.; et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, S.; Jin, L.; Li, Y.; Geng, P.; Xue, H.; Pang, H.; Xu, Q. Metal-Organic Framework-Derived Carbons for Battery Applications. Adv. Energy Mater. 2018, 8, 1800716. [Google Scholar] [CrossRef]
- Khan, M.A.N.; Klu, P.K.; Wang, C.; Zhang, W.; Luo, R.; Zhang, M.; Qi, J.; Sun, X.; Wang, L.; Li, J. Metal-organic framework-derived hollow Co3O4/carbon as efficient catalyst for peroxymonosulfate activation. Chem. Eng. J. 2019, 363, 234–246. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.; Zhu, Z.; Cui, F.; Zhu, Y.-A.; Duan, X.; Wang, S. Magnetic nitrogen-doped nanocarbons for enhanced metal-free catalytic oxidation: Integrated experimental and theoretical investigations for mechanism and application. Chem. Eng. J. 2018, 354, 507–516. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, Y.; Li, C.; Zheng, J.; Si, M.; Xiao, R.; Liao, Q.; Yang, W. Non-radical dominated catalytic degradation of chlorophenol by a structure-tailored catalyst of high nitrogen doping carbon matrix with nano-CuO. J. Environ. Chem. Eng. 2022, 10, 107559. [Google Scholar] [CrossRef]
- Li, X.; Min, X.; Hu, X.; Jiang, Z.; Li, C.; Yang, W.; Zhao, F. In-situ synthesis of highly dispersed Cu-CuxO nanoparticles on porous carbon for the enhanced persulfate activation for phenol degradation. Sep. Purif. Technol. 2021, 276, 119260. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Cai, X.; Deng, X.; Xie, Z.; Bao, S.; Shi, Y.; Lin, J.; Pang, M.; Eddaoudi, M. Synthesis of highly monodispersed Ga-soc-MOF hollow cubes, colloidosomes and nanocomposites. Chem. Commun. 2016, 52, 9901–9904. [Google Scholar] [CrossRef]
- Li, H.; Xu, S.; Du, J.; Tang, J.; Zhou, Q. Cu@Co-MOFs as a novel catalyst of peroxymonosulfate for the efficient removal of methylene blue. RSC Adv. 2019, 9, 9410–9420. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Q.; Wei, M.; Guo, S.; Fang, Y.; Ni, Z.; Yang, X.; Zhang, S.; Qiu, R. Co@CoO encapsulated with N-doped carbon nanotubes activated peroxymonosulfate for efficient purification of organic wastewater. Sep. Purif. Technol. 2022, 295, 121347. [Google Scholar] [CrossRef]
- Zhao, Y.; Riaz, M.S.; Dong, W.; Yin, Y.; Liu, Z.; Huang, F. Cu-dispersed cobalt oxides as high volumetric capacity anode materials for Li-ion storage. Energy Storage Mater. 2020, 27, 453–458. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Jiang, Z.; Li, C.; Zhao, J.; Wang, H.; Liao, Q. Structure-dependent catalysis of Co3O4 crystals in persulfate activation via nonradical pathway. Appl. Surf. Sci. 2020, 525, 146482. [Google Scholar] [CrossRef]
- Lu, Y.; Zhan, W.; He, Y.; Wang, Y.; Kong, X.; Kuang, Q.; Xie, Z.; Zheng, L. MOF-Templated Synthesis of Porous Co3O4 Concave Nanocubes with High Specific Surface Area and Their Gas Sensing Properties. ACS Appl. Mater. Interfaces 2014, 6, 4186–4195. [Google Scholar] [CrossRef]
- Hu, X.; Min, X.; Li, X.; Si, M.; Liu, L.; Zheng, J.; Yang, W.; Zhao, F. Co-Co3O4 encapsulated in nitrogen-doped carbon nanotubes for capacitive desalination: Effects of nano-confinement and cobalt speciation. J. Colloid. Interface Sci. 2022, 616, 389–400. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C.; Yan, X.; Zhang, H.; Xiao, C.; Qi, J.; Zhu, Z.; Zhou, Y.; Sun, X.; Duan, X.; et al. Rational Regulation of Co-N-C Coordination for High-Efficiency Generation of 1O2 toward Nearly 100% Selective Degradation of Organic Pollutants. Environ. Sci. Technol. 2022, 56, 8833–8843. [Google Scholar] [CrossRef]
- Dou, R.; Cheng, H.; Ma, J.; Qin, Y.; Kong, Y.; Komarneni, S. Catalytic degradation of methylene blue through activation of bisulfite with CoO nanoparticles. Sep. Purif. Technol. 2020, 239, 116561. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, J.; Li, C.; Liao, Q.; Xiao, R.; Yang, W. Strong synergistic effect of Co3O4 encapsulated in nitrogen-doped carbon nanotubes on the nonradical-dominated persulfate activation. Carbon 2020, 158, 172–183. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Yang, Y.; Feng, Y.; Wu, D.; Mao, S. Activation of persulfate with metal–organic framework-derived nitrogen-doped porous Co@C nanoboxes for highly efficient p-Chloroaniline removal. Chem. Eng. J. 2019, 358, 408–418. [Google Scholar] [CrossRef]
- Kondo, S.; Ishikawa, T.; Abe, I. Adsorption Science; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Gong, X.; Luo, W.; Guo, N.; Zhang, S.; Wang, L.; Jia, D.; Ai, L.; Feng, S. Carbon nanofiber@ZIF-8 derived carbon nanosheet composites with a core–shell structure boosting capacitive deionization performance. J. Mater. Chem. A 2021, 9, 18604–18613. [Google Scholar] [CrossRef]
- Tang, L.; Liu, Y.; Wang, J.; Zeng, G.; Deng, Y.; Dong, H.; Feng, H.; Wang, J.; Peng, B. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism. Appl. Catal. B Environ. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, Z.; Hu, X.; Li, X.; Wang, H.; Xiao, R. Enhanced activation of persulfate by nitric acid/annealing modified multi-walled carbon nanotubes via non-radical process. Chemosphere 2019, 220, 514–522. [Google Scholar] [CrossRef]
- Indrawirawan, S.; Sun, H.; Duan, X.; Wang, S. Nanocarbons in different structural dimensions (0–3D) for phenol adsorption and metal-free catalytic oxidation. Appl. Catal. B Environ. 2015, 179, 352–362. [Google Scholar] [CrossRef]
- Guan, Y.H.; Ma, J.; Ren, Y.M.; Liu, Y.L.; Xiao, J.Y.; Lin, L.Q.; Zhang, C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, C.S.; Tu, Y.J.; Huang, Y.H.; Zhang, H. Heterogeneous Degradation of Organic Pollutants by Persulfate Activated by CuO-Fe3O4: Mechanism, Stability, and Effects of pH and Bicarbonate Ions. Environ. Sci. Technol. 2015, 49, 6838–6845. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. 3D-hierarchically structured MnO2 for catalytic oxidation of phenol solutions by activation of peroxymonosulfate: Structure dependence and mechanism. Appl. Catal. B Environ. 2015, 164, 159–167. [Google Scholar] [CrossRef]
- Li, W.; Liu, B.; Wang, Z.; Wang, K.; Lan, Y.; Zhou, L. Efficient activation of peroxydisulfate (PDS) by rice straw biochar modified by copper oxide (RSBC-CuO) for the degradation of phenacetin (PNT). Chem. Eng. J. 2020, 395, 125094. [Google Scholar] [CrossRef]
- Wu, J.; Su, H.; Wang, Z.; Hou, B.; Cheng, X.; Yury, V.S.; Wang, X.; Liu, B.; Zhu, X.; Mao, Y.; et al. N/ZnFe2O4 codoped biochar as an activator for peroxydisulfate to degrade oxytetracycline: Synthesis, property and mechanism. Sep. Purif. Technol. 2022, 297, 121487. [Google Scholar] [CrossRef]
- Wu, L.; Yu, Y.; Zhang, Q.; Hong, J.; Wang, J.; She, Y. A novel magnetic heterogeneous catalyst oxygen-defective CoFe2O4−x for activating peroxymonosulfate. Appl. Surf. Sci. 2019, 480, 717–726. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Hu, J.; Qian, B.; Zeng, X.; Qi, Y.; Liu, Y.; Zhang, L.; Zhang, X. Oxygen vacant Co3O4 in situ embedded on carbon spheres: Cooperatively tuning electron transfer for boosted peroxymonosulfate activation. J. Mater. Chem. A 2021, 9, 16489–16499. [Google Scholar] [CrossRef]
- Minero, C.; Mariella, G.; Maurino, V.; Vione, D.; Pelizzetti, E. Photocatalytic transformation of organic compounds in the presence of inorganic ions. 2. Competitive reactions of phenol and alcohols an a titanium dioxide-fluoride system. Langmuir 2000, 16, 8964–8972. [Google Scholar] [CrossRef]
- Jiang, X.; Yan, X.; Hu, X.; Feng, R.; Li, T.; Wang, L. Carbon coated CoO plates/3D nickel foam: An efficient and readily recyclable catalyst for peroxymonosulfate activation. Sep. Purif. Technol. 2022, 297, 121400. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, N.; Wei, X.; Ding, Y.; Ke, Y.; Wu, P.; Liu, Z. Mechanism insight into efficient peroxydisulfate activation by novel nano zero-valent iron anchored yCo3O4 (nZVI/yCo3O4) composites. J. Hazard. Mater. 2020, 400, 123157. [Google Scholar] [CrossRef]
- Ding, Y.; Fu, L.; Peng, X.; Lei, M.; Wang, C.; Jiang, J. Copper catalysts for radical and nonradical persulfate based advanced oxidation processes: Certainties and uncertainties. Chem. Eng. J. 2022, 427, 131776. [Google Scholar] [CrossRef]
- Dong, H.Y.; Li, Y.; Wang, S.C.; Liu, W.F.; Zhou, G.M.; Xie, Y.F.; Guan, X.H. Both Fe(IV) and Radicals Are Active Oxidants in the Fe(II)/Peroxydisulfate Process. Environ. Sci. Technol. Lett. 2020, 7, 219–224. [Google Scholar] [CrossRef]
- Ding, Y.B.; Pan, C.; Peng, X.Q.; Mao, Q.H.; Xiao, Y.W.; Fu, L.B.; Huang, J. Deep mineralization of bisphenol A by catalytic peroxymonosulfate activation with nano CuO/Fe3O4 with strong Cu-Fe interaction. Chem. Eng. J. 2020, 384, 123378. [Google Scholar] [CrossRef]
- Alexopoulou, C.; Petala, A.; Frontistis, Z.; Drivas, C.; Kennou, S.; Kondarides, D.I.; Mantzavinos, D. Copper phosphide and persulfate salt: A novel catalytic system for the degradation of aqueous phase micro-contaminants. Appl. Catal. B Environ. 2019, 244, 178–187. [Google Scholar] [CrossRef]
- Chi, H.Z.; He, X.; Zhang, J.Q.; Wang, D.; Zhai, X.D.; Ma, J. Hydroxylamine enhanced degradation of naproxen in Cu2+ activated peroxymonosulfate system at acidic condition: Efficiency, mechanisms and pathway. Chem. Eng. J. 2019, 361, 764–772. [Google Scholar] [CrossRef]
- Wang, Y.R.; Tian, D.F.; Chu, W.; Li, M.R.; Lu, X.W. Nanoscaled magnetic CuFe2O4 as an activator of peroxymonosulfate for the degradation of antibiotics norfloxacin. Sep. Purif. Technol. 2019, 212, 536–544. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Li, Y.; Li, W.; Zhou, L.; Lan, Y.; Li, Y. Insight into heterogeneous catalytic degradation of sulfamethazine by peroxymonosulfate activated with CuCo2O4 derived from bimetallic oxalate. Chem. Eng. J. 2020, 384, 123257. [Google Scholar] [CrossRef]
- Hirani, R.A.K.; Asif, A.H.; Rafique, N.; Wu, H.; Shi, L.; Zhang, S.; Duan, X.; Wang, S.; Saunders, M.; Sun, H. Three-dimensional nitrogen-doped graphene oxide beads for catalytic degradation of aqueous pollutants. Chem. Eng. J. 2022, 446, 137042. [Google Scholar] [CrossRef]
- Li, C.; Wu, J.; Peng, W.; Fang, Z.; Liu, J. Peroxymonosulfate activation for efficient sulfamethoxazole degradation by Fe3O4/β-FeOOH nanocomposites: Coexistence of radical and non-radical reactions. Chem. Eng. J. 2019, 356, 904–914. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.; Deng, L. Performance of CuO/Oxone system: Heterogeneous catalytic oxidation of phenol at ambient conditions. Chem. Eng. J. 2011, 178, 239–243. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, J.; Ouyang, D.; Qian, L.; Han, L.; Chen, M. Heterogeneously catalyzed persulfate by CuMgFe layered double oxide for the degradation of phenol. Appl. Catal. A-Gen. 2017, 538, 19–26. [Google Scholar] [CrossRef]
- Lu, S.; Wang, G.; Chen, S.; Yu, H.; Ye, F.; Quan, X. Heterogeneous activation of peroxymonosulfate by LaCo1−xCuxO3 perovskites for degradation of organic pollutants. J. Hazard. Mater. 2018, 353, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Liao, Z.; Liu, Y.; Jawad, A.; Ifthikar, J.; Chen, Z. Synergistic degradation of phenols using peroxymonosulfate activated by CuO-Co3O4@MnO2 nanocatalyst. J. Hazard. Mater. 2017, 329, 262–271. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhu, X.; Wu, J.; Gao, H.; Yang, W.; Hu, X. Enhanced Heterogeneous Peroxymonosulfate Activation by MOF-Derived Magnetic Carbonaceous Nanocomposite for Phenol Degradation. Materials 2023, 16, 3325. https://doi.org/10.3390/ma16093325
Li X, Zhu X, Wu J, Gao H, Yang W, Hu X. Enhanced Heterogeneous Peroxymonosulfate Activation by MOF-Derived Magnetic Carbonaceous Nanocomposite for Phenol Degradation. Materials. 2023; 16(9):3325. https://doi.org/10.3390/ma16093325
Chicago/Turabian StyleLi, Xinyu, Xinfeng Zhu, Junfeng Wu, Hongbin Gao, Weichun Yang, and Xiaoxian Hu. 2023. "Enhanced Heterogeneous Peroxymonosulfate Activation by MOF-Derived Magnetic Carbonaceous Nanocomposite for Phenol Degradation" Materials 16, no. 9: 3325. https://doi.org/10.3390/ma16093325
APA StyleLi, X., Zhu, X., Wu, J., Gao, H., Yang, W., & Hu, X. (2023). Enhanced Heterogeneous Peroxymonosulfate Activation by MOF-Derived Magnetic Carbonaceous Nanocomposite for Phenol Degradation. Materials, 16(9), 3325. https://doi.org/10.3390/ma16093325




