Biopotentials of Collagen Scaffold Impregnated with Plant-Cell-Derived Epidermal Growth Factor in Defective Bone Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Laboratory Consumables
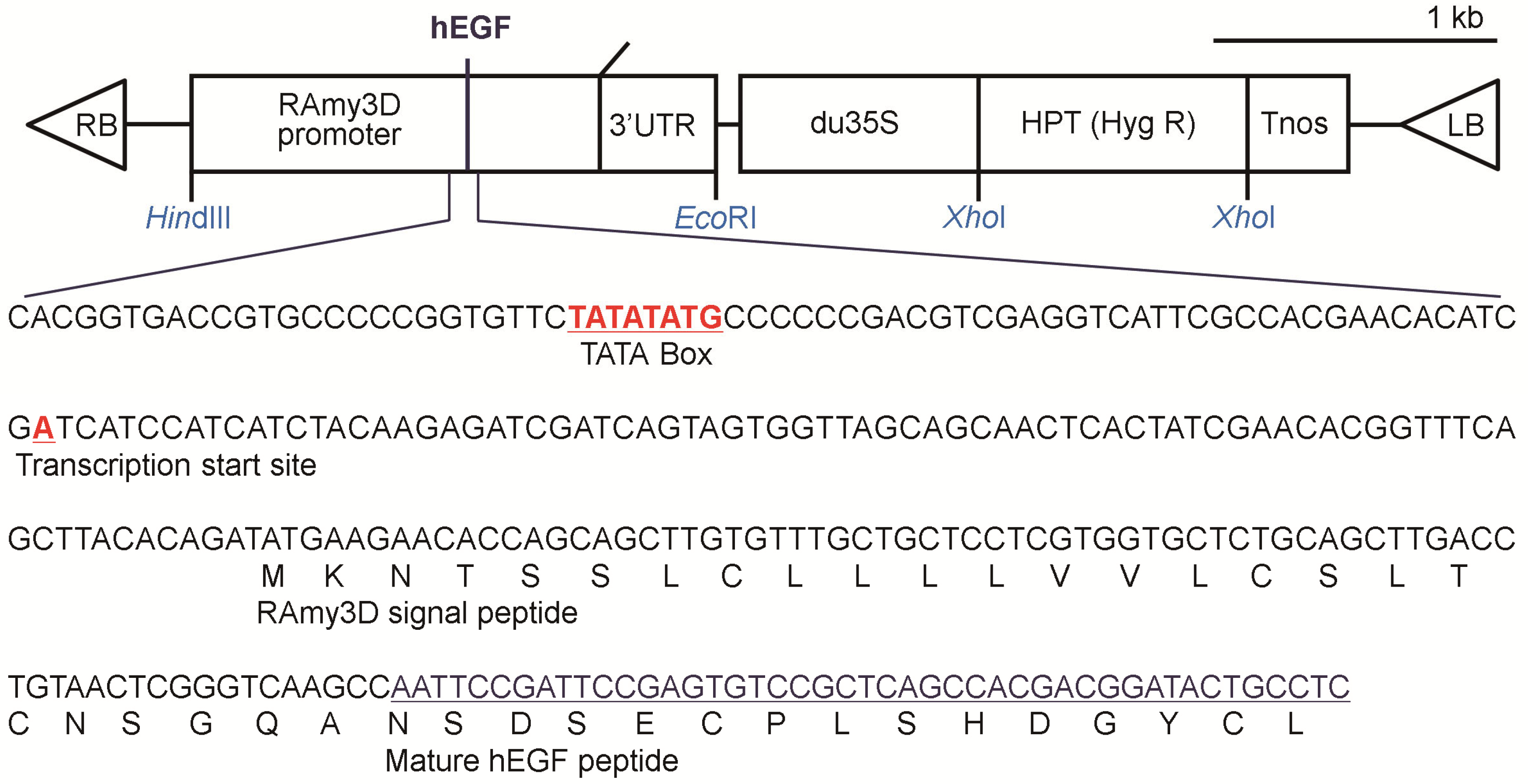
2.2. Production of p-rhEGF Using a Plant Expression System
2.3. Fabrication of ACSs
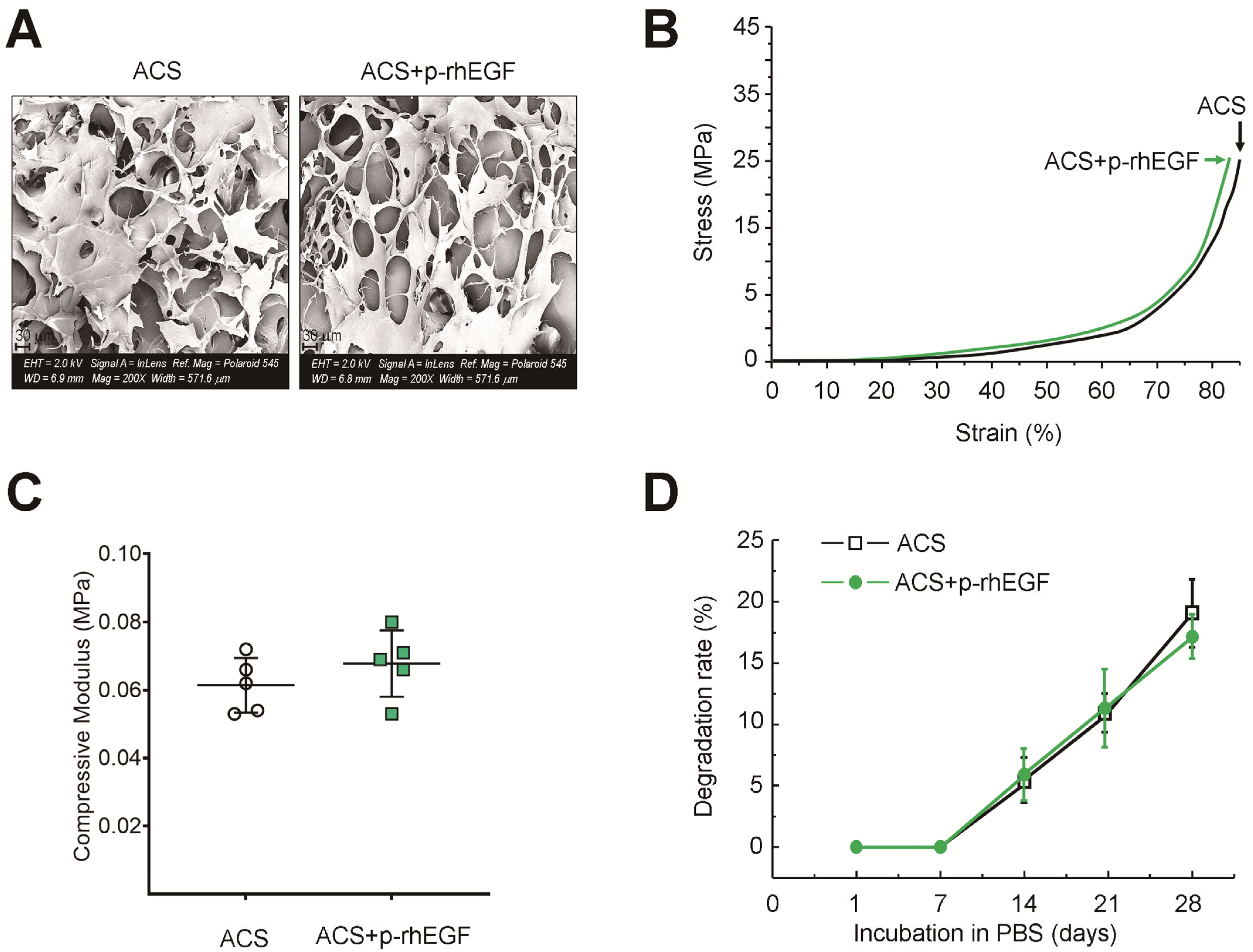
2.4. Charactization of ACSs
2.5. Animals and Ethical Statements
2.6. Creation of Calvarial Defects
2.7. Implantation of ACSs or rhEGF-Loaded ACSs into the Defects
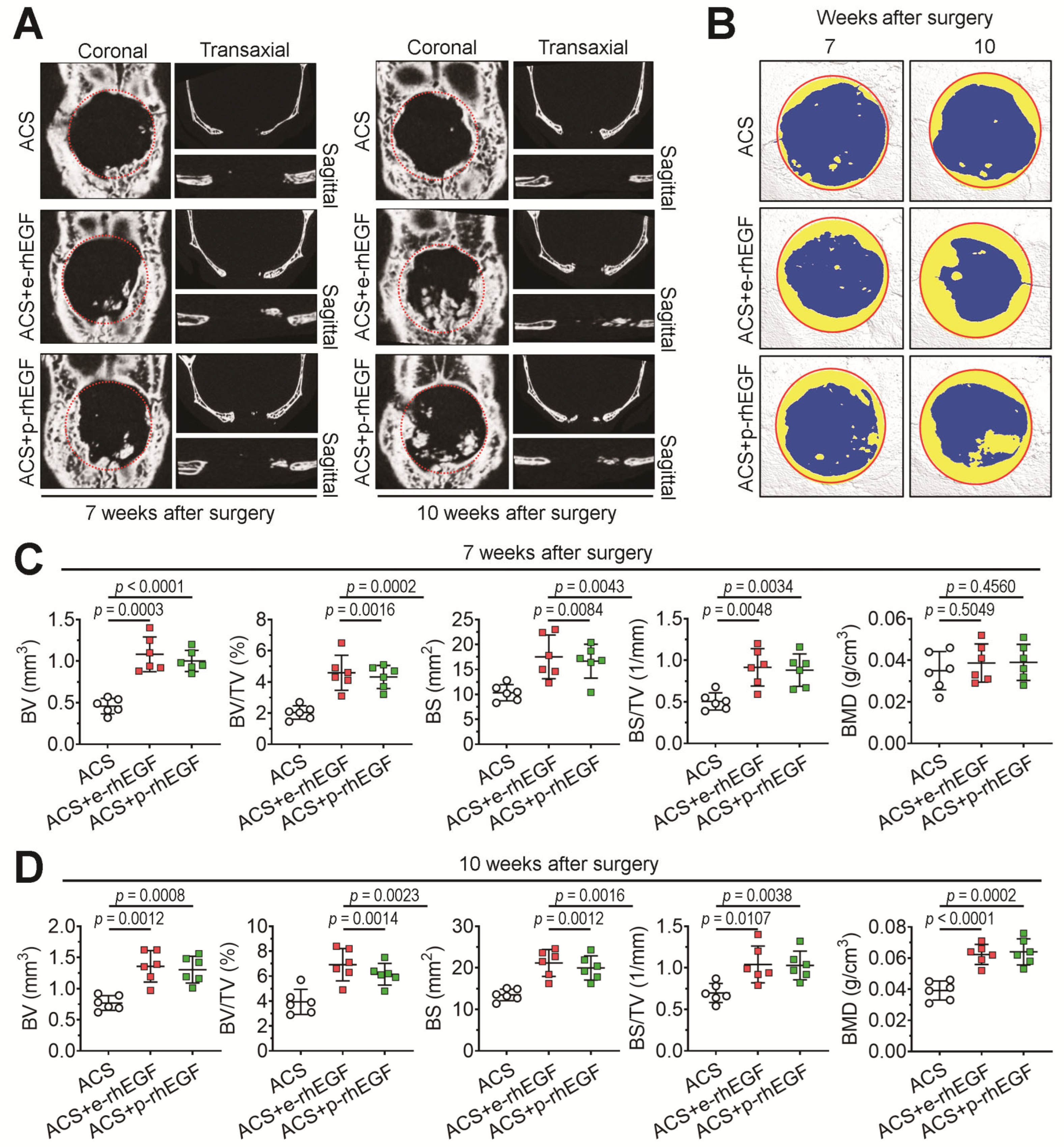
2.8. Live μCT Analysis
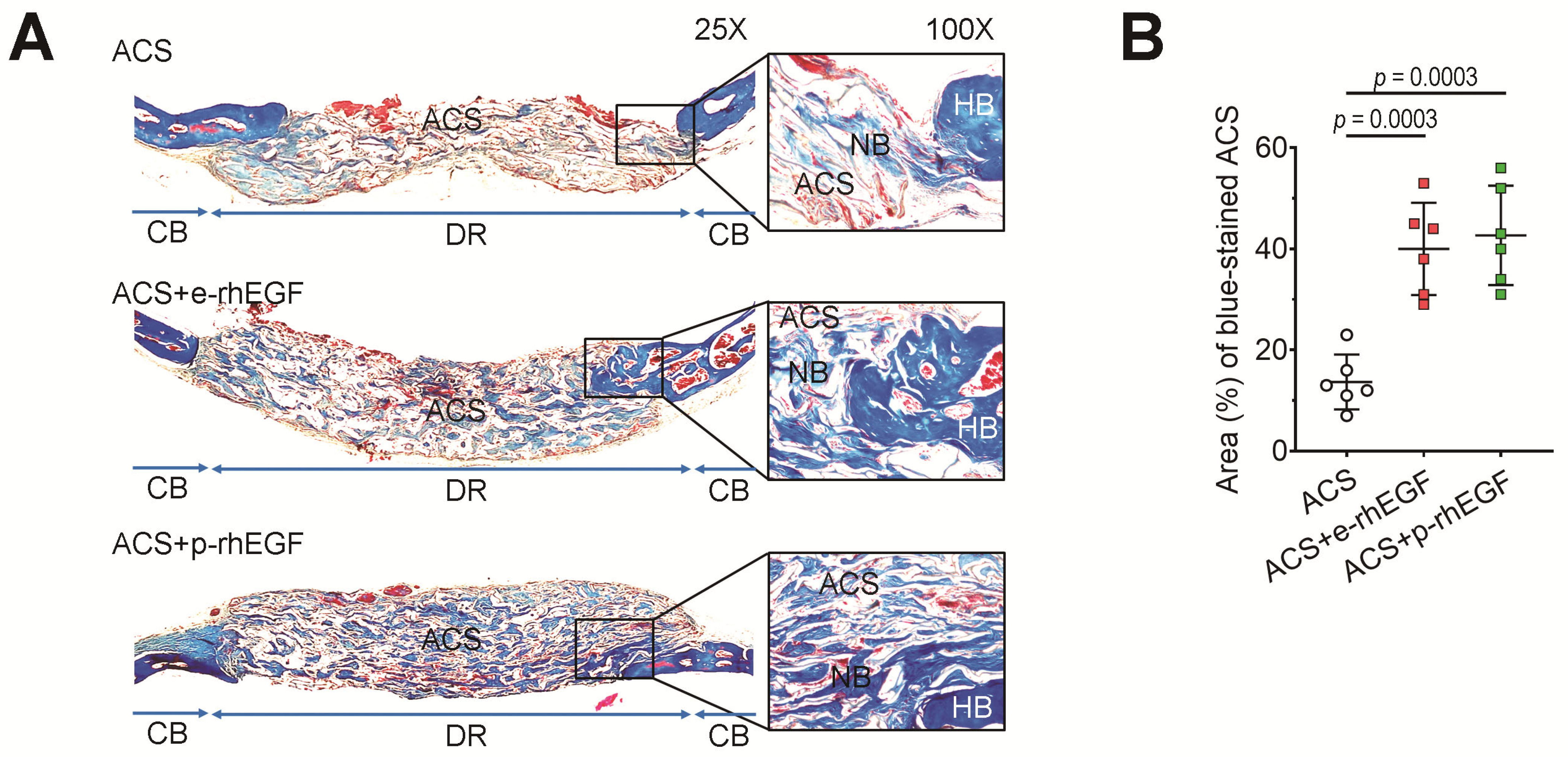
2.9. Immunohistochemistry (IHC) and Masson’s Trichrome (MT) Staining
2.10. Real-Time Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
2.11. In Vitro Evaluations on the p-rhEGF-Stimulated Cellular Responses
2.12. Statistical Analyses
3. Results
3.1. Characterization of ACSs
3.2. Implanting the p-rhEGF-Impregnated ACSs Promotes New Bone Formation in Calvarial Defects Greater Than ACSs Alone Do
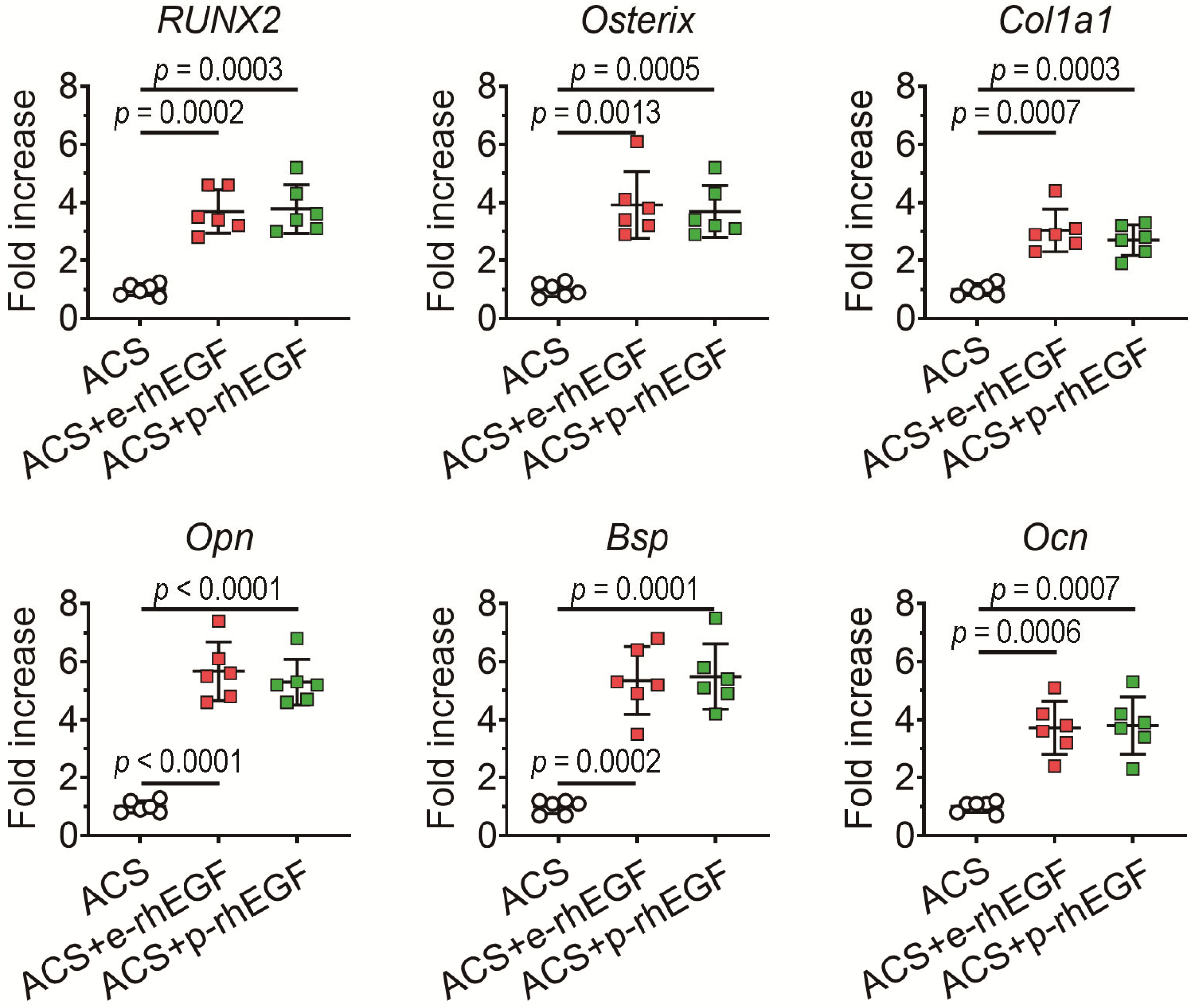
3.3. Enhanced New Bone Formation in the ACS + p-rhEGF Group Is Correlated with the Increased Expression of Osteogenic Marker Molecules
3.4. Exogenous Addition of p-rhEGF Augments Proliferation and Induction of IL-8, BMP2, and VEGF in hPDL Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sculean, A.; Stavropoulos, A.; Bosshardt, D.D. Self-regenerative capacity of intra-oral bone defects. J. Clin. Periodontol. 2019, 46, 70–81. [Google Scholar] [CrossRef] [PubMed]
- De Grado, G.F.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Engin. 2018, 9, 1–18. [Google Scholar]
- Kowalczewski, C.; Saul, J.M. Biomaterials for the delivery of growth factors and other therapeutic agents in tissue engineering approaches to bone regeneration. Front Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Zeng, F.; Harris, R.C. Epidermal growth factor, from gene organization to beside. Semin. Cell Dev. Biol. 2014, 28, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateous, S.; Rodrigues-Diez, R.; Morgado-Pascual, J.L.; Valentijn, F.; Valdivielso, J.M.; Goldschmeding, R.; Ruiz-Ortega, M. Role of epidermal growth factor receptor (EGFR) and its ligands in kidney inflammation and damage. Mediators Inflamm. 2018, 2018, 8739473. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Emans, N. Molecular farming of pharmaceutical proteins. Transgenic Res. 2000, 9, 279–299. [Google Scholar] [CrossRef]
- Shin, Y.J.; Hong, S.Y.; Kwon, T.H.; Jang, Y.S.; Yang, M.S. High level of expression of recombinant human granulocyte-macrophage colony stimulating factor in transgenic rice cell suspension culture. Biotechnol. Bioeng. 2003, 82, 778–783. [Google Scholar] [CrossRef]
- Goldstein, D.A.; Thomas, J.A. Biopharmaceuticals derived from genetically modified plants. QJM 2004, 97, 705–716. [Google Scholar] [CrossRef]
- Jin, T.; Wang, J.; Zhu, X.; Xu, Y.; Zhou, X.; Yang, L. A new transient expression system for large-scale production of recombinant proteins in plants based on air-brushing an Agrobacterium suspension. Biotechnol. Rep. 2015, 6, 36–40. [Google Scholar] [CrossRef]
- Poudel, S.B.; Bhattarai, G.; Kook, S.H.; Shin, Y.J.; Kwon, T.H.; Lee, S.Y.; Lee, J.C. Recombinant human IGF-1 produced by transgenic plant cell suspension culture enhances new bone formation in calvarial defects. Growth Horm. IGF Res. 2017, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.B.; Min, C.K.; Lee, J.H.; Shin, Y.J.; Kwon, T.H.; Jeon, Y.M.; Lee, J.C. Local supplementation with plant-derived recombinant human FGF2 protein enhances bone formation in critical-sized calvarial defects. J. Bone Miner. Metab. 2019, 37, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.S.; Yu, H.Y.; Chung, N.D.; Shin, Y.J.; Kwon, T.H.; Yang, M.S. Production of functional recombinant bovine trypsin in transgenic rice cell suspension cultures. Protein Expr. Purif. 2011, 76, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.D.; Kim, N.S.; Giap, D.V.; Jang, S.H.; Oh, S.M.; Jang, S.H.; Kim, T.G.; Jang, Y.S.; Yang, M.S. Production of functional human vascular endothelial growth factor 165 in transgenic rice cell suspension cultures. Enzym. Microb. Technol. 2014, 63, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Marmey, P.; Taylor, N.J.; Brizard, J.P.; Espinoza, C.; D’Cruz, P.; Huet, H.; Zhang, S.; de Kochko, A.; Beachy, R.N.; et al. Expression and inheritance of multiple transgenes in rice plants. Nat. Biotechnol. 1998, 16, 1060–1064. [Google Scholar] [CrossRef]
- Kim, T.G.; Baek, M.Y.; Lee, E.K.; Kwon, T.H.; Yang, M.S. Expression of human growth hormone in transgenic rice cell suspension culture. Plant Cell Rep. 2008, 27, 885–891. [Google Scholar] [CrossRef]
- Lim, S.S.; Kook, S.H.; Bhattarai, G.; Cho, E.S.; Seo, Y.K.; Lee, J.C. Local delivery of COMP-angiopoietin 1 accelerates new bone formation in rat calvarial defects. J. Biomed. Mater. Res. A 2015, 103, 2942–2951. [Google Scholar] [CrossRef]
- An, N.; Ou, J.; Jiang, D.; Zhang, L.; Liu, J.; Fu, K.; Dai, Y.; Yang, D. Expression of a functional recombinant human basic fibroblast growth factor from transgenic rice seeds. Int. J. Mol. Sci. 2013, 14, 3556–3567. [Google Scholar] [CrossRef]
- Wang, Y.P.; Wei, Z.Y.; Zhong, X.F.; Lin, C.J.; Cai, Y.H.; Ma, J.; Zhang, Y.Y.; Liu, Y.Z.; Xing, S.C. Stable expression of basic fibroblast growth factor in chloroplasts of tobacco. Int. J. Mol. Sci. 2015, 17, 19. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Murmu, A.; Singh, A.; Panda, A.K. Kinetics of inclusion body formation and its correlation with the characteristics of protein aggregates in Escherichia coli. PLoS ONE 2012, 7, e33951. [Google Scholar] [CrossRef]
- Panahi, M.; Alli, Z.; Cheng, X.; Belbaraka, L.; Belgoudi, J.; Sardana, R.; Phipps, J.; Altosaar, I. Recombinant protein expression plasmids optimized for industrial E. coli fermentation and plant systems produce biologically active human insulin-like growth factor-1 in transgenic rice and tobacco plants. Transgenic Res. 2004, 13, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Kusnadi, A.R.; Nikolov, Z.L.; Howard, J.A. Production of recombinant proteins in transgenic plants: Practical considerations. Biotechnol. Bioeng. 1997, 56, 473–484. [Google Scholar] [CrossRef]
- Daniell, H.; Ruiz, G.; Denes, B.; Sandberg, L.; Langridge, W. Optimization of codon omposition and regulatory elements for expression of human insulin like growth factor-1 in transgenic chloroplasts and evaluation of structural identity and function. BMC Biotechnol. 2009, 9, 33. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Tan, C.C.; Ku, J.T.; Hsu, W.C.; Su, S.C.; Lu, C.A.; Huang, L.F. Improving pharmaceutical protein production in Oryza sativa. Int. J. Mol. Sci. 2013, 14, 8719–8739. [Google Scholar] [CrossRef]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the spotlight back on plant suspension cultures. Front. Plant Sci. 2016, 7, 297. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, G.; Jeon, Y.M.; Choi, K.C.; Wagle, S.; Sim, H.J.; Kim, J.I.; Zhao, S.; Kim, J.G.; Cho, E.S.; Kook, S.H.; et al. Functional improvement of collagen-based bioscaffolds to enhance periodontal-defect healing via combination with dietary antioxidant and COMP-angiopoietin 1. Biomat. Adv. 2022, 135, 112673. [Google Scholar] [CrossRef]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of Runx2 in osteogenesis. Eur. Cell Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Hou, Y.; Fang, W. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am. J. Transl. Res. 2015, 7, 2527–2535. [Google Scholar]
- San Martin, I.A.; Varela, N.; Gaete, M.; Villegas, K.; Osorio, M.; Tapia, J.C.; Antonelli, M.; Mancilla, E.E.; Pereira, B.P.; Nathan, S.S.; et al. Impaired cell cycle regulation of the osteoblast-related heterodimeric transcription factor Runx2-Cbfbeta in osteosarcoma cells. J. Cell Physiol. 2009, 221, 560–571. [Google Scholar] [CrossRef]
- Lucero, C.M.; Vega, O.A.; Osorio, M.M.; Tapia, J.C.; Antonelli, M.; Stein, G.S.; van Wijnen, A.J.; Galindo, M.A. The cancer-related transcription factor Runx2 modulates cell proliferation in human osteosarcoma cell lines. J. Cell Physiol. 2013, 228, 714–723. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, X.; Xiao, G.; Kang, Y.; Partridge, N.C.; Qin, L. EGF-like ligands stimulate osteoclastogenesis by regulating expression of osteoclast regulatory factors by osteoblasts: Implications for osteolytic bone metastases. J. Biol. Chem. 2007, 282, 26656–26664. [Google Scholar] [CrossRef] [PubMed]
- Boonanantanasarn, K.; Lee, H.L.; Baek, K.; Woo, K.M.; Ryoo, H.M.; Baek, J.H.; Kim, G.S. EGF inhibits Wnt/β-catenin-induced osteoblast differentiation by promoting β-catenin degradation. J. Cell Biochem. 2015, 116, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.I.; Garant, P.R. Expression and role of epidermal growth factor receptors during differentiation of cementoblasts, osteoblasts, and periodontal ligament fibroblasts in the rat. Anat. Rec. 1996, 245, 342–360. [Google Scholar] [CrossRef]
- Tamama, K.; Kawasaki, H.; Wells, A. Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). Possible enhancement of therapeutic potential of MSC. J. Biomed. Biotechnol. 2010, 2010, 795385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Zhang, M.; Jin, M.; Bai, C.; Wang, X. Potential mechanism of interleukin-8 production from lung cancer cells: An involvement of EGF-EGFR-PI3K-Akt-Erk pathway. J. Cell Physiol. 2012, 227, 35–43. [Google Scholar] [CrossRef]
- Shen, X.H.; Xu, S.J.; Jin, C.Y.; Ding, F.; Zhou, Y.C.; Fu, G.S. Interleukin-8 prevents oxidative stress-induced human endothelial cell senescence via telomerase activation. Int. Immunopharmacol. 2013, 16, 261–267. [Google Scholar] [CrossRef]
- Teramatsu, Y.; Maeda, H.; Sugii, H.; Tomokiyo, A.; Hamano, S.; Wada, N.; Yamamoto, N.; Koori, K.; Akamine, A. Expression and effects of epidermal growth factor on human periodontal ligament cells. Cell Tissue Res. 2014, 357, 633–643. [Google Scholar] [CrossRef]
- Marquez, L.; de Abreu, F.A.; Ferreira, C.L.; Alves, G.D.; Miziara, M.N.; Alves, J.B. Enhanced bone healing of rat tooth sockets after administration of epidermal growth factor (EGF) carried by liposome. Injury 2013, 44, 558–564. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, S.J.; Baek, H.R.; Lee, K.M.; Chang, B.S.; Lee, C.K. Synergistic induction of early stage of bone formation by combination of recombinant human bone morphogenetic protein-2 and epidermal growth factor. J. Tissue Eng. Regen. Med. 2015, 9, 447–459. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, S.B.; Bhattarai, G.; Kwon, T.-H.; Lee, J.-C. Biopotentials of Collagen Scaffold Impregnated with Plant-Cell-Derived Epidermal Growth Factor in Defective Bone Healing. Materials 2023, 16, 3335. https://doi.org/10.3390/ma16093335
Poudel SB, Bhattarai G, Kwon T-H, Lee J-C. Biopotentials of Collagen Scaffold Impregnated with Plant-Cell-Derived Epidermal Growth Factor in Defective Bone Healing. Materials. 2023; 16(9):3335. https://doi.org/10.3390/ma16093335
Chicago/Turabian StylePoudel, Sher Bahadur, Govinda Bhattarai, Tae-Ho Kwon, and Jeong-Chae Lee. 2023. "Biopotentials of Collagen Scaffold Impregnated with Plant-Cell-Derived Epidermal Growth Factor in Defective Bone Healing" Materials 16, no. 9: 3335. https://doi.org/10.3390/ma16093335
APA StylePoudel, S. B., Bhattarai, G., Kwon, T.-H., & Lee, J.-C. (2023). Biopotentials of Collagen Scaffold Impregnated with Plant-Cell-Derived Epidermal Growth Factor in Defective Bone Healing. Materials, 16(9), 3335. https://doi.org/10.3390/ma16093335





