Insight into the Mechanism of Cobalt-Nickel Separation Using DFT Calculations on Ethylenediamine-Modified Silica Gel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Ethylenediamine Modified Silica Gel
2.2. Adsorption Experiment
2.3. Structural Characterization
2.4. Computational Methods
3. Results and Discussion
3.1. Ethylenediamine Modified Silica Gel
3.2. Comparison of the Adsorption Properties of Ni and Co on SG and en/SG
3.3. Co(II) and Ni(II) Stable Species in Sulfate Solution
3.4. The separation Mechanism of en/SG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, T.; Chen, J.; Li, H.; Li, K. An integrated process for the separation and recovery of valuable metals from the spent LiNi0.5Co0.2Mn0.3O2 cathode materials. Sep. Purif. Technol. 2020, 245, 116869. [Google Scholar] [CrossRef]
- Akcil, A.; Sun, Z.; Panda, S. COVID-19 disruptions to tech-metals supply are a wake up call. Nature 2020, 587, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, W.; Huang, H.; Yan, W.; Li, X.; Ning, P.; Cao, H.; Sun, Z. Recycling of spent lithium-ion batteries in view of green chemistry. Green Chem. 2021, 23, 6139–6171. [Google Scholar] [CrossRef]
- Zheng, Q.; Cao, Z.; Wu, S.; Li, Q.; Wang, M.; Guan, W.; Zhang, G. Direct Extraction of Ni(II) from Acidic Polymetallic Sulfate Media with a Novel Synergistic Extraction System of DNNSA-MSL211. ACS Sustain. Chem. Eng. 2023, 11, 3416–3428. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Huang, W.; Omran, M.; Zhang, F.; Gao, L.; Chen, G. Reductive roasting of cathode powder of spent ternary lithium-ion battery by pyrolysis of invasive plant Crofton weed. Renew. Energy 2023, 206, 86–96. [Google Scholar] [CrossRef]
- Wang, M.; Liu, K.; Xu, Z.; Dutta, S.; Valix, M.; Alessi, D.S.; Huang, L.; Zimmerman, J.B.; Tsang, D.C.W. Selective Extraction of Critical Metals from Spent Lithium-Ion Batteries. Environ. Sci. Technol. 2023, 57, 3940–3950. [Google Scholar] [CrossRef]
- Vieceli, N.; Ottink, T.; Stopic, S.; Dertmann, C.; Swiontek, T.; Vonderstein, C.; Sojka, R.; Reinhardt, N.; Ekberg, C.; Friedrich, B.; et al. Solvent extraction of cobalt from spent lithium-ion batteries: Dynamic optimization of the number of extraction stages using factorial design of experiments and response surface methodology. Sep. Purif. Technol. 2023, 307, 122793. [Google Scholar] [CrossRef]
- Rana, M.; Khan, M.I.H.; Nshizirungu, T.; Jo, Y.-T.; Park, J.-H. Green and sustainable route for the efficient leaching and recovery of valuable metals from spent Ni−Cd batteries. Chem. Eng. J. 2023, 455, 140626. [Google Scholar] [CrossRef]
- Tang, Y.-C.; Wang, J.-Z.; Shen, Y.-H. Separation of Valuable Metals in the Recycling of Lithium Batteries via Solvent Extraction. Minerals 2023, 13, 285. [Google Scholar] [CrossRef]
- Chaudhary, V.; Lakhera, P.; Kim, K.-H.; Deep, A.; Kumar, P. Insights into the Eco-Friendly Recovery Process for Valuable Metals from Waste Lithium-ion Batteries by Organic Acids Leaching. Sep. Purif. Rev. 2023, 1–18. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; He, C.; Wei, Y.; Fujita, T.; Wang, G.; Ma, S.; Yang, W. Solvent extraction and separation of cobalt from leachate of spent lithium-ion battery cathodes with N263 in nitrite media. Int. J. Miner. Metall. Mater. 2023, 30, 897–907. [Google Scholar] [CrossRef]
- Wen, J.; Lee, M.S. Recovery of Nickel and Cobalt Metal Powders from the Leaching Solution of Spent Lithium-Ion Battery by Solvent Extraction and Chemical Reduction. Miner. Process. Extr. Metall. Rev. 2023, 1–11. [Google Scholar] [CrossRef]
- Bagdat, S.; Tokay, F.; Demirci, S.; Yilmaz, S.; Sahiner, N. Removal of Cd(II), Co(II), Cr(III), Ni(II), Pb(II) and Zn(II) ions from wastewater using polyethyleneimine (PEI) cryogels. J. Environ. Manag. 2023, 329, 117002. [Google Scholar] [CrossRef] [PubMed]
- Giove, A.; El Ouardi, Y.; Sala, A.; Ibrahim, F.; Hietala, S.; Sievanen, E.; Branger, C.; Laatikainen, K. Highly selective recovery of Ni(II) in neutral and acidic media using a novel Ni(II)-ion imprinted polymer. J. Hazard. Mater. 2023, 444 Pt B, 130453. [Google Scholar] [CrossRef]
- Rahman, A.; Haque, M.A.; Ghosh, S.; Shinu, P.; Attimarad, M.; Kobayashi, G. Modified Shrimp-Based Chitosan as an Emerging Adsorbent Removing Heavy Metals (Chromium, Nickel, Arsenic, and Cobalt) from Polluted Water. Sustainability 2023, 15, 2431. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Liu, S.; Zhang, G.; Dong, L.; Gu, P.; Hou, L.A. Layered metal sulfide NMTS for rapid removal of radioactive strontium ions from aqueous solution. Sep. Purif. Technol. 2023, 310, 122887. [Google Scholar] [CrossRef]
- Jally, B.; François, M.; Kessler, M.; Laubie, B.; Simonnot, M.-O. Recovery of nickel from strongly acidic bio-ore leachate using a bispicolylamine-based chelating resin. Sep. Purif. Technol. 2022, 239, 121126. [Google Scholar] [CrossRef]
- Orooji, Y.; Han, N.; Nezafat, Z.; Shafiei, N.; Shen, Z.; Nasrollahzadeh, M.; Karimi-Maleh, H.; Luque, R.; Bokhari, A.; Klemeš, J.J. Valorisation of nuts biowaste: Prospects in sustainable bio(nano)catalysts and environmental applications. J. Clean. Prod. 2022, 347, 131220. [Google Scholar] [CrossRef]
- Irfan, D.; Tang, X.; Abdulhasanb, M.J.; Zaidi, M.; Mustafa, Y.F.; Jasem, H.; Altimari, U.S.; Chem, C. Epichlorohydrin Crosslinked 2,4-Dihydroxybenzaldehyde Schiff Base Chitosan@SrFe12O19 (EP-DBSB-CS@SrFe12O19) Magnetic Nanocomposite for Efficient Removal of Pb(II) and Cd(II) from Aqueous Solution. J. Polym. Environ. 2022, 30, 4201–4209. [Google Scholar] [CrossRef]
- Wang, Y.; Kou, X.; Zhao, J.; Xin, A. Three sources-in-one bio-MOFs doped silicone acrylic emulsion-based waterborne flame-retarding coatings with formaldehyde adsorption property. Prog. Org. Coat. 2023, 175, 107329. [Google Scholar] [CrossRef]
- Xue, Y.; Cheng, W.; Cao, M.; Gao, J.; Chen, J.; Gui, Y.; Zhu, W.; Ma, F. Development of nitric acid-modified activated carbon electrode for removal of Co2+/Mn2+/Ni2+ by electrosorption. Environ. Sci. Pollut. Res. 2022, 29, 77536–77552. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, K.; Rajesh, S.; Madhappan, S.; Gnanasekaran, L.; Guganathan, L.; Phan, T.T.V.; Kim, S.C. Selective removal of copper (II) ions from aqueous solution using pyridyl-bridged mesoporous organosilica hybrid adsorbent. Environ. Res. 2023, 224, 115439. [Google Scholar] [CrossRef]
- Piatek, J.; de Bruin-Dickason, C.N.; Jaworski, A.; Chen, J.; Budnyak, T.; Slabon, A. Glycine-functionalized silica as sobent for cobalt(II) and nickel(II) recovery. Appl. Surf. Sci. 2020, 530, 147299. [Google Scholar] [CrossRef]
- Suharso; Buhani; Sumadi. Immobilization of S. duplicatum supported silica gel matrix and its application on adsorption–desorption of Cu (II), Cd (II) and Pb (II) ions. Desalination 2010, 263, 64–69. [Google Scholar] [CrossRef]
- Lu, J.; Wu, X.; Li, Y.; Cui, W.; Liang, Y. Modified silica gel surface with chelating ligand for effective mercury ions adsorption. Surf. Interfaces 2018, 12, 108–115. [Google Scholar] [CrossRef]
- Hatay, I.; Gup, R.; Ersoz, M. Silica gel functionalized with 4-phenylacetophynone 4-aminobenzoylhydrazone: Synthesis of a new chelating matrix and its application as metal ion collector. J. Hazard. Mater. 2008, 150, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Mittal, S.; Azami, S.; Adholeya, A. Surface modified silica gel for extraction of metal ions: An environment friendly method for waste treatment. Surf. Eng. 2013, 21, 232–237. [Google Scholar] [CrossRef]
- Wang, M.; Qu, R.; Sun, C.; Yin, P.; Chen, H. Dynamic adsorption behavior and mechanism of transition metal ions on silica gels functionalized with hydroxyl- or amino-terminated polyamines. Chem. Eng. J. 2013, 221, 264–274. [Google Scholar] [CrossRef]
- Kondo, K.; Kanazawa, Y.; Matsumoto, M. Adsorption of Noble Metals Using Silica Gel Modified with Surfactant Molecular Assembly Containing an Extractant. Sep. Sci. Technol. 2015, 50, 1453–1460. [Google Scholar] [CrossRef]
- Bazargan, M.; Mirzaei, M.; Franconetti, A.; Frontera, A. On the preferences of five-membered chelate rings in coordination chemistry: Insights from the Cambridge Structural Database and theoretical calculations. Dalton Trans. 2019, 48, 5476–5490. [Google Scholar] [CrossRef]
- Han, H.; Wang, Y.; Zhang, Y.; Cong, Y.; Qin, J.; Gao, R.; Chai, C.; Song, Y. Oxygen Reduction Reaction Electrocatalysts Derived from Metalloporphyrin-Modified Meso-/Macroporous Polyaniline. Acta Phys. Chim. Sin. 2020, 37, 2008017. [Google Scholar] [CrossRef]
- Haider, M.I.; Hu, H.; Seewald, T.; Ahmed, S.; Sultan, M.; Schmidt-Mende, L.; Fakharuddin, A. Ethylenediamine Vapors-Assisted Surface Passivation of Perovskite Films for Efficient Inverted Solar Cells. Sol. RRL 2023, 2201092, 1–12. [Google Scholar] [CrossRef]
- Parlar, E.D.; Özten, Ö.; Kızılaslan, A.; Can, M. Synthesis of an ethylene diamine modified tannin polymer and recovery of gold(iii) ions from electronic wastes. React. Chem. Eng. 2023. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Chen, J.; Jiang, C.; Wu, S.; Wang, H. Effective removal of heavy metals with amino-functionalized silica gel in tea polyphenol extracts. J. Food Meas. Charact. 2020, 14, 2134–2144. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Hu, X.; Wu, Y.; Tang, X.; He, Q.; Peng, S. Enhanced selective adsorption of lead(II) from complex wastewater by DTPA functionalized chitosan-coated magnetic silica nanoparticles based on anion-synergism. J. Hazard. Mater. 2022, 422, 126856. [Google Scholar] [CrossRef]
- Arakaki, L.N.H.; Filha, V.L.S.A.; Germano, A.F.S.; Santos, S.S.G.; Fonseca, M.G.; Sousa, K.S.; Espínola, J.G.P.; Arakaki, T. Silica gel modified with ethylenediamine and succinic acid-adsorption and calorimetry of cations in aqueous solution. Thermochim. Acta 2013, 556, 34–40. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Soliman, E.M. Novel route for silylation of silica gel and aliphatic amines immobilization based on microwave-assisted solvent free synthesis and their applications for Cu(II) and Fe(III) removal from natural water samples. J. Environ. Sci. Health 2013, 48, 817–828. [Google Scholar] [CrossRef]
- Tang, Y.; Zheng, M.; Ren, C.; Li, X.; Guo, L. Visualized Reaction Tracking and Physical Property Analysis for a Picked 3D Area in a Reactive Molecular Dynamics Simulation System. Acta Phys.-Chim. Sin. 2020, 37, 2003037. [Google Scholar] [CrossRef]
- Ramarajan, D.; Tamilarasan, K.; Milanović, Ž.; Milenković, D.; Marković, Z.; Sudha, S.; Kavitha, E. Vibrational spectroscopic studies (FTIR and FT-Raman) and molecular dynamics analysis of industry inspired 3-amino-4-hydroxybenzene sulfonic acid. J. Mol. Struct. 2020, 1205, 127579. [Google Scholar] [CrossRef]
- Bharathy, G.; Prasana, J.C.; Muthu, S.; Irfan, A.; Asif, F.B.; Saral, A.; Aayisha, S.; Niranjana devi, R. Evaluation of electronic and biological interactions between N-[4-(Ethylsulfamoyl)phenyl]acetamide and some polar liquids (IEFPCM solvation model) with Fukui function and molecular docking analysis. J. Mol. Liq. 2021, 340, 117271. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q.; Hu, J.; Fu, M.; Song, Y.; Hu, H.; Hu, F. Insights into coextraction of water and ammonia with nickel(II) through structural investigation. J. Mol. Liq. 2016, 213, 23–27. [Google Scholar] [CrossRef]
- Uchikoshi, M.; Shinoda, K. Determination of structures of cobalt(II)-chloro complexes in hydrochloric acid solutions by X-ray absorption spectroscopy at 298 K. Struct. Chem. 2018, 30, 945–954. [Google Scholar] [CrossRef]
- Yuan, L.; Wen, J.; Ning, P.; Yang, H.; Sun, Z.; Cao, H. Inhibition Role of Solvation on the Selective Extraction of Co(II): Toward Eco-Friendly Separation of Ni and Co. ACS Sustain. Chem. Eng. 2022, 10, 1160–1171. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, H.; Ning, P.; Wen, J.; Sun, Z.; Cao, H. Green separation and recovery of cobalt and nickel from sulphuric acid achieved by complexation-assisted solvent extraction. Sep. Purif. Technol. 2022, 286, 120343. [Google Scholar] [CrossRef]
- Hu, F.; Hu, H.; Yang, J.; Luo, Y.; Lundstrom, M.; Ji, G.; Hu, J. Preferential extraction of Ni(II) over Co(II) by arylsulphonic acid in the presence of pyridinecarboxylate ester: Experiemntal and DFT calculations. J. Mol. Liq. 2019, 291, 111253. [Google Scholar] [CrossRef]
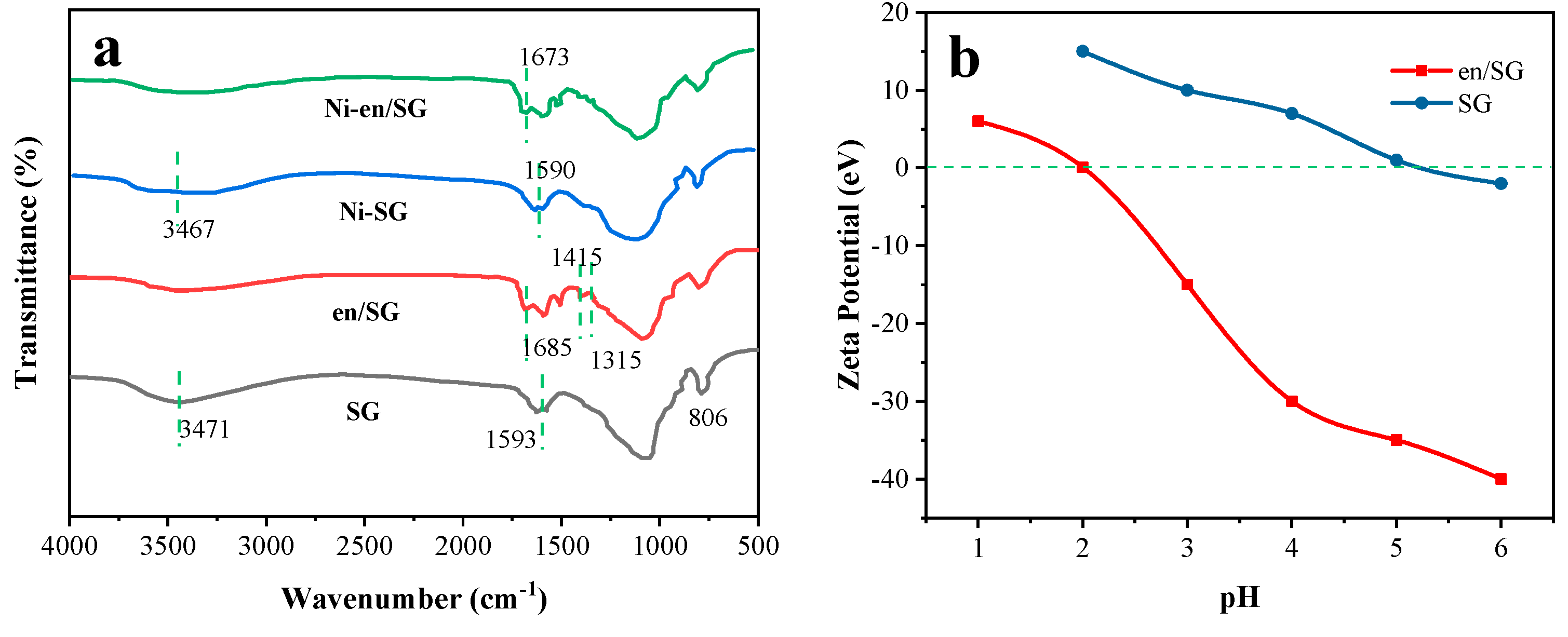
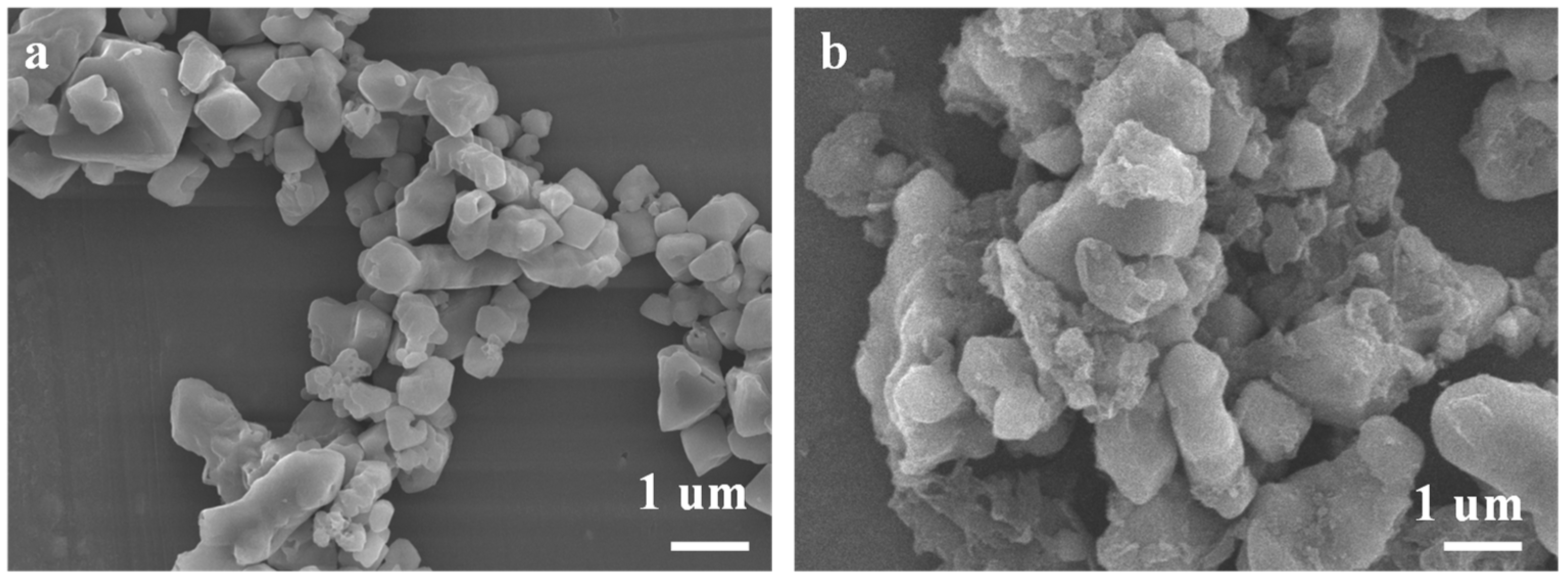


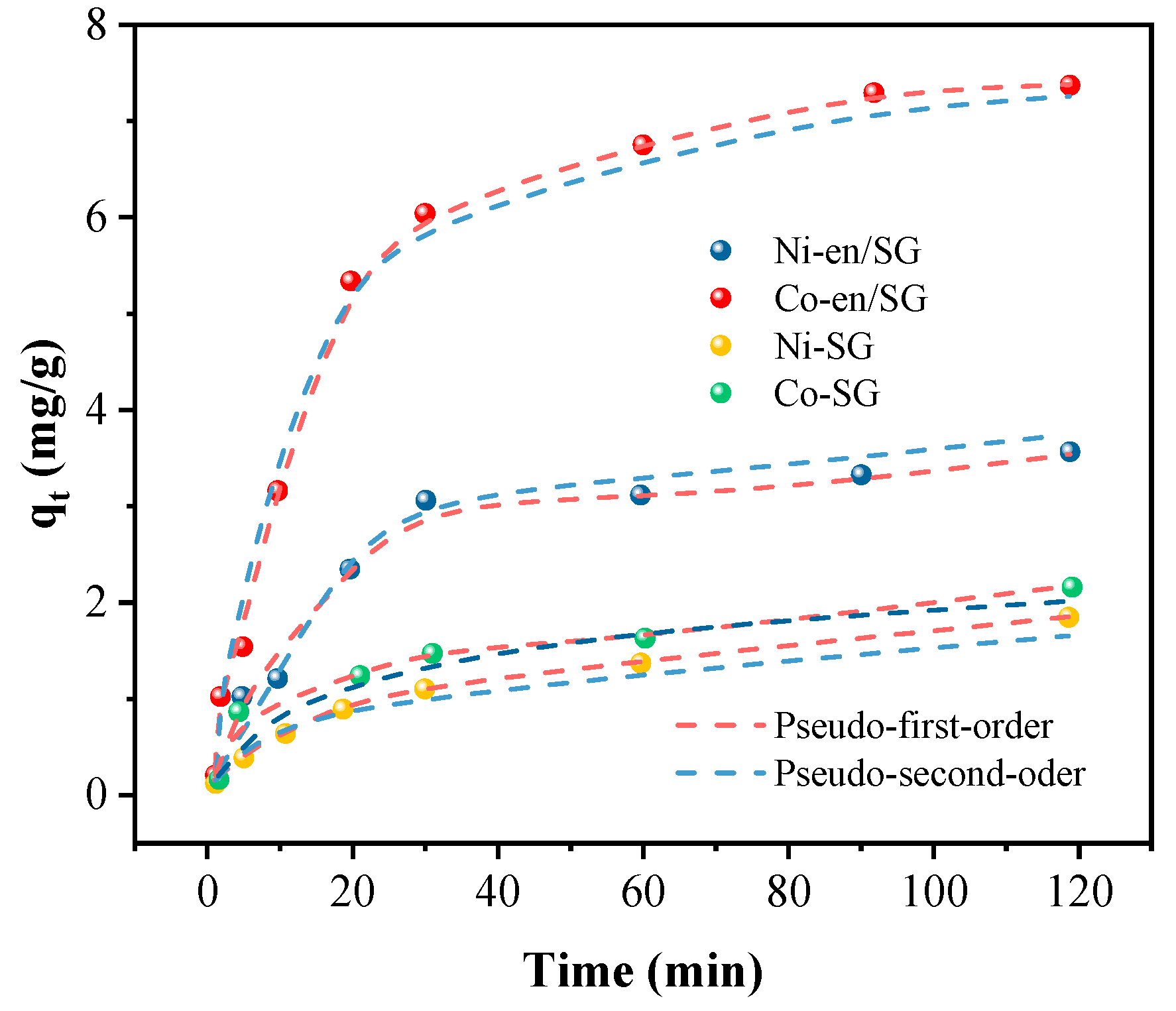


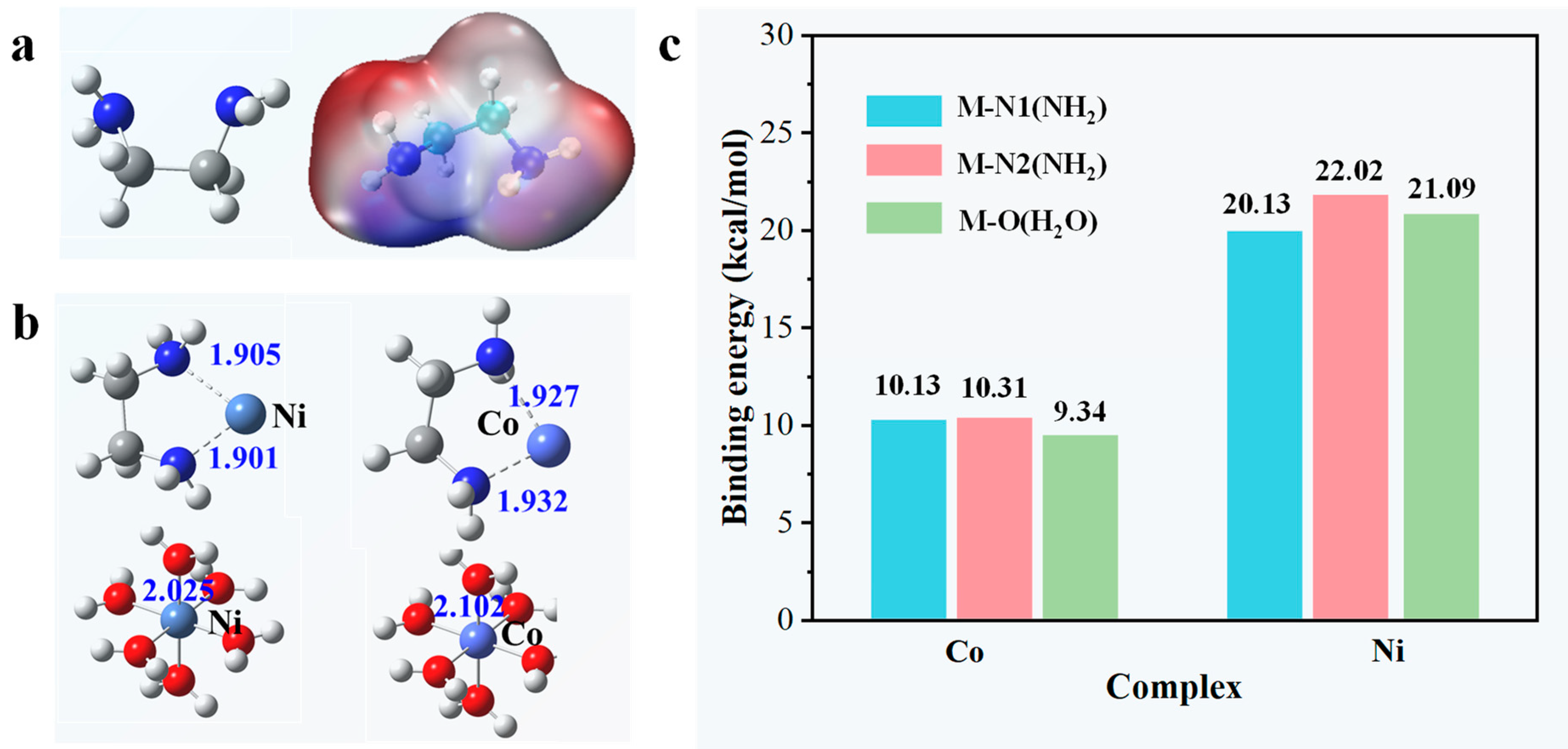
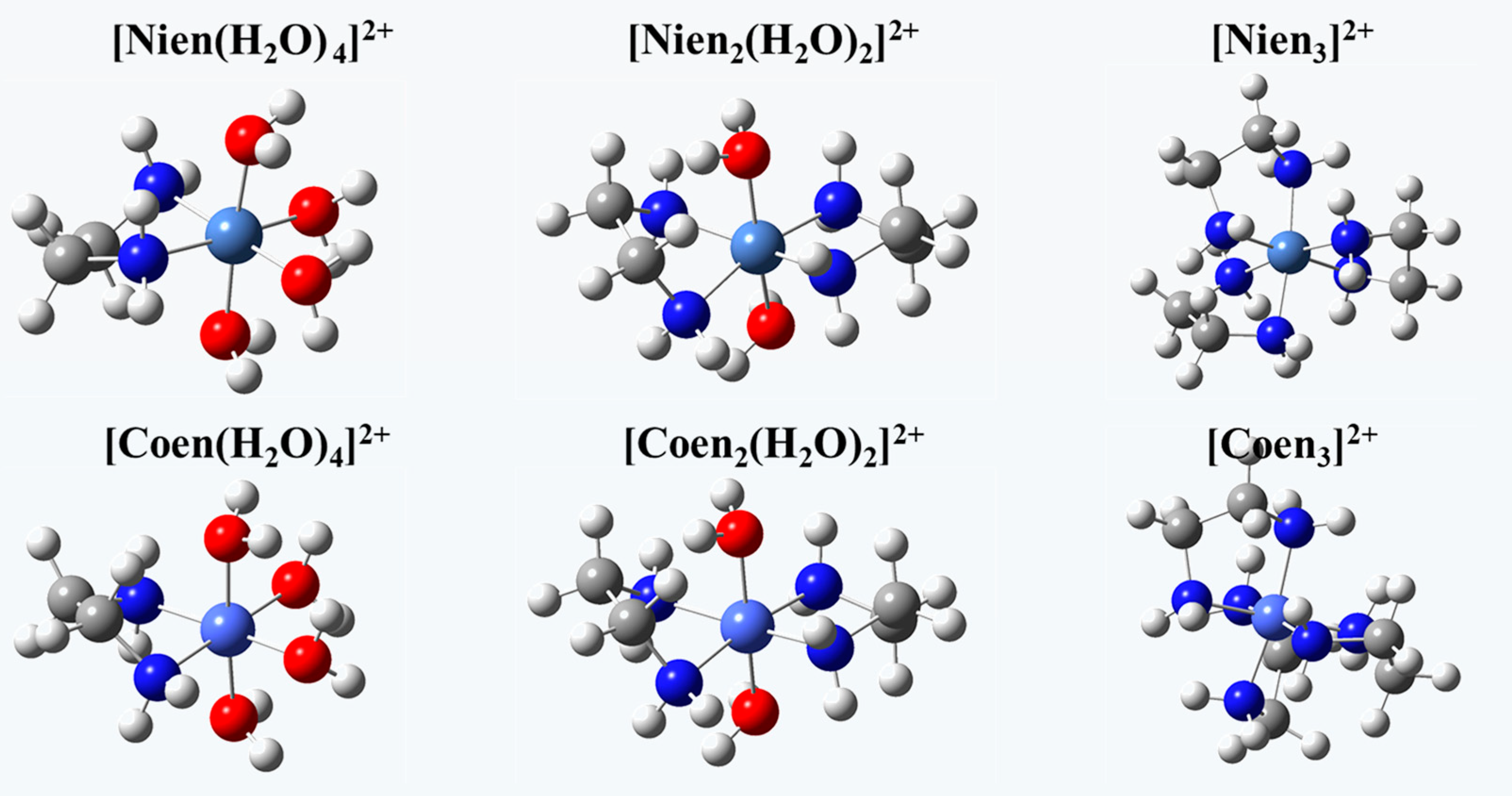
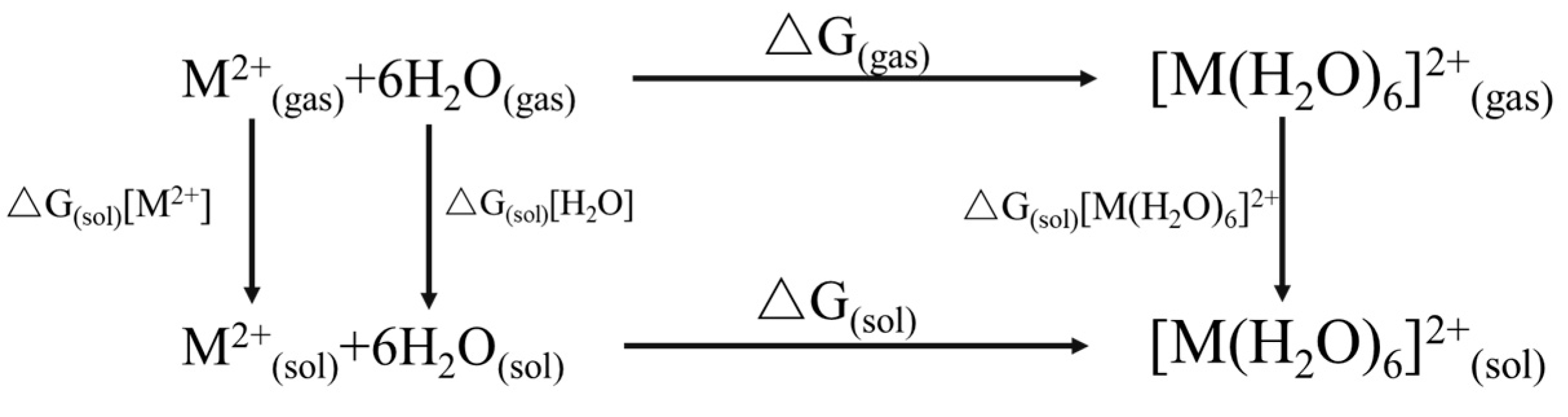

| Adsorbent | Species | pH = 2 | pH = 3 | pH = 4 | pH = 5 | pH = 6 |
|---|---|---|---|---|---|---|
| SG | Ni | 3.1% | 5.3% | 8.6% | 9.5% | 11.2% |
| Co | 4.4% | 8.1% | 10.4% | 13.4% | 17.9% | |
| en/SG | Ni | 4.5% | 6.8% | 10.2% | 12.2% | 14.3% |
| Co | 5.9% | 21.3% | 44.8% | 61.7% | 78.2% |
| M-Co | M-Ni | |
|---|---|---|
| ΔGsol(M2+): kJ/mol | −1812.91 | −1794.92 |
| ΔG1(comp): kJ/mol | −120.96 | −97.25 |
| ΔG2(comp): kJ/mol | −110.17 | −86.69 |
| ΔG3(comp): kJ/mol | −45.17 | −76.15 |
| ΔGtotal = ΔGsol + ΔG1 | −1933.87 | −1892.17 |
| en/SG | en/SG Bond Lengths (Å) | en/SG-Ni(H2O)4 Bond Lengths (Å) | en/SG-Co(H2O)4 Bond Lengths |
|---|---|---|---|
| R(Si1-O2) | 1.654 | 1.653 | 1.652 |
| R(Si8-O2) | 1.669 | 1.670 | 1.669 |
| R(Si1-O2) | 1.606 | 1.607 | 1.605 |
| R(N24-C27) | 1.457 | 1.535 | 1.522 |
| R(N32-C26) | 1.464 | 1.532 | 1.520 |
| R(M-N24) | - | 1.441 | 1.432 |
| R(M-N32) | - | 1.440 | 1.431 |
| Thermodynamic Parameters | M-Co | M-Ni |
|---|---|---|
| ΔH(comp) (KJ/mol) | −122.33 | −90.29 |
| ΔG(comp) (KJ/mol) | −134.13 | −96.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Yuan, L.; Yuan, M.; Ning, P. Insight into the Mechanism of Cobalt-Nickel Separation Using DFT Calculations on Ethylenediamine-Modified Silica Gel. Materials 2023, 16, 3445. https://doi.org/10.3390/ma16093445
Yang H, Yuan L, Yuan M, Ning P. Insight into the Mechanism of Cobalt-Nickel Separation Using DFT Calculations on Ethylenediamine-Modified Silica Gel. Materials. 2023; 16(9):3445. https://doi.org/10.3390/ma16093445
Chicago/Turabian StyleYang, Hailun, Ling Yuan, Menglei Yuan, and Pengge Ning. 2023. "Insight into the Mechanism of Cobalt-Nickel Separation Using DFT Calculations on Ethylenediamine-Modified Silica Gel" Materials 16, no. 9: 3445. https://doi.org/10.3390/ma16093445
APA StyleYang, H., Yuan, L., Yuan, M., & Ning, P. (2023). Insight into the Mechanism of Cobalt-Nickel Separation Using DFT Calculations on Ethylenediamine-Modified Silica Gel. Materials, 16(9), 3445. https://doi.org/10.3390/ma16093445








