Corrosion of Titanium Electrode Used for Solar Saline Electroflotation
Abstract
1. Introduction
2. Materials and Methods
2.1. Corrosion of Ti Rod Electrode Study
2.2. Solar Electroflotation Simple Cell
2.3. Electrodes Surface Characterization
2.4. Weigh-Loss Measurements
3. Results and Discussion
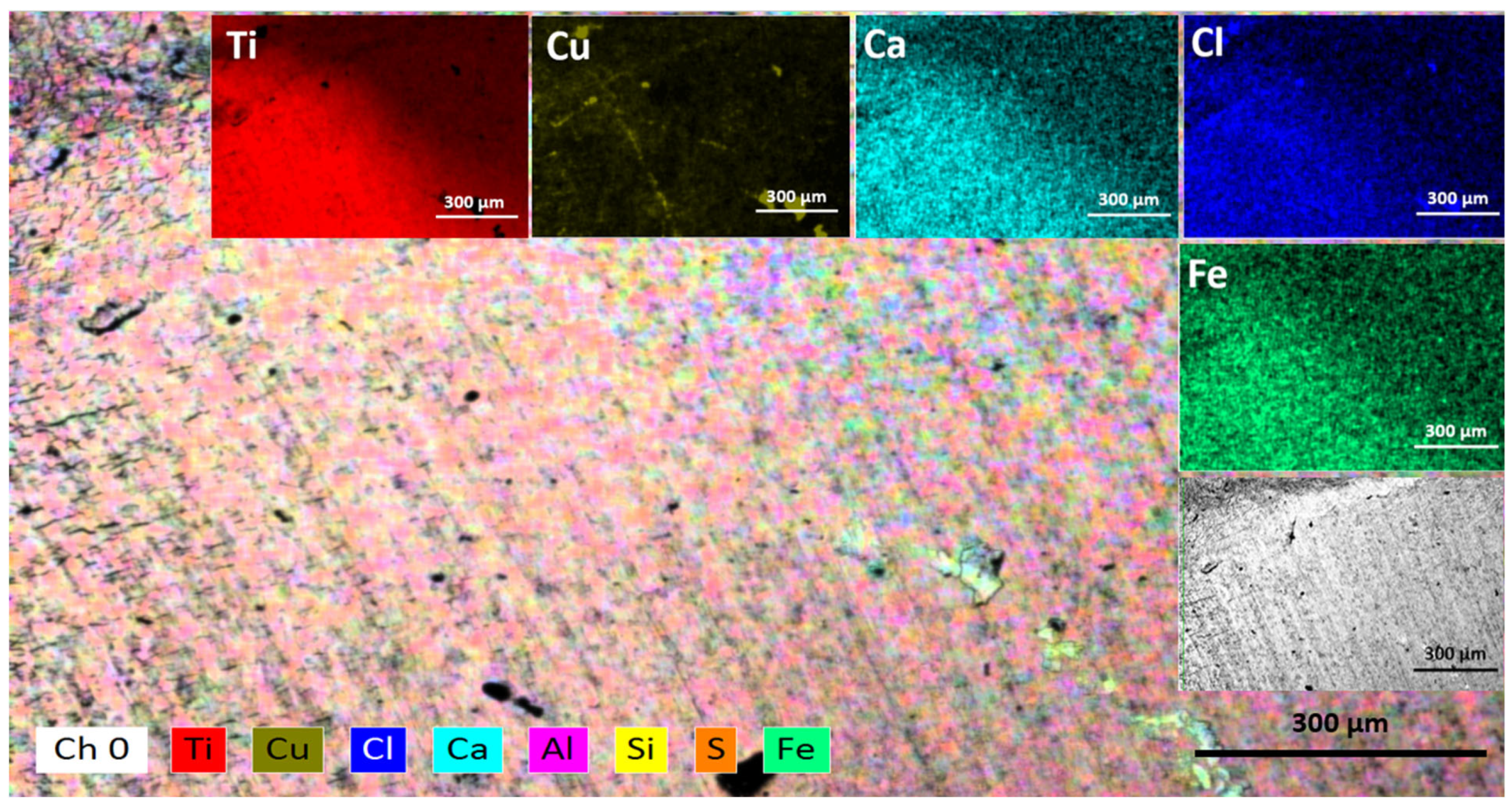
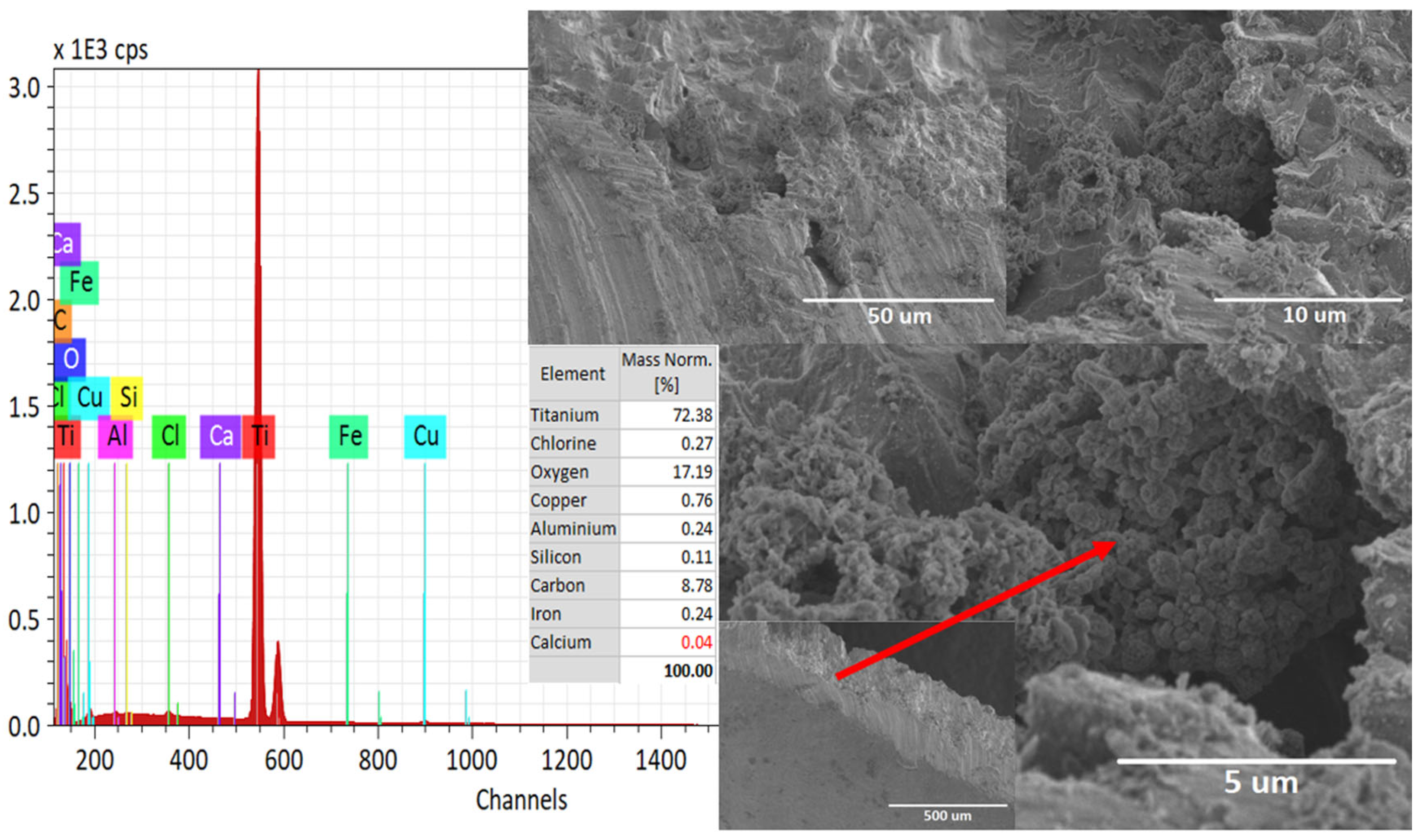
3.1. Surface and Elemental Analysis after the Corrosion Process
3.2. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Msigwa, G.; Yang, M.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Strategies to achieve a carbon neutral society: A review. Environ. Chem. Lett. 2022, 20, 2277–2310. [Google Scholar]
- Igogo, T.; Awuah-Offei, K.; Newman, A.; Lowder, T.; Engel-Cox, J. Integrating renewable energy into mining operations: Opportunities, challenges, and enabling approaches. Appl. Energy 2021, 300, 117375. [Google Scholar]
- Dalini, E.A.; Karimi, G.; Zandevakili, S.; Goodarzi, M. A Review on Environmental, Economic and Hydrometallurgical Processes of Recycling Spent Lithium-ion Batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 451–472. [Google Scholar]
- McLellan, B.C.; Corder, G.D.; Giurco, D.P.; Ishihara, K.N. Renewable energy in the minerals industry: A review of global potential. J. Clean. Prod. 2012, 32, 32–44. [Google Scholar]
- Moreno-Leiva, S.; Díaz-Ferrán, G.; Haas, J.; Telsnig, T.; Díaz-Alvarado, F.A.; Palma-Behnke, R.; Kracht, W.; Román, R.; Chudinzow, D.; Eltrop, L. Towards solar power supply for copper production in Chile: Assessment of global warming potential using a life-cycle approach. J. Clean. Prod. 2017, 164, 242–249. [Google Scholar]
- Bhaskar, R.G. Electroflotation-A Critical Review. Available online: https://www.researchgate.net/publication/256498999 (accessed on 1 August 2022).
- Kyzas, G.Z.; Matis, K.A. Electroflotation process: A review. J. Mol. Liq. 2016, 220, 657–664. [Google Scholar] [CrossRef]
- Naimi, Y.; Antar, A. Hydrogen Generation by Water Electrolysis. Adv. Hydrog. Gener. Technol. 2018, 1. [Google Scholar] [CrossRef]
- Janssen, L.J.J.; Hoogland, J.G. The effect of electrolytically evolved gas bubbles on the thickness of the diffusion layer. Electrochim. Acta 1970, 15, 1013–1023. [Google Scholar]
- Khosla, N.K.; Venkatachalam, S.; Somasundaran, P. Pulsed electrogeneration of bubbles for electroflotation. J. Appl. Electrochem. 1991, 21, 986–990. [Google Scholar]
- Paparao, J.; Murugan, S. Oxy-hydrogen gas as an alternative fuel for heat and power generation applications—A review. Int. J. Hydrogen Energy 2021, 46, 37705–37735. [Google Scholar]
- Streblau, M.; Aprahamian, B.; Simov, M.; Dimova, T. The influence of the electrolyte parameters on the efficiency of the oxyhydrogen (HHO) generator. In Proceedings of the 2014 18th International Symposium on Electrical Apparatus and Technologies (SIELA), Bourgas, Bulgaria, 29–31 May 2014; pp. 1–4. [Google Scholar] [CrossRef]
- Tadesse, B.; Albijanic, B.; Makuei, F.; Browner, R. Recovery of Fine and Ultrafine Mineral Particles by Electroflotation–A Review. Miner. Process. Extr. Met. Rev. 2019, 40, 108–122. [Google Scholar] [CrossRef]
- Raju, G.B.; Khangaonkar, P.R. Electroflotation of chalcopyrite fines with sodium diethyldithiocarbamate as collector. Int. J. Miner. Process. 1984, 13, 211–221. [Google Scholar]
- Makuei, F.; Tadesse, B.; Albijanic, B.; Browner, R. Electroflotation of ultrafine chalcopyrite particles with sodium oleate collector. Min. Eng. 2018, 120, 44–46. [Google Scholar]
- Gilberto, L.; Pino, G.; Torem, M. Electroflotation of cassiterite fines using a hydrophobic bacterium strain. Rev. Esc. De Minas Ouro Preto 2013, 66, 507–512. [Google Scholar]
- Tumsri, K.; Chavalparit, O. Optimizing Electrocoagulation-electroflotation Process for Algae Removal. In Proceedings of the 2nd International Conference on Environmental Science and Technology IPCBEE, Singapore, 26–28 February 2011; Volume 6, pp. 452–456. [Google Scholar]
- Bande, R.M.; Prasad, B.; Mishra, I.; Wasewar, K.L. Oil field effluent water treatment for safe disposal by electroflotation. Chem. Eng. J. 2008, 137, 503–509. [Google Scholar]
- Ibrahim, M.Y.; Mostafa, S.R.; Fahmy, M.F.M.; Hafez, A. Utilization of electroflotation in remediation of oily wastewater. Sep. Sci. Technol. 2001, 36, 3749–3762. [Google Scholar]
- Liu, A.; Fan, P.-P.; Han, F.; Han, H.; Li, Z.-H.; Wang, H.-F.; Fan, M.-Q. Effect of electroflotation on quartz and magnetite and its utilization on the reverse flotation of magnetic separateion concentrate. Miner. Eng. 2022, 175, 107292. [Google Scholar]
- Sarkar, M.S.K.A.; Donne, S.W.; Evans, G.M. Utilization of hydrogen in electroflotation of silica. Adv. Powder Technol. 2011, 22, 482–492. [Google Scholar]
- Llerena, C.; Ho, J.C.K.; Piron, D.L. Effects of pH on electroflotation of sphalerite. Chem. Eng. Commun. 1996, 155, 217–228. [Google Scholar]
- Akarsu, C.; Kumbur, H.; Kideys, A.E. Removal of microplastics from wastewater through electrocoagulation-electroflotation and membrane filtration processes. Water Sci. Technol. 2021, 84, 1648–1662. [Google Scholar]
- Akarsu, C.; Deniz, F. Electrocoagulation/Electroflotation Process for Removal of Organics and Microplastics in Laundry Wastewater. Clean 2021, 49, 2000146. [Google Scholar]
- Alexandrova, L.; Nedialkova, T.; Nishkov, I. Electroflotation of metal ions in waste water. Int. J. Miner. Process 1994, 41, 285–294. [Google Scholar]
- Chen, X.; Chen, G.; Yue, P.L. Novel electrode system for electroflotation of wastewater. Env. Sci. Technol. 2002, 36, 778–783. [Google Scholar]
- Matis, K.A.; Peleka, E.N. Alternative flotation techniques for wastewater treatment: Focus on electroflotation. Sep. Sci. Technol. 2010, 45, 2465–2474. [Google Scholar]
- Nunes, R.R.; Ribeiro, R.; Morão, G.M.; Rezende, M.O.O.; Moreira-Santos, M. Treatment of Wastewaters Containing Sulfonylurea Herbicides by Electroflotation: Chemical and Ecotoxicological Efficacy. Water 2022, 14, 2723. [Google Scholar]
- Mohtashami, R.; Shang, J.Q. Treatment of automotive paint wastewater in continuous-flow electroflotation reactor. J. Clean. Prod. 2019, 218, 335–346. [Google Scholar]
- dos Santos, G.N.; Felisardo, R.J.A.; Galrão, D.G.; Barbosa, M.P.R.; Santos, R.M.; da Silva, G.F.; dos Santos Freitas, L.; Dariva, S.M.E.; Garcia-Segura, S.; Cavalcanti, E.B. Electroflotation enables treatment of effluents generated during pyrolytic biomass revalorization. Sep. Purif. Technol. 2021, 277, 119458. [Google Scholar]
- Ksentini, I.; Aouadi, M.L.; Ben Bacha, H.; Ben Mansour, L. Solar energy integration in the treatment of industrial effluent by coagulation—Electroflotation. Desalination Water Treat. 2010, 20, 60–65. [Google Scholar]
- Mook, W.T.; Aroua, M.K.; Issabayeva, G. Prospective applications of renewable energy based electrochemical systems in wastewater treatment: A review. Renew. Sustain. Energy Rev. 2014, 38, 36–46. [Google Scholar]
- García-Orozco, V.M.; Linares-Hernández, I.; Natividad, R.; Balderas-Hernández, P.; Alanis-Ramírez, C.; Barrera-Díaz, C.E.; Roa-Morales, G. Solar-photovoltaic electrocoagulation of wastewater from a chocolate manufacturing industry: Anodic material effect (aluminium, copper and zinc) and life cycle assessment. J. Environ. Chem. Eng. 2022, 10, 107969. [Google Scholar]
- Hakizimana, J.N.; Najid, N.; Gourich, B.; Vial, C.; Stiriba, Y.; Naja, J. Hybrid electrocoagulation/electroflotation/electrodisinfection process as a pretreatment for seawater desalination. Chem. Eng. Sci. 2017, 170, 530–541. [Google Scholar]
- El-Ghenymy, A.; Alsheyab, M.; Khodary, A.; Sirés, I.; Abdel-Wahab, A. Corrosion behavior of pure titanium anodes in saline medium and their performance for humic acid removal by electrocoagulation. Chemosphere 2020, 246, 125674. [Google Scholar]
- Castro, S. Physico-chemical factors in flotation of Cu-Mo-Fe ores with seawater: A critical review. Physicochem. Probl. Miner. Process. 2018, 54, 1223–1236. [Google Scholar]
- Castro, S.; Laskowski, J.S. Froth Flotation in Saline Water. KONA Powder Part. J. 2011, 29, 4–15. [Google Scholar]
- Uribe, L.; Gutierrez, L.; Laskowski, J.S.; Castro, S. Role of calcium and magnesium cations in the interactions between kaolinite and chalcopyrite in seawater. Physicochem. Probl. Miner. Process. 2017, 53, 737–749. [Google Scholar]
- Li, Z.; Rao, F.; Song, S.; Li, Y.; Liu, W. Slime coating of kaolinite on chalcopyrite in saline water flotation. Int. J. Miner. Metall. Mater. 2018, 25, 481–488. [Google Scholar]
- Mu, Y.; Peng, Y. The effect of saline water on copper activation of pyrite in chalcopyrite flotation. Min. Eng. 2019, 131, 336–341. [Google Scholar]
- Galleguillos, F.; Cáceres, L.; Maxwell, L.; Soliz, Á. Electrochemical Ion Pumping Device for Blue Energy Recovery: Mixing Entropy Battery. Appl. Sci. 2020, 10, 5537. [Google Scholar]
- Bhola, R.; Bhola, S.M.; Mishra, B.; Olson, D.L. Electrochemical behavior of titanium and its alloys as dental implants in normal saline. Res. Lett. Phys. Chem. 2009, 2009, 574359. [Google Scholar]
- Chen, C.C.; Chen, J.H.; Chao, C.G.; Say, W.C. Electrochemical characteristics of surface of titanium formed by electrolytic polishing and anodizing. J. Mater. Sci. 2005, 40, 4053–4059. [Google Scholar]
- Huang, Y.Z.; Blackwood, D.J. Characterisation of titanium oxide film grown in 0.9% NaCl at different sweep rates. Electrochim. Acta 2005, 51, 1099–1107. [Google Scholar]
- Müller, K. Electroflotation From the Double Layer to Troubled Waters. In Electrochemistry in Transition; Springer: New York, NY, USA, 1992; pp. 21–37. [Google Scholar] [CrossRef]
- Mahnič-Kalamiza, S.; Miklavčič, D. Scratching the electrode surface: Insights into a high-voltage pulsed-field application from in vitro & in silico studies in indifferent fluid. Electrochim. Acta 2020, 363, 137187. [Google Scholar]
- Cáceres, L.; Frez, Y.; Galleguillos, F.; Soliz, A.; Gómez-Silva, B.; Borquez, J. Aqueous dried extract of skytanthus acutus meyen as corrosion inhibitor of carbon steel in neutral chloride solutions. Metals 2021, 11, 1992. [Google Scholar]
- Malaret, F. Exact calculation of corrosion rates by the weight-loss method. Exp. Results 2022, 3, e13. [Google Scholar]
- Robinson, F.P.A.; Scurr, W.G. The effect of boron on the corrosion resistance of austenitic stainless steels. Corrosion 1977, 33, 408–417. [Google Scholar]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Mørch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is There Anything Better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar]
- Gao, Q.; Zhang, W.; Shi, Z.; Yang, L.; Tang, Y. Structural Design and Electronic Modulation of Transition-Metal-Carbide Electrocatalysts toward Efficient Hydrogen Evolution. Adv. Mater. 2019, 31, 1802880. [Google Scholar]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-Noble Metal-based Carbon Composites in Hydrogen Evolution Reaction: Fundamentals to Applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar]
- Thomas, N.T.; Nobe, K. Kinetics of the Hydrogen Evolution Reaction on Titanium. J. Electrochem. Soc. 1970, 117, 622. [Google Scholar]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.M.; Ormellese, M.; Pedeferri, M. Corrosion of titanium: Part 1: Aggressive environments and main forms of degradation. J. Appl. Biomater. Funct. Mater. 2017, 15, e291–e302. [Google Scholar]
- Stress-Corrosion Cracking of Titanium Alloys. In Stress-Corrosion Cracking; ASM International: Singapore, 2017; pp. 271–302. [CrossRef]
- Maheshwari, S.; Li, Y.; Agrawal, N.; Janik, M.J. Chapter Three—Density Functional Theory Models for Electrocatalytic Reactions; Song, C.B.T.-A., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 63, pp. 117–167. [Google Scholar]
- Xu, Y.; Wang, C.; Huang, Y.; Fu, J. Recent advances in electrocatalysts for neutral and large-current-density water electrolysis. Nano Energy 2021, 80, 105545. [Google Scholar]
- Wang, C.; Shang, H.; Jin, L.; Xu, H.; Du, Y. Advances in hydrogen production from electrocatalytic seawater splitting. Nanoscale 2021, 13, 7897–7912. [Google Scholar]
- Lasia, A. Mechanism and kinetics of the hydrogen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 19484–19518. [Google Scholar]
- Sun, K.; Xu, W.; Lin, X.; Tian, S.; Lin, W.; Zhou, D.; Sun, X. Electrochemical Oxygen Reduction to Hydrogen Peroxide via a Two-Electron Transfer Pathway on Carbon-Based Single-Atom Catalysts. Adv. Mater. Interfaces 2021, 8, 2001360. [Google Scholar]
- Calvo, E.J. Oxygen Reduction Reaction in Nonaqueous Media; Wandelt, K.B.T.-E., Ed.; Elsevier: Singapore, 2018; pp. 831–837. [Google Scholar] [CrossRef]
- Mollica, A.; Ventura, G.; Traverso, E.; Scotto, V. Catholic behaviour of nickel and titanium in natural seawater. Int. Biodeterior. 1988, 24, 221–230. [Google Scholar]
- He, D.; Zhong, L.; Gan, S.; Xie, J.; Wang, W.; Liu, Z.; Guo, W.; Yang, X.; Niu, L. Hydrogen peroxide electrosynthesis via regulating the oxygen reduction reaction pathway on Pt noble metal with ion poisoning. Electrochim. Acta 2021, 371, 137721. [Google Scholar]
- Torresi, R.M.; Cámara, O.R.; De Pauli, C.P.; Giordano, M.C. Hydrogen evolution reaction on anodic titanium oxide films. Electrochim. Acta 1987, 32, 1291–1301. [Google Scholar]
- Harlov, D.E.; Aranovich, L. The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes: Surface, Crust, and Mantle BT—The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes: Surface, Crust, and Mantle; Harlov, D.E., Aranovich, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 1–19. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, Q.; Li, X.; Yao, F.; Xie, L.; Zhao, J.; Chen, F.; Xie, T.; Zeng, G. Electrochemically induced pitting corrosion of Ti anode: Application to the indirect reduction of bromate. Chem. Eng. J. 2016, 289, 114–122. [Google Scholar]
- Kuang, Y.; Kenney, M.J.; Meng, Y.; Hung, W.-H.; Liu, Y.; Huang, J.E.; Prasanna, R.; Li, P.; Li, Y.; Wang, L.; et al. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl. Acad. Sci. USA 2019, 116, 6624–6629. [Google Scholar]
- Ko, J.S.; Johnson, J.K.; Johnson, P.I.; Xia, Z. Decoupling Oxygen and Chlorine Evolution Reactions in Seawater using Iridium-based Electrocatalysts. ChemCatChem 2020, 12, 4526–4532. [Google Scholar]
- Cáceres, L.; Vargas, T.; Parra, M. Study of the variational patterns for corrosion kinetics of carbon steel as a function of dissolved oxygen and NaCl concentration. Electrochim. Acta 2009, 54, 7435–7443. [Google Scholar]
- Cáceres, L.; Vargas, T.; Herrera, L. Influence of pitting and iron oxide formation during corrosion of carbon steel in unbuffered NaCl solutions. Corros. Sci. 2009, 51, 971–978. [Google Scholar]
- Soliz, A.; Cáceres, L. Corrosion behavior of carbon steel in LiBr in comparison to NaCl solutions under controlled hydrodynamic conditions. Int. J. Electrochem. Sci. 2015, 10, 5673–5693. [Google Scholar]
- Soliz, A.; Guzmán, D.; Cáceres, L.; Madrid, F.M.G. Electrochemical Kinetic Analysis of Carbon Steel Powders Produced by High-Energy Ball Milling. Metals 2022, 12, 665. [Google Scholar]
- Rybalka, K.V.; Beketaeva, L.A.; Bukhan’ko, N.G.; Davydov, A.D. Dependence of corrosion current on the composition of titanium-nickel alloy in NaCl solution. Russ. J. Electrochem. 2014, 50, 1149–1156. [Google Scholar]
- Malik, A.S.; Liu, T.; Dupuis, M.; Li, R.; Li, C. Water Oxidation on TiO2: A Comparative DFT Study of 1e-, 2e-, and 4e- Processes on Rutile, Anatase, and Brookite. J. Phys. Chem. C 2020, 124, 8094–8100. [Google Scholar]





| Electrochemical Parameters | |||||||
| Ti | |||||||
| (Am−2) | (mVdec−1) | (Am−2) | (mVdec−1) | (Am−2) | (Am−2) | (mVdec−1) | |
| 1.99 × 10−9 | 238 | −4.42 × 10−7 | −168 | −13.68 | −4.5 × 10−7 | −217 | |
| Corrosion Parameters | |||||||
| Ti | |||||||
| (mV/SHE) | (Am−2) | ||||||
| −7.27 | 0.069 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madrid, F.M.G.; Arancibia-Bravo, M.; Cisterna, J.; Soliz, Á.; Salazar-Avalos, S.; Guevara, B.; Sepúlveda, F.; Cáceres, L. Corrosion of Titanium Electrode Used for Solar Saline Electroflotation. Materials 2023, 16, 3514. https://doi.org/10.3390/ma16093514
Madrid FMG, Arancibia-Bravo M, Cisterna J, Soliz Á, Salazar-Avalos S, Guevara B, Sepúlveda F, Cáceres L. Corrosion of Titanium Electrode Used for Solar Saline Electroflotation. Materials. 2023; 16(9):3514. https://doi.org/10.3390/ma16093514
Chicago/Turabian StyleMadrid, Felipe M. Galleguillos, María Arancibia-Bravo, Jonathan Cisterna, Álvaro Soliz, Sebastián Salazar-Avalos, Bastián Guevara, Felipe Sepúlveda, and Luis Cáceres. 2023. "Corrosion of Titanium Electrode Used for Solar Saline Electroflotation" Materials 16, no. 9: 3514. https://doi.org/10.3390/ma16093514
APA StyleMadrid, F. M. G., Arancibia-Bravo, M., Cisterna, J., Soliz, Á., Salazar-Avalos, S., Guevara, B., Sepúlveda, F., & Cáceres, L. (2023). Corrosion of Titanium Electrode Used for Solar Saline Electroflotation. Materials, 16(9), 3514. https://doi.org/10.3390/ma16093514









