Isothermal Experiments on Steam Oxidation of Zr−Sn−Nb Alloy at 1050 °C: Kinetics and Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Method
3. Results
3.1. Macroscopic Appearance
3.2. Oxidation Weight Gain
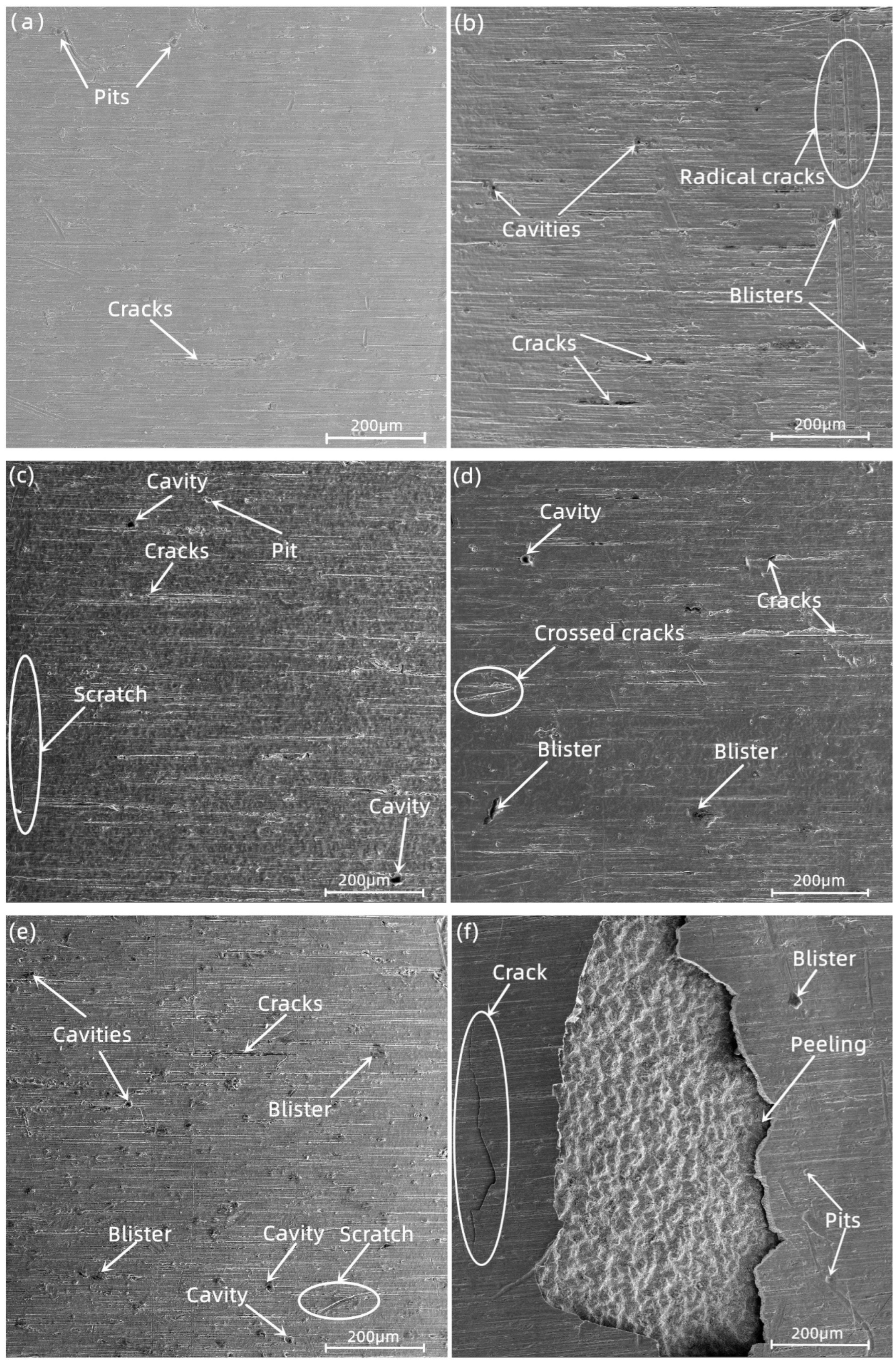
3.3. Surface Morphology
3.4. Cross-Sectional Morphology
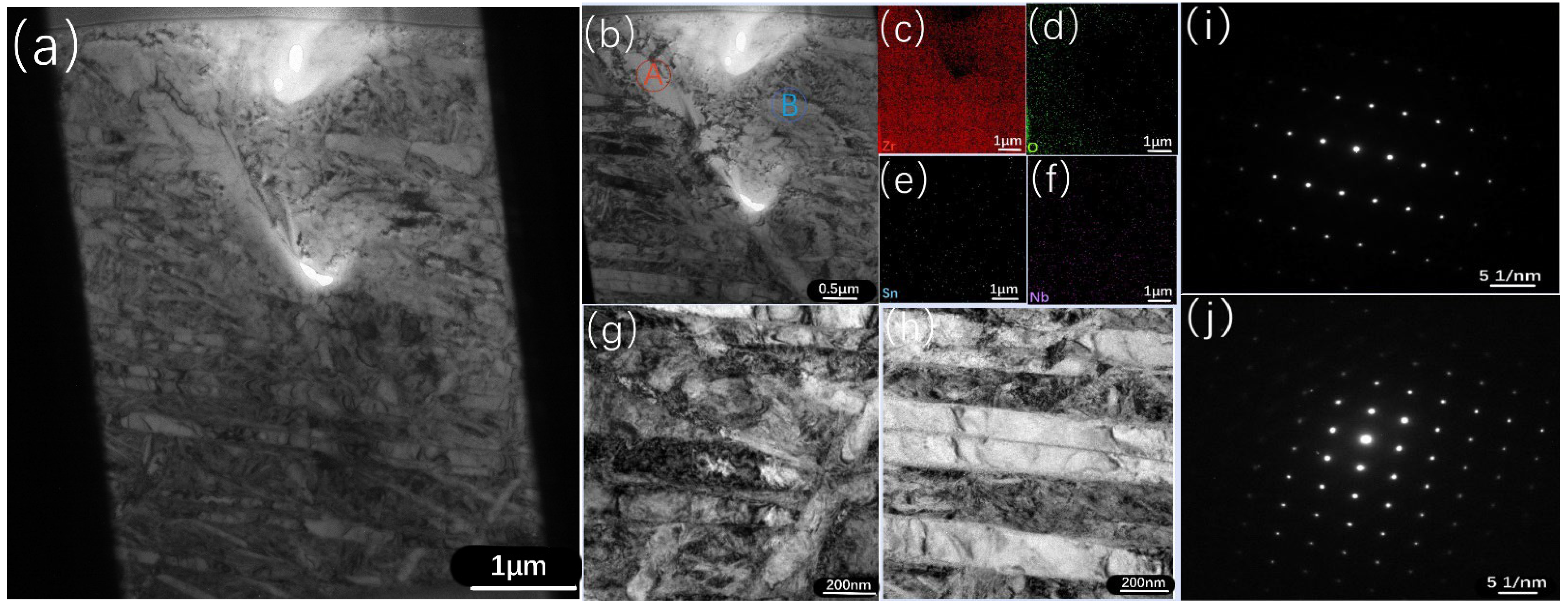
3.5. TEM Results of the Zr−Sn−Nb Alloy
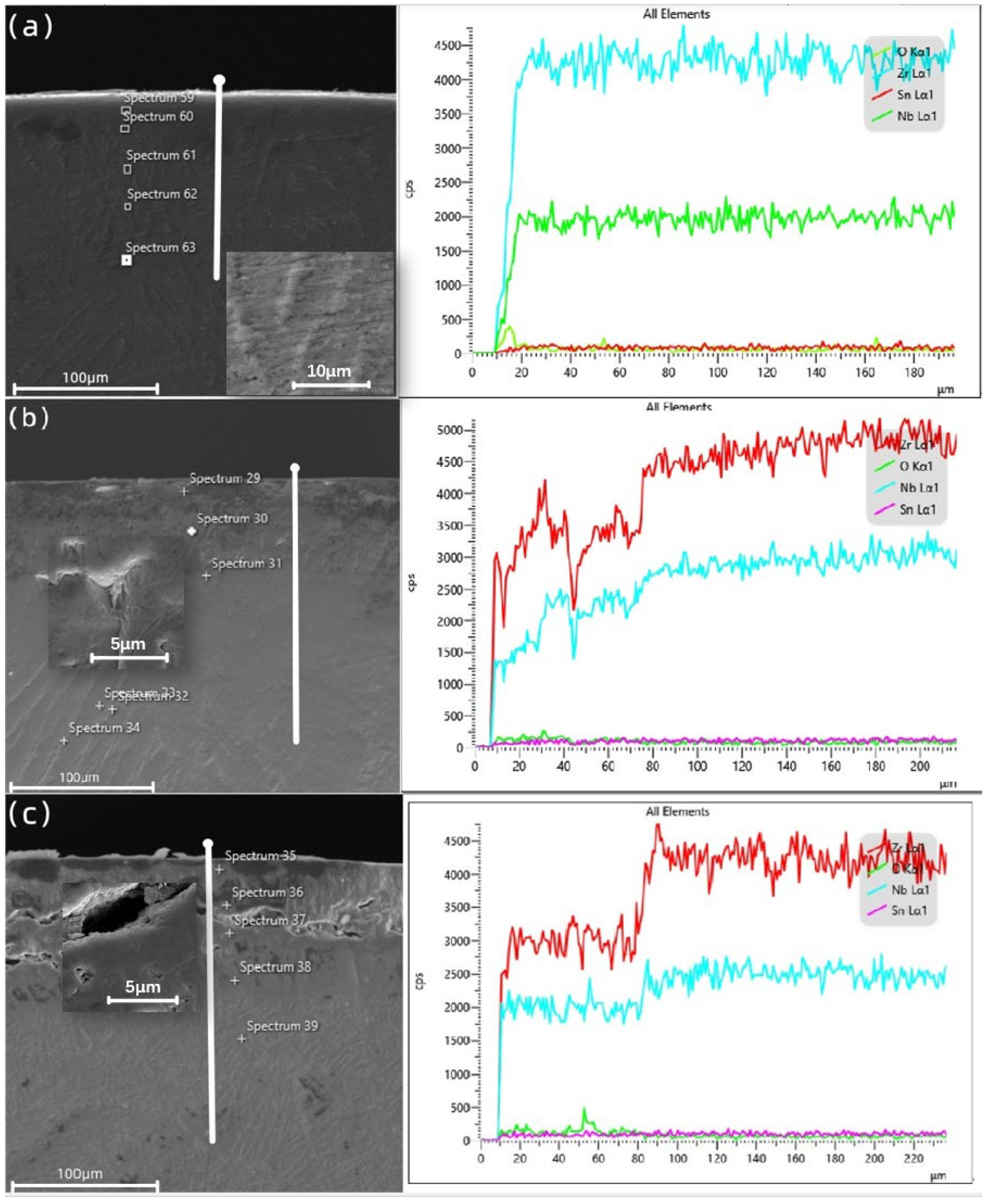
3.6. Cross-Sectional Distribution of Elements
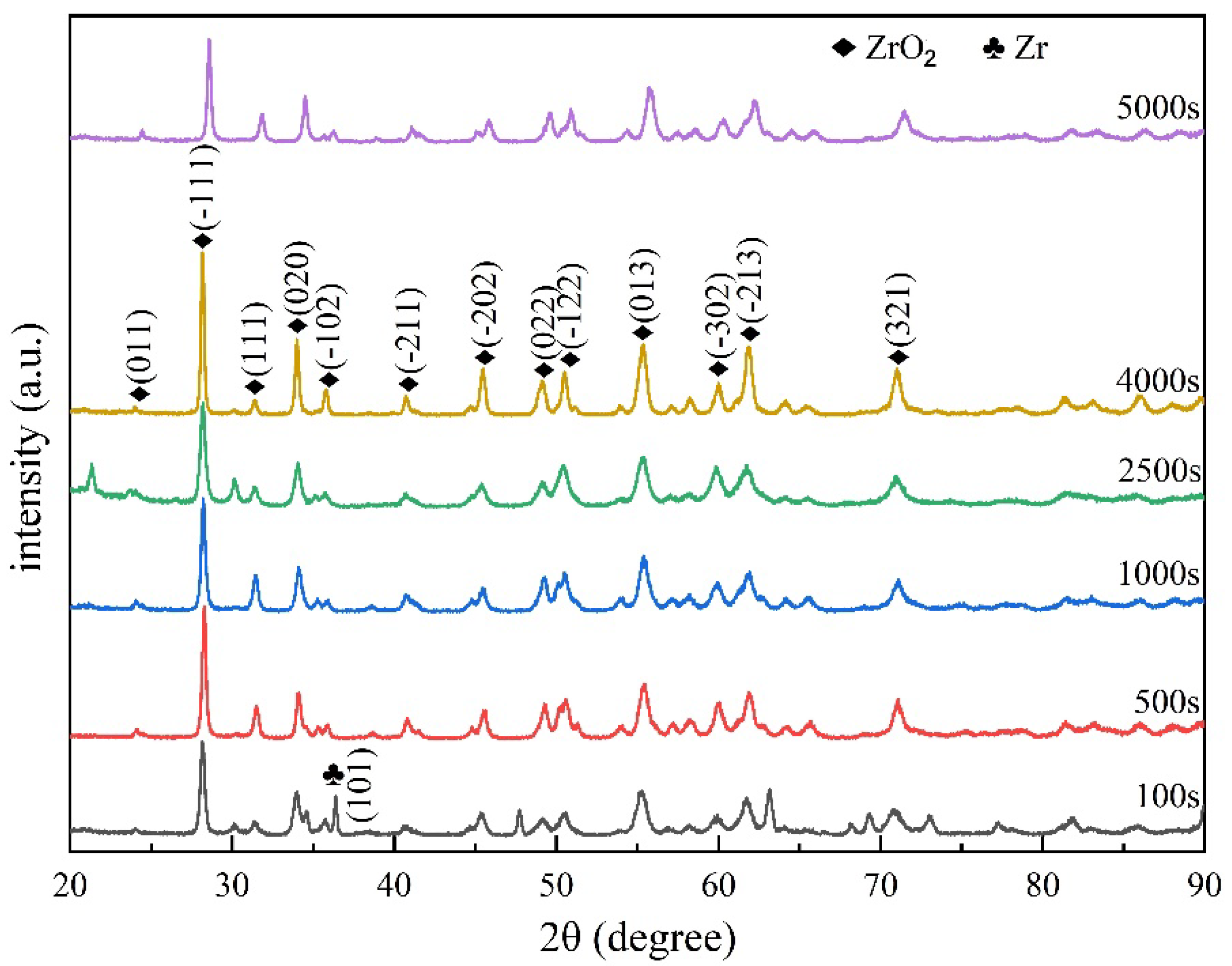
3.7. Surface XRD
4. Discussion
5. Conclusions
- (1)
- During high-temperature steam oxidation at 1050 °C, the surface of the Zr−Sn−Nb alloy undergoes a color change and local deformation, but is able to maintain an intact macrostructure for a long time until it breaks down after 4000 s of oxidation.
- (2)
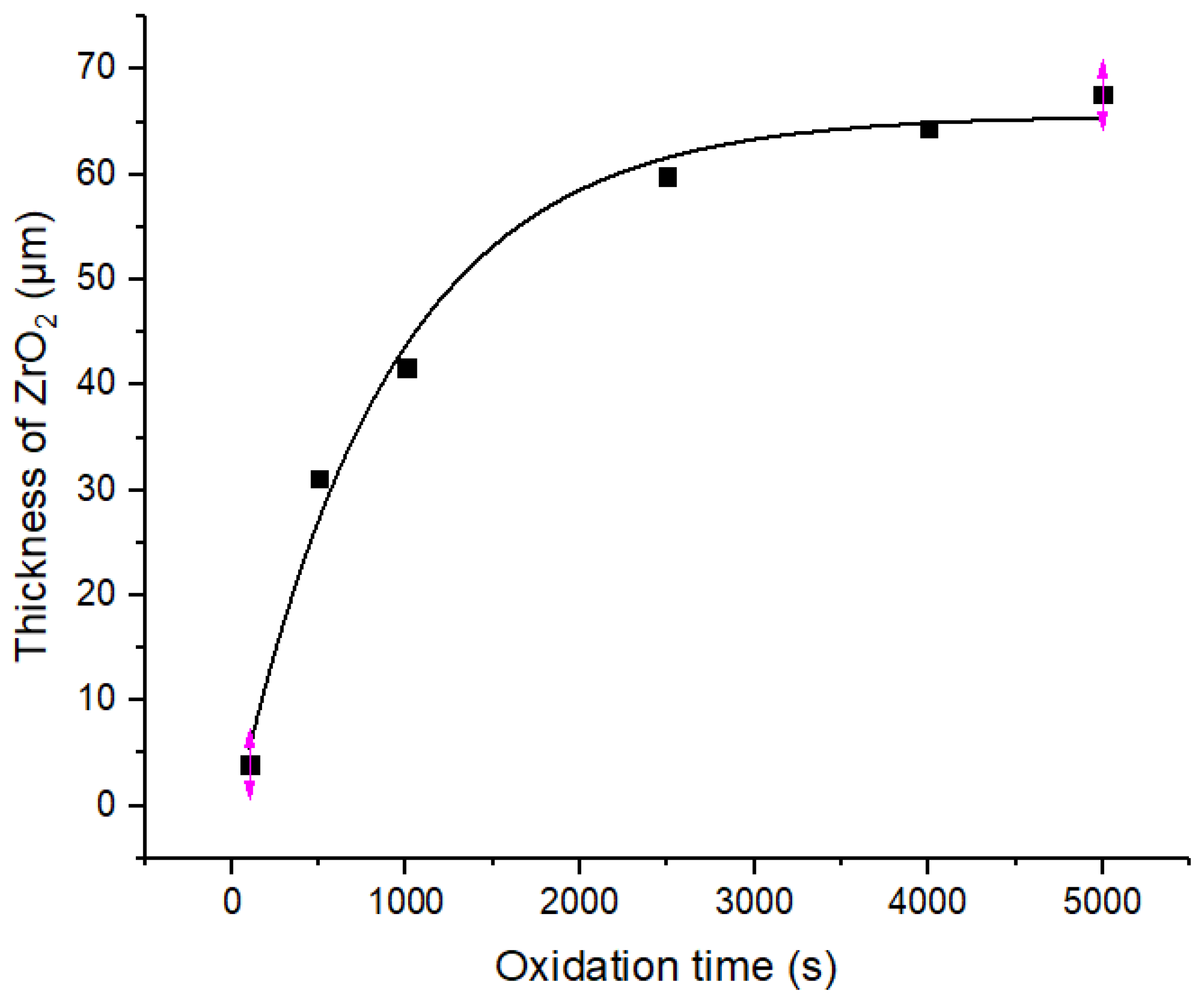
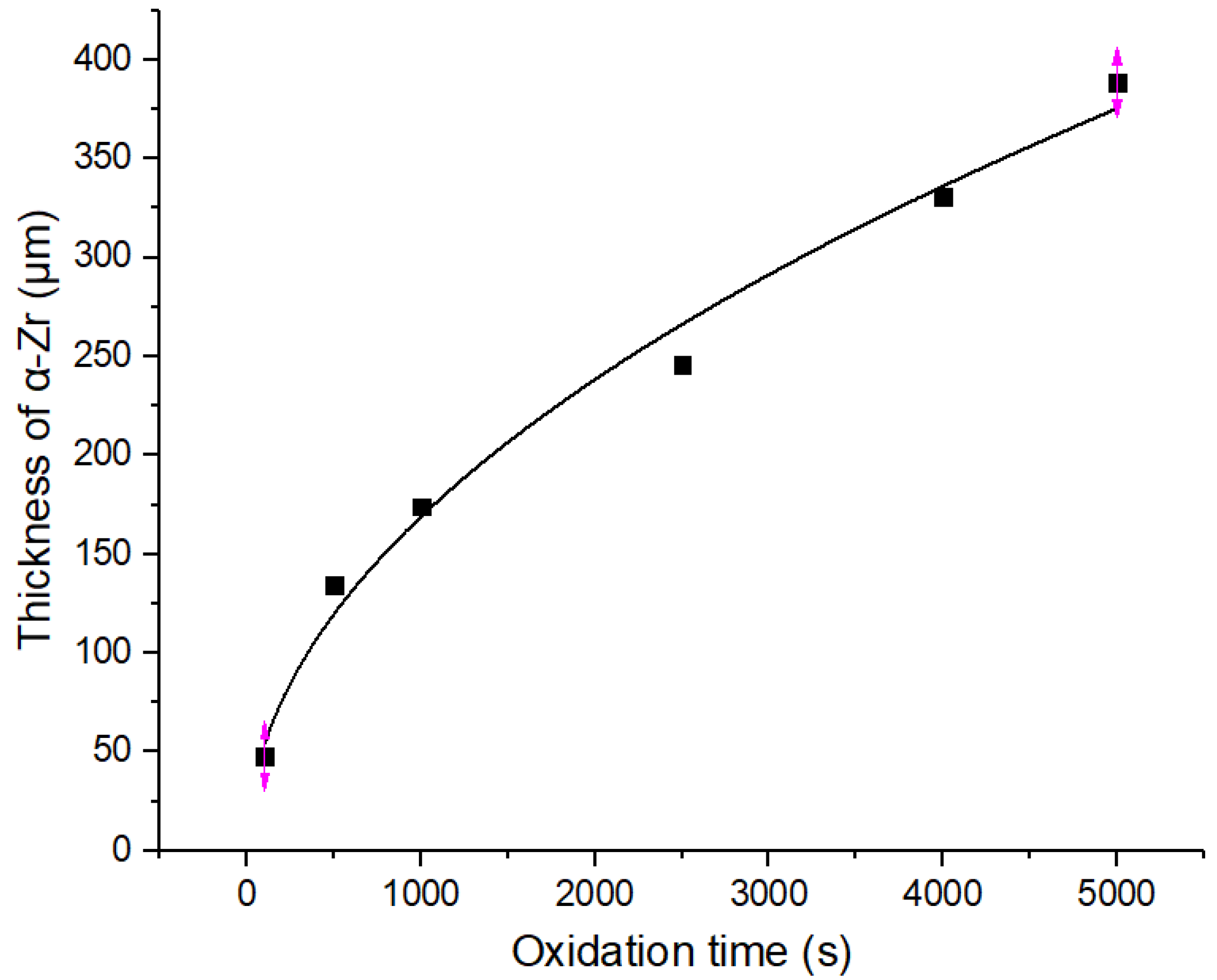
- When the Zr−Sn−Nb alloy is oxidized by steam at 1050 °C, the curve of oxidation weight gain versus time follows a parabolic law. In addition, the curves of ZrO2 thickness and α-Zr thickness also follow a parabolic law.
- (3)
- The microstructure of the Zr−Sn−Nb alloy after steam oxidation at 1050 °C consists mainly of the oxide layer (ZrO2), α-Zr(O), and prior-β. The breakdown of oxide films, the generation of micropores and cracks, and the increase in the number of cracks are related to the increase in the proportions of ZrO2 and α-Zr(O).
- (4)
- The changes in the atomic content of O, Zr, Nb, and Sn at all sections of the alloy’s cross-section become more dramatic with increasing oxidation time. As the oxidation time increases, the proportion of Nb atoms becomes greater, leading to a decrease in the oxidation resistance of the alloy.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, C.M.; Han, Y.-S.; Mok, Y.-K.; Sohn, D.-S. Study of mechanism of oxidation resistance enhancement induced by preformed oxide on zirconium alloys. Corros. Sci. 2019, 158, 108105. [Google Scholar] [CrossRef]
- Brachet, J.-C.; Rouesne, E.; Ribis, J.; Guilbert, T.; Urvoy, S.; Nony, G.; Toffolon-Masclet, C.; Le Saux, M.; Chaabane, N.; Palancher, H. High temperature steam oxidation of chromium-coated zirconium-based alloys: Kinetics and process. Corros. Sci. 2020, 167, 108537. [Google Scholar] [CrossRef]
- Sundell, G.; Thuvander, M.; Andren, H.-O. Barrier oxide chemistry and hydrogen pick-up mechanisms in zirconium alloys. Corros. Sci. 2016, 102, 490–502. [Google Scholar] [CrossRef]
- Kim, T.; Kim, J.; Choi, K.J.; Yoo, S.C.; Kim, S.; Kim, J.H. Phase transformation of oxide film in zirconium alloy in high temperature hydrogenated water. Corros. Sci. 2015, 99, 134–144. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Baek, J.H.; Kim, S.J.; Kim, H.G.; Ruhmann, H. Corrosion characteristics and oxide microstructures of Zircaloy-4 in aqueous alkali hydroxide solutions. J. Nucl. Mater. 1999, 270, 322–333. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, J.W.; Kim, H.; Lee, S.U. Prediction of ballooning and burst for nuclear fuel cladding with anisotropic creep modeling during Loss of Coolant Accident (LOCA). Nucl. Eng. Technol. 2021, 53, 3379–3397. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, R.; Zhang, Y.; Chen, P.; Lan, Y.; Yu, J.; Su, G.; Qiu, S.; Tian, W. Isothermal experiments on steam oxidation of magnetron-sputtered chromium-coated zirconium alloy cladding at 1200 °C. Corros. Sci. 2022, 206, 110544. [Google Scholar] [CrossRef]
- Hirano, M.; Yonomoto, T.; Ishigaki, M.; Watanabe, N.; Maruyama, Y.; Sibamoto, Y.; Watanabe, T.; Moriyama, K. Insights from review and analysis of the Fukushima Dai-ichi accident: Fukushima NPP accident related. J. Nucl. Sci. Technol. 2012, 49, 1–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, S.; Zhang, L.; Qiu, S.; Su, G.; Tian, W. A review on analysis of LWR severe accident. J. Nucl. Eng. Radiat. Sci. 2015, 1, 041018. [Google Scholar] [CrossRef]
- Kim, T.; Kim, S.; Lee, C.-y.; Mok, Y.-k.; Kim, J.H. In situ investigation of the oxidation of Zr–Nb–Cu alloy in high-temperature hydrogenated water. Corros. Sci. 2017, 119, 91–101. [Google Scholar] [CrossRef]
- Urbanic, V.; Heidrick, T. High-temperature oxidation of zircaloy-2 and zircaloy-4 in steam. J. Nucl. Mater. 1978, 75, 251–261. [Google Scholar] [CrossRef]
- Ballinger, R.; Dobson, W.; Biederman, R. Oxidation reaction kinetics of Zircaloy-4 in an unlimited steam environment. J. Nucl. Mater. 1976, 62, 213–220. [Google Scholar] [CrossRef]
- Suman, S. Impact of hydrogen on rupture behaviour of Zircaloy-4 nuclear fuel cladding during loss-of-coolant accident: A novel observation of failure at multiple locations. Nucl. Eng. Technol. 2021, 53, 474–483. [Google Scholar] [CrossRef]
- Amaya, M.; Nagase, F. The relationship between the amount of oxidation and activation energy on the steam oxidation reaction of Zircaloy-4 cladding. J. Nucl. Mater. 2013, 440, 457–466. [Google Scholar] [CrossRef]
- Steinbrueck, M.; da Silva, F.O.; Grosse, M. Oxidation of Zircaloy-4 in steam-nitrogen mixtures at 600–1200 °C. J. Nucl. Mater. 2017, 490, 226–237. [Google Scholar] [CrossRef]
- Nagase, F.; Otomo, T.; Uetsuka, H. Oxidation kinetics of low-Sn Zircaloy-4 at the temperature range from 773 to 1573 K. J. Nucl. Sci. Technol. 2003, 40, 213–219. [Google Scholar] [CrossRef]
- Steinbrück, M.; Schaffer, S. High-temperature oxidation of Zircaloy-4 in oxygen–nitrogen mixtures. Oxid. Met. 2016, 85, 245–262. [Google Scholar] [CrossRef]
- Steinbrück, M.; Birchley, J.; Boldyrev, A.; Goryachev, A.; Grosse, M.; Haste, T.; Hózer, Z.; Kisselev, A.; Nalivaev, V.; Semishkin, V. High-temperature oxidation and quench behaviour of Zircaloy-4 and E110 cladding alloys. Prog. Nucl. Energy 2010, 52, 19–36. [Google Scholar] [CrossRef]
- Baek, J.H.; Park, K.B.; Jeong, Y.H. Oxidation kinetics of Zircaloy-4 and Zr–1Nb–1Sn–0.1 Fe at temperatures of 700–1200 °C. J. Nucl. Mater. 2004, 335, 443–456. [Google Scholar] [CrossRef]
- Baker Jr, L.; Just, L.C. Studies of Metal-Water Reactions at High Temperatures. III. Experimental and Theoretical Studies of the Zirconium-Water Reaction; Argonne National Lab.(ANL): Argonne, IL, USA, 1962.
- Cathcart, J.; Pawel, R.; McKee, R.; Druschel, R.; Yurek, G.; Campbell, J.; Jury, S. Zirconium Metal-Water Oxidation Kinetics. IV. Reaction Rate Studies; Oak Ridge National Lab.: Oak Ridge, TN, USA, 1977.
- Yueh, H.; Colburn, D.; Comstock, R.; Dahlback, M.; Hallstadius, L.; Kesterson, R.; Shah, H. Improved ZIRLOTM Cladding Performance through Chemistry and Process Modifications; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Bossis, P.; Pecheur, D.; Hanifi, K.; Thomazet, J.; Blat, M. Comparison of the High Burn-Up Corrosion on M5 and Low Tin Zircaloy-4; ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Shishov, V.; Peregud, M.; Nikulina, A.; Kon’kov, V.; Novikov, V.; Markelov, V.; Khokhunova, T.; Kobylyansky, G.; Novoselov, A.; Ostrovsky, Z. Structure-phase state, corrosion and irradiation properties of Zr-Nb-Fe-Sn system alloys. In Proceedings of the Zirconium in the Nuclear Industry: 15th International Symposium, Sunriver, OR, USA, 24–28 June 2007. [Google Scholar]
- Zhao, W.; Liu, Y.; Jiang, H.; Peng, Q. Effect of heat treatment and Nb and H contents on the phase transformation of N18 and N36 zirconium alloys. J. Alloys Compd. 2008, 462, 103–108. [Google Scholar] [CrossRef]
- Park, J.-Y.; Yoo, S.J.; Choi, B.-K.; Jeong, Y.H. Corrosion and oxide characteristics of Zr–1.5Nb–0.4Sn–0.2Fe–0.1 Cr alloys in 360 °C pure water and LiOH solution. J. Nucl. Mater. 2008, 373, 343–350. [Google Scholar] [CrossRef]
- Jian, L. Nuclear Structure Materials; Chemistry Industry Press: Beijing, China, 2007; p. 143. [Google Scholar]
- Liu, Y.; Qiu, J.; Liu, X.; Zhao, W. Study on oxidation behavior of N18 alloy in steam at 600~1200 °C. Nucl. Power Eng. 2010, 85–88. [Google Scholar]
- Leistikow, S.; Schanz, G.; Berg, H.V. Kinetics and Morphology of the Isothermal Steam Oxidation of Zircaloy 4 at 700–1300 °C; Kernforschungszentrum Karlsruhe GmbH: Freiburg, Germany, 1978. [Google Scholar]
- Feng, Z.; Chengyu, Z.; Wanqian, Z.; Yao, Z.; Le, C.; Mei, L.; Pengcheng, S. Oxidation Behaviors of Zr-Sn-Nb Alloy in 1000 similar to 1250 degrees C Steam. Rare Met. Mater. Eng. 2021, 50, 3677–3683. [Google Scholar]




| Oxidation Time (s) | 100 | 500 | 1000 | 2500 | 4000 | 5000 |
|---|---|---|---|---|---|---|
| Weight gain (mg/cm2) | 0.20 | 0.65 | 0.98 | 1.40 | 2.10 | 2.95 |
| Oxidation Temperature (°C) | 1050 °C |
|---|---|
| Oxidation time (s) | 0 s~5000 s |
| Equation | |
| a | 0.0057 |
| b | 0.7239 |
| R-Squared | 0.9612 |
| Position | Element Content | (at%) | ||
|---|---|---|---|---|
| O | Zr | Nb | Sn | |
| Spectrum 59 | 28.00 | 69.07 | 2.41 | 0.52 |
| Spectrum 60 | 19.72 | 76.74 | 3.25 | 0.29 |
| Spectrum 61 | 20.40 | 75.97 | 3.16 | 0.47 |
| Spectrum 62 | 24.03 | 72.73 | 2.82 | 0.42 |
| Spectrum 63 | 17.65 | 78.60 | 3.22 | 0.53 |
| Position | Element Content | (at%) | ||
|---|---|---|---|---|
| O | Zr | Nb | Sn | |
| Spectrum 29 | 45.69 | 50.70 | 3.20 | 0.42 |
| Spectrum 30 | 26.94 | 59.15 | 13.53 | 0.38 |
| Spectrum 31 | 15.73 | 73.04 | 10.68 | 0.55 |
| Spectrum 32 | 13.26 | 73.81 | 12.59 | 0.33 |
| Spectrum 33 | 11.30 | 77.42 | 11.08 | 0.19 |
| Spectrum 34 | 19.01 | 68.32 | 12.24 | 0.44 |
| Position | Element Content | (at%) | ||
|---|---|---|---|---|
| O | Zr | Nb | Sn | |
| Spectrum 35 | 43.82 | 47.18 | 8.54 | 0.46 |
| Spectrum 36 | 43.19 | 48.04 | 8.27 | 0.51 |
| Spectrum 37 | 38.57 | 51.97 | 9.11 | 0.34 |
| Spectrum 38 | 16.45 | 73.43 | 9.53 | 0.60 |
| Spectrum 39 | 11.66 | 77.41 | 10.11 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, R.; Tang, D.; Yang, C.; Wang, Y.; Zhang, L.; Lei, M. Isothermal Experiments on Steam Oxidation of Zr−Sn−Nb Alloy at 1050 °C: Kinetics and Process. Materials 2023, 16, 3823. https://doi.org/10.3390/ma16103823
Jiang R, Tang D, Yang C, Wang Y, Zhang L, Lei M. Isothermal Experiments on Steam Oxidation of Zr−Sn−Nb Alloy at 1050 °C: Kinetics and Process. Materials. 2023; 16(10):3823. https://doi.org/10.3390/ma16103823
Chicago/Turabian StyleJiang, Rui, Dewen Tang, Chen Yang, Yanli Wang, Lin Zhang, and Ming Lei. 2023. "Isothermal Experiments on Steam Oxidation of Zr−Sn−Nb Alloy at 1050 °C: Kinetics and Process" Materials 16, no. 10: 3823. https://doi.org/10.3390/ma16103823
APA StyleJiang, R., Tang, D., Yang, C., Wang, Y., Zhang, L., & Lei, M. (2023). Isothermal Experiments on Steam Oxidation of Zr−Sn−Nb Alloy at 1050 °C: Kinetics and Process. Materials, 16(10), 3823. https://doi.org/10.3390/ma16103823





