One-Dimensional Mn5Si3 Nanorods: Fabrication, Microstructure, and Magnetic Properties via a Novel Casting-Extraction Route
Abstract
:1. Introduction
2. Experimental Method
2.1. Preparation Process of Mn5Si3 Nanorods
2.2. Characterization of Mn5Si3 Nanorods
3. Results and Discussion
3.1. Microstructure Characterization
3.2. Formation and Growth Mechanism of Mn5Si3 Nanorods
3.3. Magnetic Properties
3.4. Oxidation Resistance
3.5. Prospects and Advancements for Applications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Machin, A.; Fontanez, K.; Arango, J.C.; Ortiz, D.; Leon, D.J.; Pinilla, S.; Nicolosi, V.; Petrescu, F.I.; Morant, C.; Marquez, F. One-dimensional (1D) nanostructured materials for energy applications. Materials 2021, 14, 2609. [Google Scholar] [CrossRef]
- Sohn, H.; Park, C.; Oh, J.M.; Kang, S.W.; Kim, M.J. Silver nanowire networks: Mechano-electric properties and applications. Materials 2019, 12, 2526. [Google Scholar] [CrossRef] [PubMed]
- Stroe, M.; Burlanescu, T.; Paraschiv, M.; Lorinczi, A.; Matei, E.; Ciobanu, R.; Baibarac, M. Optical and structural properties of composites based on poly(urethane) and TiO2 nanowires. Materials 2023, 16, 1742. [Google Scholar] [CrossRef] [PubMed]
- Maraj, M.; Nabi, G.; Usman, K.; Wang, E.; Wei, W.; Wang, Y.; Sun, W. High quality growth of cobalt doped GaN nanowires with enhanced ferromagnetic and optical response. Materials 2020, 13, 3537. [Google Scholar] [CrossRef]
- Panzic, I.; Capan, I.; Brodar, T.; Bafti, A.; Mandic, V. Structural and electrical characterization of pure and Al-doped ZnO nanorods. Materials 2021, 14, 7454. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.; Abbas, G.; Afzaal, M.; Naz, M.Y.; Ghuffar, A.; Irfan, M.; Legutko, S.; Jozwik, J.; Zawada-Michalowska, M.; Ghanim, A.A.J.; et al. Gold nanorods for doxorubicin delivery: Numerical analysis of electric field enhancement, optical properties and drug loading/releasing efficiency. Materials 2022, 15, 1764. [Google Scholar] [CrossRef]
- Shinde, S.K.; Jalak, M.B.; Karade, S.S.; Majumder, S.; Tamboli, M.S.; Truong, N.T.N.; Maile, N.C.; Kim, D.Y.; Jagadale, A.D.; Yadav, H.M. A novel synthesized 1D nanobelt-like cobalt phosphate electrode material for excellent supercapacitor applications. Materials 2022, 15, 8235. [Google Scholar] [CrossRef]
- Saleem, M.I.; Katware, A.; Amin, A.; Jung, S.H.; Lee, J.H. YCl3-substituted CsPbI3 perovskite nanorods for efficient red-light-emitting diodes. Nanomaterials 2023, 13, 1366. [Google Scholar] [CrossRef]
- Saleem, M.I.; Yang, S.; Batool, A.; Sulaman, M.; Veeramalai, C.P.; Jiang, Y.; Tang, Y.; Cui, Y.; Tang, L.; Zou, B. CsPbI3 nanorods as the interfacial layer for high-performance, all-solution-processed self-powered photodetectors. J. Mater. Sci. Technol. 2021, 75, 196–204. [Google Scholar] [CrossRef]
- Yang, D.; Li, P.; Zou, Y.; Cao, M.; Hu, H.; Zhong, Q.; Hu, J.; Sun, B.; Duhm, S.; Xu, Y.; et al. Interfacial synthesis of monodisperse CsPbBr3 nanorods with tunable aspect ratio and clean surface for efficient light-emitting diode applications. Chem. Mater. 2019, 31, 1575–1583. [Google Scholar] [CrossRef]
- Schmitt, A.L.; Higgins, J.M.; Szczech, J.R.; Jin, S. Synthesis and applications of metal silicide nanowires. J. Mater. Chem. 2010, 20, 223–235. [Google Scholar] [CrossRef]
- Tang, S. Morphology evolution of Mn-Si composition gradient micro/nano-materials prepared by oxygen assisted chemical vapor deposition. J. Nanomater. 2018, 2018, 7547295. [Google Scholar] [CrossRef]
- Seo, K.; Yoon, H.; Ryu, S.W.; Lee, S.; Jo, Y.; Jung, M.H.; Kim, J.; Choi, Y.K.; Kim, B. Itinerant helimagnetic single-crystalline. ACS Nano 2010, 4, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.M.; Ding, R.; DeGrave, J.P.; Jin, S. Signature of helimagnetic ordering in single-crystal MnSi nanowires. Nano Lett. 2010, 10, 1605–1610. [Google Scholar] [CrossRef]
- Das, B.; Balasubramanian, B.; Manchanda, P.; Mukherjee, P.; Skomski, R.; Hadjipanayis, G.C.; Sellmyer, D.J. Mn5Si3 nanoparticles: Synthesis and size-induced ferromagnetism. Nano Lett. 2016, 16, 1132–1137. [Google Scholar] [CrossRef]
- Hortamani, M.; Sandratskii, L.; Zahn, P.; Mertig, I. Physical origin of the incommensurate spin spiral structure in Mn3Si. J. Appl. Phys. 2009, 105, 07E506. [Google Scholar] [CrossRef]
- Hamzan, N.B.; Ng, C.Y.B.; Sadri, R.; Lee, M.K.; Chang, L.J.; Tripathi, M.; Dalton, A.; Goh, B.T. Controlled physical properties and growth mechanism of manganese silicide nanorods. J. Alloys Compd. 2021, 851, 156693. [Google Scholar] [CrossRef]
- Lu, J.; Wang, L.; Zhang, J.; Li, Q.; Liu, W.; Lou, Z.; Zheng, A.; Zhou, Q. Preparation and magnetic properties of manganese silicide nanorods by a solid-state reaction route. Micro Nano Lett. 2018, 13, 341–343. [Google Scholar] [CrossRef]
- Gottschilch, M.; Gourdon, O.; Persson, J.; Cruz, C.D.L.; Petricek, V.; Brueckel, T. Study of the antiferromagnetism of Mn5Si3: An inverse magnetocaloric effect material. J. Mater. Chem. 2012, 22, 15275. [Google Scholar] [CrossRef]
- Surgers, C.; Fischer, G.; Winkel, P.; Lohneysen, H.V. Large topological Hall effect in the non-collinear phase of an antiferromagnet. Nat. Commun. 2014, 5, 3400. [Google Scholar] [CrossRef]
- Higgins, J.M.; Ding, R.; Jin, S. Synthesis and characterization of manganese-rich silicide (α-Mn5Si3, β-Mn5Si3, and β-Mn3Si) nanowires. Chem. Mater. 2011, 23, 3848–3853. [Google Scholar] [CrossRef]
- Rylkov, V.V.; Nikolaev, S.N.; Chernoglazov, K.Y.; Aronzon, B.A.; Maslakov, K.I.; Tugushev, V.V.; Kulatov, E.T.; Likhachev, I.A.; Pashaev, E.M.; Semisalova, A.S.; et al. High-temperature ferromagnetism in Si1−x Mnx (x ≈ 0.5) nonstoichiometric alloys. JETP Lett. 2012, 96, 255–262. [Google Scholar] [CrossRef]
- Rashid, H.U.; Yu, K.; Umar, M.N.; Anjum, M.N.; Khan, K.; Ahmad, N.; Jan, M.T. Catalyst role in chemical vapor deposition (CVD) process: A review. Rev. Adv. Mater. Sci. 2015, 40, 235–248. [Google Scholar]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials 2018, 11, 822. [Google Scholar] [CrossRef]
- Wang, X.; Jie, J.; Qu, J.; Liu, S.; Yin, G.; Li, T. Novel strategy for fabrication of field-emission Ti5Si3 nanowires via casting-extraction method. Mater. Lett. 2020, 279, 128353. [Google Scholar] [CrossRef]
- Li, H.; Jie, J.; Liu, S.; Zhang, Y.; Li, T. Crystal growth and morphology evolution of D88 (Mn,Fe)5Si3 phase and its influence on the mechanical and wear properties of brasses. Mater. Sci. Eng. A 2017, 704, 45–56. [Google Scholar] [CrossRef]
- Li, H.; Jie, J.; Zhang, P.; Jia, C.; Wang, T.; Li, T. Study on the formation and precipitation mechanism of Mn5Si3 phase in the MBA-2 brass alloy. Metall. Mater. Trans. A 2016, 47, 2616–2624. [Google Scholar] [CrossRef]
- Wang, X.; Jie, J.; Liu, S.; Dong, Z.; Yin, G.; Li, T. Growth mechanism of primary Ti5Si3 phases in special brasses and their effect on wear resistance. J. Mater. Sci. Tech. 2021, 61, 138–146. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeier, T.C. Smithells Metals Reference Book; Elsevier Butterworth-Heinemann: Oxford, UK, 2004. [Google Scholar]
- Davis, J.R. ASM Handbook: Properties and Selection: Nonferrous Alloys and Special Purpose Materials; ASM International: Materials Park, OH, USA, 1990; Volume 2. [Google Scholar]
- Bie, L.; Chen, X.; Liu, P.; Zhang, T.; Xu, X. Morphology evolution of Mn5Si3 phase and effect of Mn content on wear resistance of special brass. Met. Mater. Int. 2019, 26, 431–443. [Google Scholar] [CrossRef]
- Chen, S.; Mi, X.; Huang, G.; Li, Y. Effect of Ce addition on microstructure and properties of Cu–Zn–Mn–Al–based alloy. Mater. Res. Express 2018, 6, 016518. [Google Scholar] [CrossRef]
- Gou, P.; Niu, B.; Wang, N.; Dong, Z.; Li, Z.; Wang, Q.; Kang, R.; Dong, C. Composition-optimized Cu-Zn-(Mn, Fe, Si) alloy and its microstructural evolution with thermomechanical treatments. J. Mater. Eng. Perform. 2021, 31, 590–601. [Google Scholar] [CrossRef]
- Sunagawa, I. Crystals: Growth, Morphology and Perfection; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Lander, G.; Brown, P.; Forsyth, J. The antiferromagnetic structure of Mn5Si3. Pro. Phys. Soc. 1967, 91, 332. [Google Scholar] [CrossRef]
- Hartman, P. Crystal Growth: An Introduction; North–Holland: Amsterdam, The Netherlands, 1973. [Google Scholar]
- Libbrecht, K.G. The physics of snow crystals. Rep. Prog. Phys. 2005, 68, 855–895. [Google Scholar] [CrossRef]
- Cao, D.; Li, H.; Pan, L.; Li, J.; Wang, X.; Jing, P.; Cheng, X.; Wang, W.; Wang, J.; Liu, Q. High saturation magnetization of γ-Fe2O3 nano-particles by a facile one-step synthesis approach. Sci. Rep. 2016, 6, 32360. [Google Scholar] [CrossRef] [PubMed]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, O.N.; Singh, K. Shape and size-dependent magnetic properties of Fe3O4 nanoparticles synthesized using piperidine. Nanoscale Res. Lett. 2017, 12, 298. [Google Scholar] [CrossRef]
- Guimar, A.P. Principles of Nanomagnetism; Springer-Verlag: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Manikandan, D.; Murugan, R. Genesis and tuning of ferromagnetism in SnO2 semiconductor nanostructures: Comprehensive review on size, morphology, magnetic properties and DFT investigations. Prog. Mater Sci. 2022, 130, 100970. [Google Scholar] [CrossRef]
- Soumare, Y.; Garcia, C.; Maurer, T.; Chaboussant, G.; Ott, F.; Fievet, F.; Piquemal, J.Y.; Viau, G. Kinetically controlled synthesis of hexagonally close-packed cobalt nanorods with high magnetic coercivity. Adv. Funct. Mater. 2009, 19, 1971–1977. [Google Scholar] [CrossRef]
- Valdes, D.P.; Lima, E.; Zysler, R.D.; Goya, G.F.; Biasi, E.D. Role of anisotropy, frequency, and interactions in magnetic hyperthermia applications: Noninteracting nanoparticles and linear chain arrangements. Phys. Rev. Appl. 2021, 15, 044005. [Google Scholar] [CrossRef]
- Ralandinliu Kahmei, R.D.; Borah, J.P. Clustering of MnFe2O4 nanoparticles and the effect of field intensity in the generation of heat for hyperthermia application. Nanotechnology 2019, 30, 035706. [Google Scholar] [CrossRef]
- Vereda, F.; Vicente, J.D.; Hidalgo-Alvarez, R. Physical properties of elongated magnetic particles: Magnetization and friction coefficient anisotropies. Chem. Phys. Chem. 2009, 10, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Winters, B.J.; Holm, J.; Roberts, J.T. Thermal processing and native oxidation of silicon nanoparticles. J. Nanopart. Res. 2011, 13, 5473–5484. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P.; Wagner, F.E.; Berensmeier, S. Oxidation of magnetite nanoparticles: Impact on surface and crystal properties. Cryst. Eng. Comm. 2017, 19, 246–255. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, P.; Zhang, C.M. Structure, mechanical, electronic and thermodynamic properties of Mo5Si3 from first-principles calculations. Ceram. Int. 2018, 44, 12357–12362. [Google Scholar] [CrossRef]
- Seo, K.; Lee, S.; Jo, Y.; Jung, M.H.; Kim, J.; Churchill, D.G.; Kim., B. Room temperature ferromagnetism in single-crystalline Fe5Si3 nanowires. J. Phys. Chem. C 2009, 113, 6902–6905. [Google Scholar] [CrossRef]
- Hsu, H.F.; Tsai, P.C.; Lu, K.C. The physical characterization of single-crystalline chromium silicide nanowires grown by chemical vapor deposition. In Electrochemical Society Meeting Abstracts; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2018; p. 1394. [Google Scholar]

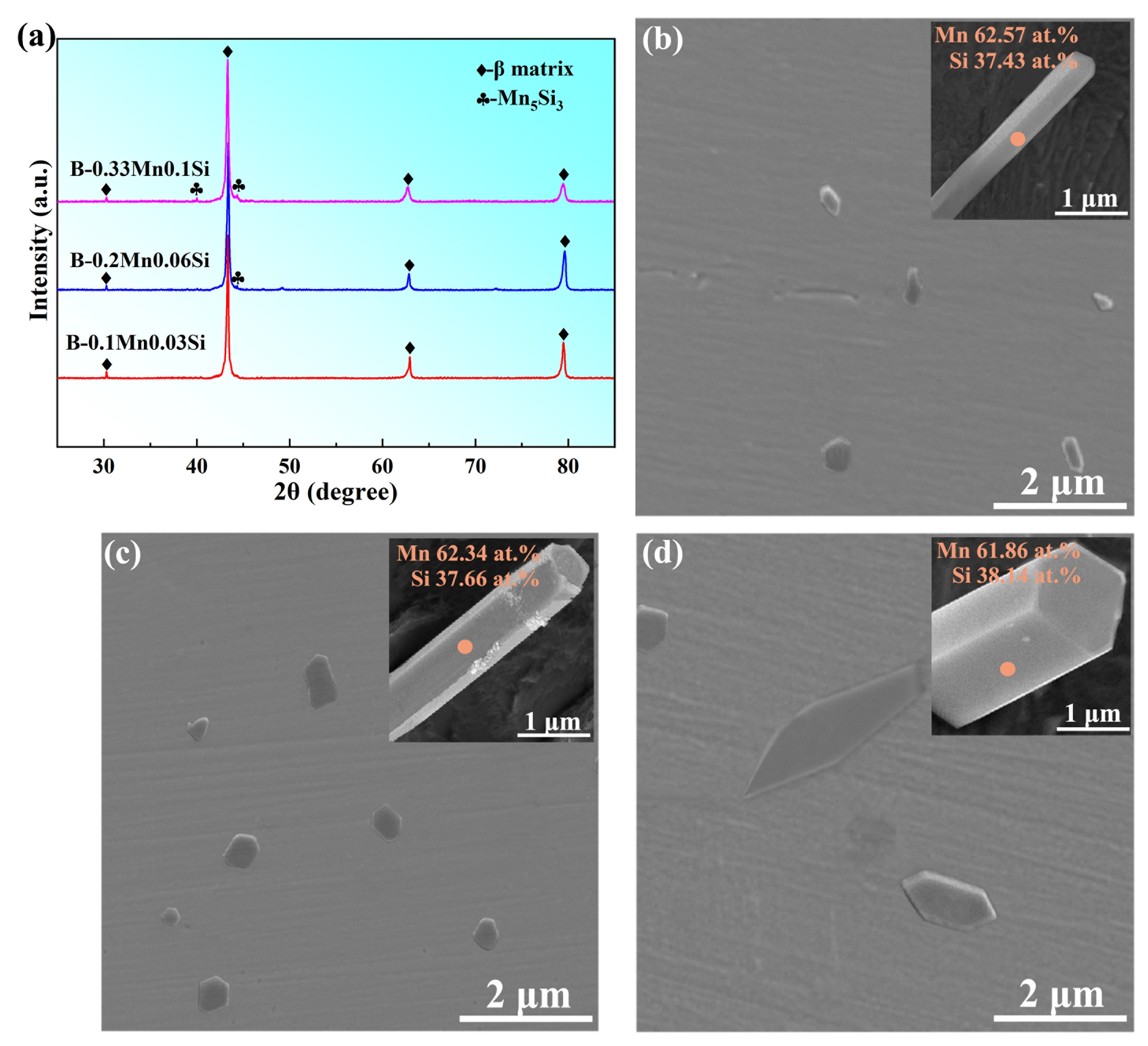



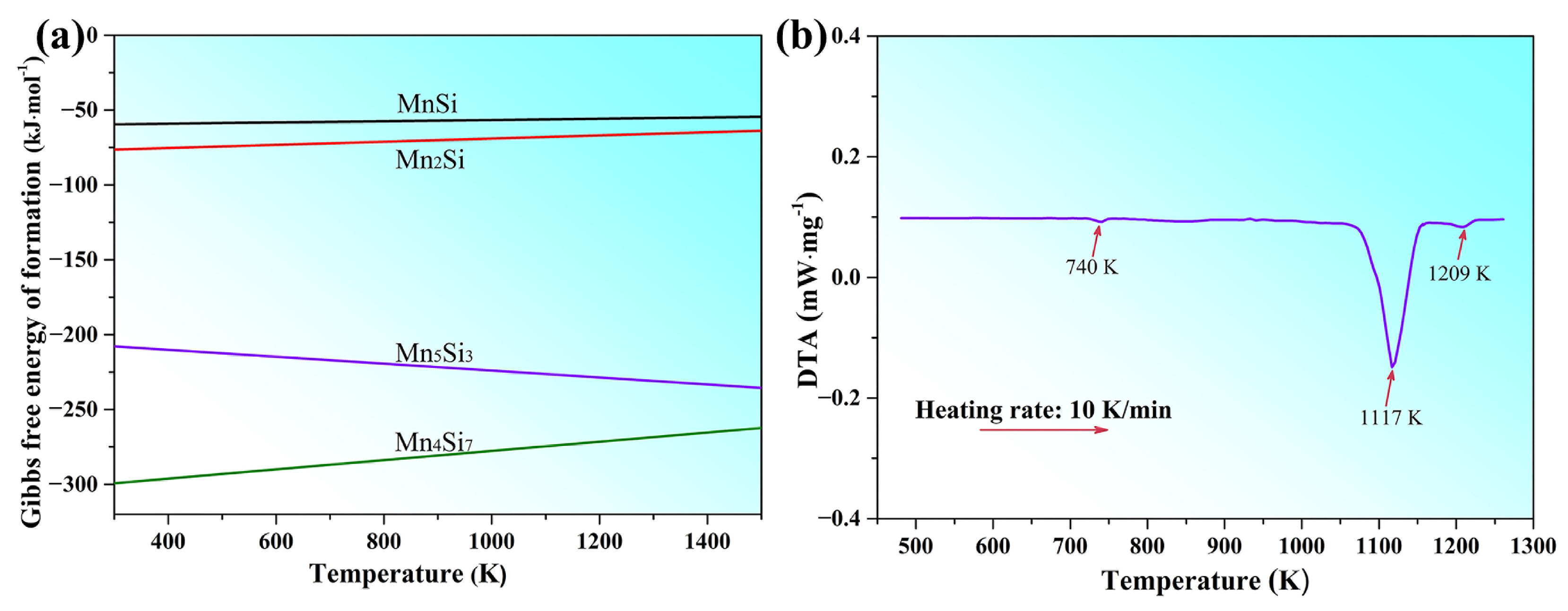

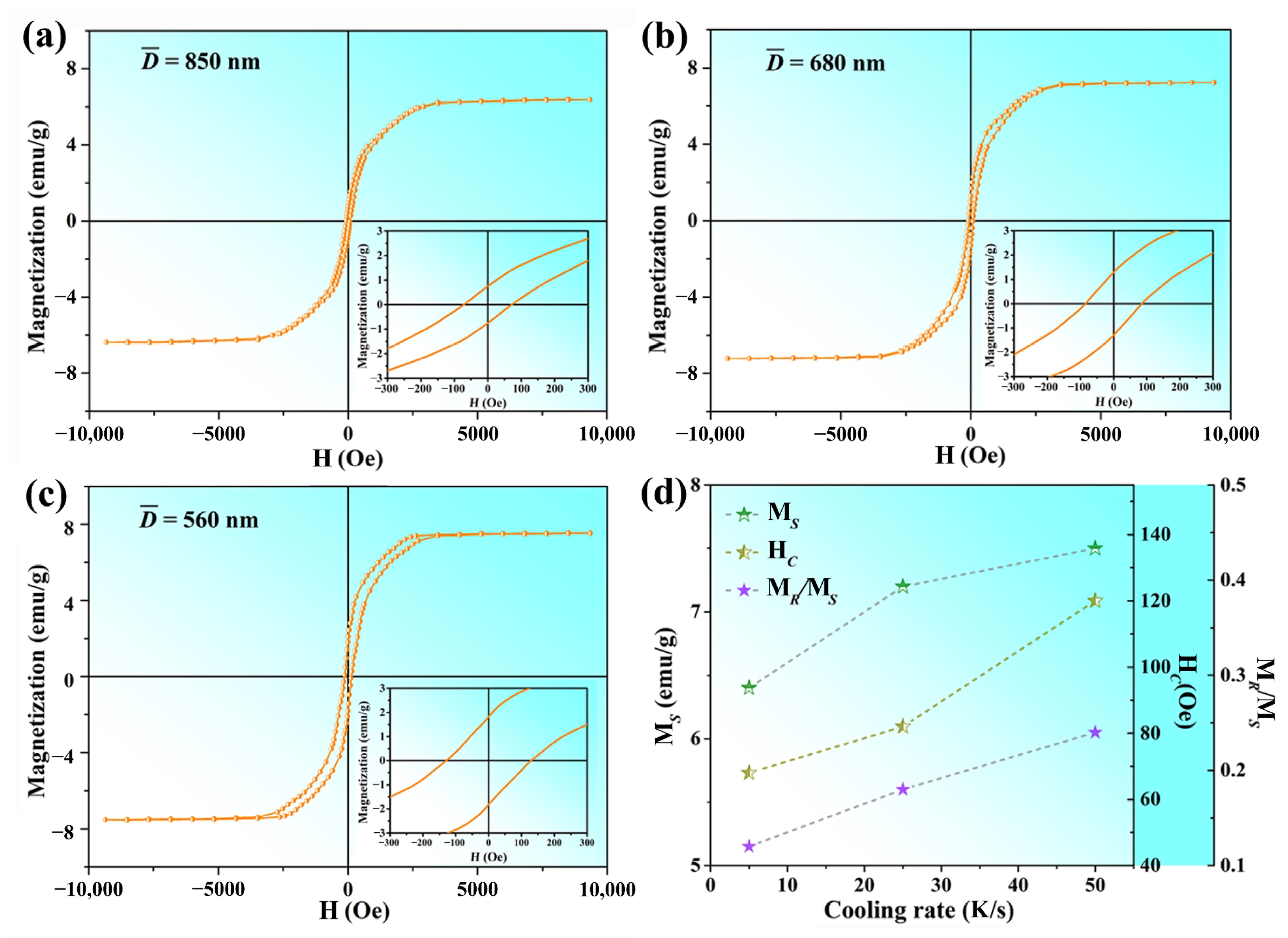
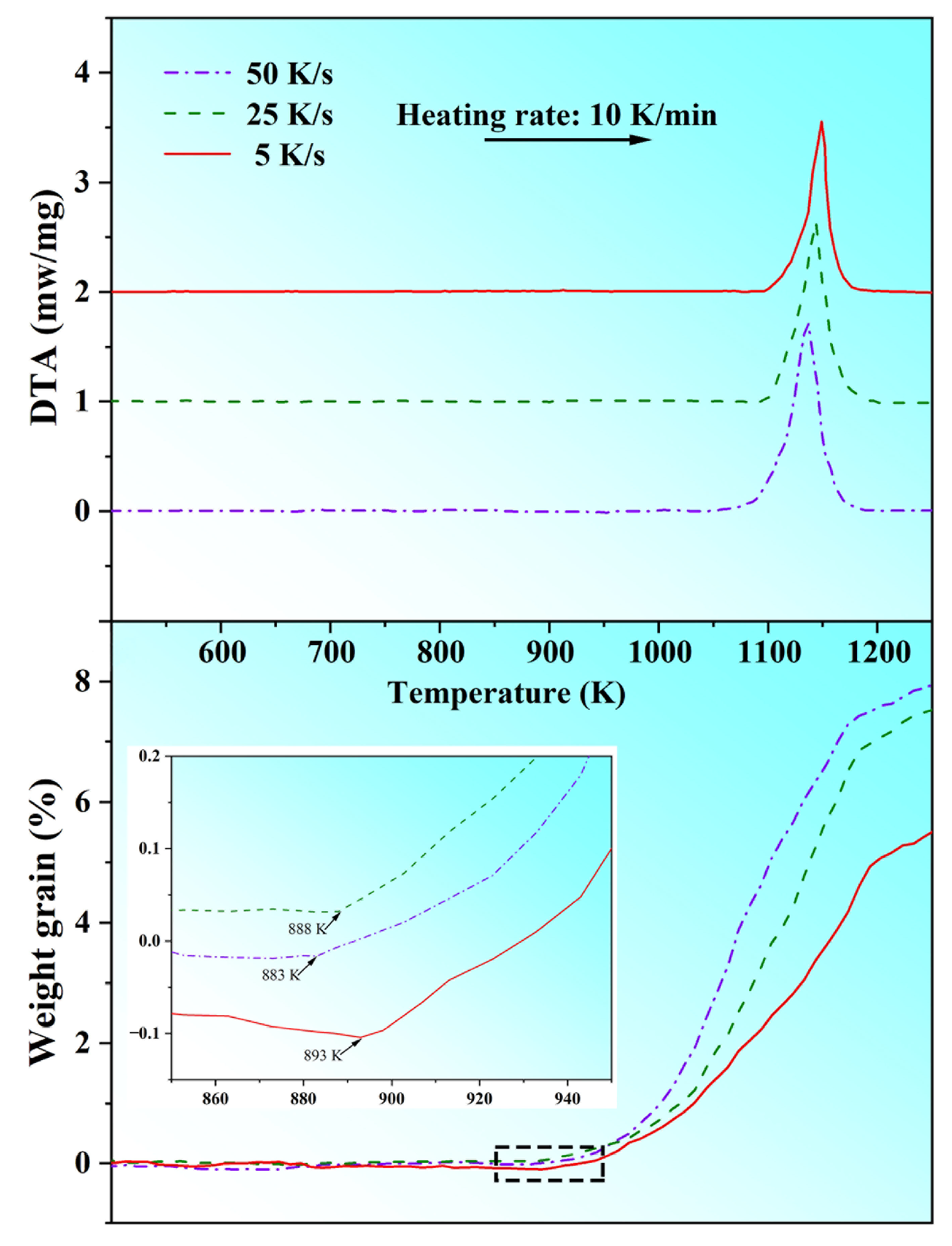
| Brass Alloy | Zn | Mn | Si | Al | Cu |
|---|---|---|---|---|---|
| B-0.1Mn-0.03Si | 35.0 | 0.10 | 0.03 | 3.0 | bal. |
| B-0.2Mn-0.06Si | 35.0 | 0.20 | 0.06 | 3.0 | bal. |
| B-0.33Mn-0.1Si | 35.0 | 0.33 | 0.10 | 3.0 | bal. |
| Sample | Preparation Method | Nanostructure | Dimension | Ms (emu/g) | Hc (Oe) | |
|---|---|---|---|---|---|---|
| Diameter | Length | |||||
| Ref. [15] | CVD | Nanorods | 200–400 nm | Several μm | 4.63 | 61.5 |
| Ref. [14] | CVD | Nanorods | ~1.82 μm | ~43.92 μm | 0.70 | 100 |
| This work | Casting-extraction | Nanorods | 400–750 nm | 2~11 μm | 7.5 | 120 |
| Ref. [12] | Magnetron sputtering | Nanoparticles | ~8.6 nm | - | ~129 | ~500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Niu, D.; Zhang, Z.; Yang, F.; Wang, H.; Cheng, W. One-Dimensional Mn5Si3 Nanorods: Fabrication, Microstructure, and Magnetic Properties via a Novel Casting-Extraction Route. Materials 2023, 16, 3540. https://doi.org/10.3390/ma16093540
Li H, Niu D, Zhang Z, Yang F, Wang H, Cheng W. One-Dimensional Mn5Si3 Nanorods: Fabrication, Microstructure, and Magnetic Properties via a Novel Casting-Extraction Route. Materials. 2023; 16(9):3540. https://doi.org/10.3390/ma16093540
Chicago/Turabian StyleLi, Hang, Dongtao Niu, Zhongtao Zhang, Fan Yang, Hongxia Wang, and Weili Cheng. 2023. "One-Dimensional Mn5Si3 Nanorods: Fabrication, Microstructure, and Magnetic Properties via a Novel Casting-Extraction Route" Materials 16, no. 9: 3540. https://doi.org/10.3390/ma16093540




