Investigating the Effect of Reflectance Tuning on Photocatalytic Dye Degradation with Biotemplated ZnO Photonic Nanoarchitectures Based on Morpho Butterfly Wings
Abstract
:1. Introduction
2. Materials and Methods
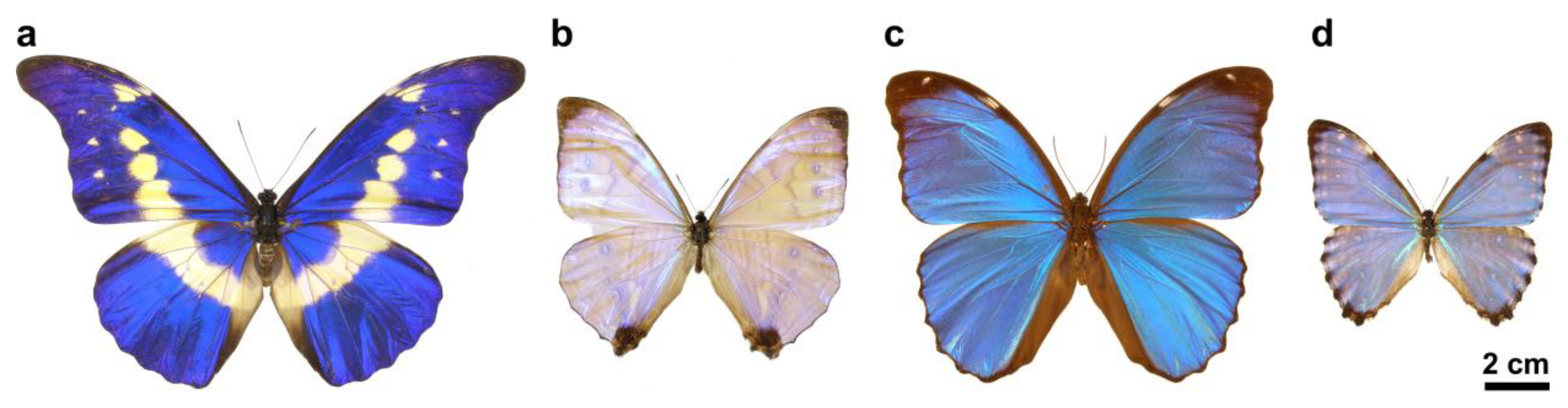
2.1. Butterflies
2.2. Atomic Layer Deposition (ALD)
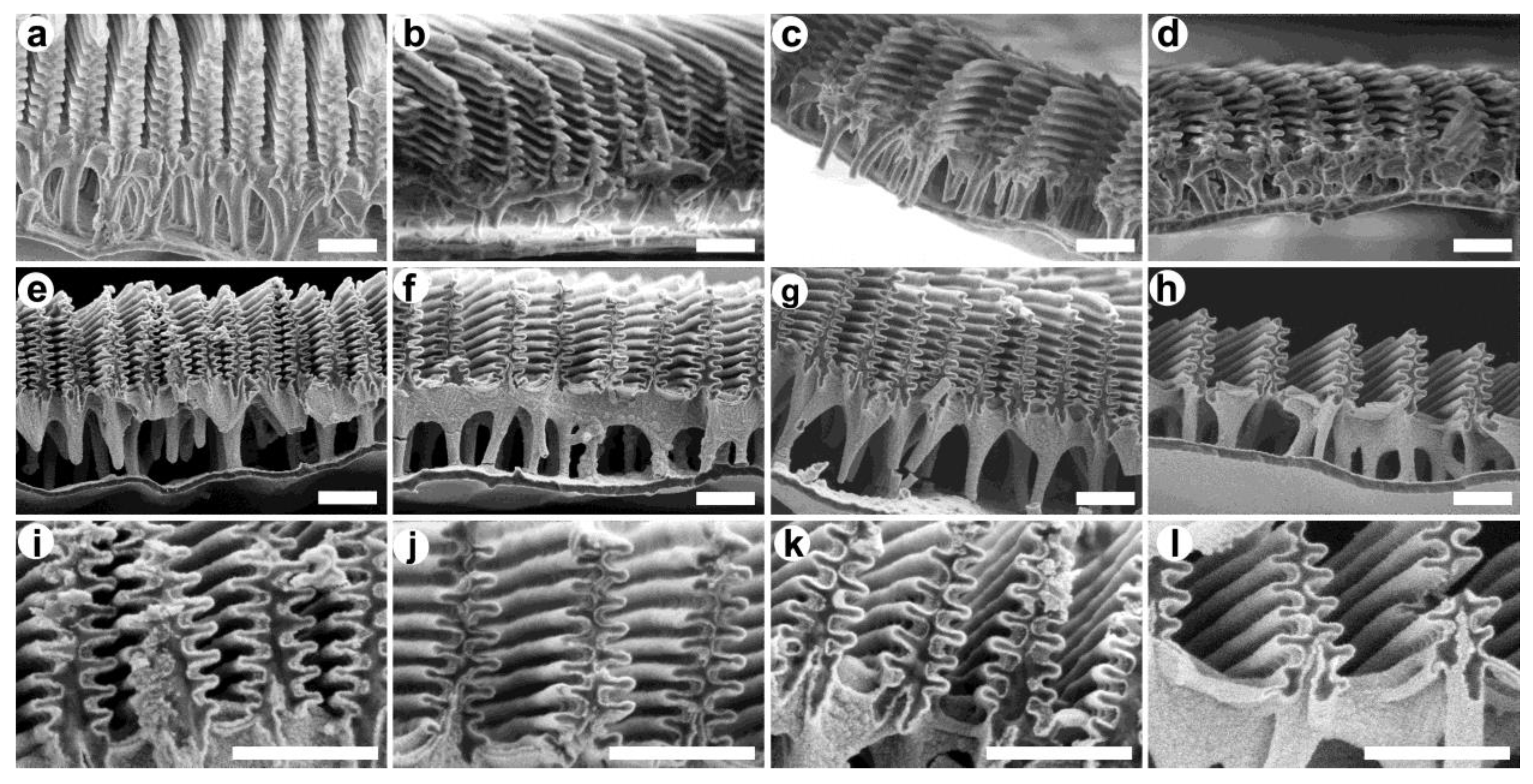
2.3. Scanning Electron Microscopy (SEM)
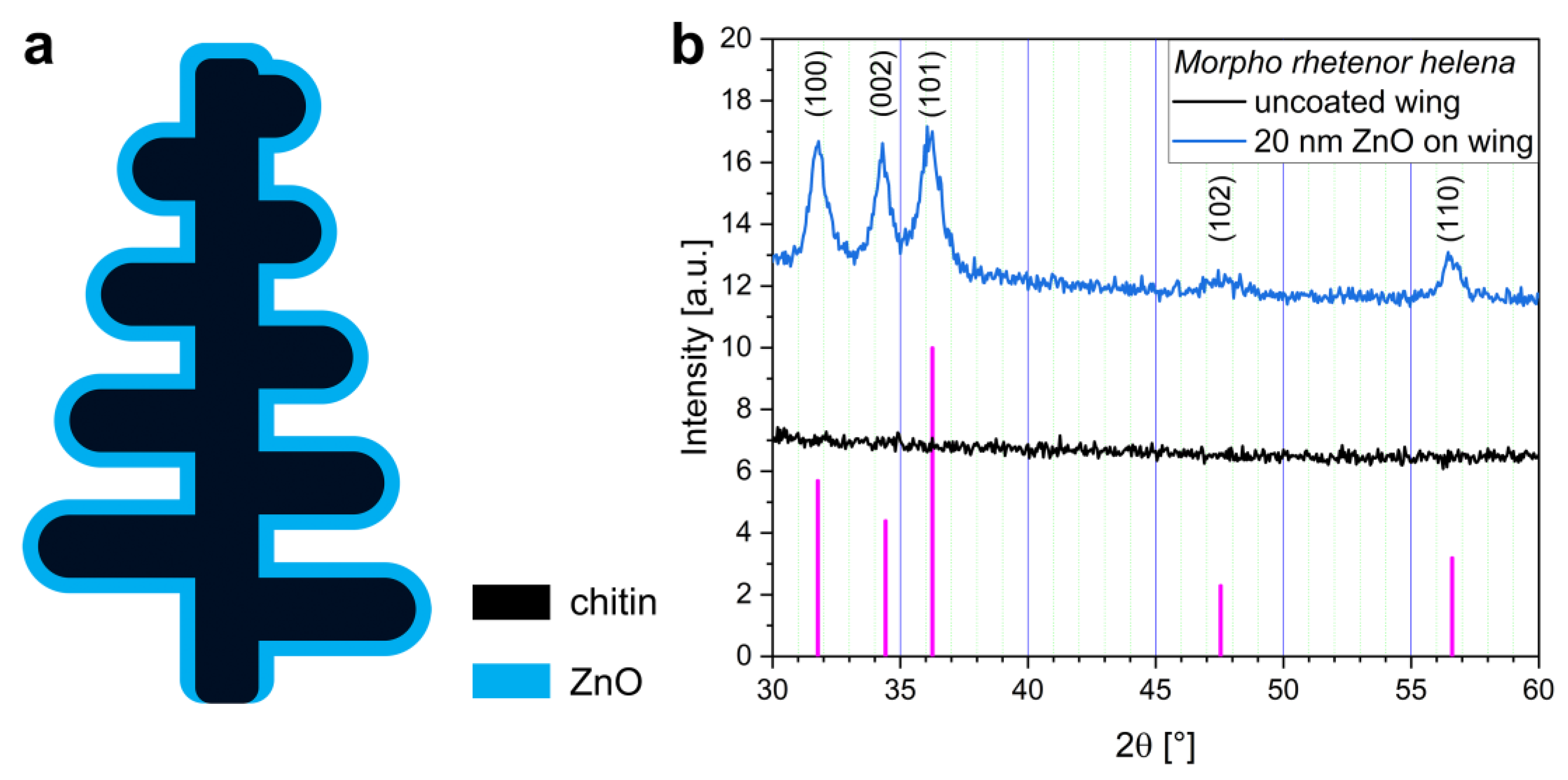
2.4. X-ray Diffraction Analysis (XRD)
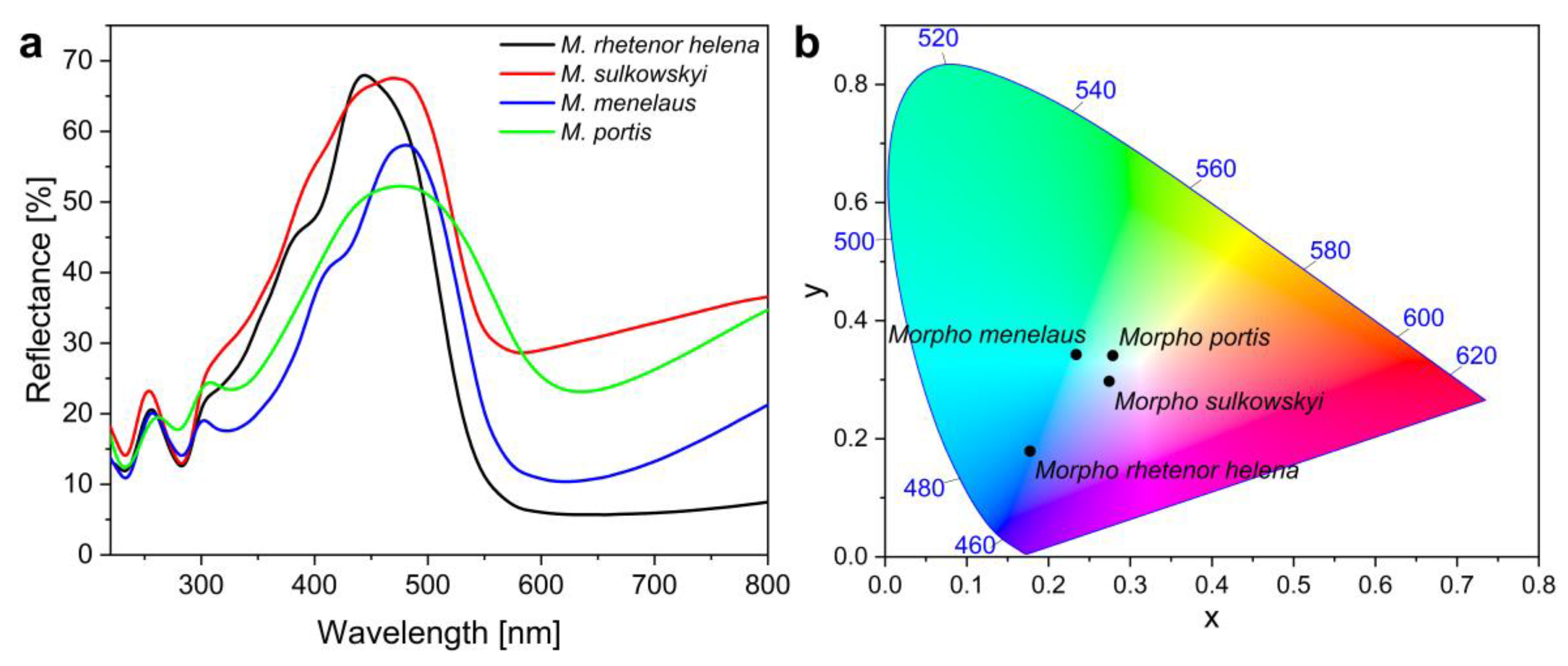
2.5. Reflectance Spectrophotometry
2.6. Photocatalytic Degradation Measurements
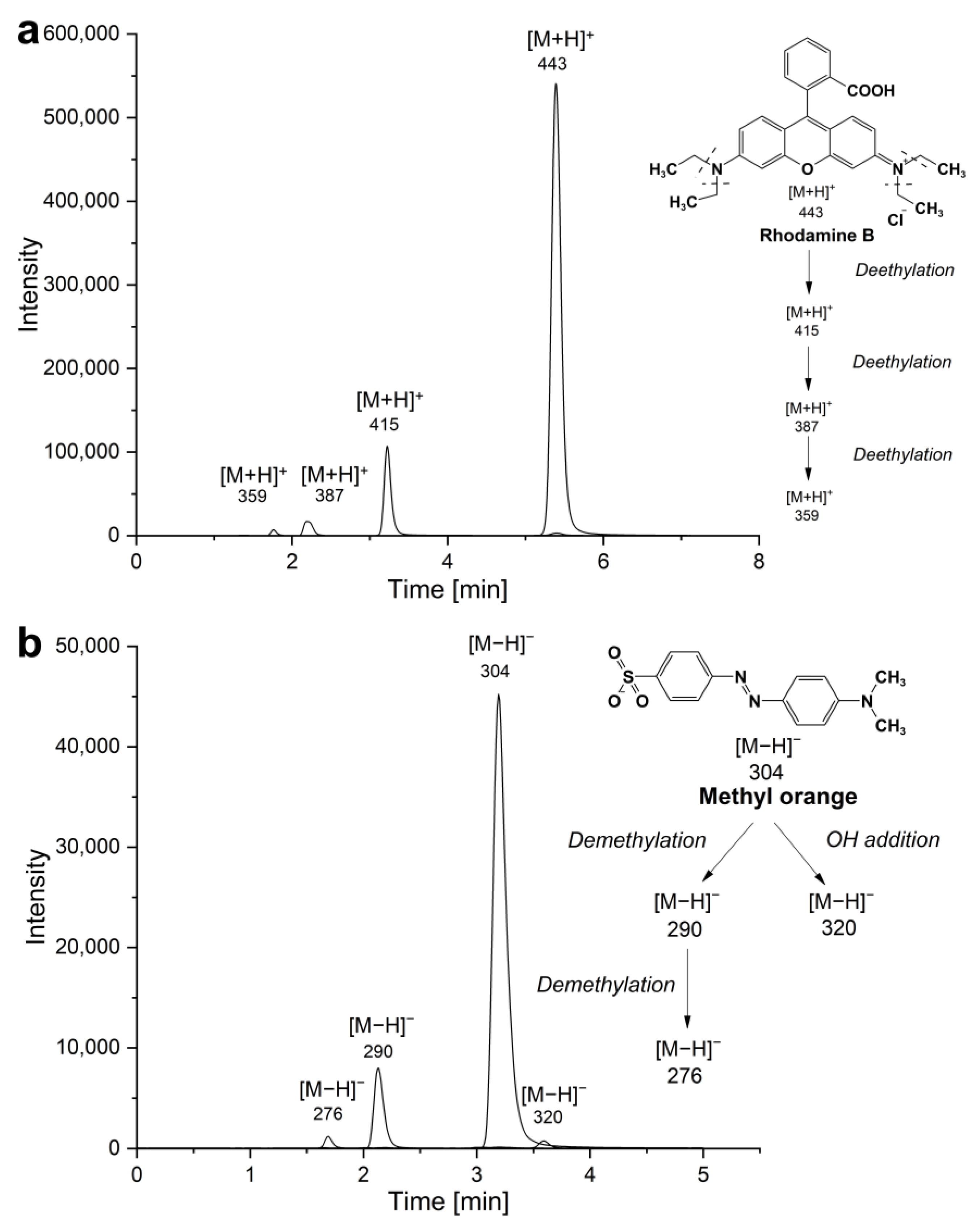
2.7. Liquid Chromatography-Mass Spectroscopy (LC-MS)
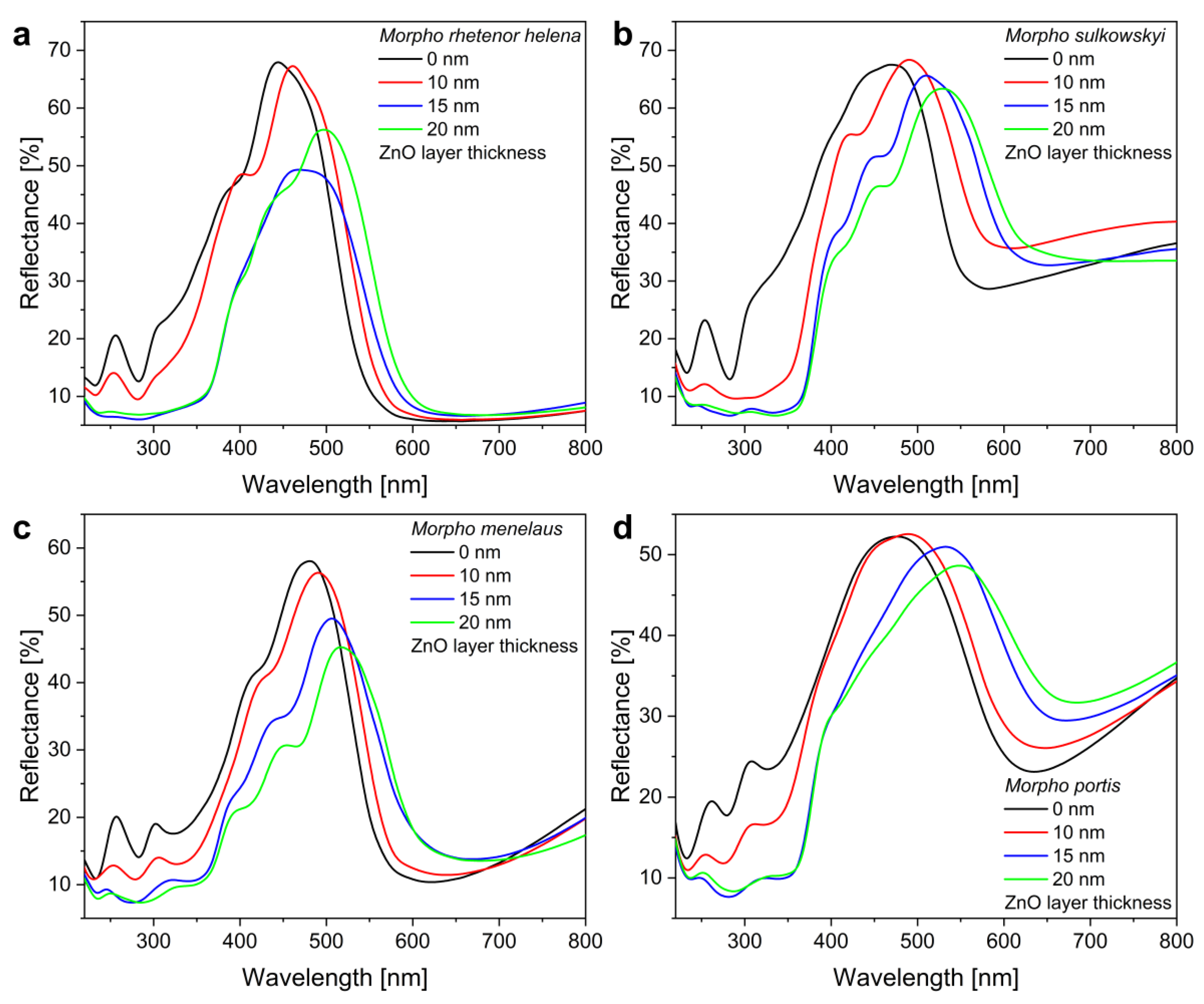
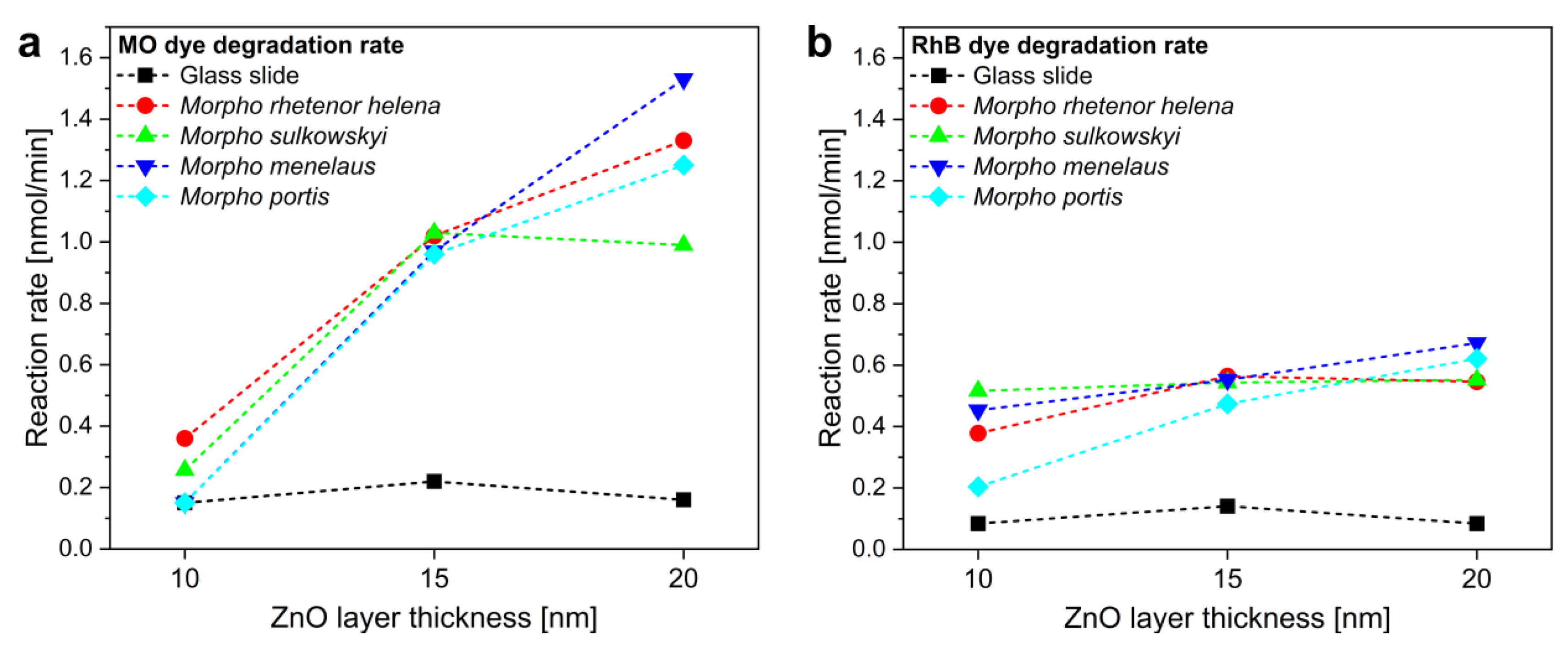
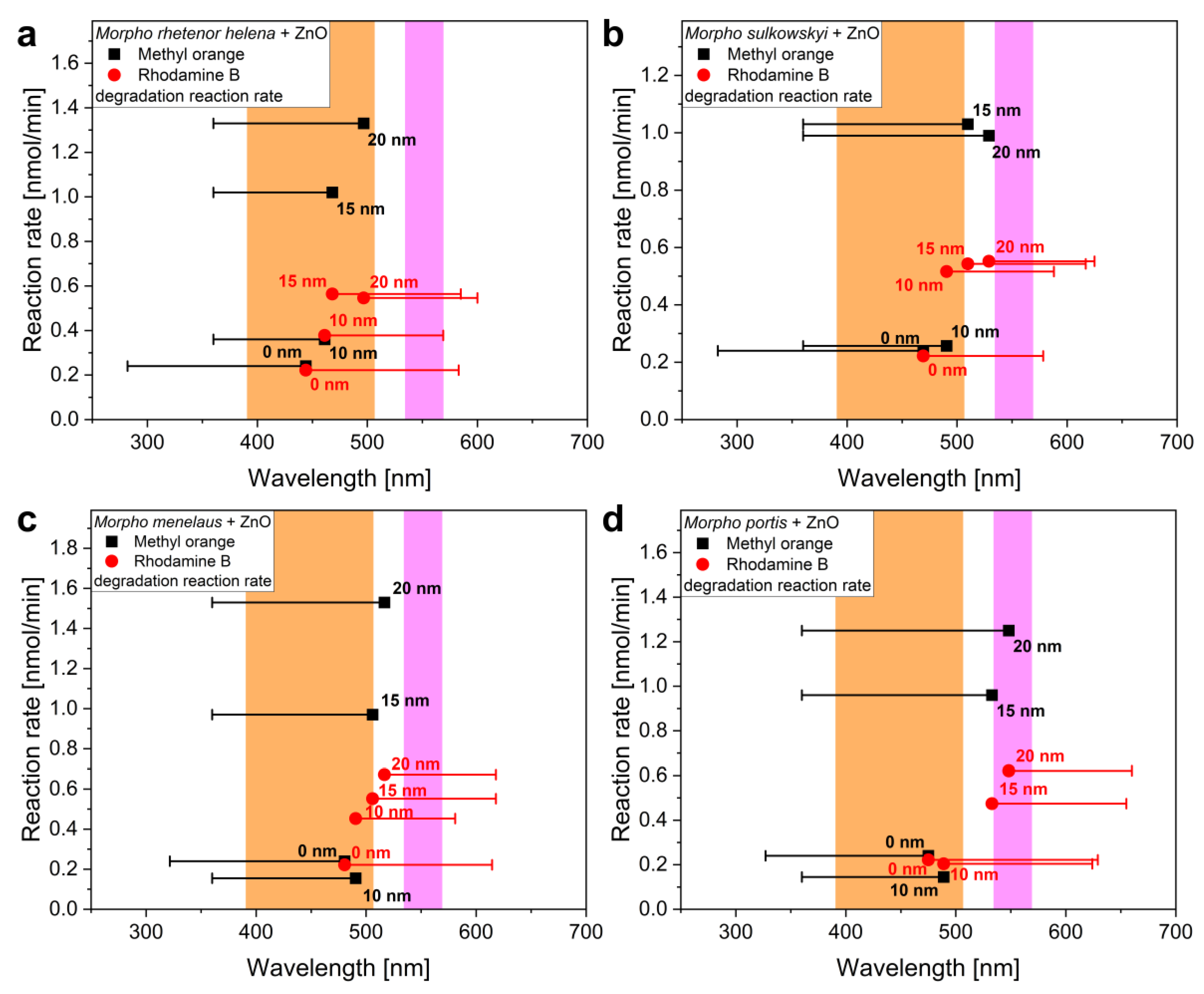
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sotiropoulou, S.; Sierra-Sastre, Y.; Mark, S.S.; Batt, C.A. Biotemplated Nanostructured Materials. Chem. Mater. 2008, 3, 821–834. [Google Scholar] [CrossRef]
- Bartl, M.H.; Galusha, J.W.; Jorgensen, M.R. Oxide-Based Photonic Crystals from Biological Templates. In Functional Metal Oxide Nanostructures; Wu, J., Cao, J., Han, W.Q., Janotti, A., Kim, H.C., Eds.; Springer: New York, NY, USA, 2012; Volume 149, pp. 175–207. [Google Scholar] [CrossRef]
- Deuerling, S.; Kugler, S.; Klotz, M.; Zollfrank, C.; Van Opdenbosch, D. A Perspective on Bio-Mediated Material Structuring. Adv. Mater. 2018, 30, 1703656. [Google Scholar] [CrossRef] [PubMed]
- Gorb, S.N. Functional Surfaces in Biology Volume 1–Little Structures with Big Effects; Springer: Dordrecht, Germany, 2009. [Google Scholar] [CrossRef]
- Biró, L.P.; Vigneron, J.P. Photonic nanoarchitectures in butterflies and beetles: Valuable sources for bioinspiration. Laser Photon. Rev. 2011, 5, 27–51. [Google Scholar] [CrossRef]
- Herrera-Beurnio, M.C.; Hidalgo-Carrillo, J.; López-Tenllado, F.J.; Martin-Gómez, J.; Estévez, R.C.; Urbano, F.J.; Marinas, A. Bio-Templating: An Emerging Synthetic Technique for Catalysts. A Review. Catalysts 2021, 11, 1364. [Google Scholar] [CrossRef]
- Galusha, J.W.; Jorgensen, M.R.; Bartl, M.H. Diamond-Structured Titania Photonic-Bandgap Crystals from Biological Templates. Adv. Mater. 2010, 22, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.R.; Bartl, M.H. Biotemplating routes to three-dimensional photonic crystals. J. Mater. Chem. 2011, 21, 10583–10591. [Google Scholar] [CrossRef]
- Rodríguez, R.E.; Agarwal, S.P.; An, S.; Kazyak, E.; Das, D.; Shang, W.; Skye, R.; Deng, T.; Dasgupta, N.P. Biotemplated Morpho Butterfly Wings for Tunable Structurally Colored Photocatalysts. ACS Appl. Mater. Interfaces 2018, 10, 4614–4621. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bhushan, B.; Tong, J. Structural coloration in nature. RSC Adv. 2013, 3, 14862–14889. [Google Scholar] [CrossRef]
- Burg, S.L.; Parnell, A.J. Self-assembling structural colour in nature. J. Phys. Condens. Matter 2018, 30, 413001. [Google Scholar] [CrossRef] [PubMed]
- McDougal, A.; Miller, B.; Singh, M.; Kolle, M. Biological growth and synthetic fabrication of structurally colored materials. J. Opt. 2019, 21, 073001. [Google Scholar] [CrossRef]
- Kemp, D.J. Female butterflies prefer males bearing bright iridescent ornamentation. Proc. R. Soc. B 2007, 274, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Kemp, D.J. Female mating biases for bright ultraviolet iridescence in the butterfly Eurema hecabe (Pieridae). Behav. Ecol. 2007, 19, 1–8. [Google Scholar] [CrossRef]
- Merilaita, S.; Tuomi, J.; Jormalainen, V. Optimization of cryptic coloration in heterogeneous habitats. Biol. J. Linn. Soc. 1999, 67, 151–161. [Google Scholar] [CrossRef]
- Suzuki, T.K.; Tomita, S.; Sezutsu, H. Multicomponent structures in camouflage and mimicry in butterfly wing patterns. J. Morphol. 2019, 280, 149–166. [Google Scholar] [CrossRef]
- Parker, A.R. 515 Million Years of Structural Colour. J. Opt. A Pure Appl. Opt. 2000, 2, R15–R28. [Google Scholar] [CrossRef]
- Joannopoulos, J.D.; Meade, R.D.; Winn, J.N. Photonic Crystals: Molding the Flow of Light; Princeton University Press: Princeton, NJ, USA, 1995. [Google Scholar]
- Lonergan, A.; O’Dwyer, C. Many Facets of Photonic Crystals: From Optics and Sensors to Energy Storage and Photocatalysis. Adv. Mater. Technol. 2022, 8, 2201410. [Google Scholar] [CrossRef]
- Kertész, K.; Baji, Z.; Deák, A.; Piszter, G.; Rázga, Z.; Bálint, Z.; Biró, L.P. Additive and subtractive modification of butterfly wing structural colors. Colloid Interface Sci. Commun. 2021, 40, 100346. [Google Scholar] [CrossRef]
- Piszter, G.; Kertész, K.; Nagy, G.; Baji, Z.; Horváth, Z.E.; Bálint, Z.; Pap, J.S.; Biró, L.P. Spectral tuning of biotemplated ZnO photonic nanoarchitectures for photocatalytic applications. R. Soc. Open Sci. 2022, 9, 220090. [Google Scholar] [CrossRef]
- Piszter, G.; Kertész, K.; Kovács, D.; Zámbó, D.; Baji, Z.; Illés, L.; Nagy, G.; Pap, J.S.; Bálint, Z.; Biró, L.P. Spectral Engineering of Hybrid Biotemplated Photonic/Photocatalytic Nanoarchitectures. Nanomaterials 2022, 12, 4490. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis by inorganic solid materials. In Advances in Inorganic Chemistry; van Eldik, R., Stochel, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 63, pp. 395–430. [Google Scholar]
- Zhou, H.; Fan, T.; Zhang, D. Biotemplated Materials for Sustainable Energy and Environment: Current Status and Challenges. ChemSusChem 2011, 4, 1344–1387. [Google Scholar] [CrossRef]
- Jamshaid, M.; Nazir, M.A.; Najam, T.; Shah, S.S.A.; Khan, H.M.; ur Rehman, A. Facile synthesis of Yb3+-Zn2+ substituted M type hexaferrites: Structural, electric and photocatalytic properties under visible light for methylene blue removal. Chem. Phys. Lett. 2022, 805, 139939. [Google Scholar] [CrossRef]
- Kumar, O.P.; Shahzad, K.; Nazir, M.A.; Farooq, N.; Malik, M.; Shah, S.S.A.; ur Rehman, A. Photo-Fenton activated C3N4x/AgOy@Co1−xBi0.1−yO7 dual s-scheme heterojunction towards degradation of organic pollutants. Opt. Mater. 2022, 126, 12199. [Google Scholar] [CrossRef]
- Shahzad, K.; Hussain, S.; Nazir, M.A.; Jamshaid, M.; ur Rehman, A.; Alkorbi, A.S.; Alsaiari, R.; Alhemiary, N.A. Versatile Ag2O and ZnO nanomaterials fabricated via annealed Ag-PMOS and ZnO-PMOS: An efficient photocatalysis tool for azo dyes. J. Mol. Liq. 2022, 356, 119036. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, H.; Wu, M.; Van der Schueren, B.; Li, Y.; Deparis, O.; Ye, J.H.; Ozin, G.; Hasan, T.; Su, B.-L. Slow Photons for Photocatalysis and Photovoltaics. Adv. Mater. 2017, 29, 1605349. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Hedrich, C.; Jiang, L.; Law, C.S.; Chirumamilla, M.; Abell, A.D.; Blick, R.H.; Zierold, R.; Santos, A. Harnessing Slow Light in Optoelectronically Engineered Nanoporous Photonic Crystals for Visible Light-Enhanced Photocatalysis. ACS Catal. 2021, 11, 12947–12962. [Google Scholar] [CrossRef]
- Deparis, O.; Mouchet, S.R.; Su, B.-L. Light harvesting in photonic crystals revisited: Why do slow photons at the blue edge enhance absorption? Phys. Chem. Chem. Phys. 2015, 17, 30525–30532. [Google Scholar] [CrossRef]
- Berthier, S. Photonique des Morphos; Springer: Paris, France, 2010; 300p. [Google Scholar]
- Giraldo, M.A.; Yoshioka, S.; Liu, C.; Stavenga, D.G. Coloration mechanisms and phylogeny of Morpho butterflies. J. Exp. Biol. 2016, 219, 3936–3944. [Google Scholar] [CrossRef] [PubMed]
- Piszter, G.; Kertész, K.; Bálint, Z.; Biró, L.P. Wide-gamut structural colors on oakblue butterflies by naturally tuned photonic nanoarchitectures. R. Soc. Open Sci. 2023, 10, 221487. [Google Scholar] [CrossRef]
- Ohno, Y. CIE Fundamentals for color measurements. In Proceedings of the International Conference on Digital Printing Technologies, Vancouver, BC, Canada, 15–20 October 2000; pp. 540–545. [Google Scholar]
- Iqbal, J.; Jilani, A.; Ziaul Hassan, P.M.; Rafique, S.; Jafer, R.; Alghamdi, A.A. ALD grown nanostructured ZnO thin films: Effect of substrate temperature on thickness and energy band gap. J. King Saud. Univ. Sci. 2016, 28, 347–354. [Google Scholar] [CrossRef]
- Leertouwer, H.L.; Wilts, B.D.; Stavenga, D.G. Refractive index and dispersion of butterfly chitin and bird keratin measured by polarizing interference microscopy. Opt. Express 2011, 19, 24061–24066. [Google Scholar] [CrossRef] [PubMed]
- Likodimos, V. Photonic Crystal-Assisted Visible Light Activated TiO2 Photocatalysis. Appl. Catal. B 2018, 230, 269–303. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, J.; Hidaka, H. Highly selective deethylation of rhodamine B: Adsorption and photooxidation pathways of the dye on the TiO2/SiO2 composite photocatalyst. Int. J. Photoenergy 2003, 5, 209–217. [Google Scholar] [CrossRef]
- Ariyanti, D.; Maillot, M.; Gao, W. TiO2 used as photocatalyst for rhodamine B degradation under solar radiation. Int. J. Mod. Phys. B 2017, 31, 1744095. [Google Scholar] [CrossRef]
- Comparelli, R.; Fanizza, E.; Curri, M.L.; Cozzoli, P.D.; Mascolo, G.; Passino, R.; Agostiano, A. Photocatalytic degradation of azo dyes by organic-capped anatase TiO2 nanocrystals immobilized onto substrates. Appl. Catal. B 2005, 55, 81–91. [Google Scholar] [CrossRef]
- Gupta, N.; Pal, B. Core–shell structure of metal loaded CdS–SiO2 hybrid nanocomposites for complete photomineralization of methyl orange by visible light. J. Mol. Catal. A Chem. 2014, 391, 158–167. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piszter, G.; Nagy, G.; Kertész, K.; Baji, Z.; Kovács, K.; Bálint, Z.; Horváth, Z.E.; Pap, J.S.; Biró, L.P. Investigating the Effect of Reflectance Tuning on Photocatalytic Dye Degradation with Biotemplated ZnO Photonic Nanoarchitectures Based on Morpho Butterfly Wings. Materials 2023, 16, 3584. https://doi.org/10.3390/ma16093584
Piszter G, Nagy G, Kertész K, Baji Z, Kovács K, Bálint Z, Horváth ZE, Pap JS, Biró LP. Investigating the Effect of Reflectance Tuning on Photocatalytic Dye Degradation with Biotemplated ZnO Photonic Nanoarchitectures Based on Morpho Butterfly Wings. Materials. 2023; 16(9):3584. https://doi.org/10.3390/ma16093584
Chicago/Turabian StylePiszter, Gábor, Gergely Nagy, Krisztián Kertész, Zsófia Baji, Krisztina Kovács, Zsolt Bálint, Zsolt Endre Horváth, József Sándor Pap, and László Péter Biró. 2023. "Investigating the Effect of Reflectance Tuning on Photocatalytic Dye Degradation with Biotemplated ZnO Photonic Nanoarchitectures Based on Morpho Butterfly Wings" Materials 16, no. 9: 3584. https://doi.org/10.3390/ma16093584




