Rational Design of Flexible Mechanical Force Sensors for Healthcare and Diagnosis
Abstract
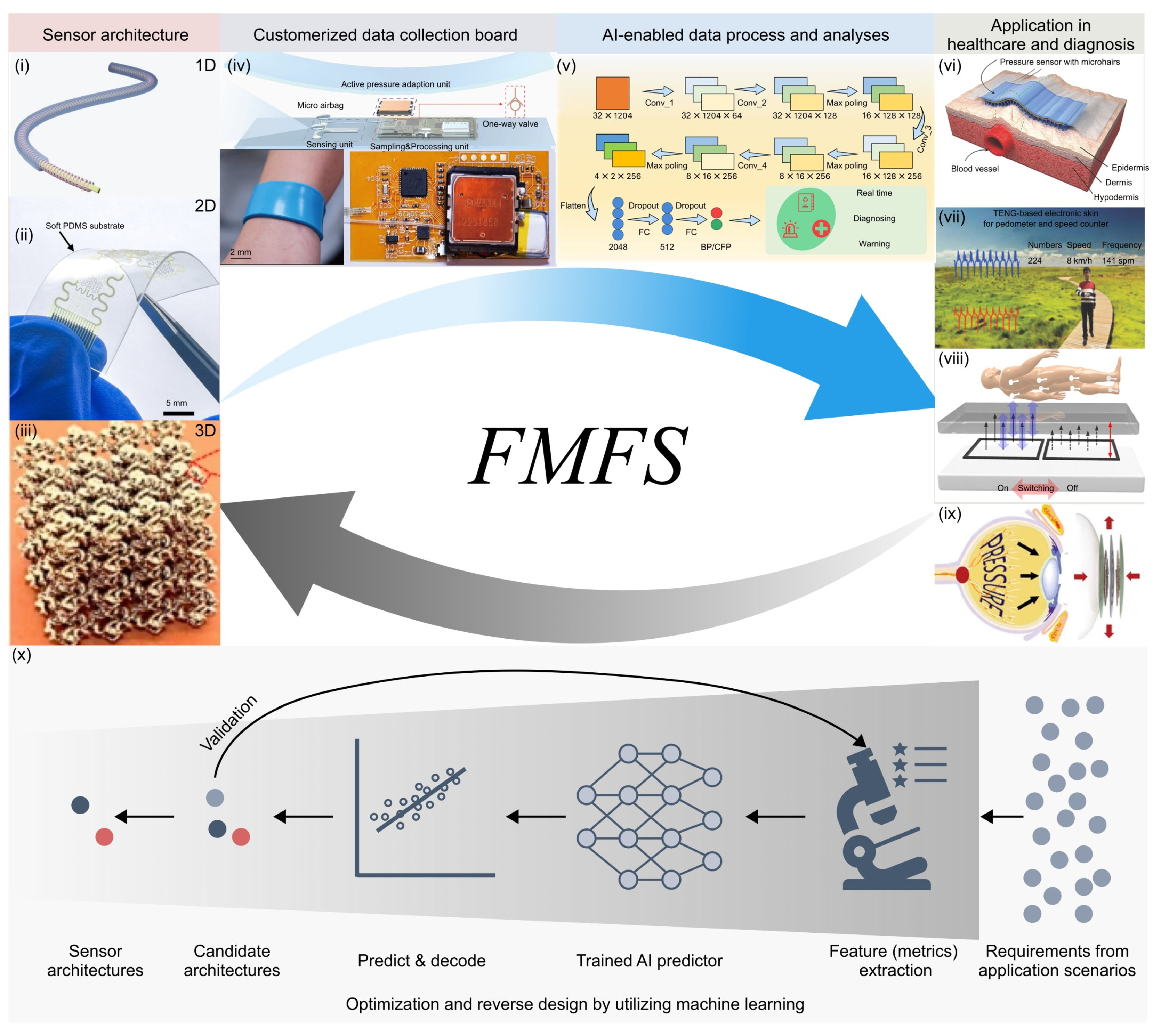
:1. Introduction
2. Fundamentals of Mechanical Force Sensors
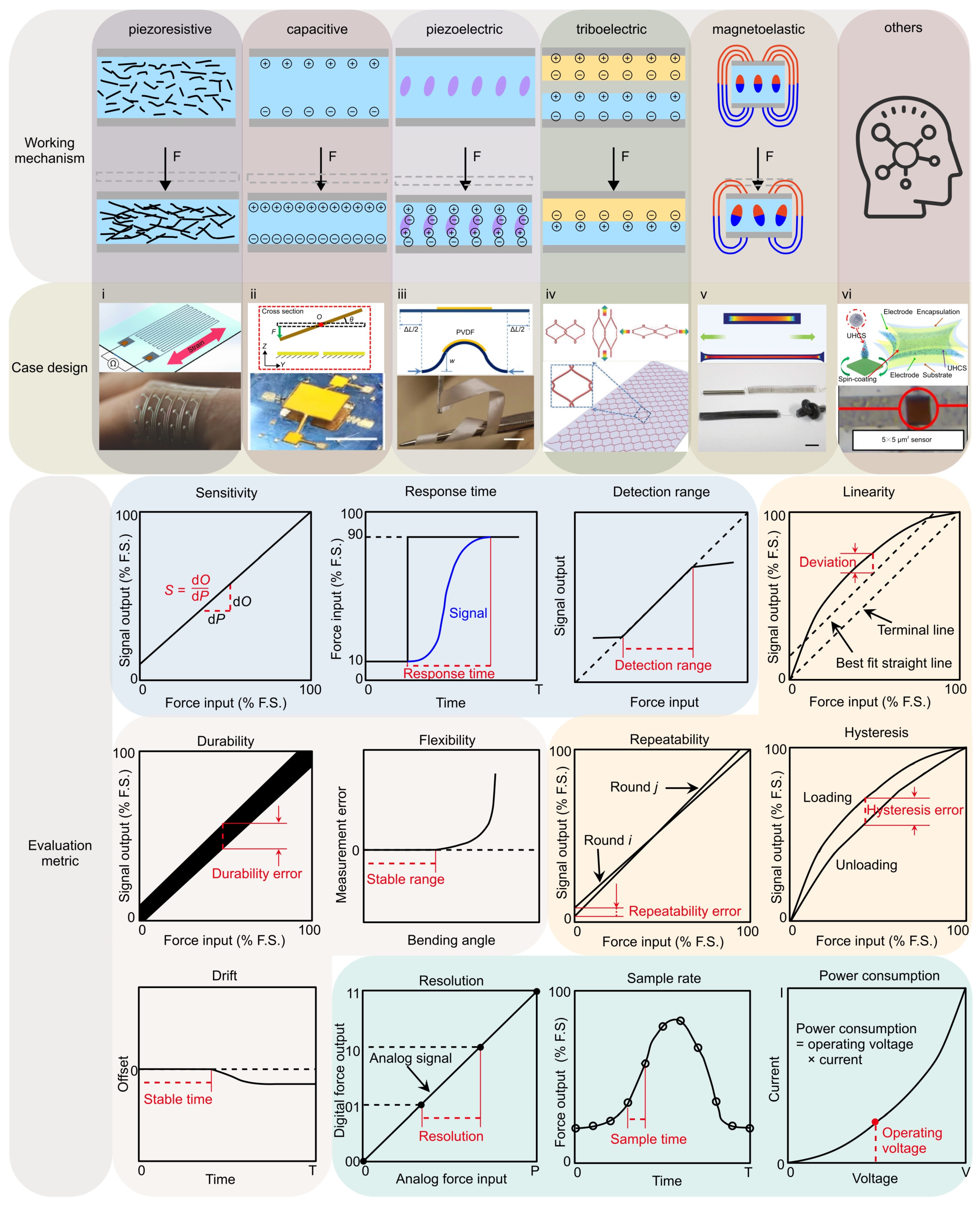
2.1. Classification of Mechanical Force Sensors
- (1)
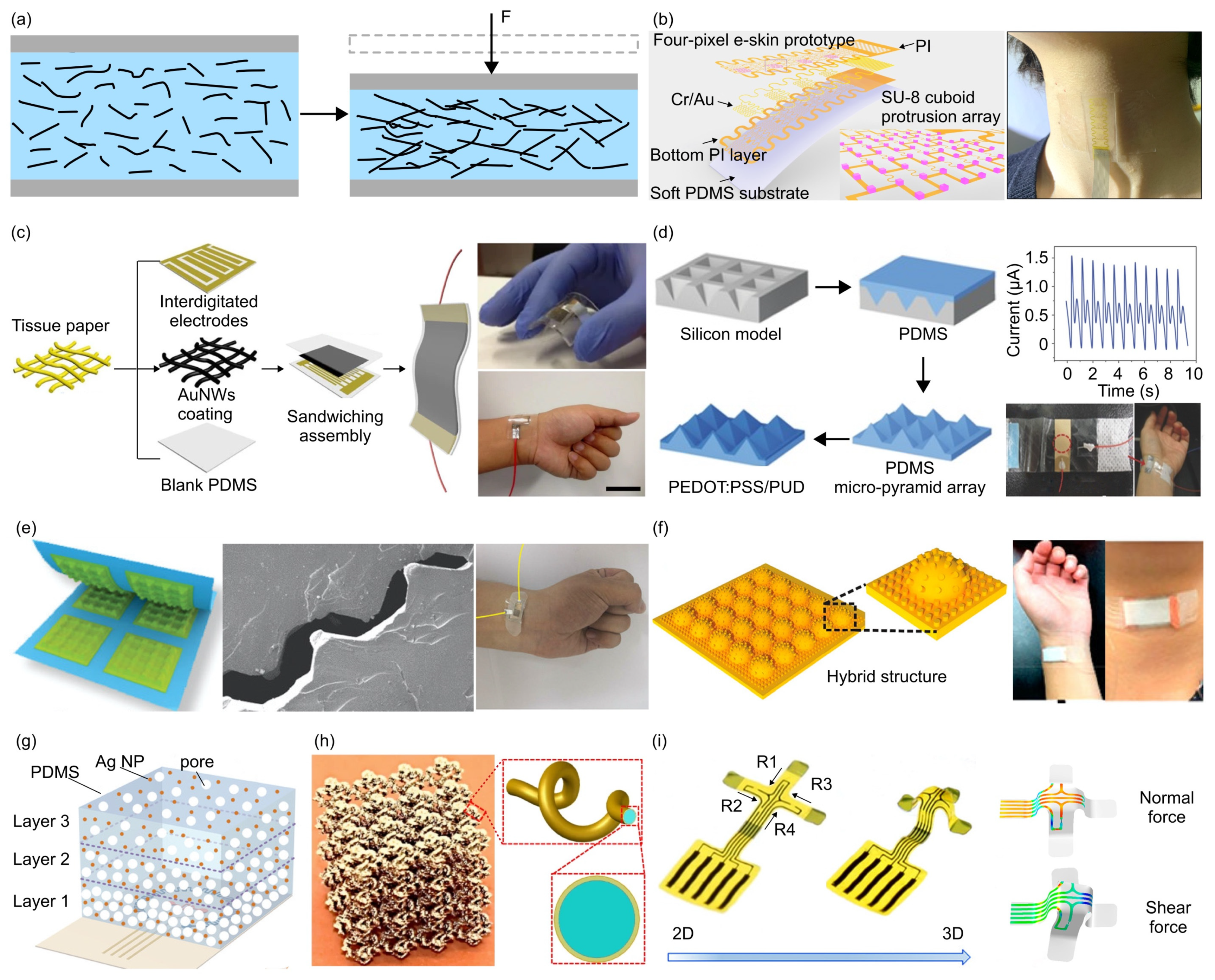
- The piezoresistive effect is a phenomenon in which the electrical resistance of a material changes in response to applied mechanical force, originating from alterations in the material’s crystal structure when subjected to mechanical deformations. Due to their simple design and ease of read-out, piezoresistive force sensors were widely investigated. Typically, metals and semiconductors are used as the sensing material, while polymers serve as the soft substrate. For example, Valentine et al. fabricated a biocompatible and highly stretchable force sensor through hybrid 3D printing [123]. The developed Ag thermoplastic polyurethane (TPU) inks could produce mechanically robust, stretchable conductors, resulting in a consistent and repeatable electrical response in the fabricated force sensor.
- (2)
- The capacitive force sensor relies on changes in capacitance to achieve measurements of mechanical force. This type of force sensor typically consists of two conductive plates separated by a dielectric material. Usually, conductive materials like metal, carbon nanotubes, graphene, or conductive polymers can be exploited as the electrodes, while air gaps or non-conductive polymers serve as the dielectric material. For example, Ye and Zhang et al. utilized controlled compressive buckling assembly strategy to fabricate a 3D seesaw-like capacitive sensor with a tunable sensitivity [75].
- (3)
- The piezoelectric effect is an interesting phenomenon observed in specific materials that can produce an electric charge when subjected to mechanical loadings. This usually occurs as a result of the reconfiguration of charged atoms or molecules within the material. Commonly used piezoelectric materials include lead zirconate titanate (PZT) ceramics, lead magnesium niobate-lead titanate (PMN-PT) ceramics, and polyvinylidene fluoride (PVDF). For instance, Persano et al. developed a large-area, flexible piezoelectric material comprising sheets of electrospun fibers made from the polymer poly(vinylidenefluoride-co-trifluoroethylene) (P(VDF-TrFe)) [163]. The force sensor based on such fiber arrays exhibits an ultra-high sensitivity, allowing measurement of very low pressures (e.g., 0.1 Pa).
- (4)
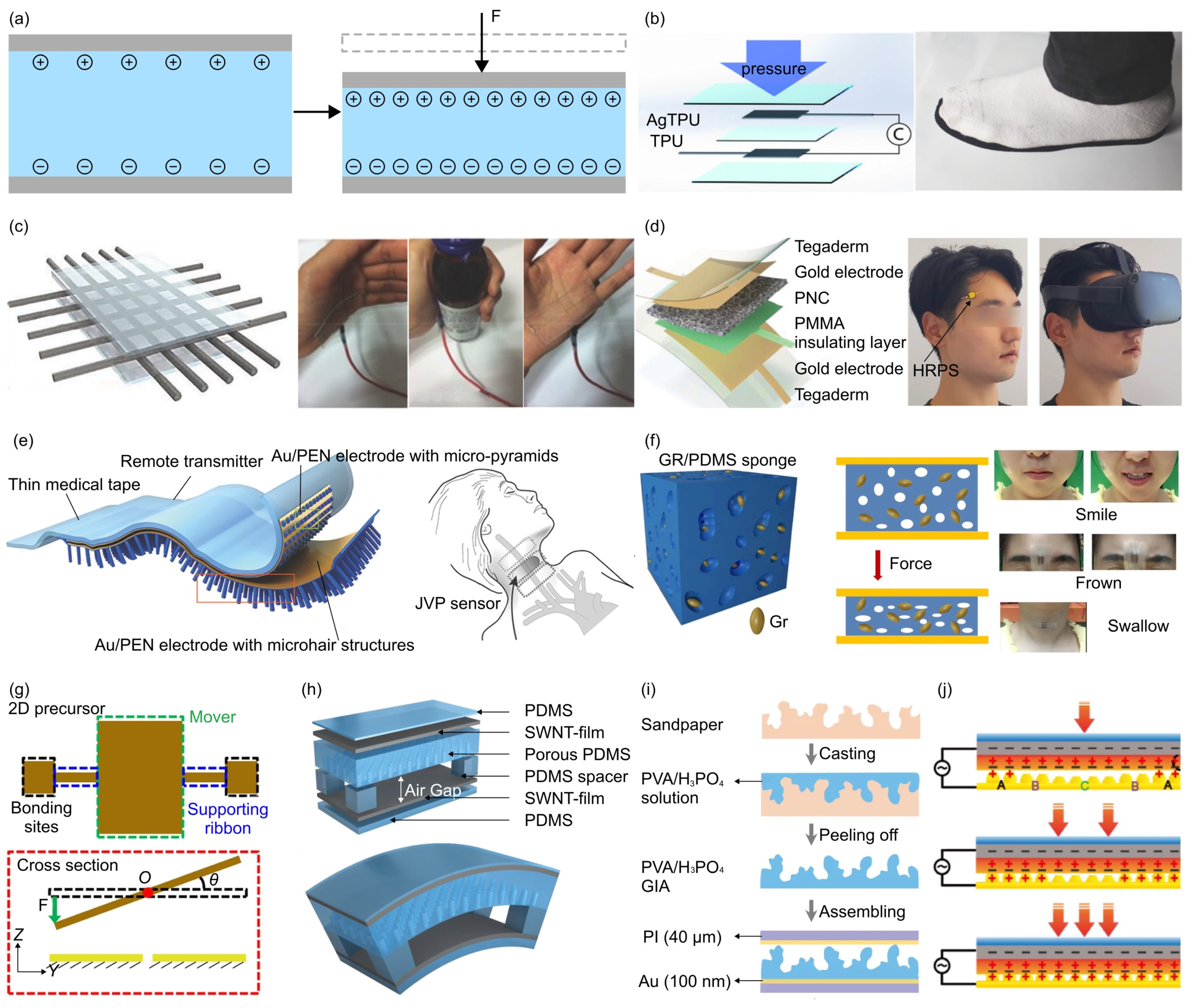
- The triboelectric effect is a phenomenon where specific materials become electrically charged upon contact and subsequent separation. To develop triboelectric force sensors, the researchers usually use copper, silver, carbon, polymer hybrid electrode materials, and flexible ion-gel electrode materials as the electrode materials. For example, Dong et al. reported a stretchable and washable skin-inspired triboelectric nanogenerator through embedding the planar and designable conductive yarn network into the flexible elastomer [164].
- (5)
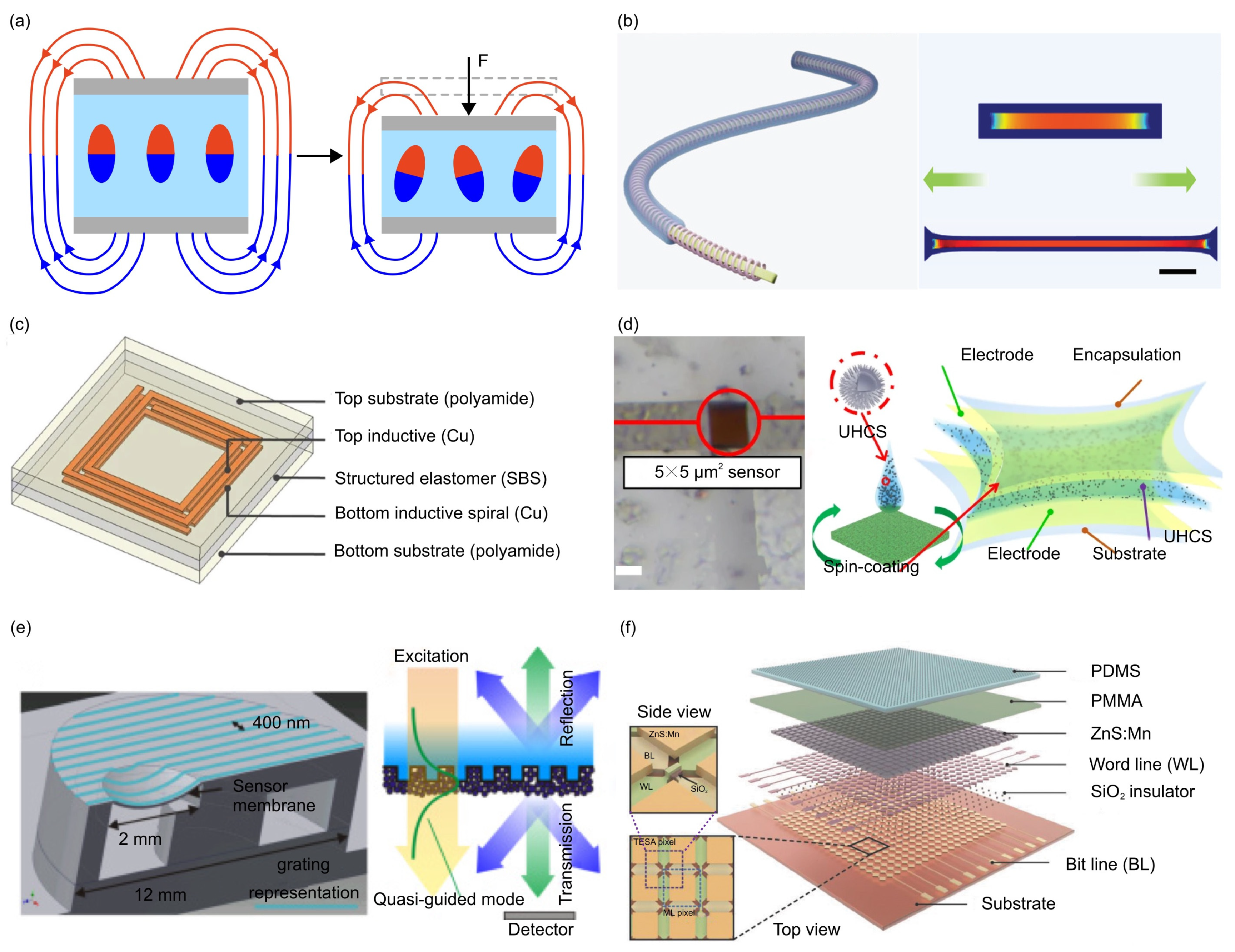
- The magnetoelastic effect is a phenomenon in which the mechanical properties of a material change in response to an applied magnetic field. Typically, magnetoelastic materials include ferromagnetic materials (e.g., iron, nickel, and cobalt-based alloys), ferrimagnetic materials (e.g., magnetite and yttrium iron garnet) and soft magnetostrictive composites (e.g., blended NdFeB-Ecoflex). For example, Zhang et al. developed an innovative force sensor based on a flexible NdFeB magnet. This sensor exhibits a high stretchability (>150%), a rapid response time (~30 milliseconds), and an outstanding linearity (R2 > 0.98) [165].
- (6)
- Other physics phenomenon adopted in designing force sensors includes the Fowler–Nordheim tunnelling effect, optical grating, resonant circuit, among others. Such unusual sensing mechanisms can offer excellent sensing performance in certain aspects of mechanical force sensors. For example, the Fowler–Nordheim tunneling effect is a quantum mechanical phenomenon in which electrons tunnel through a potential energy barrier when exposed to a strong electric field. Based on this mechanism, Shi et al. fabricated a flexible pressure sensor with ultrahigh sensitivity (260.3 kPa−1 at 1 Pa), sensing density, and transparency [166].
2.2. Evaluation Metrics of Force Sensors
- (1)
- Sensitivity is defined as the change of the observed physical quantity given a unit change of the force. The specific expression for the force sensor can be written as:
- (2)
- Response time is defined as the time consumption that the sensor needs to apply the changes made in the input force to the output signal. Typically, we use the time it takes for the sensor to rise from 10% to 90% of its full-scale range as the response time. The specific expression is given by:
- (3)
- Detection range refers to the span of force within which the force sensor can provide precise and meaningful force measurements, as written by:
- (4)
- Linearity is defined as the straightness of the output at various equally spaced force points during a loading process, and the error
- (5)
- Hysteresis is defined as the maximum difference in output at any measurement value within the sensor’s specified range, when approaching the point first with increasing and then with decreasing the applied force. The specific expression can be written as:
- (6)
- Repeatability refers to the consistency of the sensor’s readings when the same magnitude of force is applied multiple times. The difference in the output signals between two measurements can be utilized to quantify the repeatability of the force sensor, i.e.,
- (7)
- Flexibility usually refers to the capability of enduring large degrees of bending and twisting deformations, without evident changes in the sensor performance. Typically, the measurement error under different bending (or twisting) angles (α) could be used to characterize the sensor’s flexibility, as follows:
- (8)
- Durability in the context of mechanical force sensors denotes their capability of enduring repeated or extended exposure to forces without experiencing notable degradation or damage. Typically, the durability of sensors encompasses two aspects: (1) the stability of the sensor itself under constant environmental influences, and (2) the stability of the evaluation metrics under various environments. The former is mainly determined by the oxidation/corrosion resistance (without force loading) or fatigue property (with force loading) of the component material, over a long period (e.g., months or even years). The latter is usually a short process (several minutes or hours) and contingent upon the service environment (e.g., temperature, humidity, and mechanical stress), because environmental factors highly impact the accuracy and reliability of force measurements. A durable force sensor should display minimal degradation in its measurement capabilities even after undergoing prolonged cyclic loading or rapid environmental changes.
- (9)
- Drift is characterized by the gradual degradation of the sensor, leading to deviations from its originally calibrated state. The drift of force sensor is typically induced by temperature/humidity variations, the aging of sensor components, or other environmental conditions.
- (10)
- Resolution refers to the smallest force increase that the sensor can measure. Typically, signal acquisition boards are utilized to collect analog signals from the force sensors and convert them into digital signals. A higher resolution means that the force sensing system can detect more subtle force variations.
- (11)
- Sample rate, another system-level performance metric, refers to the rate at which the data is read and displayed. A high sample rate can improve the measurement accuracy of the force sensing system, especially for the measurement of dynamic forces.
- (12)
- Power consumption refers to the amount of energy used per unit time, and is usually defined by the product of operating voltage and current [167].
3. Structures and Applications for Different Types of Force Sensors
3.1. Piezoresistive Force Sensors
3.2. Capacitive Force Sensors
3.3. Piezoelectric and Triboelectric Force Sensors

3.4. Mechanical Force Sensors Based on Magnetoelastic Effect and Other Physics Phenomenon
4. Summary and Perspectives
4.1. Developing Novel Sensor Architectures
4.2. Intelligent Mechanical Force Sensing Systems with Integrated Hardware and Software
4.3. Problem-Driven Design of Flexible Mechanical Force Sensors
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 21 December 2023).
- Chan, M.; Estève, D.; Fourniols, J.-Y.; Escriba, C.; Campo, E. Smart Wearable Systems: Current Status and Future Challenges. Artif. Intell. Med. 2012, 56, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Luo, G.; Ji, Z.; Bo, R.; Xue, Z.; Yan, D.; Zhang, F.; Bai, K.; Liu, J.; Cheng, X.; et al. Highly-Integrated, Miniaturized, Stretchable Electronic Systems Based on Stacked Multilayer Network Materials. Sci. Adv. 2022, 8, eabm3785. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, X.; Liu, J.; Ye, D.; Duan, Y.; Li, K.; Yin, Z.; Huang, Y. Flexible Metamaterial Electronics. Adv. Mater. 2022, 34, 2200070. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, H.; Xiao, L.; Duan, Y.; Zhu, H.; Bian, J.; Ye, D.; Yin, Z. Assembly and Applications of 3D Conformal Electronics on Curvilinear Surfaces. Mater. Horiz. 2019, 6, 642–683. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Xu, Z.; Liang, L.; Tian, Y.; Li, L.; Wu, H.; Zhong, S. Cuffless Blood Pressure Monitoring: Academic Insights and Perspectives Analysis. Micromachines 2022, 13, 1225. [Google Scholar] [CrossRef]
- Zazoum, B.; Batoo, K.M.; Khan, M.A.A. Recent Advances in Flexible Sensors and Their Applications. Sensors 2022, 22, 4653. [Google Scholar] [CrossRef] [PubMed]
- Shumba, A.-T.; Montanaro, T.; Sergi, I.; Bramanti, A.; Ciccarelli, M.; Rispoli, A.; Carrizzo, A.; De Vittorio, M.; Patrono, L. Wearable Technologies and AI at the Far Edge for Chronic Heart Failure Prevention and Management: A Systematic Review and Prospects. Sensors 2023, 23, 6896. [Google Scholar] [CrossRef] [PubMed]
- Ochieze, C.; Zare, S.; Sun, Y. Wearable Upper Limb Robotics for Pervasive Health: A Review. Prog. Biomed. Eng. 2023, 5, 032003. [Google Scholar] [CrossRef]
- Mantovani, E.; Demrozi, F.; Hertz, D.L.; Turetta, C.; Ferro, O.; Argyriou, A.A.; Pravadelli, G.; Tamburin, S. Wearables, Sensors, and Smart Devices for the Detection and Monitoring of Chemotherapy-Induced Peripheral Neurotoxicity: Systematic Review and Directions for Future Research. J. Peripher. Nerv. Syst. 2022, 27, 238–258. [Google Scholar] [CrossRef]
- Costin, H.-N.; Sanei, S. Intelligent Biosignal Processing in Wearable and Implantable Sensors. Biosensors 2022, 12, 396. [Google Scholar] [CrossRef]
- Beduk, T.; Beduk, D.; Hasan, M.R.; Guler Celik, E.; Kosel, J.; Narang, J.; Salama, K.N.; Timur, S. Smartphone-Based Multiplexed Biosensing Tools for Health Monitoring. Biosensors 2022, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Montiel, V.R.-V.; Vargas, E.; Teymourian, H.; Wang, J. Wearable and Mobile Sensors for Personalized Nutrition. ACS Sens. 2021, 6, 1745–1760. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A Review on Electrospun Nanofibers Based Advanced Applications: From Health Care to Energy Devices. Polymers 2021, 13, 3746. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Vizza, P.; Calabrese, B.; Ielpo, N. Biopotential Signal Monitoring Systems in Rehabilitation: A Review. Sensors 2021, 21, 7172. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, K.; Tokito, S. Organic-Transistor-Based Biosensors Interfaced with Human Skin for Non-Invasive Perspiration Analysis. Sens. Actuators B Chem. 2021, 349, 130778. [Google Scholar] [CrossRef]
- Komolafe, A.; Zaghari, B.; Torah, R.; Weddell, A.S.; Khanbareh, H.; Tsikriteas, Z.M.; Vousden, M.; Wagih, M.; Jurado, U.T.; Shi, J.; et al. E-Textile Technology Review–From Materials to Application. IEEE Access 2021, 9, 97152–97179. [Google Scholar] [CrossRef]
- Khan, A.S.; Khan, F.U. Experimentation of a Wearable Self-Powered Jacket Harvesting Body Heat for Wearable Device Applications. J. Sens. 2021, 2021, e9976089. [Google Scholar] [CrossRef]
- Hasan, M.N.; Sahlan, S.; Osman, K.; Mohamed Ali, M.S. Energy Harvesters for Wearable Electronics and Biomedical Devices. Adv. Mater. Technol. 2021, 6, 2000771. [Google Scholar] [CrossRef]
- Farahmand Nejad, M.A.; Ranjbar, S.; Parolo, C.; Nguyen, E.P.; Álvarez-Diduk, R.; Hormozi-Nezhad, M.R.; Merkoçi, A. Electrochromism: An Emerging and Promising Approach in (Bio)Sensing Technology. Mater. Today 2021, 50, 476–498. [Google Scholar] [CrossRef]
- Silvera-Tawil, D.; Hussain, M.S.; Li, J. Emerging Technologies for Precision Health: An Insight into Sensing Technologies for Health and Wellbeing. Smart Health 2020, 15, 100100. [Google Scholar] [CrossRef]
- Gu, J.-W.; Lee, J.-H.; Kang, S.-K. 3D Electronic Sensors for Bio-Interfaced Electronics and Soft Robotics. Adv. Sens. Res. 2023, 2, 2300013. [Google Scholar] [CrossRef]
- Di Patrizio Stanchieri, G.; De Marcellis, A.; Faccio, M.; Palange, E.; Guler, U. A New Light-to-Frequency Analog Front-End Circuit for Optical Sensing in Biomedical Applications. In Proceedings of the 2021 IEEE Biomedical Circuits and Systems Conference (BioCAS), Berlin, Germany, 7–9 October 2021; pp. 1–5. [Google Scholar]
- Azimi, B.; Milazzo, M.; Lazzeri, A.; Berrettini, S.; Uddin, M.J.; Qin, Z.; Buehler, M.J.; Danti, S. Electrospinning Piezoelectric Fibers for Biocompatible Devices. Adv. Healthc. Mater. 2020, 9, 1901287. [Google Scholar] [CrossRef]
- Naveed, S.; Jinzhong, M.; Shahzad, S.; Wu, X.; Ren, T. Flexible Planar Capacitive Devices for Hydration and Sweat Sensing. Flex. Print. Electron. 2023, 8, 025009. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kariper, İ.A. Production and Applications of Flexible/Wearable Triboelectric Nanogenerator (TENGS). Synth. Met. 2021, 273, 116692. [Google Scholar] [CrossRef]
- Balayan, S.; Chauhan, N.; Chandra, R.; Kuchhal, N.K.; Jain, U. Recent Advances in Developing Biosensing Based Platforms for Neonatal Sepsis. Biosens. Bioelectron. 2020, 169, 112552. [Google Scholar] [CrossRef]
- Hussin, H.; Soin, N.; Hatta, S.F.W.M.; Rezali, F.A.M.; Wahab, Y.A. Review—Recent Progress in the Diversity of Inkjet-Printed Flexible Sensor Structures in Biomedical Engineering Applications. J. Electrochem. Soc. 2021, 168, 077508. [Google Scholar] [CrossRef]
- Mahmudiono, T.; Olegovich Bokov, D.; Abdalkareem Jasim, S.; Kamal Abdelbasset, W.; Dinora, M. Khashirbaeva State-of-the-Art of Convenient and Low-Cost Electrochemical Sensor for Food Contamination Detection: Technical and Analytical Overview. Microchem. J. 2022, 179, 107460. [Google Scholar] [CrossRef]
- Arya, S.S.; Dias, S.B.; Jelinek, H.F.; Hadjileontiadis, L.J.; Pappa, A.-M. The Convergence of Traditional and Digital Biomarkers through AI-Assisted Biosensing: A New Era in Translational Diagnostics? Biosens. Bioelectron. 2023, 235, 115387. [Google Scholar] [CrossRef]
- Chaudhary, V.; Khanna, V.; Ahmed Awan, H.T.; Singh, K.; Khalid, M.; Mishra, Y.K.; Bhansali, S.; Li, C.-Z.; Kaushik, A. Towards Hospital-on-Chip Supported by 2D MXenes-Based 5th Generation Intelligent Biosensors. Biosens. Bioelectron. 2023, 220, 114847. [Google Scholar] [CrossRef]
- Ray, T.R.; Choi, J.; Bandodkar, A.J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J.A. Bio-Integrated Wearable Systems: A Comprehensive Review. Chem. Rev. 2019, 119, 5461–5533. [Google Scholar] [CrossRef]
- Castaneda, D.; Esparza, A.; Ghamari, M.; Soltanpur, C.; Nazeran, H. A Review on Wearable Photoplethysmography Sensors and Their Potential Future Applications in Health Care. Int. J. Biosens. Bioelectron. 2018, 4, 195. [Google Scholar]
- Nair, V.; Dalrymple, A.N.; Yu, Z.; Balakrishnan, G.; Bettinger, C.J.; Weber, D.J.; Yang, K.; Robinson, J.T. Miniature Battery-Free Bioelectronics. Science 2023, 382, eabn4732. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.; Kim, J.S.; Kim, S.; Kang, H.; Kim, D.J.; Choi, H.; Roe, D.G.; Choi, Y.J.; Lee, S.G.; Cho, J.H.; et al. Ion Trap and Release Dynamics Enables Nonintrusive Tactile Augmentation in Monolithic Sensory Neuron. Sci. Adv. 2023, 9, eadi3827. [Google Scholar] [CrossRef] [PubMed]
- Franklin, D.; Tzavelis, A.; Lee, J.Y.; Chung, H.U.; Trueb, J.; Arafa, H.; Kwak, S.S.; Huang, I.; Liu, Y.; Rathod, M.; et al. Synchronized Wearables for the Detection of Haemodynamic States via Electrocardiography and Multispectral Photoplethysmography. Nat. Biomed. Eng. 2023, 7, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; He, J.; Zhang, L.; Yang, F.; Zhang, D. A Novel Pressure Sensor Design for Low Pressure Ranges. In Proceedings of the 2014 IEEE International Conference on Electron Devices and Solid-State Circuits, Chengdu, China, 18–20 June 2014; pp. 1–2. [Google Scholar]
- Xu, T.; Wang, H.; Xia, Y.; Zhao, Z.; Huang, M.; Wang, J.; Zhao, L.; Zhao, Y.; Jiang, Z. Piezoresistive Pressure Sensor with High Sensitivity for Medical Application Using Peninsula-Island Structure. Front. Mech. Eng. 2017, 12, 546–553. [Google Scholar] [CrossRef]
- Basov, M.; Prigodskiy, D. Development of High-Sensitivity Piezoresistive Pressure Sensors for −0.5… + 0.5 kPa. J. Micromechan. Microeng. 2020, 30, 105006. [Google Scholar] [CrossRef]
- Basov, M. Pressure Sensor with Novel Electrical Circuit Utilizing Bipolar Junction Transistor. In Proceedings of the 2021 IEEE Sensors, Virtual, 31 October–4 November 2021; pp. 1–4. [Google Scholar]
- Fiorillo, A.S.; Critello, C.D.; Pullano, S.A. Theory, Technology and Applications of Piezoresistive Sensors: A Review. Sens. Actuators Phys. 2018, 281, 156–175. [Google Scholar] [CrossRef]
- Basov, M. Ultra-High Sensitivity MEMS Pressure Sensor Utilizing Bipolar Junction Transistor for Pressures Ranging From −1 to 1 kPa. IEEE Sens. J. 2021, 21, 4357–4364. [Google Scholar] [CrossRef]
- Takahashi, H.; Takei, Y.; Noda, K.; Matsumoto, K.; Shimoyama, I. Triaxial Piezoresistive Force Sensor Probe with High Sensitivity and Stiffness Using 3D Notch Structure. J. Micromechan. Microeng. 2023, 33, 125005. [Google Scholar] [CrossRef]
- Malhotra, N.; Hoang, K.; Desai, J.P. Towards the Development of a MEMS-Based Force Sensor for In Vivo Tumor Tissue Demarcation. In Proceedings of the 2023 International Symposium on Medical Robotics (ISMR), Atlanta, GA, USA, 19–21 April 2023; pp. 1–6. [Google Scholar]
- Sitaramgupta V, V.S.N.; Sakorikar, T.; Pandya, H.J. An MEMS-Based Force Sensor: Packaging and Proprioceptive Force Recognition Through Vibro-Haptic Feedback for Catheters. IEEE Trans. Instrum. Meas. 2022, 71, 3141168. [Google Scholar] [CrossRef]
- Lanzolla, A.M.L.; Attivissimo, F.; Percoco, G.; Ragolia, M.A.; Stano, G.; Di Nisio, A. Additive Manufacturing for Sensors: Piezoresistive Strain Gauge with Temperature Compensation. Appl. Sci. 2022, 12, 8607. [Google Scholar] [CrossRef]
- Dózsa, T.; Radó, J.; Volk, J.; Kisari, Á.; Soumelidis, A.; Kovács, P. Road Abnormality Detection Using Piezoresistive Force Sensors and Adaptive Signal Models. IEEE Trans. Instrum. Meas. 2022, 71, 3194900. [Google Scholar] [CrossRef]
- Yeh, S.-K.; Hsieh, M.-L.; Fang, W. CMOS-Based Tactile Force Sensor: A Review. IEEE Sens. J. 2021, 21, 12563–12577. [Google Scholar] [CrossRef]
- Tiwari, B.; Billot, M.; Clévy, C.; Agnus, J.; Piat, E.; Lutz, P. A Two-Axis Piezoresistive Force Sensing Tool for Microgripping. Sensors 2021, 21, 6059. [Google Scholar] [CrossRef] [PubMed]
- Sitaramgupta V, V.S.N.; B.S, A.; Behera, B.; Padmanabhan, D.; Pandya, H.J. A Ring-Shaped MEMS-Based Piezoresistive Force Sensor for Cardiac Ablation Catheters. IEEE Sens. J. 2021, 21, 26042–26049. [Google Scholar] [CrossRef]
- Nguyen, T.-V.; Mizuki, Y.; Tsukagoshi, T.; Takahata, T.; Ichiki, M.; Shimoyama, I. MEMS-Based Pulse Wave Sensor Utilizing a Piezoresistive Cantilever. Sensors 2020, 20, 1052. [Google Scholar] [CrossRef] [PubMed]
- Miani, T.; Verdot, T.; Berthelot, A.; Maspero, F.; Koumela, A.; Robert, P.; Langfelder, G.; Arcamone, J.; Sansa, M. Resonant Accelerometers Based on Nanomechanical Piezoresistive Transduction. In Proceedings of the 2021 IEEE 34th International Conference on Micro Electro Mechanical Systems (MEMS), Virtual, 25–29 January 2021; pp. 192–195. [Google Scholar]
- Pommois, R.; Furusawa, G.; Kosuge, T.; Yasunaga, S.; Hanawa, H.; Takahashi, H.; Kan, T.; Aoyama, H. Micro Water Flow Measurement Using a Temperature-Compensated MEMS Piezoresistive Cantilever. Micromachines 2020, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Lamba, M.; Chaudhary, H.; Singh, K. Analytical Study of MEMS/NEMS Force Sensor for Microbotics Applications. IOP Conf. Ser. Mater. Sci. Eng. 2019, 594, 012021. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, X.; Nguyen, X.A.; Shi, Q.; Lin, F.; Chauhan, S.; Ge, Z.; Cheng, W. Hierarchically Resistive Skins as Specific and Multimetric On-Throat Wearable Biosensors. Nat. Nanotechnol. 2023, 18, 889–897. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Zhou, J.; Huang, X.; Xu, L.; Jia, S.; Gao, Z.; Yao, K.; Li, D.; Zhang, B.; et al. Thin, Soft, Wearable System for Continuous Wireless Monitoring of Artery Blood Pressure. Nat. Commun. 2023, 14, 5009. [Google Scholar] [CrossRef]
- Oh, Y.S.; Kim, J.-H.; Xie, Z.; Cho, S.; Han, H.; Jeon, S.W.; Park, M.; Namkoong, M.; Avila, R.; Song, Z.; et al. Battery-Free, Wireless Soft Sensors for Continuous Multi-Site Measurements of Pressure and Temperature from Patients at Risk for Pressure Injuries. Nat. Commun. 2021, 12, 5008. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.; Safeea, M.; Neto, P. A Flexible Piezoresistive/Self-Capacitive Hybrid Force and Proximity Sensor to Interface Collaborative Robots. IEEE Trans. Ind. Inform. 2023, 19, 2485–2495. [Google Scholar] [CrossRef]
- Pang, C.; Lee, G.-Y.; Kim, T.; Kim, S.M.; Kim, H.N.; Ahn, S.-H.; Suh, K.-Y. A Flexible and Highly Sensitive Strain-Gauge Sensor Using Reversible Interlocking of Nanofibres. Nat. Mater. 2012, 11, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, X.; Xu, J.; Fang, Y.; Chen, G.; Song, Y.; Li, S.; Chen, J. Giant Magnetoelastic Effect in Soft Systems for Bioelectronics. Nat. Mater. 2021, 20, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Mannsfeld, S.C.B.; Tee, B.C.-K.; Stoltenberg, R.M.; Chen, C.V.H.-H.; Barman, S.; Muir, B.V.O.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly Sensitive Flexible Pressure Sensors with Microstructured Rubber Dielectric Layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A Stretchable Carbon Nanotube Strain Sensor for Human-Motion Detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef]
- Han, M.; Chen, L.; Aras, K.; Liang, C.; Chen, X.; Zhao, H.; Li, K.; Faye, N.R.; Sun, B.; Kim, J.-H.; et al. Catheter-Integrated Soft Multilayer Electronic Arrays for Multiplexed Sensing and Actuation during Cardiac Surgery. Nat. Biomed. Eng. 2020, 4, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.W.; Han, M.; Xie, Z.; Chung, H.U.; Lee, J.Y.; Avila, R.; Yohay, J.; Chen, X.; Liang, C.; Patel, M.; et al. Wireless Sensors for Continuous, Multimodal Measurements at the Skin Interface with Lower Limb Prostheses. Sci. Transl. Med. 2020, 12, eabc4327. [Google Scholar] [CrossRef]
- Wang, C.; Cai, M.; Hao, Z.; Nie, S.; Liu, C.; Du, H.; Wang, J.; Chen, W.; Song, J. Stretchable, Multifunctional Epidermal Sensor Patch for Surface Electromyography and Strain Measurements. Adv. Intell. Syst. 2021, 3, 2100031. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, Y.; Zhu, P.; Xiao, J.; Nie, G. Highly Stretchable and Rehealable Wearable Strain Sensor Based on Dynamic Covalent Thermoset and Liquid Metal. Smart Mater. Struct. 2021, 30, 105001. [Google Scholar] [CrossRef]
- Shi, C.; Zou, Z.; Lei, Z.; Zhu, P.; Nie, G.; Zhang, W.; Xiao, J. Stretchable, Rehealable, Recyclable, and Reconfigurable Integrated Strain Sensor for Joint Motion and Respiration Monitoring. Research 2021, 2021, 9846036. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gao, L.; Zhao, H.; Huang, H.; Wang, Y.; Chen, G.; Qin, Y.; Zhao, N.; Xu, D.; Duan, L.; et al. Stretchable and Anti-Impact Iontronic Pressure Sensor with an Ultrabroad Linear Range for Biophysical Monitoring and Deep Learning-Aided Knee Rehabilitation. Microsyst. Nanoeng. 2021, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.J.; Srivastava, S.; Tawale, J.S.; Gupta, B.K. Three-Dimensional CNT-rGO/PDMS Porous Scaffold Derived Supercompressible Lightweight Body-Mounted Piezoresistive Force Sensor for Human Motion Monitoring. Colloids Surf. Physicochem. Eng. Asp. 2023, 675, 131993. [Google Scholar] [CrossRef]
- Tannarana, M.; Solanki, G.K.; Bhakhar, S.A.; Patel, K.D.; Pathak, V.M.; Pataniya, P.M. 2D-SnSe2 Nanosheet Functionalized Piezo-Resistive Flexible Sensor for Pressure and Human Breath Monitoring. ACS Sustain. Chem. Eng. 2020, 8, 7741–7749. [Google Scholar] [CrossRef]
- Muzaffar, S.; Elfadel, I.M. Self-Synchronized, Continuous Body Weight Monitoring Using Flexible Force Sensors and Ground Reaction Force Signal Processing. IEEE Sens. J. 2020, 20, 10886–10897. [Google Scholar] [CrossRef]
- Kim, M.; Kaliannagounder, V.K.; Unnithan, A.R.; Park, C.H.; Kim, C.S.; Ramachandra Kurup Sasikala, A. Development of In-Situ Poled Nanofiber Based Flexible Piezoelectric Nanogenerators for Self-Powered Motion Monitoring. Appl. Sci. 2020, 10, 3493. [Google Scholar] [CrossRef]
- Jugade, S.S.; Kulkarni, S.M. PDMS–ZnO Flexible Piezoelectric Composites for Measurement of Muscle Activity. Bull. Mater. Sci. 2020, 43, 209. [Google Scholar] [CrossRef]
- Yan, D.; Chang, J.; Zhang, H.; Liu, J.; Song, H.; Xue, Z.; Zhang, F.; Zhang, Y. Soft Three-Dimensional Network Materials with Rational Bio-Mimetic Designs. Nat. Commun. 2020, 11, 1180. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, F.; Shen, Z.; Cao, S.; Jin, T.; Guo, X.; Li, Z.; Lin, L.; Zhang, Y. Tunable Seesaw-like 3D Capacitive Sensor for Force and Acceleration Sensing. NPJ Flex. Electron. 2021, 5, 28. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, H.; Mo, J.; Chen, S.; Xie, Y.; Ma, S.; Chen, R.; Luo, T.; Ling, W.; Qin, L.; et al. Finger-Inspired Rigid-Soft Hybrid Tactile Sensor with Superior Sensitivity at High Frequency. Nat. Commun. 2022, 13, 5076. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, M.; Shan, X.; Lee, C. Augmented Tactile-Perception and Haptic-Feedback Rings as Human-Machine Interfaces Aiming for Immersive Interactions. Nat. Commun. 2022, 13, 5224. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Jiao, Z.; Nie, S.; Wang, C.; Zou, J.; Song, J. A Multifunctional Electronic Skin Based on Patterned Metal Films for Tactile Sensing with a Broad Linear Response Range. Sci. Adv. 2021, 7, eabl8313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, X.; Wu, J.; Fang, D.; Zhang, Y. Soft Mechanical Metamaterials with Unusual Swelling Behavior and Tunable Stress-Strain Curves. Sci. Adv. 2018, 4, eaar8535. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.-L.; Wang, Y.-S.; Wang, Y.-F.; Zhou, H.-T.; Yuan, S.-M. Design of Acoustic/Elastic Phase Gradient Metasurfaces: Principles, Functional Elements, Tunability, and Coding. Appl. Mech. Rev. 2022, 74, 020801. [Google Scholar] [CrossRef]
- Xiong, W.; Guo, D.; Yang, Z.; Zhu, C.; Huang, Y. Conformable, Programmable and Step-Linear Sensor Array for Large-Range Wind Pressure Measurement on Curved Surface. Sci. China Technol. Sci. 2020, 63, 2073–2081. [Google Scholar] [CrossRef]
- Yin, S.; Jia, Z.; Li, X.; Zhu, J.; Xu, Y.; Li, T. Machine-Learning-Accelerated Design of Functional Structural Components in Deep-Sea Soft Robots. Extreme Mech. Lett. 2022, 52, 101635. [Google Scholar] [CrossRef]
- Cao, X.; Zhuo, J.; Zou, W.; Li, X.; Ruan, D.; Yang, X.; Zhou, F.; Li, T. A Two-Stage Magnetically Enhanced Buoyancy Adjustment Actuator Based on Dielectric Elastomer. J. Appl. Mech. 2023, 91, 021008. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Chernozem, R.V.; Pariy, I.O.; Surmeneva, M.A. A Review on Piezo- and Pyroelectric Responses of Flexible Nano- and Micropatterned Polymer Surfaces for Biomedical Sensing and Energy Harvesting Applications. Nano Energy 2021, 79, 105442. [Google Scholar] [CrossRef]
- Rahmani, P.; Shojaei, A. A Review on the Features, Performance and Potential Applications of Hydrogel-Based Wearable Strain/Pressure Sensors. Adv. Colloid Interface Sci. 2021, 298, 102553. [Google Scholar] [CrossRef]
- Yousuf, M.; Beigh, N.T.; Arya, D.S.; Garg, M.; Mallick, D.; Singh, P. A Sensitive and Flexible Poroelastic Barium Titanate Matrix for Pressure Sensing Applications. IEEE Sens. Lett. 2023, 7, 3240424. [Google Scholar] [CrossRef]
- Mahata, C.; Algadi, H.; Lee, J.; Kim, S.; Lee, T. Biomimetic-Inspired Micro-Nano Hierarchical Structures for Capacitive Pressure Sensor Applications. Measurement 2020, 151, 107095. [Google Scholar] [CrossRef]
- Amoli, V.; Kim, S.Y.; Kim, J.S.; Choi, H.; Koo, J.; Kim, D.H. Biomimetics for High-Performance Flexible Tactile Sensors and Advanced Artificial Sensory Systems. J. Mater. Chem. C 2019, 7, 14816–14844. [Google Scholar] [CrossRef]
- Chhetry, A.; Sharma, S.; Yoon, H.; Ko, S.; Park, J.Y. Enhanced Sensitivity of Capacitive Pressure and Strain Sensor Based on CaCu3Ti4O12 Wrapped Hybrid Sponge for Wearable Applications. Adv. Funct. Mater. 2020, 30, 1910020. [Google Scholar] [CrossRef]
- Hashim, A. Fabrication and Characteristics of Flexible, Lightweight, and Low-Cost Pressure Sensors Based on PVA/SiO2/SiC Nanostructures. J. Mater. Sci. Mater. Electron. 2021, 32, 2796–2804. [Google Scholar] [CrossRef]
- Yogeswaran, N.; Hosseini, E.S.; Dahiya, R. Graphene Based Low Voltage Field Effect Transistor Coupled with Biodegradable Piezoelectric Material Based Dynamic Pressure Sensor. ACS Appl. Mater. Interfaces 2020, 12, 54035–54040. [Google Scholar] [CrossRef]
- Pataniya, P.M.; Bhakhar, S.A.; Tannarana, M.; Zankat, C.; Patel, V.; Solanki, G.K.; Patel, K.D.; Jha, P.K.; Late, D.J.; Sumesh, C.K. Highly Sensitive and Flexible Pressure Sensor Based on Two-Dimensional MoSe2 Nanosheets for Online Wrist Pulse Monitoring. J. Colloid Interface Sci. 2021, 584, 495–504. [Google Scholar] [CrossRef]
- Masihi, S.; Panahi, M.; Maddipatla, D.; Hanson, A.J.; Bose, A.K.; Hajian, S.; Palaniappan, V.; Narakathu, B.B.; Bazuin, B.J.; Atashbar, M.Z. Highly Sensitive Porous PDMS-Based Capacitive Pressure Sensors Fabricated on Fabric Platform for Wearable Applications. ACS Sens. 2021, 6, 938–949. [Google Scholar] [CrossRef]
- Palaniappan, V.; Masihi, S.; Panahi, M.; Maddipatla, D.; Bose, A.K.; Zhang, X.; Narakathu, B.B.; Bazuin, B.J.; Atashbar, M.Z. Laser-Assisted Fabrication of a Highly Sensitive and Flexible Micro Pyramid-Structured Pressure Sensor for E-Skin Applications. IEEE Sens. J. 2020, 20, 7605–7613. [Google Scholar] [CrossRef]
- Kumaresan, Y.; Ozioko, O.; Dahiya, R. Multifunctional Electronic Skin With a Stack of Temperature and Pressure Sensor Arrays. IEEE Sens. J. 2021, 21, 26243–26251. [Google Scholar] [CrossRef]
- Nguyen, D.-N.; Moon, W. Piezoelectric Polymer Microfiber-Based Composite for the Flexible Ultra-Sensitive Pressure Sensor. J. Appl. Polym. Sci. 2020, 137, 48884. [Google Scholar] [CrossRef]
- Sengupta, D.; Kamat, A.M.; Smit, Q.; Jayawardhana, B.; Kottapalli, A.G.P. Piezoresistive 3D Graphene–PDMS Spongy Pressure Sensors for IoT Enabled Wearables and Smart Products. Flex. Print. Electron. 2022, 7, 015004. [Google Scholar] [CrossRef]
- Dzedzickis, A.; Sutinys, E.; Bucinskas, V.; Samukaite-Bubniene, U.; Jakstys, B.; Ramanavicius, A.; Morkvenaite-Vilkonciene, I. Polyethylene-Carbon Composite (Velostat®) Based Tactile Sensor. Polymers 2020, 12, 2905. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, Y.; Ma, S.; Ozioko, O.; Dahiya, R. Soft Capacitive Pressure Sensor With Enhanced Sensitivity Assisted by ZnO NW Interlayers and Airgap. IEEE Sens. J. 2022, 22, 3974–3982. [Google Scholar] [CrossRef]
- Choi, H.B.; Oh, J.; Kim, Y.; Pyatykh, M.; Chang Yang, J.; Ryu, S.; Park, S. Transparent Pressure Sensor with High Linearity over a Wide Pressure Range for 3D Touch Screen Applications. ACS Appl. Mater. Interfaces 2020, 12, 16691–16699. [Google Scholar] [CrossRef]
- Thouti, E.; Nagaraju, A.; Chandran, A.; Prakash, P.V.B.S.S.; Shivanarayanamurthy, P.; Lal, B.; Kumar, P.; Kothari, P.; Panwar, D. Tunable Flexible Capacitive Pressure Sensors Using Arrangement of Polydimethylsiloxane Micro-Pyramids for Bio-Signal Monitoring. Sens. Actuators Phys. 2020, 314, 112251. [Google Scholar] [CrossRef]
- Jeong, Y.; Park, J.; Lee, J.; Kim, K.; Park, I. Ultrathin, Biocompatible, and Flexible Pressure Sensor with a Wide Pressure Range and Its Biomedical Application. ACS Sens. 2020, 5, 481–489. [Google Scholar] [CrossRef]
- Sharma, S.; Chhetry, A.; Sharifuzzaman, M.; Yoon, H.; Park, J.Y. Wearable Capacitive Pressure Sensor Based on MXene Composite Nanofibrous Scaffolds for Reliable Human Physiological Signal Acquisition. ACS Appl. Mater. Interfaces 2020, 12, 22212–22224. [Google Scholar] [CrossRef]
- Bălţatu, M.S.; Vizureanu, P.; Goanţă, V.; Ţugui, C.A.; Voiculescu, I. Mechanical Tests for Ti-Based Alloys as New Medical Materials. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012029. [Google Scholar] [CrossRef]
- Jimenez-Marcos, C.; Mirza-Rosca, J.C.; Baltatu, M.S.; Vizureanu, P. Experimental Research on New Developed Titanium Alloys for Biomedical Applications. Bioengineering 2022, 9, 686. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Geanta, V.; Baltatu, S.; Focsaneanu, S.; Earar, K. Microstructural Analysis of Biodegradable Mg-0.9Ca-1.2Zr Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2016, 147, 012033. [Google Scholar] [CrossRef]
- Bo, R.; Xu, S.; Yang, Y.; Zhang, Y. Mechanically-Guided 3D Assembly for Architected Flexible Electronics. Chem. Rev. 2023, 123, 11137–11189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, F.; Yan, Z.; Ma, Q.; Li, X.; Huang, Y.; Rogers, J.A. Printing, Folding and Assembly Methods for Forming 3D Mesostructures in Advanced Materials. Nat. Rev. Mater. 2017, 2, 17019. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Z.; Nan, K.; Xiao, D.; Liu, Y.; Luan, H.; Fu, H.; Wang, X.; Yang, Q.; Wang, J.; et al. A Mechanically Driven Form of Kirigami as a Route to 3D Mesostructures in Micro/Nanomembranes. Proc. Natl. Acad. Sci. USA 2015, 112, 11757–11764. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Liu, Y.; Ma, C.; Cheng, H.-C.; He, Q.; Wu, H.; Wang, C.; Lin, C.-Y.; Huang, Y.; Duan, X. Sensitive Pressure Sensors Based on Conductive Microstructured Air-Gap Gates and Two-Dimensional Semiconductor Transistors. Nat. Electron. 2020, 3, 59–69. [Google Scholar] [CrossRef]
- Rwei, P.; Qian, C.; Abiri, A.; Zhou, Y.; Chou, E.-F.; Tang, W.C.; Khine, M. Soft Iontronic Capacitive Sensor for Beat-to-Beat Blood Pressure Measurements. Adv. Mater. Interfaces 2022, 9, 2200294. [Google Scholar] [CrossRef]
- Pang, C.; Koo, J.H.; Nguyen, A.; Caves, J.M.; Kim, M.-G.; Chortos, A.; Kim, K.; Wang, P.J.; Tok, J.B.-H.; Bao, Z. Highly Skin-Conformal Microhairy Sensor for Pulse Signal Amplification. Adv. Mater. 2015, 27, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Ma, W.; Qiu, L.; Xia, K.; Zhang, Y.; Lu, H.; Zhu, M.; Liang, X.; Wu, X.-E.; et al. Monitoring Blood Pressure and Cardiac Function without Positioning via a Deep Learning–Assisted Strain Sensor Array. Sci. Adv. 2023, 9, eadh0615. [Google Scholar] [CrossRef]
- Tian, Y.; Han, J.; Yang, J.; Wu, H.; Bai, H. A Highly Sensitive Graphene Aerogel Pressure Sensor Inspired by Fluffy Spider Leg. Adv. Mater. Interfaces 2021, 8, 2100511. [Google Scholar] [CrossRef]
- Moghadam, B.H.; Hasanzadeh, M.; Simchi, A. Self-Powered Wearable Piezoelectric Sensors Based on Polymer Nanofiber–Metal–Organic Framework Nanoparticle Composites for Arterial Pulse Monitoring. ACS Appl. Nano Mater. 2020, 3, 8742–8752. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, W.; Guo, L.; Liu, Z.; Cai, P.; Cui, Y.; Wang, T.; Wang, C.; Zhu, M.; Zhou, Y.; et al. Haptically Quantifying Young’s Modulus of Soft Materials Using a Self-Locked Stretchable Strain Sensor. Adv. Mater. 2021, 2104078. [Google Scholar] [CrossRef]
- Yang, C.; Huang, X.; Li, X.; Yang, C.; Zhang, T.; Wu, Q.; Liu, D.; Lin, H.; Chen, W.; Hu, N.; et al. Wearable and Implantable Intraocular Pressure Biosensors: Recent Progress and Future Prospects. Adv. Sci. 2021, 8, 2002971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Medow, J.E.; Iskandar, B.J.; Wang, F.; Shokoueinejad, M.; Koueik, J.; Webster, J.G. Invasive and Noninvasive Means of Measuring Intracranial Pressure: A Review. Physiol. Meas. 2017, 38, R143. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, G.E.; Shen, B.Y.; Aref, A.A. The Sensimed Triggerfish Contact Lens Sensor: Efficacy, Safety, and Patient Perspectives. Clin. Ophthalmol. 2017, 11, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Boutry, C.M.; Nguyen, A.; Lawal, Q.O.; Chortos, A.; Rondeau-Gagné, S.; Bao, Z. A Sensitive and Biodegradable Pressure Sensor Array for Cardiovascular Monitoring. Adv. Mater. 2015, 27, 6954–6961. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-L.; Lee, C.-H.; Hsieh, C.-H. Digitizing Abdominal Palpation with a Pressure Measurement and Positioning Device. PeerJ 2020, 8, e10511. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zhong, J.; Liu, H.; Ma, Y.; Liu, N.; Song, Y.; Liang, J.; Shao, Z.; Sun, Y.; Dong, Y.; et al. Human Pulse Diagnosis for Medical Assessments Using a Wearable Piezoelectret Sensing System. Adv. Funct. Mater. 2018, 28, 1803413. [Google Scholar] [CrossRef]
- Valentine, A.D.; Busbee, T.A.; Boley, J.W.; Raney, J.R.; Chortos, A.; Kotikian, A.; Berrigan, J.D.; Durstock, M.F.; Lewis, J.A. Hybrid 3D Printing of Soft Electronics. Adv. Mater. 2017, 29, 1703817. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, D.; Ramalingame, R.; Palaniyappan, S.; Wagner, G.; Kanoun, O. Flexible Ultra-Thin Nanocomposite Based Piezoresistive Pressure Sensors for Foot Pressure Distribution Measurement. Sensors 2021, 21, 6082. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Yao, G.; Lou, W.; Zhang, T.; Zhang, Z.; Xie, M.; Gan, X.; Pan, T.; Gao, M.; et al. Effective Orthodontic Tooth Movement via an Occlusion-Activated Electromechanical Synergistic Dental Aligner. ACS Nano 2023, 17, 16757–16769. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, X.; Chen, S.-C.; Zhao, N. Emerging Technologies of Flexible Pressure Sensors: Materials, Modeling, Devices, and Manufacturing. Adv. Funct. Mater. 2019, 29, 1808509. [Google Scholar] [CrossRef]
- Nie, Z.; Kwak, J.W.; Han, M.; Rogers, J.A. Mechanically Active Materials and Devices for Bio-Interfaced Pressure Sensors—A Review. Adv. Mater. 2022, 2022, 2205609. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Wang, Y.; Xia, K.; Yin, Z.; Wang, H.; Zhang, M.; Liang, X.; Lu, H.; Zhu, M.; et al. Physical Sensors for Skin-Inspired Electronics. InfoMat 2020, 2, 184–211. [Google Scholar] [CrossRef]
- Guo, W.-T.; Tang, X.-G.; Tang, Z.; Sun, Q.-J. Recent Advances in Polymer Composites for Flexible Pressure Sensors. Polymers 2023, 15, 2176. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.; Lee, J.; Bae, K.; Sim, S.; Kim, J. Recent Progress in Flexible Tactile Sensors for Human-Interactive Systems: From Sensors to Advanced Applications. Adv. Mater. 2021, 33, 2005902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jin, T.; Xue, Z.; Zhang, Y. Recent Progress in Three-Dimensional Flexible Physical Sensors. Int. J. Smart Nano Mater. 2022, 13, 17–41. [Google Scholar] [CrossRef]
- Mishra, R.B.; El-Atab, N.; Hussain, A.M.; Hussain, M.M. Recent Progress on Flexible Capacitive Pressure Sensors: From Design and Materials to Applications. Adv. Mater. Technol. 2021, 6, 2001023. [Google Scholar] [CrossRef]
- Meng, K.; Xiao, X.; Wei, W.; Chen, G.; Nashalian, A.; Shen, S.; Xiao, X.; Chen, J. Wearable Pressure Sensors for Pulse Wave Monitoring. Adv. Mater. 2022, 34, 2109357. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.; Tang, W.; Chen, J.; Zhu, Z.; Li, L.; Zhou, N.; Kang, X.; Xu, D.; Wang, L.; et al. Flexible Pressure Sensors Based on Bionic Microstructures: From Plants to Animals. Adv. Mater. Interfaces 2022, 9, 2101312. [Google Scholar] [CrossRef]
- Tai, G.; Wei, D.; Su, M.; Li, P.; Xie, L.; Yang, J. Force-Sensitive Interface Engineering in Flexible Pressure Sensors: A Review. Sensors 2022, 22, 2652. [Google Scholar] [CrossRef]
- Mishra, S.; Mohanty, S.; Ramadoss, A. Functionality of Flexible Pressure Sensors in Cardiovascular Health Monitoring: A Review. ACS Sens. 2022, 7, 2495–2520. [Google Scholar] [CrossRef]
- He, R.; Liu, H.; Niu, Y.; Zhang, H.; Genin, G.M.; Xu, F. Flexible Miniaturized Sensor Technologies for Long-Term Physiological Monitoring. NPJ Flex. Electron. 2022, 6, 20. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, T.; Li, Q.; Chen, Y.; Qiu, X.; Liu, J.; Bian, Y.; Xuan, F. Flexible Microstructured Pressure Sensors: Design, Fabrication and Applications. Nanotechnology 2022, 33, 322002. [Google Scholar] [CrossRef] [PubMed]
- Anwer, A.H.; Khan, N.; Ansari, M.Z.; Baek, S.-S.; Yi, H.; Kim, S.; Noh, S.M.; Jeong, C. Recent Advances in Touch Sensors for Flexible Wearable Devices. Sensors 2022, 22, 4460. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-D.; Lee, J.S. Recent Development of Flexible Tactile Sensors and Their Applications. Sensors 2022, 22, 50. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Dinh, T.; Phan, H.-P.; Pham, T.A.; Dau, V.T.; Nguyen, N.-T.; Dao, D.V. Advances in Ultrasensitive Piezoresistive Sensors: From Conventional to Flexible and Stretchable Applications. Mater. Horiz. 2021, 8, 2123–2150. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, H.; Vaithiyanathan, D.; Kumar, H. A Review on 3D Printed Force Sensors. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1104, 012013. [Google Scholar] [CrossRef]
- Li, G.; Chen, D.; Li, C.; Liu, W.; Liu, H. Engineered Microstructure Derived Hierarchical Deformation of Flexible Pressure Sensor Induces a Supersensitive Piezoresistive Property in Broad Pressure Range. Adv. Sci. 2020, 7, 2000154. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Mao, Y.; Zhou, Q.; Zhou, J.; Chen, G.; Gao, Y.; Tian, Y.; Wen, W.; Zhou, B. Facile Preparation of Hybrid Structure Based on Mesodome and Micropillar Arrays as Flexible Electronic Skin with Tunable Sensitivity and Detection Range. ACS Appl. Mater. Interfaces 2019, 11, 28060–28071. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, M.-S.; Kim, K.; Ji, S.; Kim, Y.-T.; Park, J.; Na, K.; Bae, K.-H.; Kyun Kim, H.; et al. Wearable Smart Sensor Systems Integrated on Soft Contact Lenses for Wireless Ocular Diagnostics. Nat. Commun. 2017, 8, 14997. [Google Scholar] [CrossRef]
- Wang, H.; Fu, T.; Du, Y.; Gao, W.; Huang, K.; Liu, Z.; Chandak, P.; Liu, S.; Van Katwyk, P.; Deac, A.; et al. Scientific Discovery in the Age of Artificial Intelligence. Nature 2023, 620, 47–60. [Google Scholar] [CrossRef]
- Kim, J.; Chou, E.-F.; Le, J.; Wong, S.; Chu, M.; Khine, M. Soft Wearable Pressure Sensors for Beat-to-Beat Blood Pressure Monitoring. Adv. Healthc. Mater. 2019, 8, 1900109. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Sun, J.; Liu, H.; Bao, R.; Yu, R.; Zhai, J.; Pan, C.; Wang, Z.L. Skin-Inspired Highly Stretchable and Conformable Matrix Networks for Multifunctional Sensing. Nat. Commun. 2018, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Du, H.; Hou, X.; Lu, P.; Wang, L.; Huang, J.; Bai, N.; Wu, Z.; Fang, N.X.; Guo, C.F. Skin-Electrode Iontronic Interface for Mechanosensing. Nat. Commun. 2021, 12, 4731. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Gao, S.; Yue, W.; Li, Y.; Zhou, W.; Liu, H. Highly Morphology-Controllable and Highly Sensitive Capacitive Tactile Sensor Based on Epidermis-Dermis-Inspired Interlocked Asymmetric-Nanocone Arrays for Detection of Tiny Pressure. Small 2020, 16, 1904774. [Google Scholar] [CrossRef]
- Curry, E.J.; Ke, K.; Chorsi, M.T.; Wrobel, K.S.; Miller, A.N.; Patel, A.; Kim, I.; Feng, J.; Yue, L.; Wu, Q.; et al. Biodegradable Piezoelectric Force Sensor. Proc. Natl. Acad. Sci. USA 2018, 115, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, C.; Pan, H.; Yang, Y.; Chen, G.; Zhao, X.; Li, W.; Gong, Q.; Xie, G.; Zhou, Y.; et al. Muscle Fibers Inspired High-Performance Piezoelectric Textiles for Wearable Physiological Monitoring. Adv. Funct. Mater. 2021, 31, 2010962. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Y.; Ouyang, H.; Shi, B.; Li, N.; Jiang, D.; Xie, F.; Qu, D.; Zou, Y.; Huang, Y.; et al. Transcatheter Self-Powered Ultrasensitive Endocardial Pressure Sensor. Adv. Funct. Mater. 2019, 29, 1807560. [Google Scholar] [CrossRef]
- Ouyang, H.; Liu, Z.; Li, N.; Shi, B.; Zou, Y.; Xie, F.; Ma, Y.; Li, Z.; Li, H.; Zheng, Q.; et al. Symbiotic Cardiac Pacemaker. Nat. Commun. 2019, 10, 1821. [Google Scholar] [CrossRef]
- Xia, K.; Chen, X.; Shen, X.; Li, S.; Yin, Z.; Zhang, M.; Liang, X.; Zhang, Y. Carbonized Chinese Art Paper-Based High-Performance Wearable Strain Sensor for Human Activity Monitoring. ACS Appl. Electron. Mater. 2019, 1, 2415–2421. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Lai, X.; Gao, T.; Yang, J.; Zeng, X. Thiolated Graphene@Polyester Fabric-Based Multilayer Piezoresistive Pressure Sensors for Detecting Human Motion. ACS Appl. Mater. Interfaces 2018, 10, 41784–41792. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, H.; Tao, L.; Li, Y.; Wang, X.; Deng, N.; Yang, Y.; Ren, T.-L. Flexible, Highly Sensitive, and Wearable Pressure and Strain Sensors with Graphene Porous Network Structure. ACS Appl. Mater. Interfaces 2016, 8, 26458–26462. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zhang, F.; Di, C.; Zhu, D. Advances of Flexible Pressure Sensors toward Artificial Intelligence and Health Care Applications. Mater. Horiz. 2015, 2, 140–156. [Google Scholar] [CrossRef]
- Salvatore, G.A.; Münzenrieder, N.; Kinkeldei, T.; Petti, L.; Zysset, C.; Strebel, I.; Büthe, L.; Tröster, G. Wafer-Scale Design of Lightweight and Transparent Electronics That Wraps around Hairs. Nat. Commun. 2014, 5, 2982. [Google Scholar] [CrossRef] [PubMed]
- Sekitani, T.; Someya, T. Stretchable, Large-Area Organic Electronics. Adv. Mater. 2010, 22, 2228–2246. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Z.L. Air/Liquid-Pressure and Heartbeat-Driven Flexible Fiber Nanogenerators as a Micro/Nano-Power Source or Diagnostic Sensor. Adv. Mater. 2011, 23, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Kuang, S.Y.; Li, H.Y.; Wang, Z.L.; Zhu, G. Large-Area Integrated Triboelectric Sensor Array for Wireless Static and Dynamic Pressure Detection and Mapping. Small 2020, 16, 1906352. [Google Scholar] [CrossRef] [PubMed]
- Persano, L.; Dagdeviren, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Rogers, J.A. High Performance Piezoelectric Devices Based on Aligned Arrays of Nanofibers of Poly(Vinylidenefluoride-Co-Trifluoroethylene). Nat. Commun. 2013, 4, 1633. [Google Scholar] [CrossRef]
- Dong, K.; Wu, Z.; Deng, J.; Wang, A.C.; Zou, H.; Chen, C.; Hu, D.; Gu, B.; Sun, B.; Wang, Z.L. A Stretchable Yarn Embedded Triboelectric Nanogenerator as Electronic Skin for Biomechanical Energy Harvesting and Multifunctional Pressure Sensing. Adv. Mater. 2018, 30, 1804944. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, Y.; Hu, C.; Zhang, M.; Zhu, W.; Bowen, C.R.; Han, Y.; Yang, Y. Self-Powered Stretchable Sensor Arrays Exhibiting Magnetoelasticity for Real-Time Human–Machine Interaction. Adv. Mater. 2022, 2022, 2203786. [Google Scholar] [CrossRef]
- Shi, L.; Li, Z.; Chen, M.; Qin, Y.; Jiang, Y.; Wu, L. Quantum Effect-Based Flexible and Transparent Pressure Sensors with Ultrahigh Sensitivity and Sensing Density. Nat. Commun. 2020, 11, 3529. [Google Scholar] [CrossRef]
- Wang, X.; Meng, X.; Zhu, Y.; Ling, H.; Chen, Y.; Li, Z.; Hartel, M.C.; Dokmeci, M.R.; Zhang, S.; Khademhosseini, A. A Sub-1-V, Microwatt Power-Consumption Iontronic Pressure Sensor Based on Organic Electrochemical Transistors. IEEE Electron Device Lett. 2021, 42, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.-L.; Shim, M.-B.; Lee, B.-S.; Jeon, S.; Ko, D.-S.; Kang, T.-H.; Bae, J.; Lee, S.H.; Byun, K.-E.; Im, J.; et al. Highly Stretchable Resistive Pressure Sensors Using a Conductive Elastomeric Composite on a Micropyramid Array. Adv. Mater. 2014, 26, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, R. High Sensitivity and Broad Range Flexible Pressure Sensor Using Multilayered Porous PDMS/AgNP Sponge. Adv. Mater. Technol. 2019, 4, 1900414. [Google Scholar] [CrossRef]
- Choi, H.; Sun, J.; Ren, B.; Cha, S.; Lee, J.; Lee, B.-M.; Park, J.-J.; Choi, J.-H.; Park, J.-J. 3D Textile Structure-Induced Local Strain for a Highly Amplified Piezoresistive Performance of Carbonized Cellulose Fabric Based Pressure Sensor for Human Healthcare Monitoring. Chem. Eng. J. 2022, 450, 138193. [Google Scholar] [CrossRef]
- Chen, M.; Luo, W.; Xu, Z.; Zhang, X.; Xie, B.; Wang, G.; Han, M. An Ultrahigh Resolution Pressure Sensor Based on Percolative Metal Nanoparticle Arrays. Nat. Commun. 2019, 10, 4024. [Google Scholar] [CrossRef]
- Gong, S.; Schwalb, W.; Wang, Y.; Chen, Y.; Tang, Y.; Si, J.; Shirinzadeh, B.; Cheng, W. A Wearable and Highly Sensitive Pressure Sensor with Ultrathin Gold Nanowires. Nat. Commun. 2014, 5, 3132. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, N.; Li, L.; Hu, X.; Zou, Z.; Wang, J.; Luo, S.; Gao, Y. A Highly Flexible and Sensitive Piezoresistive Sensor Based on MXene with Greatly Changed Interlayer Distances. Nat. Commun. 2017, 8, 1207. [Google Scholar] [CrossRef]
- Pan, L.; Chortos, A.; Yu, G.; Wang, Y.; Isaacson, S.; Allen, R.; Shi, Y.; Dauskardt, R.; Bao, Z. An Ultra-Sensitive Resistive Pressure Sensor Based on Hollow-Sphere Microstructure Induced Elasticity in Conducting Polymer Film. Nat. Commun. 2014, 5, 3002. [Google Scholar] [CrossRef]
- Lee, D.-H.; Chuang, C.-H.; Shaikh, M.O.; Dai, Y.-S.; Wang, S.-Y.; Wen, Z.-H.; Yen, C.-K.; Liao, C.-F.; Pan, C.-T. Flexible Piezoresistive Tactile Sensor Based on Polymeric Nanocomposites with Grid-Type Microstructure. Micromachines 2021, 12, 452. [Google Scholar] [CrossRef]
- Wang, X.; Tao, L.; Yuan, M.; Wang, Z.; Yu, J.; Xie, D.; Luo, F.; Chen, X.; Wong, C. Sea Urchin-like Microstructure Pressure Sensors with an Ultra-Broad Range and High Sensitivity. Nat. Commun. 2021, 12, 1776. [Google Scholar] [CrossRef]
- Won, S.M.; Wang, H.; Kim, B.H.; Lee, K.; Jang, H.; Kwon, K.; Han, M.; Crawford, K.E.; Li, H.; Lee, Y.; et al. Multimodal Sensing with a Three-Dimensional Piezoresistive Structure. ACS Nano 2019, 13, 10972–10979. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Lee, D.-G.; Park, J.; Ko, H.; Lim, H. Piezoresistive Tactile Sensor Discriminating Multidirectional Forces. Sensors 2015, 15, 25463–25473. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zheng, W.; Wang, Y.; Xu, D.; Zhao, N.; Qin, Y.; Yuan, Y.; Fan, Z.; Nan, X.; Duan, Q.; et al. Flexible Tensile Strain-Pressure Sensor with an off-Axis Deformation-Insensitivity. Nano Energy 2022, 99, 107384. [Google Scholar] [CrossRef]
- Lamba, M.; Mittal, N.; Singh, K.; Chaudhary, H. Design Analysis of Polysilicon Piezoresistors PDMS (Polydimethylsiloxane) Microcantilever Based MEMS Force Sensor. Int. J. Mod. Phys. B 2020, 34, 2050072. [Google Scholar] [CrossRef]
- Yiming, B.; Han, Y.; Han, Z.; Zhang, X.; Li, Y.; Lian, W.; Zhang, M.; Yin, J.; Sun, T.; Wu, Z.; et al. A Mechanically Robust and Versatile Liquid-Free Ionic Conductive Elastomer. Adv. Mater. 2021, 33, 2006111. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Tian, Y.; Sun, S.; Hu, J.; Wang, Y.; Zhang, Z.; Liu, A.; Cheng, H.; Gao, W.; Zhang, W.; et al. Bioinspired, Multifunctional Dual-Mode Pressure Sensors as Electronic Skin for Decoding Complex Loading Processes and Human Motions. Nano Energy 2020, 78, 105337. [Google Scholar] [CrossRef]
- Fekiri, C.; Kim, H.C.; Lee, I.H. 3D-Printable Carbon Nanotubes-Based Composite for Flexible Piezoresistive Sensors. Materials 2020, 13, 5482. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Yuan, W.; Li, Y.; Gao, P.; Tiwari, N.; Chen, X.; Wang, Z.; Niu, G.; Cheng, H. Wearable Pressure Sensors Based on MXene/Tissue Papers for Wireless Human Health Monitoring. ACS Appl. Mater. Interfaces 2021, 13, 60531–60543. [Google Scholar] [CrossRef]
- Kamat, A.M.; Pei, Y.; Jayawardhana, B.; Kottapalli, A.G.P. Biomimetic Soft Polymer Microstructures and Piezoresistive Graphene MEMS Sensors Using Sacrificial Metal 3D Printing. ACS Appl. Mater. Interfaces 2021, 13, 1094–1104. [Google Scholar] [CrossRef]
- Hao, D.; Yang, R.; Yi, N.; Cheng, H. Highly Sensitive Piezoresistive Pressure Sensors Based on Laser-Induced Graphene with Molybdenum Disulfide Nanoparticles. Sci. China Technol. Sci. 2021, 64, 2408–2414. [Google Scholar] [CrossRef]
- Lamba, M.; Chaudhary, H.; Singh, K.; Keshyep, P.; Kumar, V. Graphene Piezoresistive Flexible MEMS Force Sensor for Bi-Axial Micromanipulation Applications. Microsyst. Technol. 2022, 28, 1687–1699. [Google Scholar] [CrossRef]
- Paul, S.J.; Sharma, I.; Elizabeth, I.; Gahtori, B.; Manikandan, M.R.; Titus, S.S.; Chandra, P.; Gupta, B.K. A Comparative Study of Compressible and Conductive Vertically Aligned Carbon Nanotube Forest in Different Polymer Matrixes for High-Performance Piezoresistive Force Sensors. ACS Appl. Mater. Interfaces 2020, 12, 16946–16958. [Google Scholar] [CrossRef] [PubMed]
- Lamba, M.; Chaudhary, H.; Singh, K. Optimized Analysis of Sensitivity and Non-Linearity for PDMS–Graphene MEMS Force Sensor. IETE J. Res. 2022, 68, 4453–4467. [Google Scholar] [CrossRef]
- Seesaard, T.; Wongchoosuk, C. Fabric-Based Piezoresistive Ti3AlC2/PEDOT:PSS Force Sensor for Wearable E-Textile Applications. Org. Electron. 2023, 122, 106894. [Google Scholar] [CrossRef]
- Arias-Ferreiro, G.; Lasagabáster-Latorre, A.; Ares-Pernas, A.; Dopico-García, M.S.; Pereira, N.; Costa, P.; Lanceros-Mendez, S.; Abad, M.-J. Flexible 3D Printed Acrylic Composites Based on Polyaniline/Multiwalled Carbon Nanotubes for Piezoresistive Pressure Sensors. Adv. Electron. Mater. 2022, 8, 2200590. [Google Scholar] [CrossRef]
- Abolpour Moshizi, S.; Azadi, S.; Belford, A.; Razmjou, A.; Wu, S.; Han, Z.J.; Asadnia, M. Development of an Ultra-Sensitive and Flexible Piezoresistive Flow Sensor Using Vertical Graphene Nanosheets. Nano-Micro Lett. 2020, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Yellapantula, K.; Devaraj, H.; Assadian, M.; Stuart, L.; Lo, C.-Y.; Gan, W.C.; Aw, K. Soft and Flexible Sensor Array Using Carbon Black Pillars for Object Recognition via Pressure Mapping. Measurement 2020, 159, 107781. [Google Scholar] [CrossRef]
- Yang, Z.; Pang, Y.; Han, X.; Yang, Y.; Ling, J.; Jian, M.; Zhang, Y.; Yang, Y.; Ren, T.-L. Graphene Textile Strain Sensor with Negative Resistance Variation for Human Motion Detection. ACS Nano 2018, 12, 9134–9141. [Google Scholar] [CrossRef]
- Yan, C.; Wang, J.; Kang, W.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Highly Stretchable Piezoresistive Graphene–Nanocellulose Nanopaper for Strain Sensors. Adv. Mater. 2014, 26, 2022–2027. [Google Scholar] [CrossRef]
- Ma, Q.; Cheng, H.; Jang, K.-I.; Luan, H.; Hwang, K.-C.; Rogers, J.A.; Huang, Y.; Zhang, Y. A Nonlinear Mechanics Model of Bio-Inspired Hierarchical Lattice Materials Consisting of Horseshoe Microstructures. J. Mech. Phys. Solids 2016, 90, 179–202. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Z.; Qi, H.J.; Wang, T.J. A Micro-Structure Based Constitutive Model for Anisotropic Stress–Strain Behaviors of Artery Tissues. Int. J. Solids Struct. 2018, 139–140, 55–64. [Google Scholar] [CrossRef]
- Shuai, Y.; Zhao, J.; Bo, R.; Lan, Y.; Lv, Z.; Zhang, Y. A Wrinkling-Assisted Strategy for Controlled Interface Delamination in Mechanically-Guided 3D Assembly. J. Mech. Phys. Solids 2023, 173, 105203. [Google Scholar] [CrossRef]
- Qin, R.; Hu, M.; Li, X.; Yan, L.; Wu, C.; Liu, J.; Gao, H.; Shan, G.; Huang, W. A Highly Sensitive Piezoresistive Sensor Based on MXenes and Polyvinyl Butyral with a Wide Detection Limit and Low Power Consumption. Nanoscale 2020, 12, 17715–17724. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Wang, L.; Wang, Q.; Deng, J.; Wang, Y.; Lu, P.; Huang, J.; Li, G.; Zhang, Y.; Yang, J.; et al. Graded Intrafillable Architecture-Based Iontronic Pressure Sensor with Ultra-Broad-Range High Sensitivity. Nat. Commun. 2020, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Wang, L.; Xue, Y.; Wang, Y.; Hou, X.; Li, G.; Zhang, Y.; Cai, M.; Zhao, L.; Guan, F.; et al. Graded Interlocks for Iontronic Pressure Sensors with High Sensitivity and High Linearity over a Broad Range. ACS Nano 2022, 16, 4338–4347. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.-H.; Huh, H.; Li, Z.; Lu, N. Soft Capacitive Pressure Sensors: Trends, Challenges, and Perspectives. ACS Nano 2022, 16, 3442–3448. [Google Scholar] [CrossRef]
- Ha, K.-H.; Zhang, W.; Jang, H.; Kang, S.; Wang, L.; Tan, P.; Hwang, H.; Lu, N. Highly Sensitive Capacitive Pressure Sensors over a Wide Pressure Range Enabled by the Hybrid Responses of a Highly Porous Nanocomposite. Adv. Mater. 2021, 33, 2103320. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, S.; Park, H.W.; Park, D.H.; Jeong, Y.; Kim, D.H. Highly Sensitive and Multimodal All-Carbon Skin Sensors Capable of Simultaneously Detecting Tactile and Biological Stimuli. Adv. Mater. 2015, 27, 4178–4185. [Google Scholar] [CrossRef]
- Kou, H.; Zhang, L.; Tan, Q.; Liu, G.; Dong, H.; Zhang, W.; Xiong, J. Wireless Wide-Range Pressure Sensor Based on Graphene/PDMS Sponge for Tactile Monitoring. Sci. Rep. 2019, 9, 3916. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Vosgueritchian, M.; Cheon, S.; Kim, H.; Koo, J.H.; Kim, T.R.; Lee, S.; Schwartz, G.; Chang, H.; et al. Stretchable Energy-Harvesting Tactile Electronic Skin Capable of Differentiating Multiple Mechanical Stimuli Modes. Adv. Mater. 2014, 26, 7324–7332. [Google Scholar] [CrossRef]
- Tee, B.C.-K.; Chortos, A.; Dunn, R.R.; Schwartz, G.; Eason, E.; Bao, Z. Tunable Flexible Pressure Sensors Using Microstructured Elastomer Geometries for Intuitive Electronics. Adv. Funct. Mater. 2014, 24, 5427–5434. [Google Scholar] [CrossRef]
- Yang, R.; Dutta, A.; Li, B.; Tiwari, N.; Zhang, W.; Niu, Z.; Gao, Y.; Erdely, D.; Xin, X.; Li, T.; et al. Iontronic Pressure Sensor with High Sensitivity over Ultra-Broad Linear Range Enabled by Laser-Induced Gradient Micro-Pyramids. Nat. Commun. 2023, 14, 2907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Hou, X.; Li, G.; Wang, L.; Bai, N.; Cai, M.; Zhao, L.; Wang, Y.; Zhang, J.; et al. Highly Stable Flexible Pressure Sensors with a Quasi-Homogeneous Composition and Interlinked Interfaces. Nat. Commun. 2022, 13, 1317. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Lu, T.; Xu, J.; Wang, Z.; Xu, M.; Wang, T.J. Soft Sensor for Measuring Wind Pressure. Int. J. Mech. Sci. 2018, 141, 386–392. [Google Scholar] [CrossRef]
- Liang, Y.; Wan, B.; Li, G.; Xie, Y.; Li, T. A Novel Transparent Dielectric Elastomer Sensor for Compressive Force Measurements. In Proceedings of the Electroactive Polymer Actuators and Devices (EAPAD) 2016, SPIE, Las Vegas, NV, USA, 21–24 March 2016; Volume 9798, pp. 579–584. [Google Scholar]
- Cheng, W.; Wang, X.; Xiong, Z.; Liu, J.; Liu, Z.; Jin, Y.; Yao, H.; Wong, T.-S.; Ho, J.S.; Tee, B.C.K. Frictionless Multiphasic Interface for Near-Ideal Aero-Elastic Pressure Sensing. Nat. Mater. 2023, 22, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, X.; Song, Y.; Chen, J.; Zhu, Y. Flexible Iontronic Sensors with High-Precision and High-Sensitivity Detection for Pressure and Temperature. Compos. Commun. 2023, 39, 101544. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Chu, K.; Wang, X.; Hu, Z.; Su, H. Carbon Black/PDMS Based Flexible Capacitive Tactile Sensor for Multi-Directional Force Sensing. Sensors 2022, 22, 628. [Google Scholar] [CrossRef] [PubMed]
- Oprel, J.; Wolterink, G.; Schilder, J.; Krijnen, G. Novel 3D Printed Capacitive Shear Stress Sensor. Addit. Manuf. 2023, 73, 103674. [Google Scholar] [CrossRef]
- Farman, M.; Surendra; Prajesh, R.; Upadhyay, A.K.; Kumar, P.; Thouti, E. All-Polydimethylsiloxane-Based Highly Flexible and Stable Capacitive Pressure Sensors with Engineered Interfaces for Conformable Electronic Skin. ACS Appl. Mater. Interfaces 2023, 15, 34195–34205. [Google Scholar] [CrossRef]
- Thouti, E.; Chauhan, K.; Prajesh, R.; Farman, M.; Maurya, R.K.; Sharma, P.; Nagaraju, A. Flexible Capacitive Pressure Sensors Using Microdome like Structured Polydimethylsiloxane Dielectric Layers. Sens. Actuators Phys. 2022, 335, 113393. [Google Scholar] [CrossRef]
- Sriphan, S.; Charoonsuk, T.; Khaisaat, S.; Sawanakarn, O.; Pharino, U.; Phunpruch, S.; Maluangnont, T.; Vittayakorn, N. Flexible Capacitive Sensor Based on 2D-Titanium Dioxide Nanosheets/Bacterial Cellulose Composite Film. Nanotechnology 2021, 32, 155502. [Google Scholar] [CrossRef] [PubMed]
- Ruth, S.R.A.; Feig, V.R.; Kim, M.; Khan, Y.; Phong, J.K.; Bao, Z. Flexible Fringe Effect Capacitive Sensors with Simultaneous High-Performance Contact and Non-Contact Sensing Capabilities. Small Struct. 2021, 2, 2000079. [Google Scholar] [CrossRef]
- Palaniappan, V.; Panahi, M.; Maddipatla, D.; Zhang, X.; Masihi, S.; Emani, H.R.K.M.; Narakathu, B.B.; Bazuin, B.J.; Atashbar, M.Z. Flexible M-Tooth Hybrid Micro-Structure-Based Capacitive Pressure Sensor With High Sensitivity and Wide Sensing Range. IEEE Sens. J. 2021, 21, 26261–26268. [Google Scholar] [CrossRef]
- Ntagios, M.; Dervin, S.; Dahiya, R. 3D Printed Capacitive Pressure Sensing Sole for Anthropomorphic Robots. In Proceedings of the 2021 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Virtual, 20–23 June 2021; pp. 1–4. [Google Scholar]
- Moheimani, R.; Aliahmad, N.; Aliheidari, N.; Agarwal, M.; Dalir, H. Thermoplastic Polyurethane Flexible Capacitive Proximity Sensor Reinforced by CNTs for Applications in the Creative Industries. Sci. Rep. 2021, 11, 1104. [Google Scholar] [CrossRef]
- Maddipatla, D.; Zhang, X.; Bose, A.K.; Masihi, S.; Narakathu, B.B.; Bazuin, B.J.; Williams, J.D.; Mitchell, M.F.; Atashbar, M.Z. A Polyimide Based Force Sensor Fabricated Using Additive Screen-Printing Process for Flexible Electronics. IEEE Access 2020, 8, 207813–207821. [Google Scholar] [CrossRef]
- Pruvost, M.; Smit, W.J.; Monteux, C.; Poulin, P.; Colin, A. Polymeric Foams for Flexible and Highly Sensitive Low-Pressure Capacitive Sensors. NPJ Flex. Electron. 2019, 3, 7. [Google Scholar] [CrossRef]
- Devaraj, H.; Schober, R.; Picard, M.; Teo, M.Y.; Lo, C.-Y.; Gan, W.C.; Aw, K.C. Highly Elastic and Flexible Multi-Layered Carbon Black/Elastomer Composite Based Capacitive Sensor Arrays for Soft Robotics. Meas. Sens. 2019, 2–4, 100004. [Google Scholar] [CrossRef]
- Zhi, C.; Shi, S.; Si, Y.; Fei, B.; Huang, H.; Hu, J. Recent Progress of Wearable Piezoelectric Pressure Sensors Based on Nanofibers, Yarns, and Their Fabrics via Electrospinning. Adv. Mater. Technol. 2023, 8, 2201161. [Google Scholar] [CrossRef]
- Gu, L.; Liu, J.; Cui, N.; Xu, Q.; Du, T.; Zhang, L.; Wang, Z.; Long, C.; Qin, Y. Enhancing the Current Density of a Piezoelectric Nanogenerator Using a Three-Dimensional Intercalation Electrode. Nat. Commun. 2020, 11, 1030. [Google Scholar] [CrossRef]
- Renteria, A.; Balcorta, V.H.; Marquez, C.; Rodriguez, A.A.; Renteria-Marquez, I.; Regis, J.; Wilburn, B.; Patterson, S.; Espalin, D.; Tseng, T.-L.; et al. Direct Ink Write Multi-Material Printing of PDMS-BTO Composites with MWCNT Electrodes for Flexible Force Sensors. Flex. Print. Electron. 2022, 7, 015001. [Google Scholar] [CrossRef]
- Park, J.; Ghosh, R.; Song, M.S.; Hwang, Y.; Tchoe, Y.; Saroj, R.K.; Ali, A.; Guha, P.; Kim, B.; Kim, S.-W.; et al. Individually Addressable and Flexible Pressure Sensor Matrixes with ZnO Nanotube Arrays on Graphene. NPG Asia Mater. 2022, 14, 40. [Google Scholar] [CrossRef]
- Taleb, S.; Badillo, M.; Flores-Ruiz, F.J.; Acuautla, M. From Synthesis to Application: High-Quality Flexible Piezoelectric Sensors Fabricated from Tetragonal BaTiO3/P(VDF-TrFE) Composites. Sens. Actuators Phys. 2023, 361, 114585. [Google Scholar] [CrossRef]
- Kumaresan, Y.; Ma, S.; Shakthivel, D.; Dahiya, R. AlN Ultra-Thin Chips Based Flexible Piezoelectric Tactile Sensors. In Proceedings of the 2021 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Virtual, 20–23 June 2021; pp. 1–4. [Google Scholar]
- Guzmán Sierra, D.L.; Bdikin, I.; Tkach, A.; Vilarinho, P.M.; Nunes, C.; Ferreira, P. Flexible Piezoelectric Chitosan and Barium Titanate Biocomposite Films for Sensor Applications. Eur. J. Inorg. Chem. 2021, 2021, 792–803. [Google Scholar] [CrossRef]
- Sappati, K.K.; Bhadra, S. Flexible Piezoelectric 0–3 PZT-PDMS Thin Film for Tactile Sensing. IEEE Sens. J. 2020, 20, 4610–4617. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Manjakkal, L.; Shakthivel, D.; Dahiya, R. Glycine–Chitosan-Based Flexible Biodegradable Piezoelectric Pressure Sensor. ACS Appl. Mater. Interfaces 2020, 12, 9008–9016. [Google Scholar] [CrossRef] [PubMed]
- Chamankar, N.; Khajavi, R.; Yousefi, A.A.; Rashidi, A.; Golestanifard, F. A Flexible Piezoelectric Pressure Sensor Based on PVDF Nanocomposite Fibers Doped with PZT Particles for Energy Harvesting Applications. Ceram. Int. 2020, 46, 19669–19681. [Google Scholar] [CrossRef]
- Brahem, M.; Chouchane, M.; Amamou, A. Active Vibration Control of a Rotor Bearing System Using Flexible Piezoelectric Patch Actuators. J. Intell. Mater. Syst. Struct. 2020, 31, 1284–1297. [Google Scholar] [CrossRef]
- Signore, M.A.; Rescio, G.; De Pascali, C.; Iacovacci, V.; Dario, P.; Leone, A.; Quaranta, F.; Taurino, A.; Siciliano, P.; Francioso, L. Fabrication and Characterization of AlN-Based Flexible Piezoelectric Pressure Sensor Integrated into an Implantable Artificial Pancreas. Sci. Rep. 2019, 9, 17130. [Google Scholar] [CrossRef]
- Navaraj, W.; Dahiya, R. Fingerprint-Enhanced Capacitive-Piezoelectric Flexible Sensing Skin to Discriminate Static and Dynamic Tactile Stimuli. Adv. Intell. Syst. 2019, 1, 1900051. [Google Scholar] [CrossRef]
- Natta, L.; Mastronardi, V.M.; Guido, F.; Algieri, L.; Puce, S.; Pisano, F.; Rizzi, F.; Pulli, R.; Qualtieri, A.; De Vittorio, M. Soft and Flexible Piezoelectric Smart Patch for Vascular Graft Monitoring Based on Aluminum Nitride Thin Film. Sci. Rep. 2019, 9, 8392. [Google Scholar] [CrossRef]
- Lee, K.Y.; Yoon, H.-J.; Jiang, T.; Wen, X.; Seung, W.; Kim, S.-W.; Wang, Z.L. Fully Packaged Self-Powered Triboelectric Pressure Sensor Using Hemispheres-Array. Adv. Energy Mater. 2016, 6, 1502566. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Deng, J.; Zhang, S.L.; Niu, S.; Guo, H.; Wang, Z.L. Single-Thread-Based Wearable and Highly Stretchable Triboelectric Nanogenerators and Their Applications in Cloth-Based Self-Powered Human-Interactive and Biomedical Sensing. Adv. Funct. Mater. 2017, 27, 1604462. [Google Scholar] [CrossRef]
- Yao, G.; Xu, L.; Cheng, X.; Li, Y.; Huang, X.; Guo, W.; Liu, S.; Wang, Z.L.; Wu, H. Bioinspired Triboelectric Nanogenerators as Self-Powered Electronic Skin for Robotic Tactile Sensing. Adv. Funct. Mater. 2020, 30, 1907312. [Google Scholar] [CrossRef]
- Fang, Y.; Zou, Y.; Xu, J.; Chen, G.; Zhou, Y.; Deng, W.; Zhao, X.; Roustaei, M.; Hsiai, T.K.; Chen, J. Ambulatory Cardiovascular Monitoring via a Machine-Learning-Assisted Textile Triboelectric Sensor. Adv. Mater. 2021, 33, 2104178. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, Y.; Bai, L.; Jiang, Y.; Zhang, J.; Zhao, C.; Li, T.; Yu, H.; Song, G.; Zhang, N.; et al. Crumpled Graphene Triboelectric Nanogenerators: Smaller Devices with Higher Output Performance. Adv. Mater. Technol. 2017, 2, 1700044. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, B.; Wei, G.; Wu, J.M.; Han, W.; Yang, Y. Polyimide/Graphene Nanocomposite Foam-Based Wind-Driven Triboelectric Nanogenerator for Self-Powered Pressure Sensor. Adv. Mater. Technol. 2019, 4, 1800723. [Google Scholar] [CrossRef]
- Lei, H.; Chen, Y.; Gao, Z.; Wen, Z.; Sun, X. Advances in Self-Powered Triboelectric Pressure Sensors. J. Mater. Chem. A 2021, 9, 20100–20130. [Google Scholar] [CrossRef]
- Guo, L.; Wu, G.; Wang, Q.; Li, T.; Yao, B.; Zou, Y.; Xu, M. Advances in Triboelectric Pressure Sensors. Sens. Actuators Phys. 2023, 355, 114331. [Google Scholar] [CrossRef]
- Zhang, W.; Xi, Y.; Wang, E.; Qu, X.; Yang, Y.; Fan, Y.; Shi, B.; Li, Z. Self-Powered Force Sensors for Multidimensional Tactile Sensing. ACS Appl. Mater. Interfaces 2022, 14, 20122–20131. [Google Scholar] [CrossRef]
- Syamini, J.; Chandran, A. Mylar Interlayer-Mediated Performance Enhancement of a Flexible Triboelectric Nanogenerator for Self-Powered Pressure Sensing Application. ACS Appl. Electron. Mater. 2023, 5, 1002–1012. [Google Scholar] [CrossRef]
- Rahman, M.T.; Rahman, M.S.; Kumar, H.; Kim, K.; Kim, S. Metal-Organic Framework Reinforced Highly Stretchable and Durable Conductive Hydrogel-Based Triboelectric Nanogenerator for Biomotion Sensing and Wearable Human-Machine Interfaces. Adv. Funct. Mater. 2023, 33, 2303471. [Google Scholar] [CrossRef]
- Bagchi, B.; Datta, P.; Fernandez, C.S.; Gupta, P.; Jaufuraully, S.; David, A.L.; Siassakos, D.; Desjardins, A.; Tiwari, M.K. Flexible Triboelectric Nanogenerators Using Transparent Copper Nanowire Electrodes: Energy Harvesting, Sensing Human Activities and Material Recognition. Mater. Horiz. 2023, 10, 3124–3134. [Google Scholar] [CrossRef] [PubMed]
- Varghese, H.; Abdul Hakkeem, H.M.; Farman, M.; Thouti, E.; Pillai, S.; Chandran, A. Self-Powered Flexible Triboelectric Touch Sensor Based on Micro-Pyramidal PDMS Films and Cellulose Acetate Nanofibers. Results Eng. 2022, 16, 100550. [Google Scholar] [CrossRef]
- Sukumaran, C.; Viswanathan, P.; Munirathinam, P.; Chandrasekhar, A. A Flexible and Wearable Joint Motion Sensor Using Triboelectric Nanogenerators for Hand Gesture Monitoring. Int. J. Nanotechnol. 2021, 18, 697–704. [Google Scholar] [CrossRef]
- Maharjan, P.; Bhatta, T.; Salauddin, M.; Rasel, M.S.; Rahman, M.T.; Rana, S.M.S.; Park, J.Y. A Human Skin-Inspired Self-Powered Flex Sensor with Thermally Embossed Microstructured Triboelectric Layers for Sign Language Interpretation. Nano Energy 2020, 76, 105071. [Google Scholar] [CrossRef]
- Fan, F.-R.; Tian, Z.-Q.; Wang, Z.L. Flexible Triboelectric Generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, G.; Zhou, Y.; Nashalian, A.; Xu, J.; Tat, T.; Song, Y.; Libanori, A.; Xu, S.; Li, S.; et al. Giant Magnetoelastic Effect Enabled Stretchable Sensor for Self-Powered Biomonitoring. ACS Nano 2022, 16, 6013–6022. [Google Scholar] [CrossRef]
- Jeong, H.; Cui, Y.; Tentzeris, M.M.; Lim, S. Hybrid (3D and Inkjet) Printed Electromagnetic Pressure Sensor Using Metamaterial Absorber. Addit. Manuf. 2020, 35, 101405. [Google Scholar] [CrossRef]
- Chen, L.Y.; Tee, B.C.-K.; Chortos, A.L.; Schwartz, G.; Tse, V.; Lipomi, D.J.; Wong, H.-S.P.; McConnell, M.V.; Bao, Z. Continuous Wireless Pressure Monitoring and Mapping with Ultra-Small Passive Sensors for Health Monitoring and Critical Care. Nat. Commun. 2014, 5, 5028. [Google Scholar] [CrossRef]
- Karrock, T.; Gerken, M. Pressure Sensor Based on Flexible Photonic Crystal Membrane. Biomed. Opt. Express 2015, 6, 4901–4911. [Google Scholar] [CrossRef]
- Wang, X.; Que, M.; Chen, M.; Han, X.; Li, X.; Pan, C.; Wang, Z.L. Full Dynamic-Range Pressure Sensor Matrix Based on Optical and Electrical Dual-Mode Sensing. Adv. Mater. 2017, 29, 1605817. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Liu, Z.; Bai, W.; Liu, Y.; Yan, Y.; Xue, Y.; Kandela, I.; Pezhouh, M.; MacEwan, M.R.; Huang, Y.; et al. Bioresorbable Optical Sensor Systems for Monitoring of Intracranial Pressure and Temperature. Sci. Adv. 2019, 5, eaaw1899. [Google Scholar] [CrossRef]
- Qiu, J.; Li, L.; Sun, J.; Peng, J.; Shi, P.; Zhang, R.; Dong, Y.; Lam, K.; Lo, F.P.-W.; Xiao, B.; et al. Large AI Models in Health Informatics: Applications, Challenges, and the Future. IEEE J. Biomed. Health Inform. 2023, 27, 3316750. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Cheng, X.; Xu, S.; Lai, Y.; Zhang, Y. Deep Learning Aided Inverse Design of the Buckling-Guided Assembly for 3D Frame Structures. J. Mech. Phys. Solids 2023, 179, 105398. [Google Scholar] [CrossRef]
- Cheng, X.; Fan, Z.; Yao, S.; Jin, T.; Lv, Z.; Lan, Y.; Bo, R.; Chen, Y.; Zhang, F.; Shen, Z.; et al. Programming 3D Curved Mesosurfaces Using Microlattice Designs. Science 2023, 379, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, E.; Espinosa, H.D. Recent Advances and Applications of Machine Learning in Experimental Solid Mechanics: A Review. Appl. Mech. Rev. 2023, 75, 061001. [Google Scholar] [CrossRef]
- Bastek, J.-H.; Kochmann, D.M. Inverse Design of Nonlinear Mechanical Metamaterials via Video Denoising Diffusion Models. Nat. Mach. Intell. 2023, 5, 1466–1475. [Google Scholar] [CrossRef]
- Stender, M.; Ohlsen, J.; Geisler, H.; Chabchoub, A.; Hoffmann, N.; Schlaefer, A. Up-Net: A Generic Deep Learning-Based Time Stepper for Parameterized Spatio-Temporal Dynamics. Comput. Mech. 2023, 71, 1227–1249. [Google Scholar] [CrossRef]
- Haghighat, E.; Raissi, M.; Moure, A.; Gomez, H.; Juanes, R. A Physics-Informed Deep Learning Framework for Inversion and Surrogate Modeling in Solid Mechanics. Comput. Methods Appl. Mech. Eng. 2021, 379, 113741. [Google Scholar] [CrossRef]
- Bessa, M.A.; Glowacki, P.; Houlder, M. Bayesian Machine Learning in Metamaterial Design: Fragile Becomes Supercompressible. Adv. Mater. 2019, 31, 1904845. [Google Scholar] [CrossRef]
- Regenwetter, L.; Nobari, A.H.; Ahmed, F. Deep Generative Models in Engineering Design: A Review. J. Mech. Des. 2022, 144, 071704. [Google Scholar] [CrossRef]
- Kalina, K.A.; Linden, L.; Brummund, J.; Kästner, M. FEANN: An Efficient Data-Driven Multiscale Approach Based on Physics-Constrained Neural Networks and Automated Data Mining. Comput. Mech. 2023, 71, 827–851. [Google Scholar] [CrossRef]
- Maurizi, M.; Gao, C.; Berto, F. Inverse Design of Truss Lattice Materials with Superior Buckling Resistance. NPJ Comput. Mater. 2022, 8, 247. [Google Scholar] [CrossRef]
- Kumar, S.; Tan, S.; Zheng, L.; Kochmann, D.M. Inverse-Designed Spinodoid Metamaterials. NPJ Comput. Mater. 2020, 6, 73. [Google Scholar] [CrossRef]
- Bastek, J.-H.; Kumar, S.; Telgen, B.; Glaesener, R.N.; Kochmann, D.M. Inverting the Structure–Property Map of Truss Metamaterials by Deep Learning. Proc. Natl. Acad. Sci. USA 2022, 119, e2111505119. [Google Scholar] [CrossRef] [PubMed]
- Alderete, N.A.; Pathak, N.; Espinosa, H.D. Machine Learning Assisted Design of Shape-Programmable 3D Kirigami Metamaterials. NPJ Comput. Mater. 2022, 8, 191. [Google Scholar] [CrossRef]
- van Mastrigt, R.; Dijkstra, M.; van Hecke, M.; Coulais, C. Machine Learning of Implicit Combinatorial Rules in Mechanical Metamaterials. Phys. Rev. Lett. 2022, 129, 198003. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, E. Mechanical MNIST: A Benchmark Dataset for Mechanical Metamodels. Extreme Mech. Lett. 2020, 36, 100659. [Google Scholar] [CrossRef]
- Prume, E.; Reese, S.; Ortiz, M. Model-Free Data-Driven Inference in Computational Mechanics. Comput. Methods Appl. Mech. Eng. 2023, 403, 115704. [Google Scholar] [CrossRef]
- Dalklint, A.; Wallin, M.; Bertoldi, K.; Tortorelli, D. Tunable Phononic Bandgap Materials Designed via Topology Optimization. J. Mech. Phys. Solids 2022, 163, 104849. [Google Scholar] [CrossRef]
- Trent, S.; Renno, J.; Sassi, S.; Mohamed, M.S. Using Image Processing Techniques in Computational Mechanics. Comput. Math. Appl. 2023, 136, 1–24. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, Y. Rational Design of Flexible Mechanical Force Sensors for Healthcare and Diagnosis. Materials 2024, 17, 123. https://doi.org/10.3390/ma17010123
Zhang H, Zhang Y. Rational Design of Flexible Mechanical Force Sensors for Healthcare and Diagnosis. Materials. 2024; 17(1):123. https://doi.org/10.3390/ma17010123
Chicago/Turabian StyleZhang, Hang, and Yihui Zhang. 2024. "Rational Design of Flexible Mechanical Force Sensors for Healthcare and Diagnosis" Materials 17, no. 1: 123. https://doi.org/10.3390/ma17010123
APA StyleZhang, H., & Zhang, Y. (2024). Rational Design of Flexible Mechanical Force Sensors for Healthcare and Diagnosis. Materials, 17(1), 123. https://doi.org/10.3390/ma17010123





