Improved Soil Amendment by Integrating Metal Complexes and Biodegradable Complexing Agents in Superabsorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Analytical Methods
2.3. Adsorption and Kinetic Equilibrium
2.4. Instruments
3. Results and Discussion
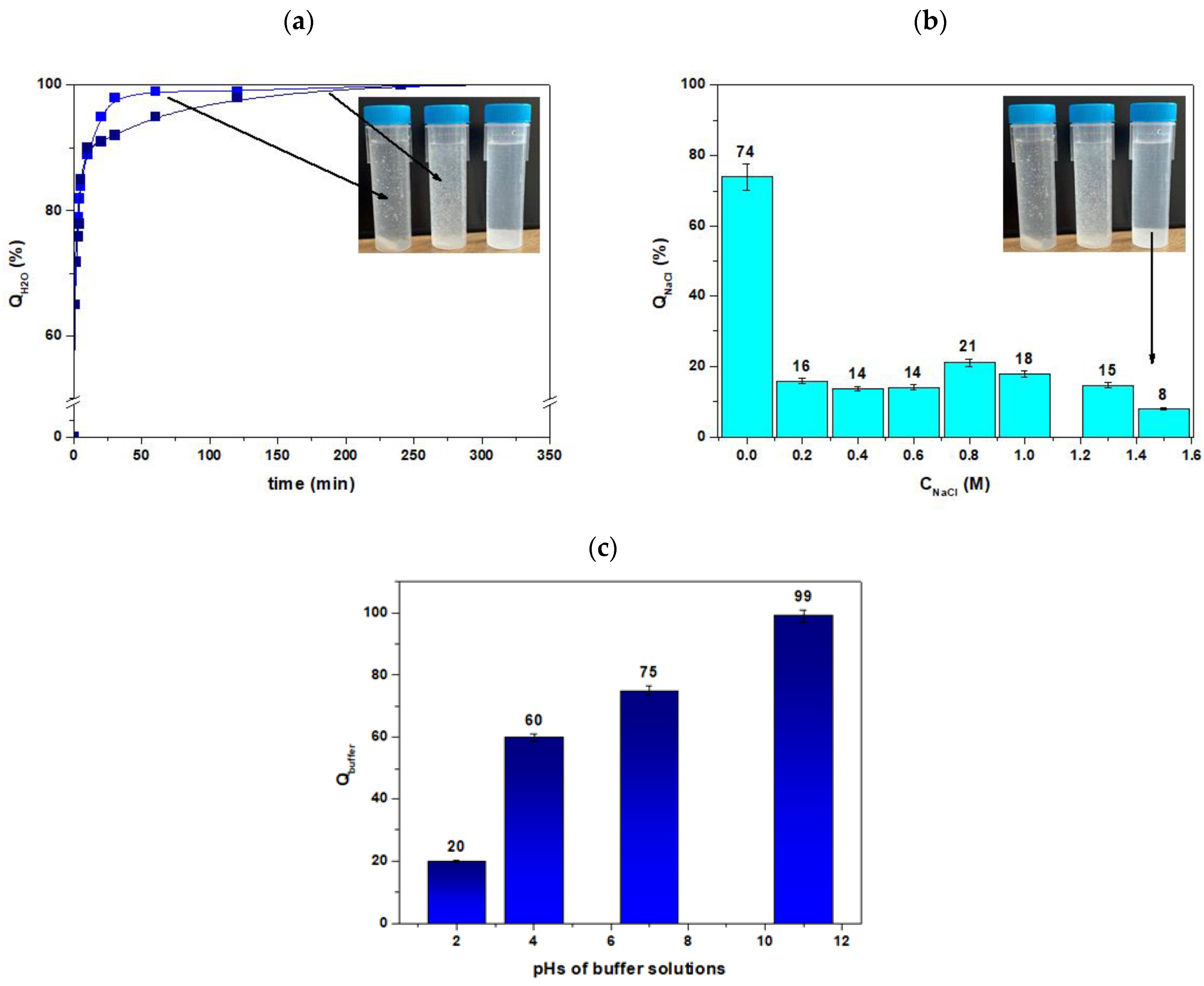
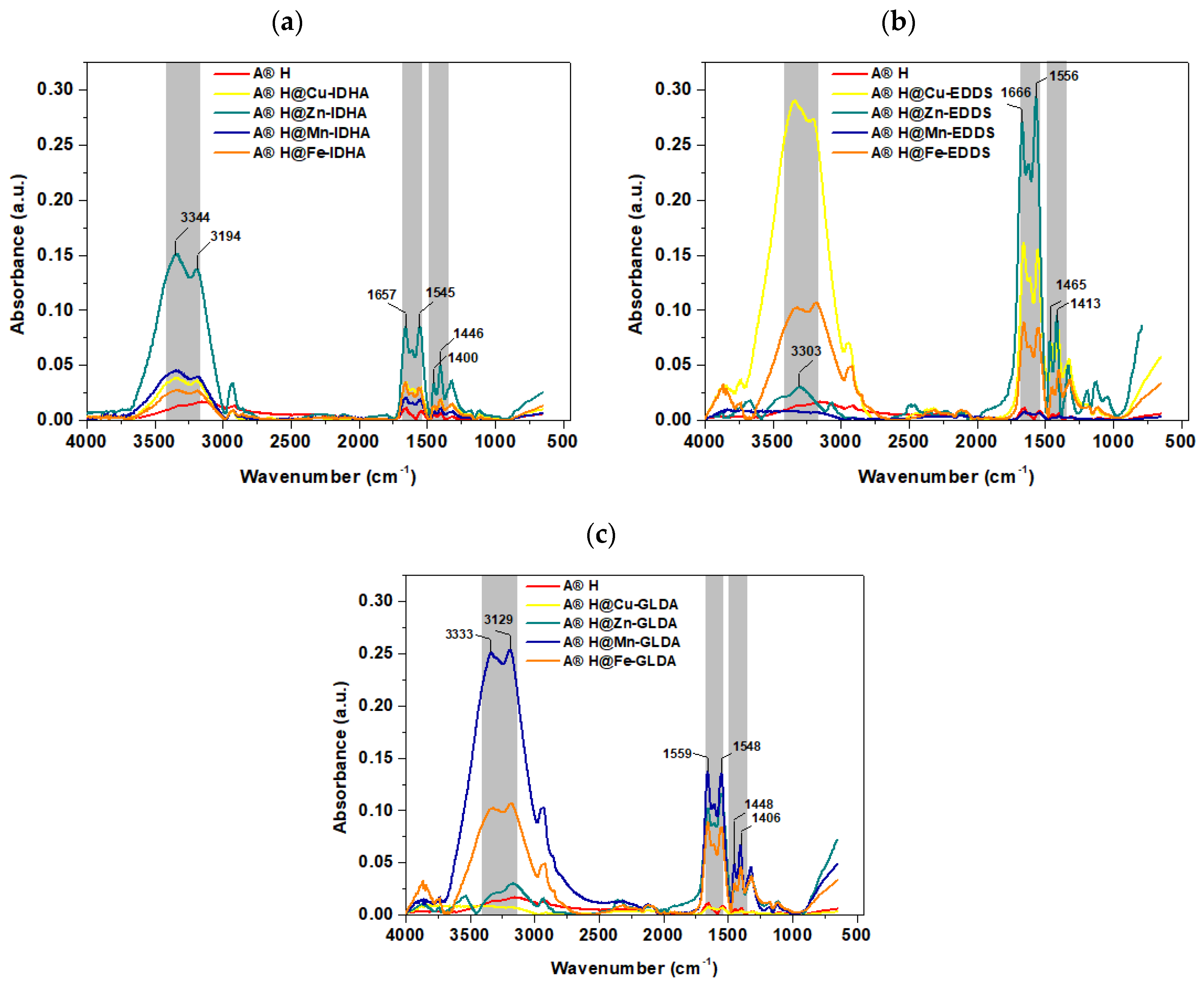
3.1. Agro® Hydrogel Characterization
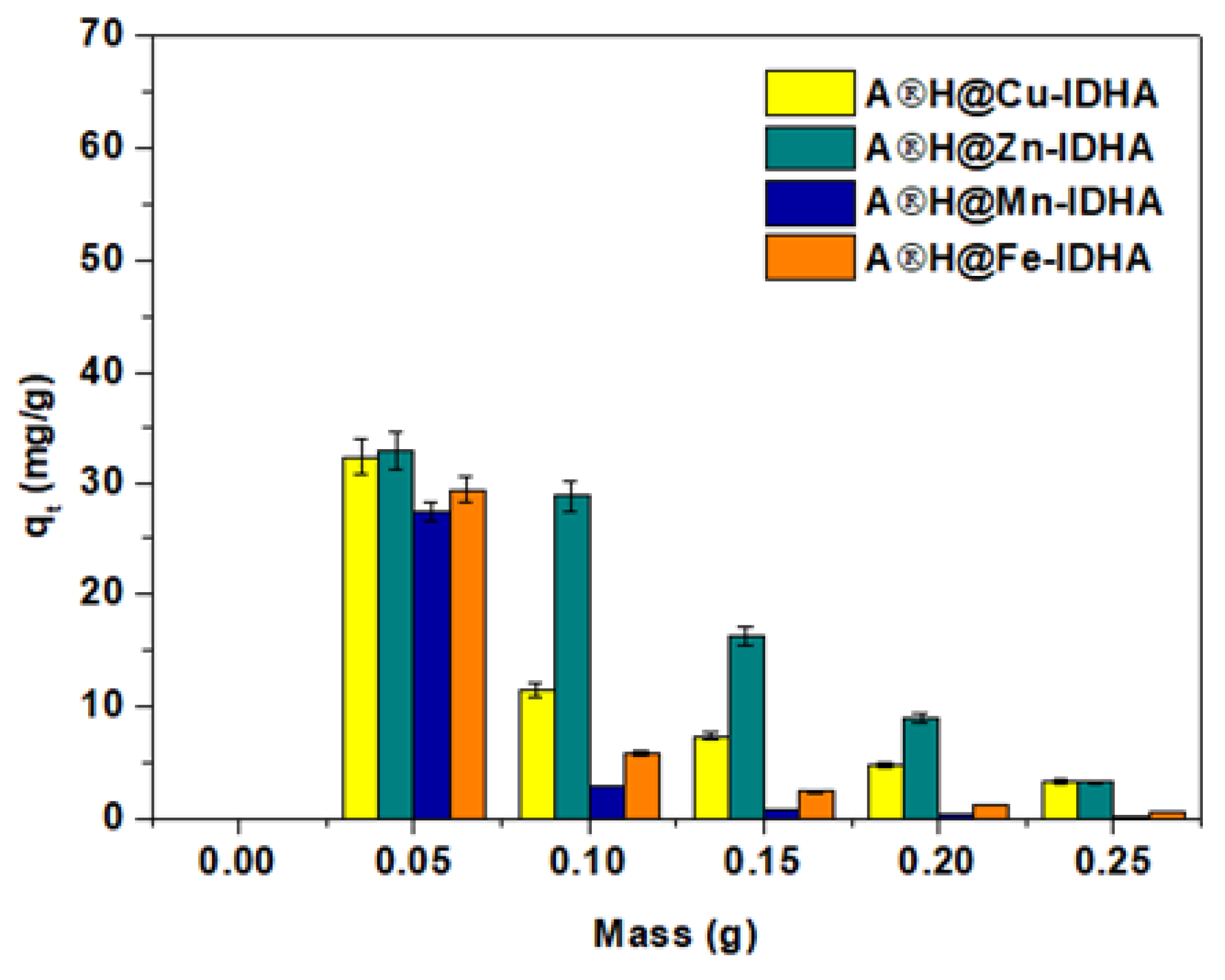
3.2. Effect of Adsorbent Mass
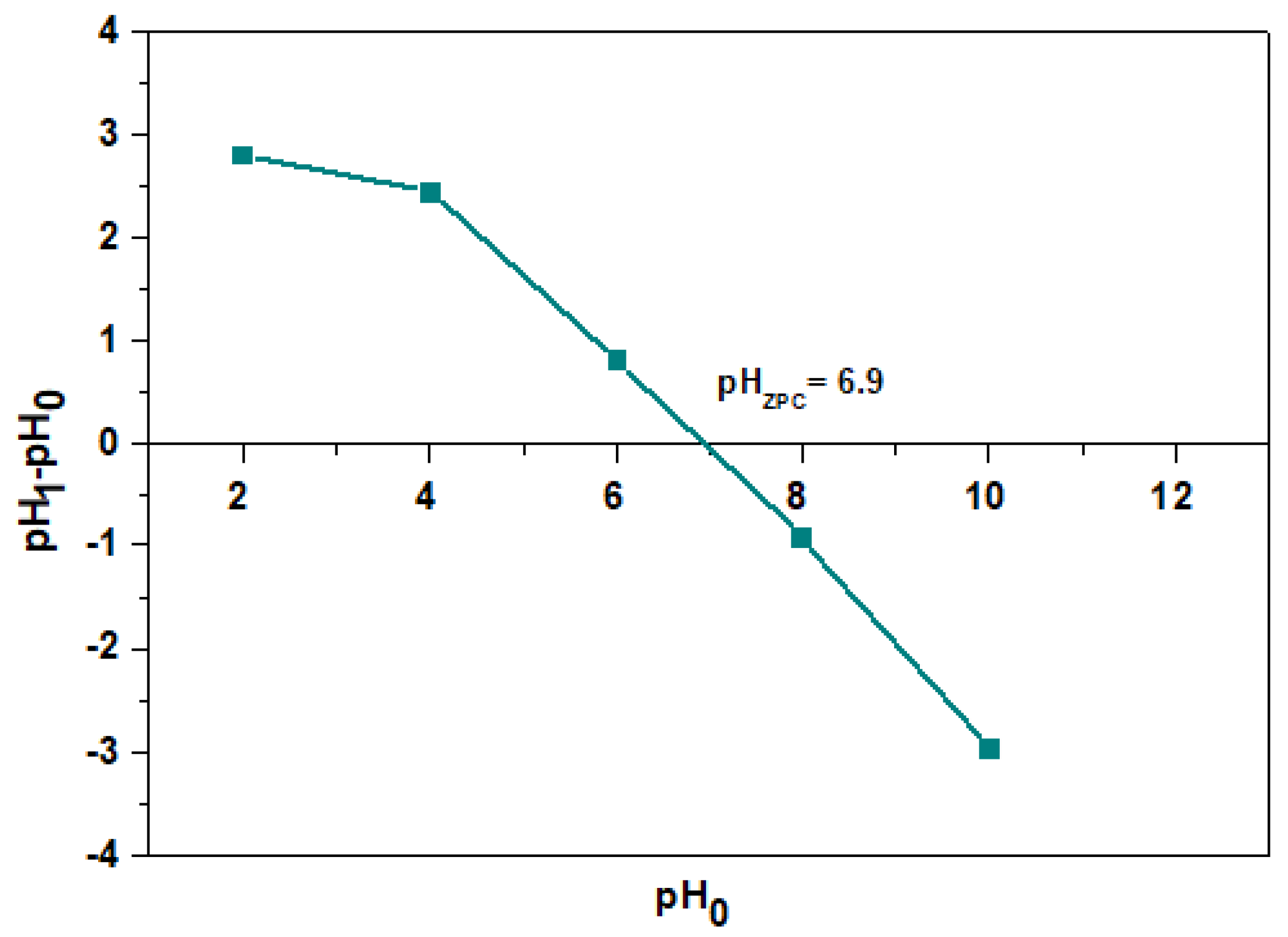
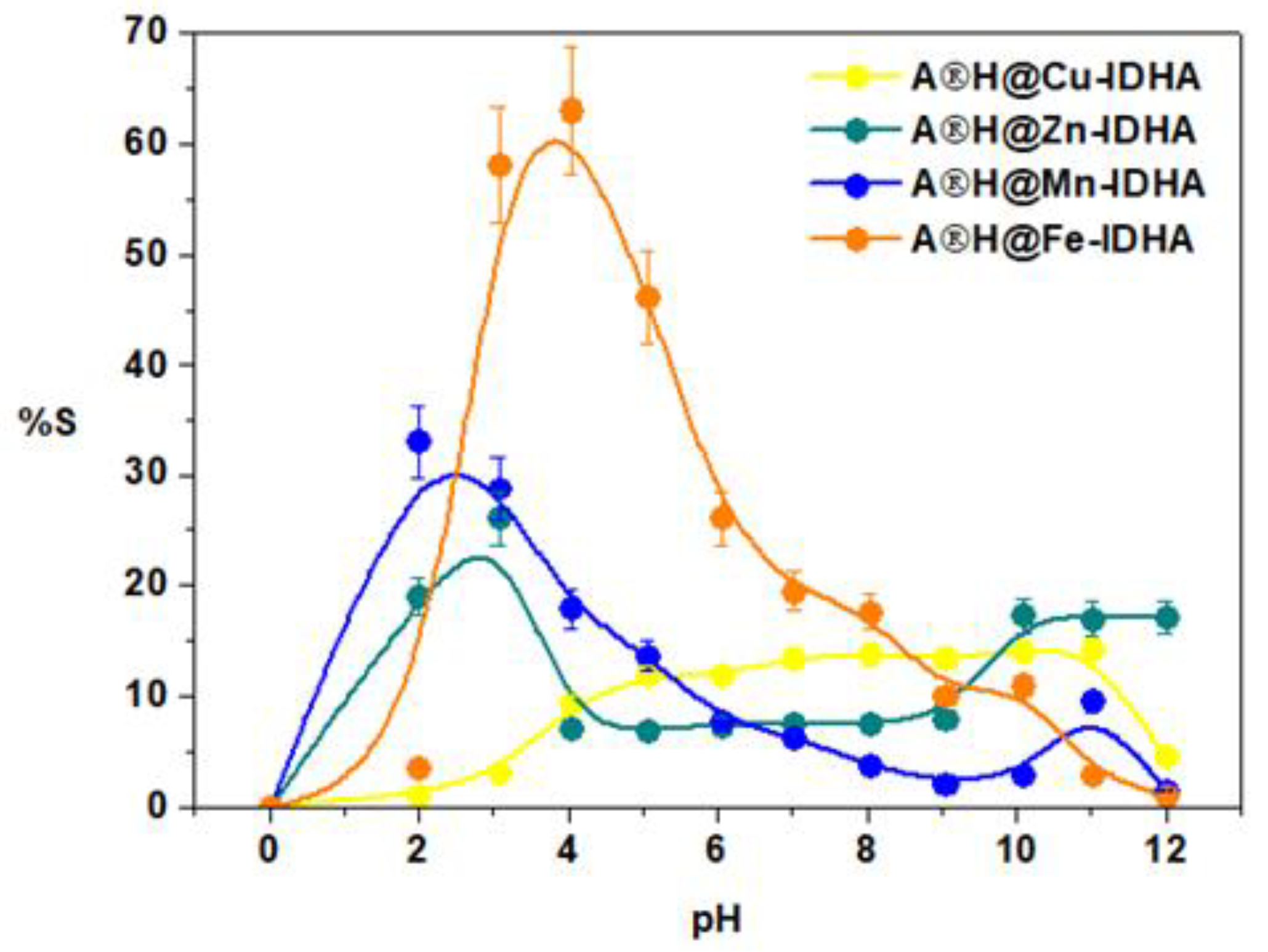
3.3. Effect of Initial pH
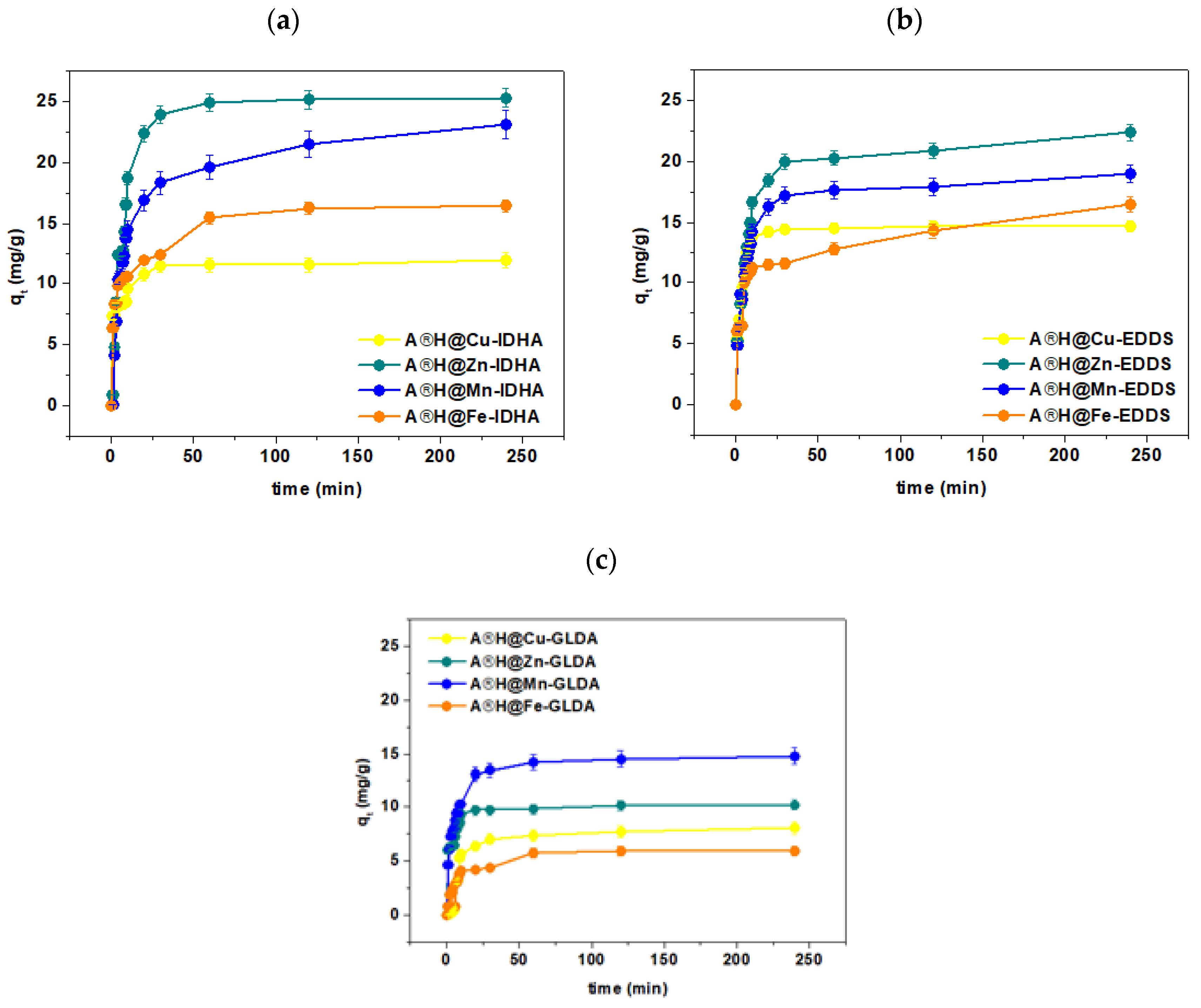
3.4. Kinetic Studies
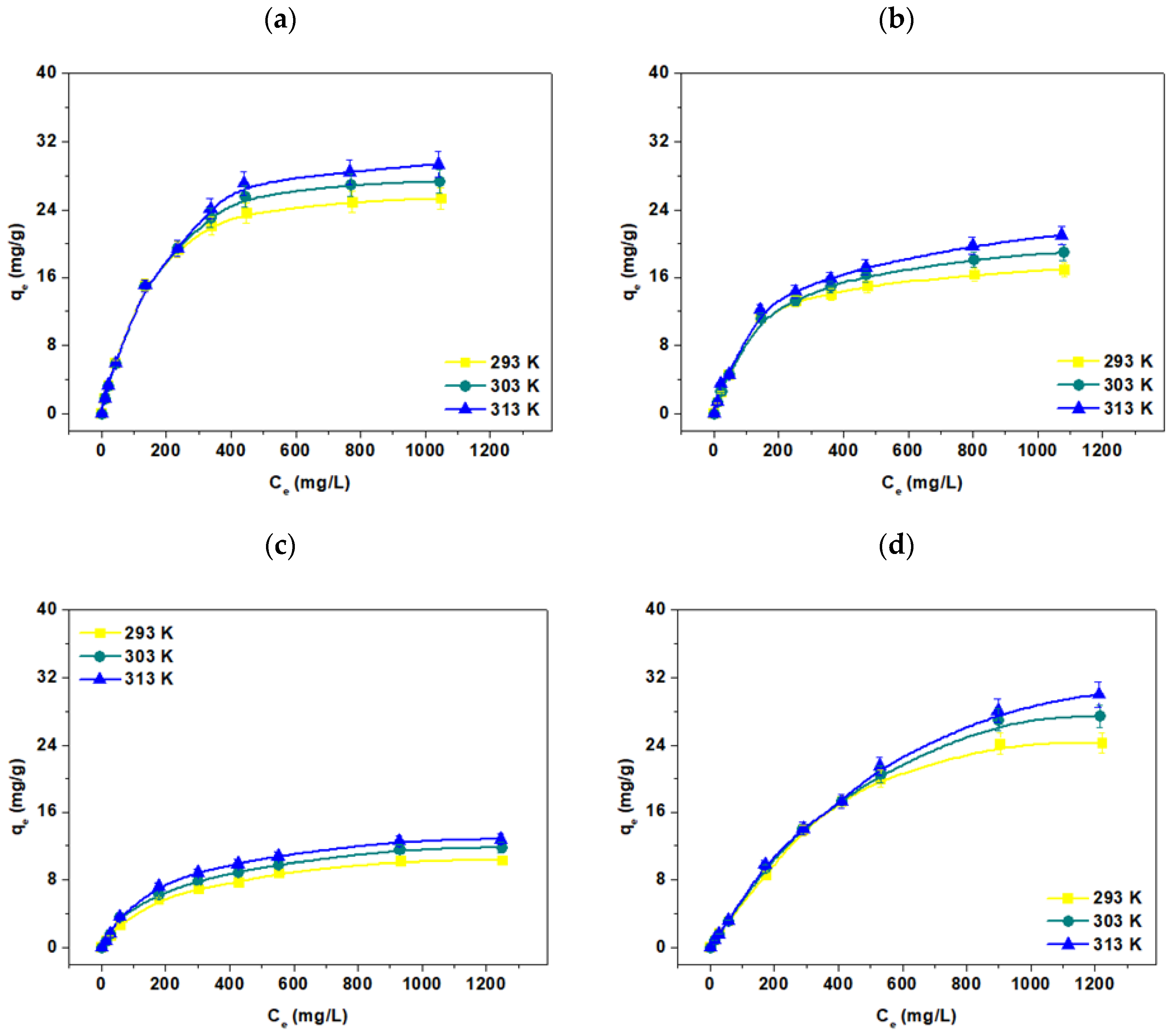
3.5. Temperature Effect, Adsorption and Thermodynamic Calculations
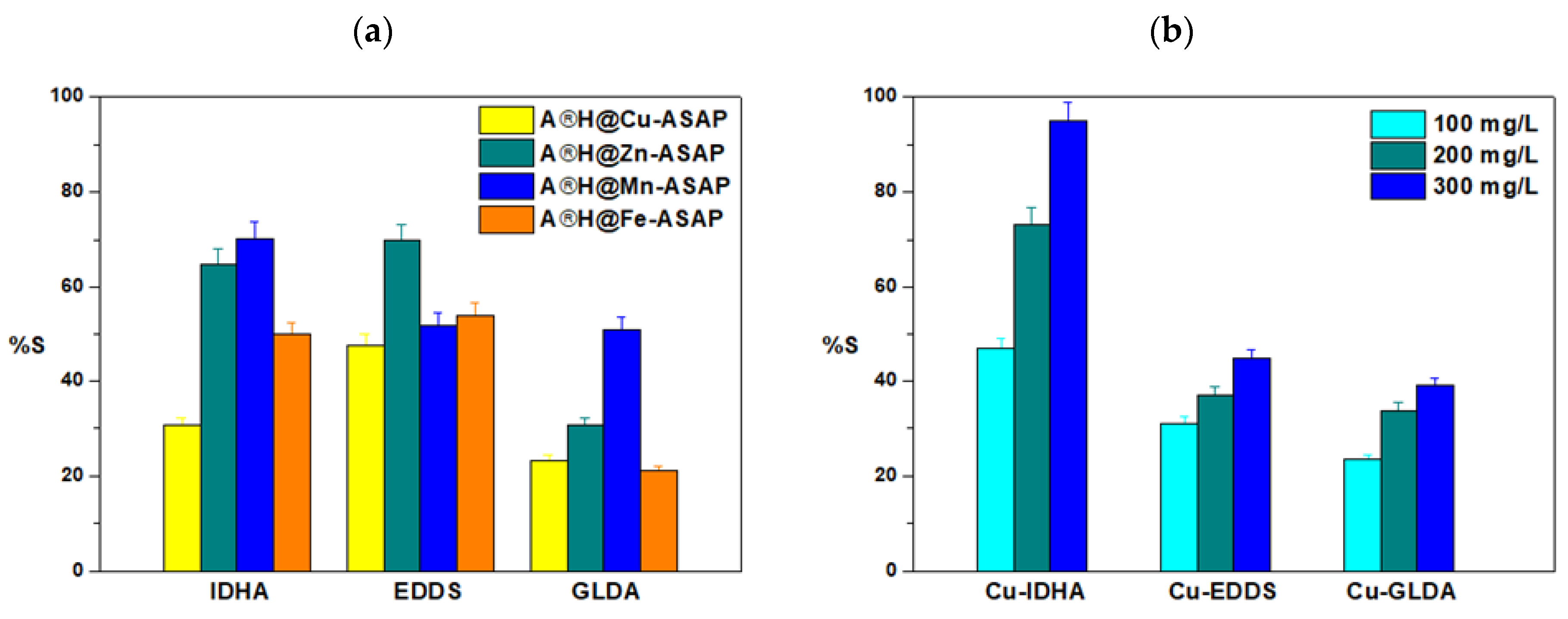
3.6. Desorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rani, D.P. An analytical study on performance of soyabean crop in India. Int. J. Res. Appl. Sci. Technol. 2022, 10, 1936–1940. [Google Scholar] [CrossRef]
- Lipowczan, A.; Trochimczuk, A. Phosphates-containing interpenetrating polymer networks (IPNs) acting as slow release fertilizer hydrogels (SRFHs) suitable for agricultural applications. Materials 2021, 14, 2893. [Google Scholar] [CrossRef]
- Piwowar, A. Consumption of mineral fertilizers in the Polish agriculture—Trends and directions of changes. Agric. Res. 2022, 11, 477–478. [Google Scholar] [CrossRef]
- Stephens, E.C.; Jones, A.D.; Parsons, D. Agricultural systems sesearch and global food security in The 21st Century: An overview and roadmap for future opportunities. Agric. Syst. 2018, 163, 1–6. [Google Scholar] [CrossRef]
- Ogino, C.M.; Junior, G.C.; Popova, N.D.; Martines Filho, J.G. Purchase Power, price and mineral fertilizers consumption: An analysis for central Brazil. Rev. Econ. Sociol. Rural. 2020, 59, 1–19. [Google Scholar] [CrossRef]
- Randive, K.; Raut, T.; Jawadand, S. An overview of the global fertilizer trends and India’s position in 2020. Miner. Econ. 2021, 34, 371–384. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]
- Thakur, M.K.; Gupta, R.K.; Thakur, V.K. Surface modification of mellulose using silane coupling agent. Carbohydr. Polym. 2014, 111, 849–855. [Google Scholar] [CrossRef]
- Bai, C.; Zhang, S.; Huang, L.; Wang, H.; Wang, W.; Ye, Q. Starch-based hydrogel loading with carbendazim for controlled-release and water absorption. Carbohydr. Polym. 2015, 125, 376–383. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A mini-review on chitosan-based hydrogels with potential for sustainable agricultural applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Kennedy, J.F.; Fayaz, U.; Khan, S.A. Recent insights into polysaccharide-based hydrogels and their potential applications in food sector: A review. Int. J. Biol. Macromol. 2022, 213, 987–1006. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Li, W.; Liu, Z.; Liu, Y.; Wei, H.; Li, J. Synthesis and characterization of double-network hydrogels based on sodium alginate and halloysite for slow release fertilizers. Int. J. Biol. Macromol. 2020, 164, 557–565. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.H.A.; Pereira, A.G.B.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogel composite made of cellulose nanofibrils and chitosan-graft-poly(acrylic acid). Carbohydr. Polym. 2012, 87, 2038–2045. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent trends in hydrogels based on psyllium polysaccharide: A review. J. Clean. Prod. 2014, 82, 1–15. [Google Scholar] [CrossRef]
- Chen, M.; Wang, W.; Fang, J.; Guo, P.; Liu, X.; Li, G.; Li, Z.; Wang, X.; Li, J.; Lei, K. Environmentally adaptive polysaccharide-based hydrogels and their applications in extreme conditions: A review. Int. J. Biol. Macromol. 2023, 241, 124496. [Google Scholar] [CrossRef]
- Khare, A.R.; Peppas, N.A. Release behavior of bioactive agents from pH-sensitive hydrogels. J. Biomater. Sci. Polym. Ed. 1993, 4, 275–289. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Controlled drug release from hydrogel-based matrices: Experiments and modeling. Int. J. Pharm. 2015, 486, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Gong, E.; Liu, B.; Zhou, L.; Che, C.; Hu, S.; Zhang, Z.; Liu, J.; Shi, J. Hydrogel-mediated drug delivery for treating stroke. Chin. Chem. Lett. 2023, 34, 108205. [Google Scholar] [CrossRef]
- Rizwan, M.; Rubina Gilani, S.; Iqbal Durani, A.; Naseem, S. Materials diversity of hydrogel: Synthesis, polymerization process and soil conditioning properties in agricultural field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef]
- Durpekova, S.; Bergerova, E.D.; Hanusova, D.; Dusankova, M.; Sedlarik, V. Eco-friendly whey/polysaccharide-based hydrogel with poly(lactic acid) for improvement of agricultural soil quality and plant growth. Int. J. Biol. Macromol. 2022, 212, 85–96. [Google Scholar] [CrossRef]
- Jumpapaeng, P.; Suwanakood, P.; Nanan, S.; Saengsuwan, S. Novel biodegradable nanocomposite hydrogels based on biopolymers and various montmorillonite contents as high-strength coating membranes for efficient slow-release fertilizers. J. Ind. Eng. Chem. 2023, 127, 191–209. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Suwanakood, P.; Saengsuwan, S. Biodegradable hydrogels of cassava starch-g-polyacrylic acid/natural rubber/polyvinyl alcohol as environmentally friendly and highly efficient coating material for slow-release urea fertilizers. J. Ind. Eng. Chem. 2021, 101, 237–252. [Google Scholar] [CrossRef]
- Dong, Z.; Qu, N.; Jiang, Q.; Han, Z.; Sun, L.; Zhang, T.; Liang, D.; Shi, Y.; Cheng, Z. Preparation and properties of multifunctional eco-friendly slow-release urea fertilizer encapsulated by diatomite filter aid waste-based superabsorbent. Prog. Org. Coat. 2023, 183, 107747. [Google Scholar] [CrossRef]
- Khushbu; Warkar, S.G.; Kumar, A. Synthesis and assessment of carboxymethyl tamarind kernel gum based novel superabsorbent hydrogels for agricultural applications. Polymer 2019, 182, 121823. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Ni, B.; Xie, L.; Zhang, X. Preparation and properties of novel slow-release pk agrochemical formulations based on carboxymethylcellulose-graft-poly(acrylic acid-co-itaconic acid) superabsorbents. J. Macromol. Sci. Part A Pure Appl. Chem. 2011, 48, 806–811. [Google Scholar] [CrossRef]
- Chang, L.; Xu, L.; Liu, Y.; Qiu, D.; Nadim, E.; Paraskar, P.; Murphy, E.J.; Hesabi, M.; Major, I.; Rizwan, M.; et al. Biodegradable superabsorbent hydrogel increases water retention properties of growing media and plant growth. J. Adv. Res. 2021, 4, 100202. [Google Scholar] [CrossRef]
- Kołodyńska, D. Complexing Agents. Kirk-Othmer Encycl. Chem. Technol. 2019, 1–26. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Drozd, A.; Ju, Y. Superabsorbents and their application for heavy metal ion removal in the presence of edds. Polymers 2021, 13, 3688. [Google Scholar] [CrossRef]
- Kołodyńska, D. The effect of the novel complexing agent in removal of heavy metal ions from waters and waste waters. Chem. Eng. J. 2010, 165, 835–845. [Google Scholar] [CrossRef]
- EU Integrated Pollution Prevention and Control, Best Available Techniques (BAT) Reference Document for the Slaughterhouses, Animal Byproducts and Edible Co-products Industries. 2021. Available online: https://Eippcb.Jrc.Ec.Europa.Eu/Sites/Default/Files/2021-06/SA-BREF-20210629.Pdf (accessed on 10 November 2023).
- Wang, X.; Hou, H.; Li, Y.; Wang, Y.; Hao, C.; Ge, C. A novel semi-IPN hydrogel: Preparation, swelling properties and adsorption studies of Co(II). J. Ind. Eng. Chem. 2016, 41, 82–90. [Google Scholar] [CrossRef]
- Masuda, Y.; Tanaka, T.; Nakanishi, T. Ion-specific swelling behavior of poly(vinyl alcohol) gel prepared by γ-ray irradiation. Colloid Polym. Sci. 2001, 279, 1241–1244. [Google Scholar] [CrossRef]
- Liang, R.; Liu, M. Preparation and properties of a double-coated slow-release and water-retention urea fertilizer. J. Agric. Food Chem. 2006, 54, 1392–1398. [Google Scholar] [CrossRef]
- Wei, B.; Jiang, J.; Gao, C.; Zhang, L.; Zhan, Y.; Jiang, S.; Li, Y.; Sun, S.; Xie, J.; Fan, X. Revealing channel controlled nutrient release mechanism of bio-oil polymer coated controlled-release fertilizer. Ind. Crop. Prod. 2021, 173, 114096. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, Y. Preparation and performance of degradable slow-release fertilizer coating material by a new ionic crosslinked hydrogel material. J. Environ. Chem. Eng. 2023, 11, 110785. [Google Scholar] [CrossRef]
- Xie, L.; Liu, M.; Ni, B.; Zhang, X.; Wang, Y. Slow-release nitrogen and boron fertilizer from a functional superabsorbent for-mulation based on wheat straw and attapulgite. Chem. Eng. J. 2011, 167, 342–348. [Google Scholar] [CrossRef]
- Bagheri Marandi, G.R.M.G.; Esfandiari, K.; Biranvand, F.; Babapour, M.; Sadeh, S. pH Sensitivity and swelling behavior of partially hydrolyzed formaldehyde-crosslinked poly(acrylamide) superabsorbent hydrogels. J. Appl. Polym. Sci. 2008, 109, 1083–1092. [Google Scholar] [CrossRef]
- Park, H.; Robinson, J.R. Mechanisms of mucoadhesion of poly(acrylic acid) hydrogels. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1987, 4, 457–464. [Google Scholar] [CrossRef]
- Motekaitis, R.J.; Martell, A.E. The iron(III) and iron(II) complexes of nitrilotriacetic acid. J. Coord. Chem. 1994, 31, 67–78. [Google Scholar] [CrossRef]
- Truong, N.D.; Medjahdi, G.; Sarazin, D.; François, J. Effect of the carboxylate group distribution on potentiometric titration of acrylamide-acrylic acid copolymers. Polym. Bull. 1990, 24, 101–106. [Google Scholar] [CrossRef]
- He, G.; Ke, W.; Chen, X.; Kong, Y.; Zheng, H.; Yin, Y.; Cai, W. Preparation and properties of quaternary ammonium chitosan-g-poly(acrylic acid-co-acrylamide) superabsorbent hydrogels. React. Funct. Polym. 2017, 111, 14–21. [Google Scholar] [CrossRef]
- Asemave, K. Greener Chelators for recovery of metals and other applications. Org. Med. Chem. Int. J. 2018, 6, 555694. [Google Scholar] [CrossRef]
- Milosavljević, N.B.; Ristić, M.D.; Perić-Grujić, A.A.; Filipović, J.M.; Štrbac, S.B.; Rakočević, Z.L.; Krušić, M.T.K. Removal of Cu2+ ions using hydrogels of chitosan, itaconic and methacrylic acid: FTIR, SEM/EDX, AFM, kinetic and equilibrium study. Colloids Surf. A Physicochem. Eng. Asp. 2011, 388, 59–69. [Google Scholar] [CrossRef]
- Maleki, L.; Edlund, U.; Albertsson, A.C. Synthesis of full interpenetrating hemicellulose hydrogel networks. Carbohydr. Polym. 2017, 170, 254–263. [Google Scholar] [CrossRef]
- Orozco-Guareño, E.; Santiago-Gutiérrez, F.; Morán-Quiroz, J.L.; Hernandez-Olmos, S.L.; Soto, V.; de la Cruz, W.; Manríquez, R.; Gomez-Salazar, S. Removal of Cu(II) ions from aqueous streams using poly(acrylic acid-co-acrylamide) hydrogels. J. Colloid Interface Sci. 2010, 349, 583–593. [Google Scholar] [CrossRef]
- Chen, J.J.; Ahmad, A.L.; Ooi, B.S. Poly(N-isopropylacrylamide-co-acrylic acid) hydrogels for copper ion adsorption: Equilibrium isotherms, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2013, 1, 339–348. [Google Scholar] [CrossRef]
- Modrzejewska, Z.; Rogacki, G.; Sujka, W.; Zarzycki, R. Sorption of copper by chitosan hydrogel: Kinetics and equilibrium. Chem. Eng. Process. Process. Intensif. 2016, 109, 104–113. [Google Scholar] [CrossRef]
- Shehzad, H.; Farooqi, Z.H.; Ahmed, E.; Sharif, A.; Ajmal, M.; Razzaq, S.; Naseer, M.U.; Nazir, M.A.; Batool, M.; Akram, T.; et al. Effective biosorption of Cu(II) using hybrid biocomposite based on N-maleated chitosan/calcium algi-nate/titania: Equilibrium sorption, kinetic and thermodynamic studies. Int. J. Biol. Macromol. 2022, 216, 676–685. [Google Scholar] [CrossRef]
- Abid, O.; Ahmed, E.; Shehzad, H.; Sharif, A.; Farooqi, Z.H.; Liu, Z.; Zhou, L.; Ouyang, J.; Begum, R.; Irfan, A.; et al. Competitive recovery of copper ions using ethyl acetoacetate modified chitosan/organo-functionalized alginate hydrogel beads: Kinetics and isothermal sorption studies. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132019. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Alijani, N. An all-biopolymer self-assembling hydrogel film consisting of chitosan and carboxymethyl guar gum: A novel bio-based composite adsorbent for Cu2+ adsorption from aqueous solution. Int. J. Biol. Macromol. 2023, 242, 124878. [Google Scholar] [CrossRef]
- Kołodyńska, D. Adsorption characteristics of chitosan modified by chelating agents of a new generation. Chem. Eng. J. 2012, 179, 33–43. [Google Scholar] [CrossRef]
- Wang, J.; Ding, L.; Wei, J.; Liu, F. Adsorption of copper ions by ion-imprinted simultaneous interpenetrating network hydrogel: Thermodynamics, morphology and mechanism. Appl. Surf. Sci. 2014, 305, 412–418. [Google Scholar] [CrossRef]
- Salih, S.S.; Ghosh, T.K. Adsorption of Zn(II) ions by chitosan coated diatomaceous earth. Int. J. Biol. Macromol. 2018, 106, 602–610. [Google Scholar] [CrossRef]
- Ju, W.; Duan, C.; Liu, L.; Jin, X.; Bravo-Ruiseco, G.; Mei, Y.; Fang, L. Reduction of Cu and nitrate leaching risk associated with EDDS-enhanced phytoextraction process by exogenous inoculation of plant growth promoting rhizobacteria. Chemosphere 2022, 287, 132288. [Google Scholar] [CrossRef]



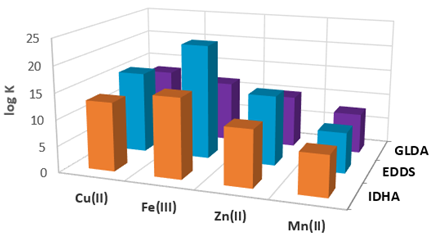 | |||
| M(II)/(III) | IDHA | EDDS | GLDA |
| Cr(III) | 9.6 | n.a. | n.a. |
| Hg(II) | 14.9 | n.a. | 14.3 |
| Mg(II) | 6.9 | 6.0 | 6.1 |
| Ni(II) | 12.2 | 16.7 | 10.9 |
| Pb(II) | 11.0 | 12.7 | 10.5 |
| Complexing Agent | M(II)/(III) | Pseudo-First Order | Pseudo-Second Order | Intraparticle Diffusion | |||||
|---|---|---|---|---|---|---|---|---|---|
| qe1 mg/g | k1 1/min | R2 | qe2 mg/g | k2 g/mg min | R2 | ki mg/g min | R2 | ||
| IDHA | Cu(II) | 3.62 | 0.025 | 0.7009 | 12.12 | 0.026 | 0.9998 | 3.14 | 0.7666 |
| Zn(II) | 14.02 | 0.045 | 0.8879 | 25.91 | 0.008 | 0.9994 | 6.14 | 0.8711 | |
| Mn(II) | 13.53 | 0.020 | 0.8437 | 23.61 | 0.005 | 0.9991 | 0.90 | 0.8567 | |
| Fe(III) | 8.16 | 0.031 | 0.9836 | 16.82 | 0.011 | 0.9991 | 4.14 | 0.9284 | |
| EDDS | Cu(II) | 4.48 | 0.074 | 0.9385 | 14.74 | 0.069 | 0.9999 | 4.71 | 0.9962 |
| Zn(II) | 10.51 | 0.020 | 0.7056 | 22.64 | 0.008 | 0.9993 | 4.90 | 0.9869 | |
| Mn(II) | 4.85 | 0.016 | 0.9523 | 15.96 | 0.021 | 0.9989 | 4.62 | 0.9813 | |
| Fe(III) | 7.54 | 0.011 | 0.7214 | 16.39 | 0.009 | 0.9935 | 0.44 | 0.9893 | |
| GLDA | Cu(II) | 5.80 | 0.029 | 0.7944 | 8.74 | 0.006 | 0.9318 | 0.24 | 0.8519 |
| Zn(II) | 2.85 | 0.034 | 0.8346 | 10.31 | 0.047 | 0.9999 | 2.57 | 0.7728 | |
| Mn(II) | 6.61 | 0.031 | 0.8646 | 15.07 | 0.016 | 0.9999 | 3.52 | 0.9704 | |
| Fe(III) | 5.01 | 0.060 | 0.9751 | 6.23 | 0.015 | 0.9773 | 0.27 | 0.8876 | |
| Isotherm Models | Parameters | Cu(II) | Zn(II) | Mn(II) | Fe(III) |
|---|---|---|---|---|---|
| Langmuir | q0 | 35.33 | 56.01 | 60.77 | 43.69 |
| KL | 0.012 | 0.017 | 0.019 | 0.013 | |
| R2 | 0.9957 | 0.9988 | 0.9970 | 0.9942 | |
| Freundlich | KF | 3.42 | 5.43 | 6.59 | 3.65 |
| 1/n | 0.34 | 0.36 | 0.34 | 0.37 | |
| R2 | 0.9810 | 0.8992 | 0.9336 | 0.9277 | |
| Temkin | KT | 0.256 | 0.346 | 0.483 | 0.282 |
| bT | 5.864 | 8.693 | 9.667 | 7.282 | |
| R2 | 0.9895 | 0.9871 | 0.9691 | 0.9942 | |
| Dubinin-Raduszkiewicz | qm | 53.48 | 100.87 | 104.83 | 85.70 |
| β | 0.0040 | 0.0046 | 0.0043 | 0.0048 | |
| Ea | 10.71 | 10.42 | 10.83 | 10,19 | |
| R2 | 0.9962 | 0.9467 | 0.9697 | 0.9633 |
| T [K] | ΔGo [kJ/mol] | ΔHo [kJ/mol] | ΔSo [kJ/mol K] | R2 |
|---|---|---|---|---|
| Cu(II)-IDHA = 1:1 | ||||
| 293 | −7.35 | 5.88 | −12.3 | 1.0000 |
| 313 | −8.26 | |||
| 333 | −9.16 | |||
| Zn(II)-IDHA = 1:1 | ||||
| 293 | −9.02 | 2.62 | −17.7 | 0.9999 |
| 313 | −9.81 | |||
| 333 | −10.61 | |||
| Mn(II)-IDHA = 1:1 | ||||
| 293 | −9.45 | 3.05 | −14.8 | 1.0000 |
| 313 | −10.30 | |||
| 333 | −11.15 | |||
| Fe(III)-IDHA = 1:1 | ||||
| 293 | −8.40 | 4.44 | −13.6 | 0.9999 |
| 313 | −9.27 | |||
| 333 | −10.15 | |||
| Desorbing Agent | Desorption Efficiency % |
|---|---|
| 0.1 M HNO3 | 98.0 |
| 0.1 M HCl | 98.4 |
| 1 M HNO3 | 100.0 |
| 1 M HCl | 100.0 |
| distilled water | 46.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozd, A.; Ju, Y.; Kołodyńska, D. Improved Soil Amendment by Integrating Metal Complexes and Biodegradable Complexing Agents in Superabsorbents. Materials 2024, 17, 141. https://doi.org/10.3390/ma17010141
Drozd A, Ju Y, Kołodyńska D. Improved Soil Amendment by Integrating Metal Complexes and Biodegradable Complexing Agents in Superabsorbents. Materials. 2024; 17(1):141. https://doi.org/10.3390/ma17010141
Chicago/Turabian StyleDrozd, Alicja, Yongming Ju, and Dorota Kołodyńska. 2024. "Improved Soil Amendment by Integrating Metal Complexes and Biodegradable Complexing Agents in Superabsorbents" Materials 17, no. 1: 141. https://doi.org/10.3390/ma17010141







