Construction of a ZnO Heterogeneous Structure Using Co3O4 as a Co-Catalyst to Enhance Photoelectrochemical Performance
Abstract
:1. Introduction
2. Experimental Sections
2.1. Chemicals
2.2. Synthesis
2.2.1. Spin Coating Technique for Deposition of ZnO Seed Layer
2.2.2. Chemical Bath Deposition of ZnO
2.2.3. Deposition of Cobalt Oxide on the Surface of Zinc Oxide
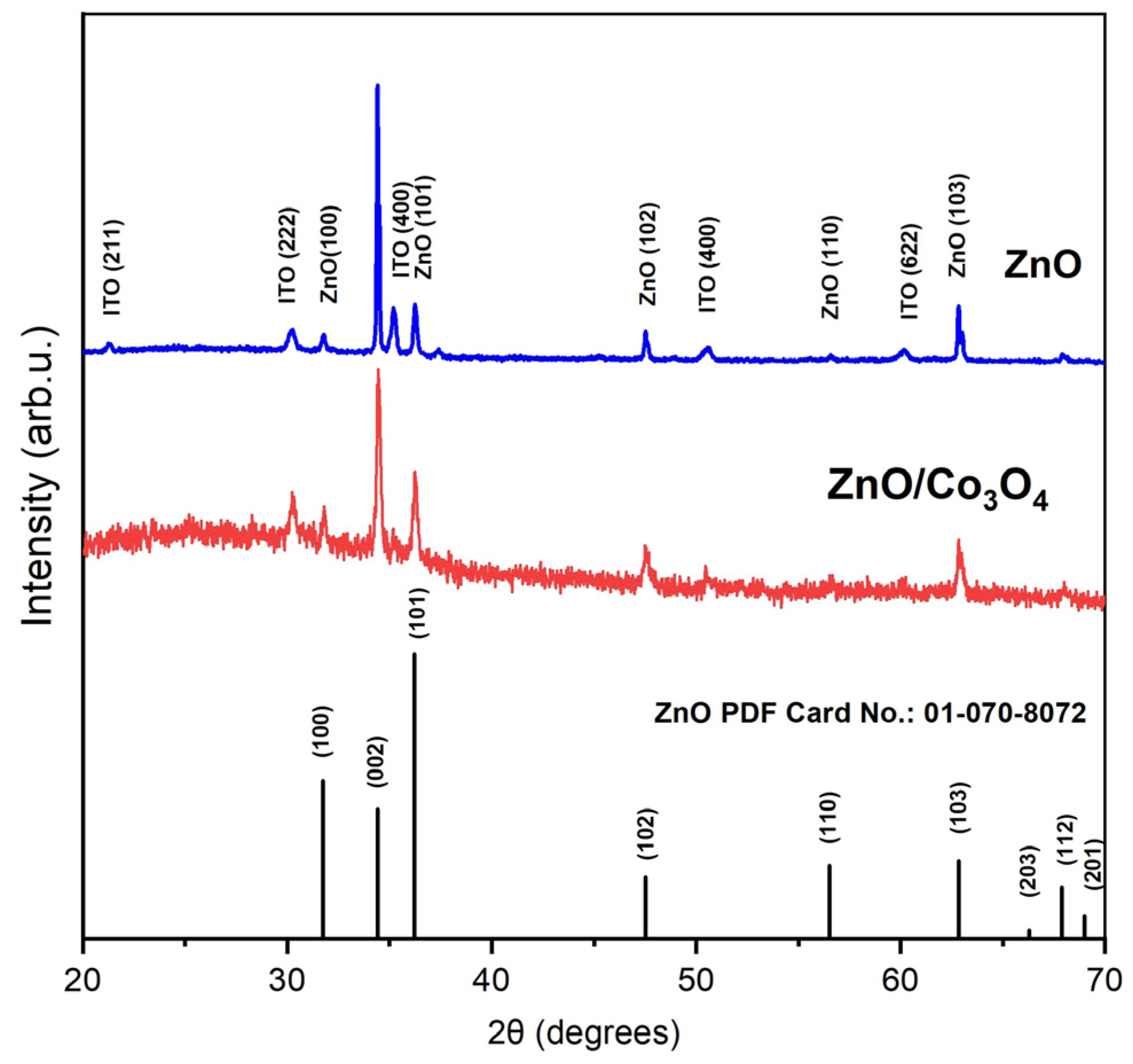
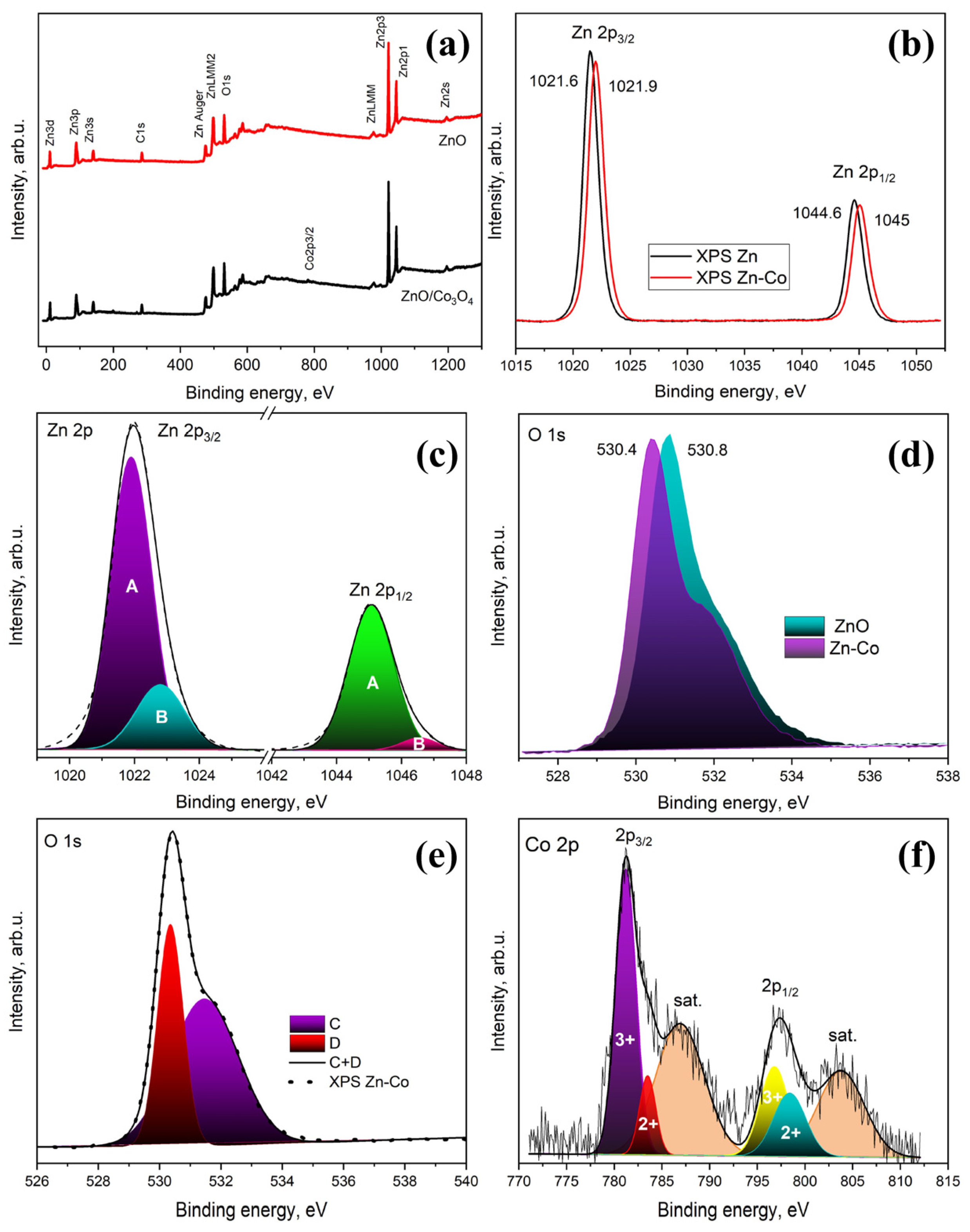
3. Material Characterization
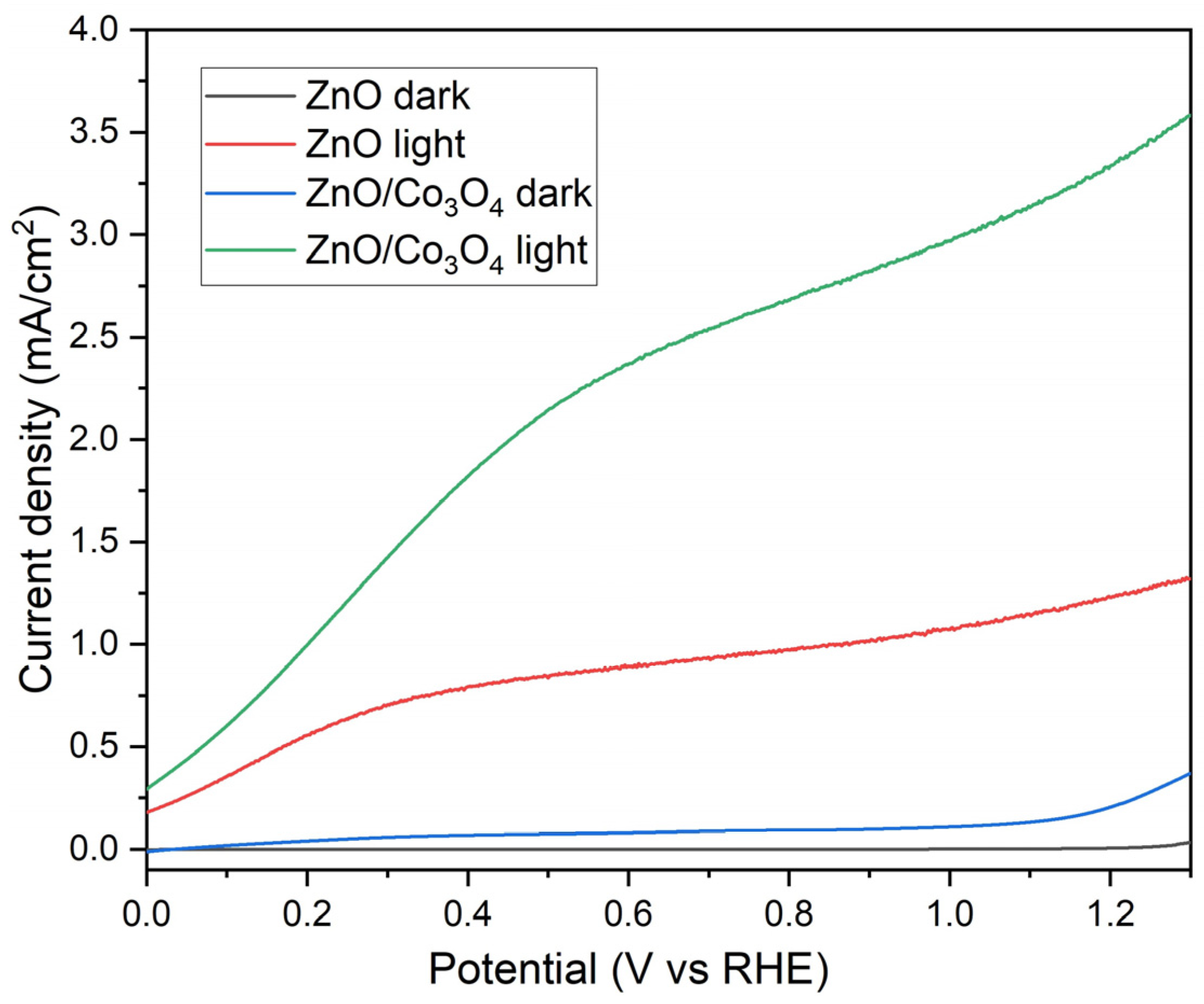
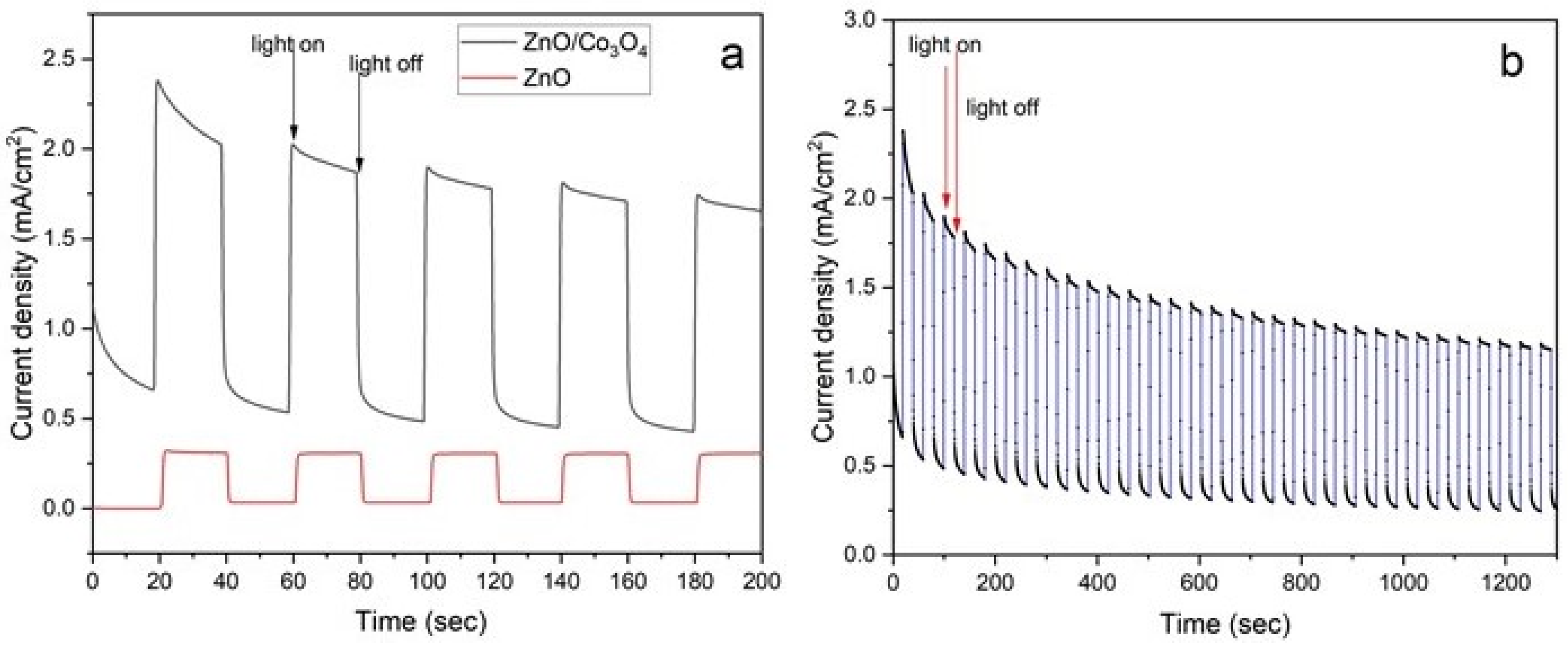
4. Photoelectrochemical Measurements
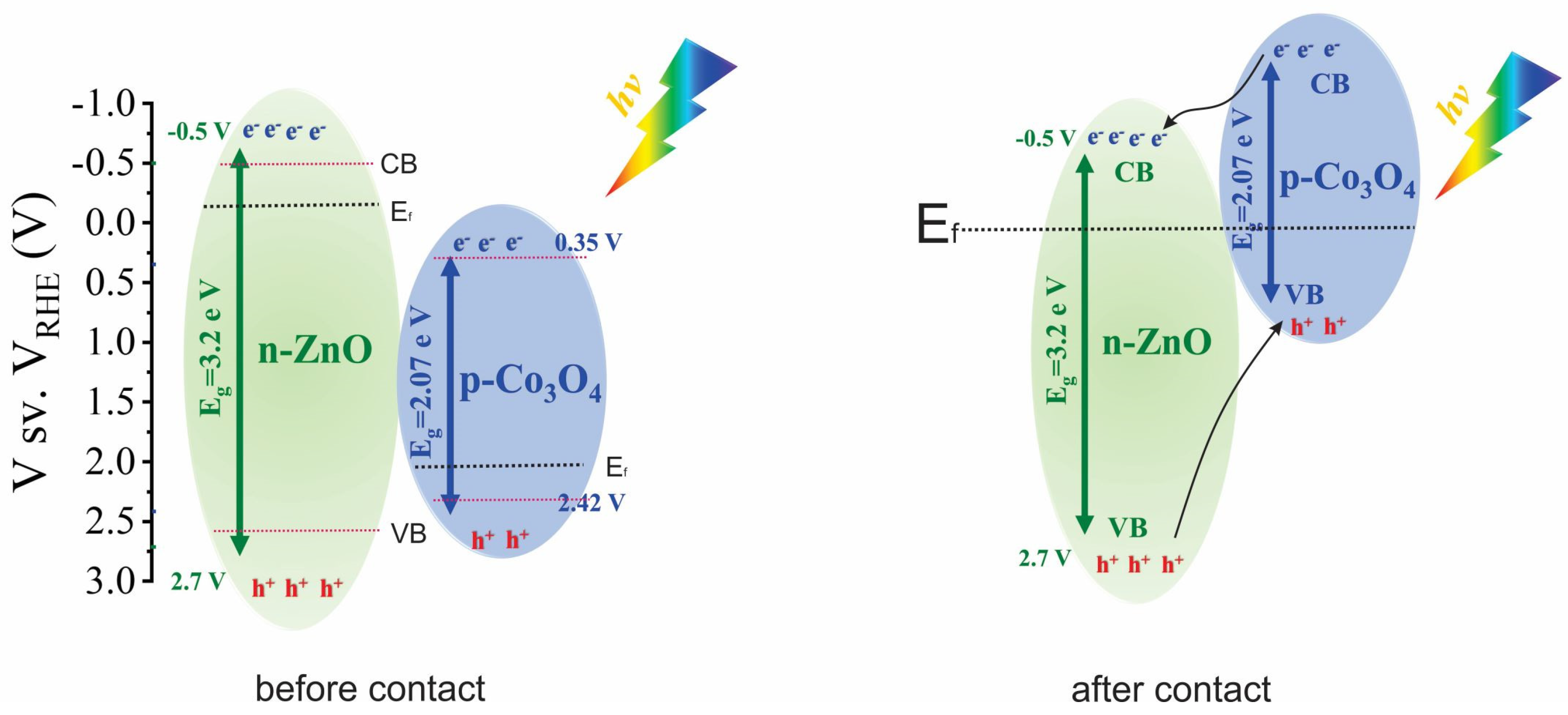
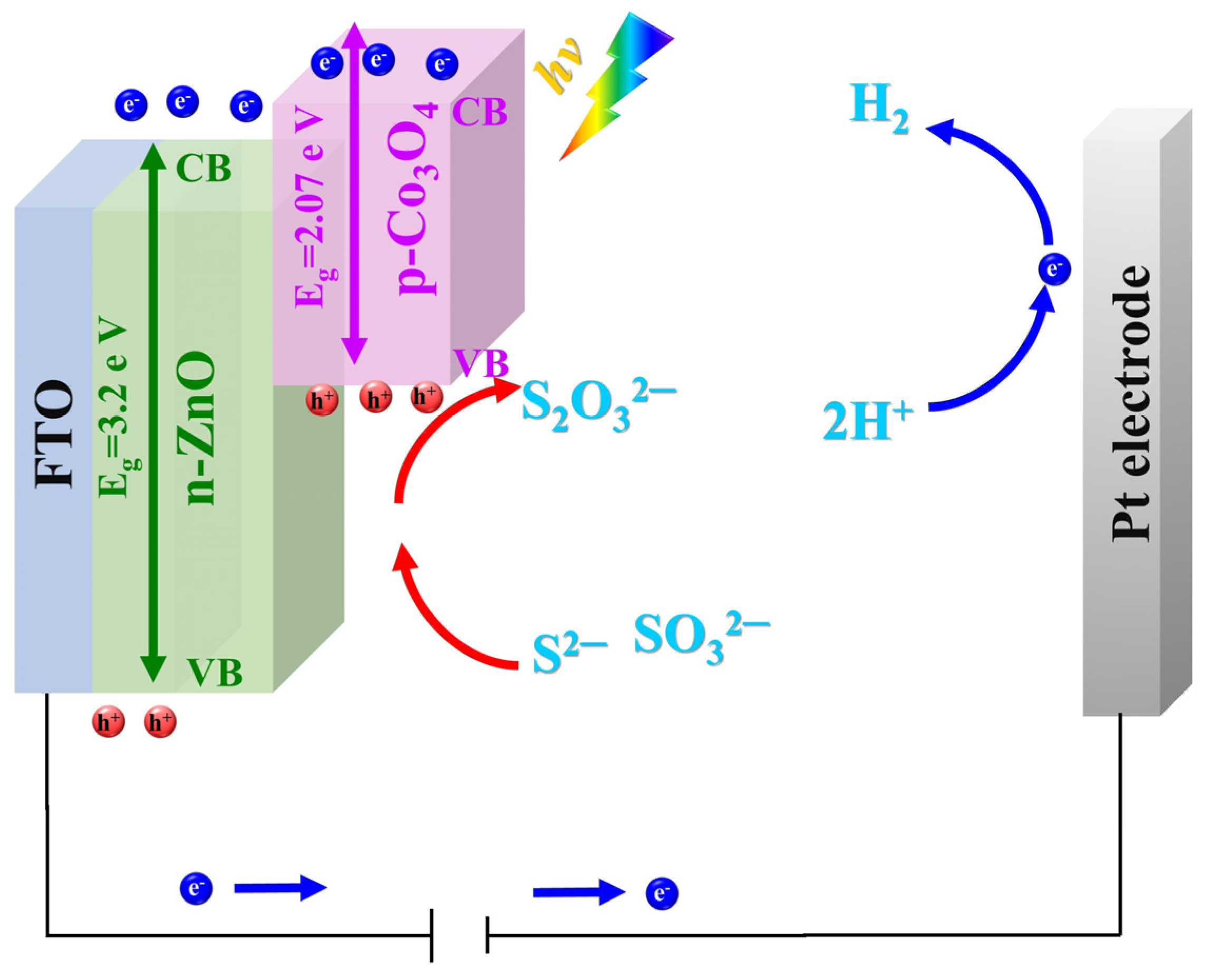
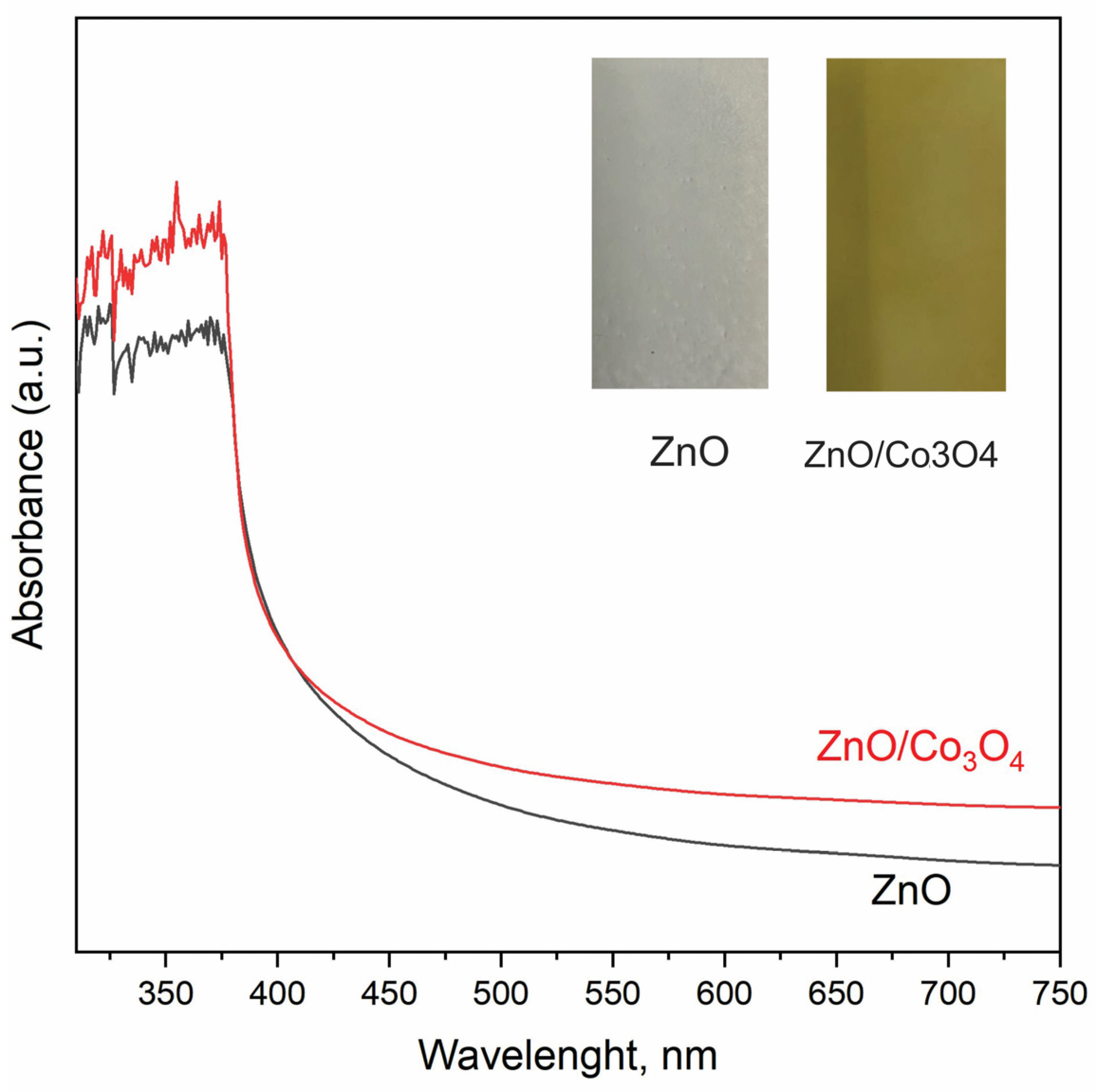
5. Results and Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Niu, Z.; Zhao, J.; Xue, L.; Fu, X.; Long, J. Recent advancements in photoelectrochemical water splitting for hydrogen production. Electrochem. Energy Rev. 2023, 6, 14. [Google Scholar] [CrossRef]
- Bozheyev, F. Transition metal dichalcogenide thin films for solar hydrogen production. Curr. Opin. Electrochem. 2022, 34, 100995. [Google Scholar] [CrossRef]
- Bozheyev, F.; Ellmer, K. Thin film transition metal dichalcogenide photoelectrodes for solar hydrogen evolution: A review. J. Mater. Chem. A 2022, 10, 9327–9347. [Google Scholar] [CrossRef]
- Bozheyev, F.; Akinoglu, E.M.; Wu, L.; Lu, H.; Nemkayeva, R.; Xue, Y.; Jin, M.; Giersig, M. Band gap optimization of tin tungstate thin films for solar water oxidation. Int. J. Hydrogen Energy 2020, 45, 8676–8685. [Google Scholar] [CrossRef]
- Mussabek, G.; Zhylkybayeva, N.; Baktygerey, S.; Yermukhamed, D.; Taurbayev, Y.; Sadykov, G.; Zaderko, A.N.; Lisnyak, V.V. Preparation and characterization of hybrid nanopowder based on nanosilicon decorated with carbon nanostructures. Appl. Nanosci. 2022, 13, 6709–6718. [Google Scholar] [CrossRef]
- Ravi, P.; Rao, V.N.; Shankar, M.; Sathish, M. CuOCr2O3 core-shell structured co-catalysts on TiO2 for efficient photocatalytic water splitting using direct solar light. Int. J. Hydrogen Energy 2018, 43, 3976–3987. [Google Scholar] [CrossRef]
- Tiwari, A.; Duvva, N.; Rao, V.N.; Venkatakrishnan, S.M.; Giribabu, L.; Pal, U. Tetrathiafulvalene scaffold-based sensitizer on hierarchical porous TiO2: Efficient light-harvesting material for hydrogen production. J. Phys. Chem. C 2018, 123, 70–81. [Google Scholar] [CrossRef]
- Xie, X.; Wang, R.; Ma, Y.; Chen, J.; Cui, Q.; Shi, Z.; Li, Z.; Xu, C. Photothermal-effect-enhanced photoelectrochemical water splitting in MXene-nanosheet-modified ZnO nanorod arrays. ACS Appl. Nano Mater. 2022, 5, 11150–11159. [Google Scholar] [CrossRef]
- Liccardo, L.; Lushaj, E.; Dal Compare, L.; Moretti, E.; Vomiero, A. Nanoscale ZnO/α-Fe2O3 Heterostructures: Toward Efficient and Low-Cost Photoanodes for Water Splitting. Small Sci. 2022, 2, 2100104. [Google Scholar] [CrossRef]
- Abdullin, K.A.; Gabdullin, M.T.; Gritsenko, L.; Ismailov, D.V.; Kalkozova, Z.K.; Kumekov, S.; Mukash, Z.O.; Sazonov, A.Y.; Terukov, E. Electrical, optical, and photoluminescence properties of ZnO films subjected to thermal annealing and treatment in hydrogen plasma. Semiconductors 2016, 50, 1010–1014. [Google Scholar] [CrossRef]
- Abdullin, K.A.; Gritsenko, L.; Kumekov, S.; Markhabaeva, A.; Terukov, E. Effect of heat and plasma treatments on the photoluminescence of zinc-oxide films. Semiconductors 2018, 52, 177–183. [Google Scholar] [CrossRef]
- Kedruk, Y.; Baigarinova, G.; Gritsenko, L.; Cicero, G.; Abdullin, K.A. Facile Low-Cost Synthesis of Highly Photocatalitycally Active Zinc Oxide Powders. Front. Mater. 2022, 9, 869493. [Google Scholar] [CrossRef]
- Rehman, S.; Ullah, R.; Butt, A.; Gohar, N. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Prikhodko, O.Y.; Aitzhanov, M.; Gusseinov, N.; Kalkozova, Z.K.; Dikhanbaev, K.; Markhabayeva, A.; Myrzabekova, M.; Nemkayeva, R.; Mukhametkarimov, Y.S. Photocatalytic activity of liquid-phase exfoliated gallium selenide flakes. Chalcogenide Lett. 2021, 18, 777–781. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Chen, C.-C. Degradation pathways of ethyl violet by photocatalytic reaction with ZnO dispersions. J. Mol. Catal. A Chem. 2007, 264, 82–92. [Google Scholar] [CrossRef]
- Kansal, S.; Singh, M.; Sud, D. Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts. J. Hazard. Mater. 2007, 141, 581–590. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M.B. Hexagonal ZnO nanorods assembled flowers for photocatalytic dye degradation: Growth, structural and optical properties. Superlattices Microstruct. 2013, 64, 495–506. [Google Scholar] [CrossRef]
- Harathi, N.; Bollu, M.; Pasupuleti, K.S.; Tauanov, Z.; Peta, K.R.; Kim, M.-D.; Reddeppa, M.; Sarkar, A.; Rao, V.N. PrGO decorated TiO2 nanoplates hybrid nanocomposite for augmented NO2 gas detection with faster gas kinetics under UV light irradiation. Sens. Actuators B Chem. 2022, 358, 131503. [Google Scholar] [CrossRef]
- Shahzad, S.; Javed, S.; Usman, M. A review on synthesis and optoelectronic applications of nanostructured ZnO. Front. Mater. 2021, 8, 613825. [Google Scholar] [CrossRef]
- Redkin, A.N.; Yakimov, E.E.; Evstafieva, M.V.; Yakimov, E.B. Grown and characterization of ZnO aligned nanorod arrays for sensor applications. Energies 2021, 14, 3750. [Google Scholar] [CrossRef]
- A Barzinjy, A. Synthesis, properties and uses of ZnO nanorods: A mini review. Int. Nano Lett. 2021, 12, 153–168. [Google Scholar]
- Nandanapalli, K.R.; Mudusu, D.; Yu, J.-S.; Lee, S. Stable and sustainable photoanodes using zinc oxide and cobalt oxide chemically gradient nanostructures for water-splitting applications. J. Colloid Interface Sci. 2020, 558, 9–20. [Google Scholar] [CrossRef]
- Tahira, A.; Ibupoto, Z.H.; Nafady, A.; Willander, M.; Nur, O. Efficient and Stable Co3O4/ZnO Nanocomposite for Photochemical Water Splitting. J. Clust. Sci. 2021, 33, 387–394. [Google Scholar] [CrossRef]
- Tien, T.-M.; Chen, E.L. A Novel ZnO/Co3O4 Nanoparticle for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts 2023, 13, 852. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, W.; Cheng, Y.F. Preparation of Co3O4@ZnO core-shell nanocomposites with intrinsic pn junction as high-performance photoelectrodes for photoelectrochemical cathodic protection under visible light. Appl. Surf. Sci. 2019, 476, 815–821. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Yang, B.; Zhou, Y.; Zhang, Z.; Wang, Y. Photo-deposition of ZnO/Co3O4 core-shell nanorods with pn junction for efficient oxygen evolution reaction. J. Solid State Electrochem. 2019, 23, 3287–3297. [Google Scholar] [CrossRef]
- Abdullin, K.A.; Zhumagulov, S.; Ismailova, G.; Kalkozova, Z.K.; Kudryashov, V.; Serikkanov, A. Synthesis of Heterogeneous ZnO/Co3O4 Nanostructures by Chemical Deposition from Solutions. Tech. Phys. 2020, 65, 1139–1143. [Google Scholar] [CrossRef]
- Xi, F.; Bozheyev, F.; Han, X.; Rusu, M.; Rappich, J.; Abdi, F.F.; Bogdanoff, P.; Kaltsoyannis, N.; Fiechter, S. Enhancing Hydrogen Evolution Reaction via Synergistic Interaction between the [Mo3S13] 2–Cluster Co-Catalyst and WSe2 Photocathode. ACS Appl. Mater. Interfaces 2022, 14, 52815–52824. [Google Scholar] [CrossRef]
- Markhabayeva, A.A.; Moniruddin, M.; Dupre, R.; Abdullin, K.A.; Nuraje, N. Designing of WO3@Co3O4 heterostructures to enhance photoelectrochemical performances. J. Phys. Chem. A 2019, 124, 486–491. [Google Scholar] [CrossRef]
- McPeak, K.M.; Le, T.P.; Britton, N.G.; Nickolov, Z.S.; Elabd, Y.A.; Baxter, J.B. Chemical bath deposition of ZnO nanowires at near-neutral pH conditions without hexamethylenetetramine (HMTA): Understanding the role of HMTA in ZnO nanowire growth. Langmuir 2011, 27, 3672–3677. [Google Scholar] [CrossRef] [PubMed]
- Ashfold, M.N.; Doherty, R.P.; Ndifor-Angwafor, N.G.; Riley, D.J.; Sun, Y. The kinetics of the hydrothermal growth of ZnO nanostructures. Thin Solid Film. 2007, 515, 8679–8683. [Google Scholar] [CrossRef]
- Kaphle, A.; Reed, T.; Apblett, A.; Hari, P. Doping efficiency in cobalt-doped ZnO nanostructured materials. J. Nanomater. 2019, 2019, 7034620. [Google Scholar] [CrossRef]
- Kalita, A.; Kalita, M.P. Effects of size reduction on microstructural, optical, vibrational, magnetic and photocatalytic properties of ZnO nanocrystals. Mater. Charact. 2018, 137, 109–118. [Google Scholar] [CrossRef]
- Abdullin, K.A.; Gabdullin, M.T.; Zhumagulov, S.K.; Ismailova, G.A.; Gritsenko, L.V.; Kedruk, Y.Y.; Mirzaeian, M. Stabilization of the surface of ZnO films and elimination of the aging effect. Materials 2021, 14, 6535. [Google Scholar] [CrossRef] [PubMed]
- Kaningini, A.G.; Azizi, S.; Sintwa, N.; Mokalane, K.; Mohale, K.C.; Mudau, F.N.; Maaza, M. Effect of Optimized Precursor Concentration, Temperature, and Doping on Optical Properties of ZnO Nanoparticles Synthesized via a Green Route Using Bush Tea (Athrixia phylicoides DC.) Leaf Extracts. ACS Omega 2022, 7, 31658–31666. [Google Scholar] [CrossRef]
- Lestari, A.; Iwan, S.; Djuhana, D.; Imawan, C.; Harmoko, A.; Fauzia, V. Effect of precursor concentration on the structural and optical properties of ZnO nanorods prepared by hydrothermal method. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; p. 020027. [Google Scholar]
- Horachit, C.; Moonnoi, S.; Ruankham, P.; Choopun, S.; Intaniwet, A. Effects of precursor concentration on hydrothermally grown ZnO nanorods as electron transporting layer in perovskite solar cells. Mater. Today Proc. 2019, 17, 1217–1223. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Kuryliszyn-Kudelska, I.; Hadžić, B.; Sibera, D.; Romčević, M.; Romčević, N.; Narkiewicz, U.; Łojkowski, W.; Arciszewska, M.; Dobrowolski, W. Magnetic properties of ZnO(Co) nanocrystals. J. Alloys Compd. 2013, 561, 247–251. [Google Scholar] [CrossRef]
- Diallo, A.; Beye, A.; Doyle, T.B.; Park, E.; Maaza, M. Green synthesis of Co3O4 nanoparticles via Aspalathus linearis: Physical properties. Green Chem. Lett. Rev. 2015, 8, 30–36. [Google Scholar] [CrossRef]
- Tortosa, M.; Manjón, F.; Mollar, M.; Marí, B. ZnO-based spinels grown by electrodeposition. J. Phys. Chem. Solids 2012, 73, 1111–1115. [Google Scholar] [CrossRef]
- Pawlak, D.A.; Ito, M.; Oku, M.; Shimamura, K.; Fukuda, T. Interpretation of XPS O (1s) in mixed oxides proved on mixed perovskite crystals. J. Phys. Chem. B 2002, 106, 504–507. [Google Scholar] [CrossRef]
- Li, M.; Meng, G.; Huang, Q.; Zhang, S. Improved sensitivity of polychlorinated-biphenyl-orientated porous-ZnO surface photovoltage sensors from chemisorption-formed ZnO-CuPc composites. Sci. Rep. 2014, 4, 4284. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.; Mahajan, P.; Mahajan, S.; Khosla, A.; Datt, R.; Gupta, V.; Young, S.-J.; Oruganti, S.K. influence of processing parameters to control morphology and optical properties of Sol-Gel synthesized ZnO nanoparticles. ECS J. Solid State Sci. Technol. 2021, 10, 023002. [Google Scholar] [CrossRef]
- Benrezgua, E.; Deghfel, B.; Zoukel, A.; Basirun, W.J.; Amari, R.; Boukhari, A.; Yaakob, M.K.; Kheawhom, S.; Mohamad, A.A. Synthesis and properties of copper doped zinc oxide thin films by sol-gel, spin coating and dipping: A characterization review. J. Mol. Struct. 2022, 1267, 133639. [Google Scholar] [CrossRef]
- Gong, H.; Cao, Y.; Zhang, Y.; Zhang, Y.; Liu, K.; Cao, H.; Yan, H. The synergetic effect of dual co-catalysts on the photocatalytic activity of square-like WO3 with different exposed facets. RSC Adv. 2017, 7, 19019–19025. [Google Scholar] [CrossRef]
- Ivanishcheva, A.P.; Sysoev, V.V.; Abdullin, K.A.; Nesterenko, A.V.; Khubezhov, S.A.; Petrov, V.V. The Application of Combined Visible and Ultraviolet Irradiation to Improve the Functional Characteristics of Gas Sensors Based on ZnO/SnO2 and ZnO/Au Nanorods. Chemosensors 2023, 11, 200. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Akter, M.S.; Haque, M.A.; Khan, G.A.; Alam, M.S. Synthesis of Co3O4/WO3 nanoheterojunction photocatalyst for the decomposition of organic pollutants under visible light irradiation. J. Clust. Sci. 2013, 24, 701–713. [Google Scholar] [CrossRef]
- Zhao, X.; Ji, H.; Jia, Q.; Wang, M. A nanoscale Co3O4–WO3 p–n junction sensor with enhanced acetone responsivity. J. Mater. Sci. Mater. Electron. 2015, 26, 8217–8223. [Google Scholar] [CrossRef]
- Prabhu, Y.T.; Rao, V.N.; Shankar, M.V.; Sreedhar, B.; Pal, U. The facile hydrothermal synthesis of CuO@ZnO heterojunction nanostructures for enhanced photocatalytic hydrogen evolution. New J. Chem. 2019, 43, 6794–6805. [Google Scholar] [CrossRef]
- Govatsi, K.; Antonelou, A.; Sygellou, L.; Neophytides, S.G.; Yannopoulos, S.N. Hybrid ZnO/MoS2 Core/Sheath Heterostructures for Photoelectrochemical Water Splitting. Appl. Nano 2021, 2, 148–161. [Google Scholar] [CrossRef]
- Kim, H.; Yong, K. Highly efficient photoelectrochemical hydrogen generation using a quantum dot coupled hierarchical ZnO nanowires array. ACS Appl. Mater. Interfaces 2013, 5, 13258–13264. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tovar, R.; Fernández-Domene, R.M.; Montañés, M.; Sanz-Marco, A.; Garcia-Anton, J. ZnO/ZnS heterostructures for hydrogen production by photoelectrochemical water splitting. RSC Adv. 2016, 6, 30425–30435. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Gao, R.; Zhang, Y.-C.; Pan, L.; Huang, C.; Liu, K.; Chang, X.-Y.; Zhang, X.; Zou, J.-J. Polarization-Enhanced direct Z-scheme ZnO-WO3−x nanorod arrays for efficient piezoelectric-photoelectrochemical Water splitting. Appl. Catal. B Environ. 2019, 259, 118079. [Google Scholar] [CrossRef]
- Cai, J.; Cao, J.; Tao, H.; Li, R.; Huang, M. Three-dimensional ZnO@TiO2 core-shell nanostructures decorated with plasmonic Au nanoparticles for promoting photoelectrochemical water splitting. Int. J. Hydrogen Energy 2021, 46, 36201–36209. [Google Scholar] [CrossRef]
- Hernández, S.; Cauda, V.; Chiodoni, A.; Dallorto, S.; Sacco, A.; Hidalgo, D.; Celasco, E.; Pirri, C.F. Optimization of 1D ZnO@TiO2 core–shell nanostructures for enhanced photoelectrochemical water splitting under solar light illumination. ACS Appl. Mater. Interfaces 2014, 6, 12153–12167. [Google Scholar] [CrossRef]
- Bagal, I.V.; Jun, S.; Choi, M.; Abdullah, A.; Waseem, A.; Ahn, S.; Kulkarni, M.A.; Cho, Y.-H.; Ryu, S.-W. Investigation of charge carrier dynamics in beaded ZnO nanowire decorated with SnS2/IrOx cocatalysts for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2023, 613, 156091. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, L.; Chen, C. Yttrium doping enhances the photoelectrochemical water splitting performance of ZnO nanorod array films. J. Alloys Compd. 2022, 896, 163144. [Google Scholar] [CrossRef]


| Metal Oxides | Electrolyte | Light Source | Photocurrent Enhancement at 1.23 V vs. RHE (Ma/Cm2) | |
|---|---|---|---|---|
| ZnO/Co3O4 | 0.5 M Na2S/Na2SO3 | Xe lamp 300 W | 3.46 | This work |
| ZnO/CoO | 0.03 M borax | Xe lamp 300 W | 1.26 | [23] |
| ZnO/WO3−x | 1 M Na2SO4 | Xe arc lamp 300 W | 2.39 | [55] |
| ZnO NSs-NRs@TiO2-Au | 0.5 M Na2SO4 | - | 1.73 | [56] |
| ZnO@TiO2 | 0.1 M NaOH | Xe lamp 450 W | 0.40 | [57] |
| SnS2/b-ZnO | 0.25 M Na2S/ 0.35 M Na2SO3 | Xe arc lamp 300 W | 0.58 | [58] |
| Y-ZnO | 0.1 M Na2SO4 | Xe arc lamp 500 W | 0.50 | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markhabayeva, A.A.; Kalkozova, Z.K.; Nemkayeva, R.; Yerlanuly, Y.; Anarova, A.S.; Tulegenova, M.A.; Tulegenova, A.T.; Abdullin, K.A. Construction of a ZnO Heterogeneous Structure Using Co3O4 as a Co-Catalyst to Enhance Photoelectrochemical Performance. Materials 2024, 17, 146. https://doi.org/10.3390/ma17010146
Markhabayeva AA, Kalkozova ZK, Nemkayeva R, Yerlanuly Y, Anarova AS, Tulegenova MA, Tulegenova AT, Abdullin KA. Construction of a ZnO Heterogeneous Structure Using Co3O4 as a Co-Catalyst to Enhance Photoelectrochemical Performance. Materials. 2024; 17(1):146. https://doi.org/10.3390/ma17010146
Chicago/Turabian StyleMarkhabayeva, Aiymkul A., Zhanar K. Kalkozova, Renata Nemkayeva, Yerassyl Yerlanuly, Assiya S. Anarova, Malika A. Tulegenova, Aida T. Tulegenova, and Khabibulla A. Abdullin. 2024. "Construction of a ZnO Heterogeneous Structure Using Co3O4 as a Co-Catalyst to Enhance Photoelectrochemical Performance" Materials 17, no. 1: 146. https://doi.org/10.3390/ma17010146
APA StyleMarkhabayeva, A. A., Kalkozova, Z. K., Nemkayeva, R., Yerlanuly, Y., Anarova, A. S., Tulegenova, M. A., Tulegenova, A. T., & Abdullin, K. A. (2024). Construction of a ZnO Heterogeneous Structure Using Co3O4 as a Co-Catalyst to Enhance Photoelectrochemical Performance. Materials, 17(1), 146. https://doi.org/10.3390/ma17010146







