Antimicrobial Cu-Doped TiO2 Coatings on the β Ti-30Nb-5Mo Alloy by Micro-Arc Oxidation
Abstract
:1. Introduction
2. Materials and Methods
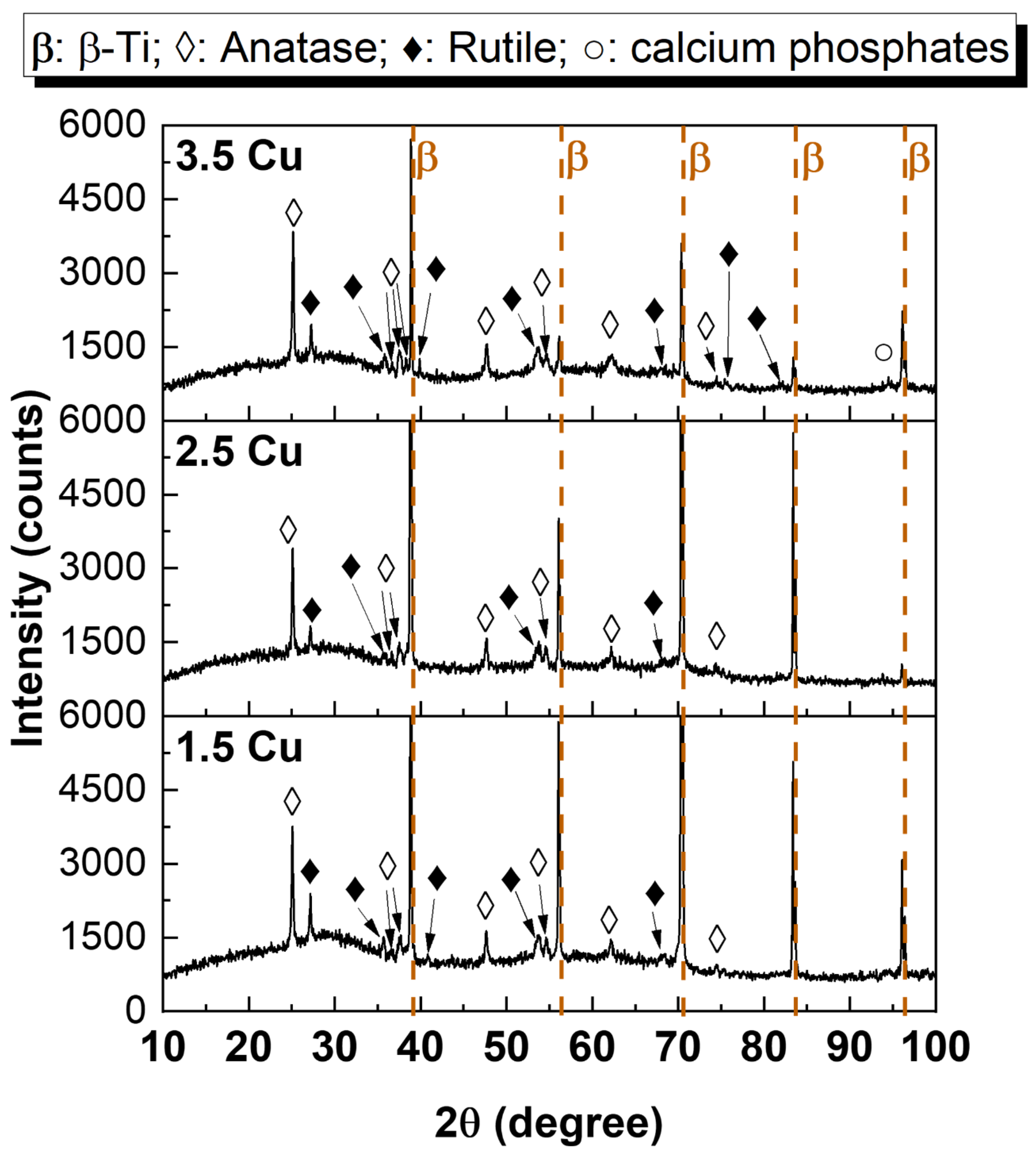
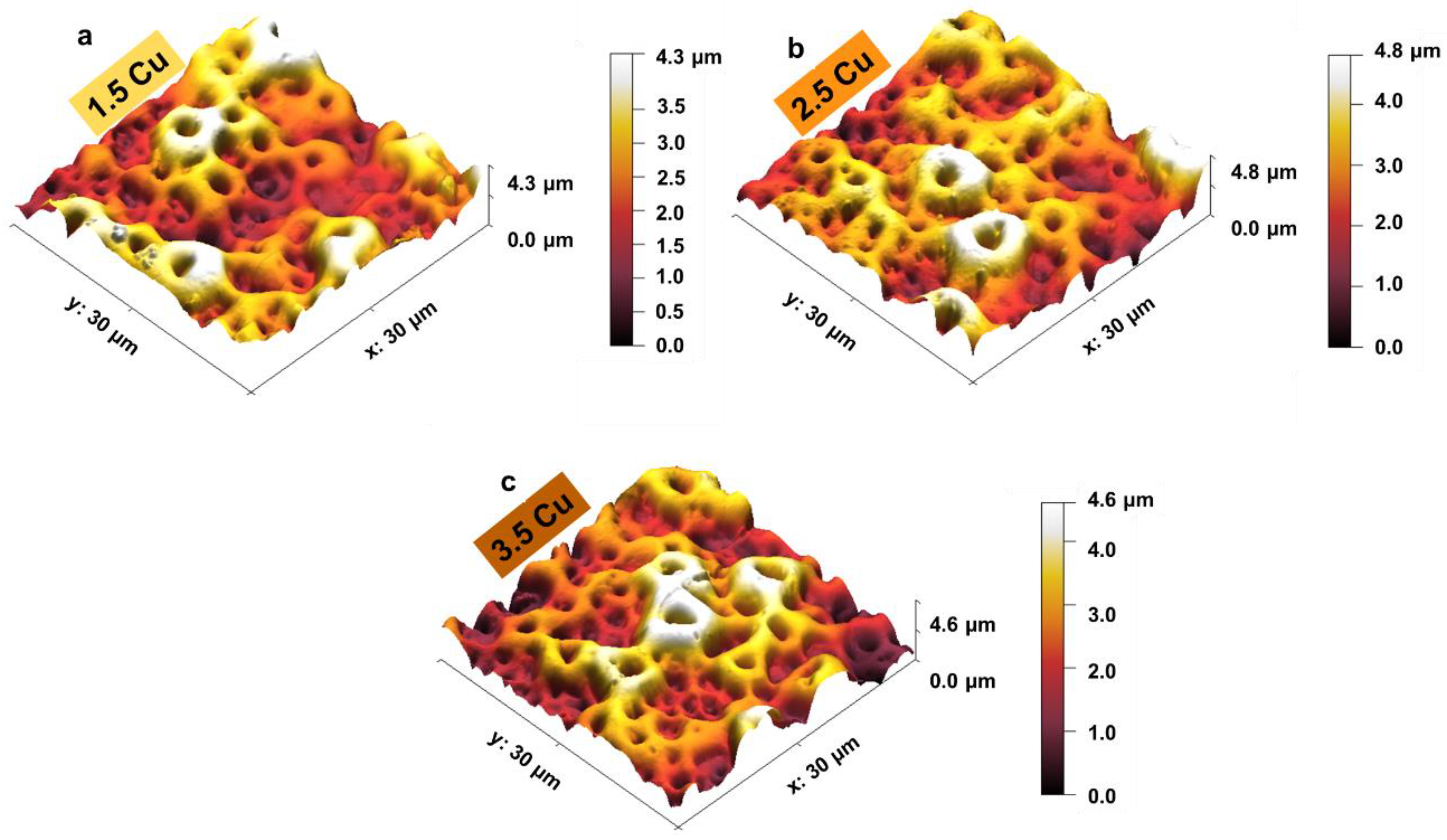
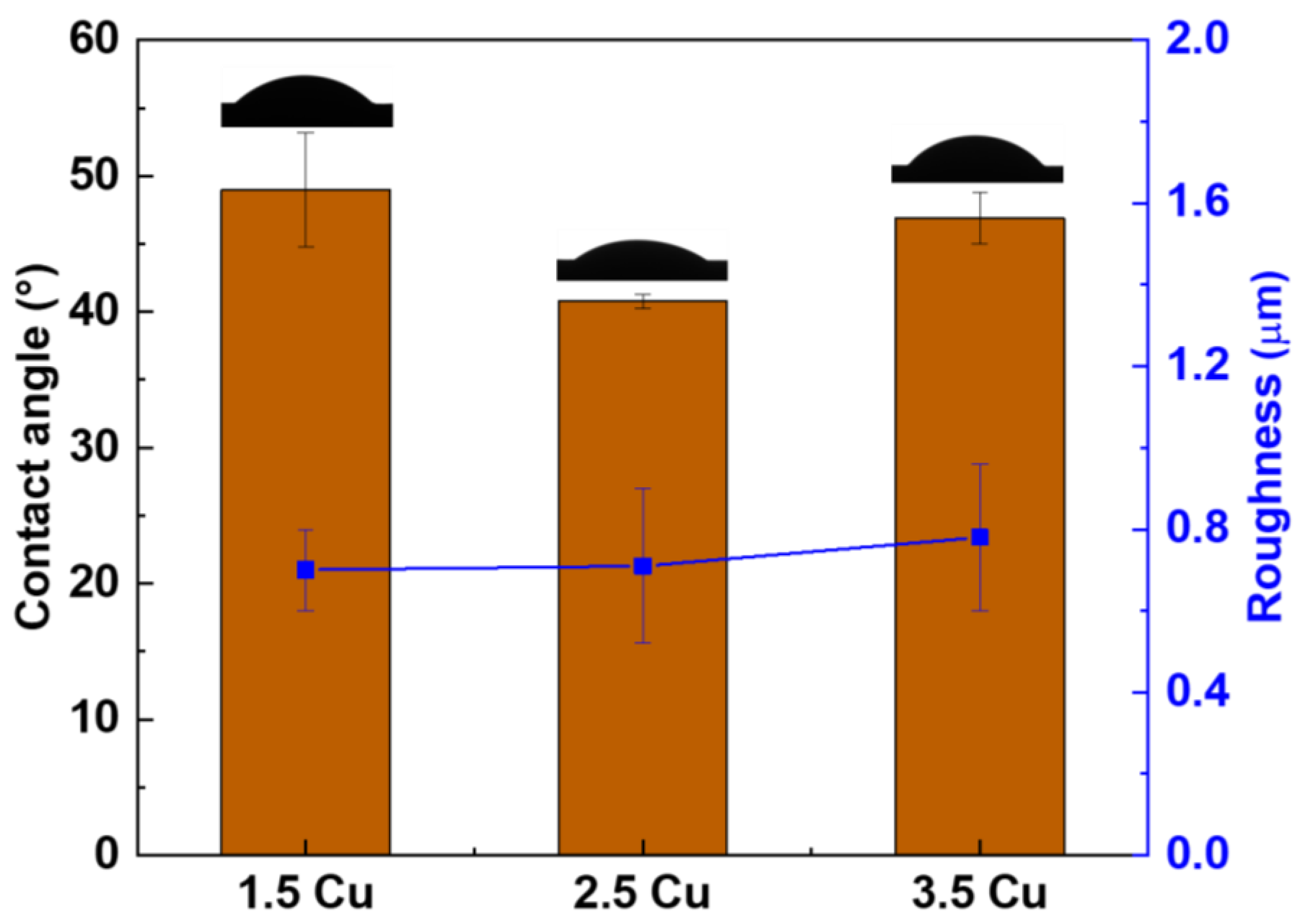
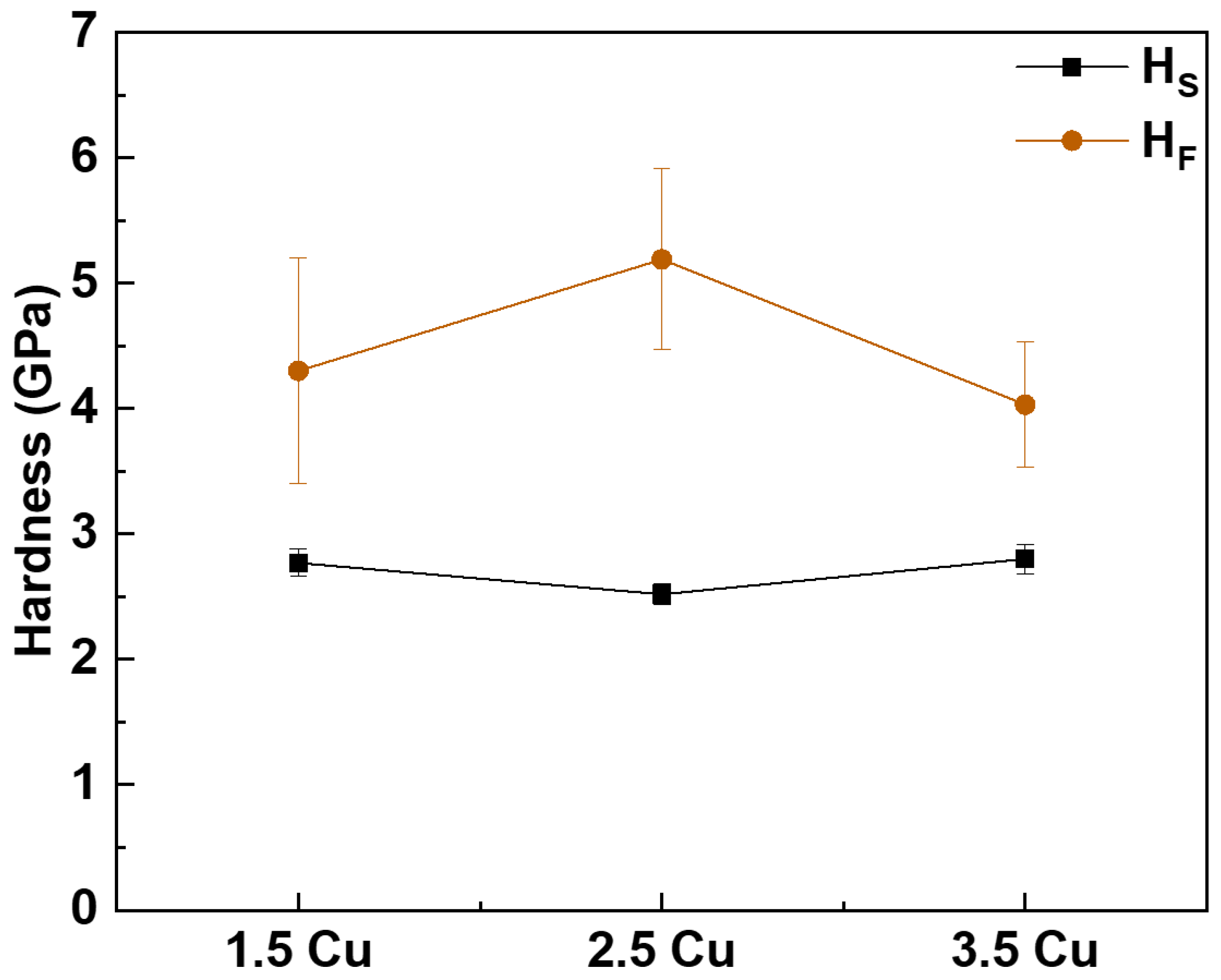
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Takeuchi, Y.; Imai, H.; Kawai, T.; Yoneyama, T. Application of titanium and titanium alloys to fixed dental prostheses. J. Prosthodont. Res. 2019, 63, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Cui, Y.-W.; Zhang, L.-C. Recent Development in Beta Titanium Alloys for Biomedical Applications. Metals 2020, 10, 1139. [Google Scholar] [CrossRef]
- Babilas, D.; Urbańczyk, E.; Sowa, M.; Maciej, A.; Korotin, D.M.; Zhidkov, I.S.; Basiaga, M.; Krok-Borkowicz, M.; Szyk-Warszyńska, L.; Pamuła, E.; et al. On the electropolishing and anodic oxidation of Ti-15Mo alloy. Electrochim. Acta 2016, 205, 256–265. [Google Scholar] [CrossRef]
- Inaekyan, K.; Brailovski, V.; Prokoshkin, S.; Pushin, V.; Dubinskiy, S.; Sheremetyev, V. Comparative study of structure formation and mechanical behavior of age-hardened Ti–Nb–Zr and Ti–Nb–Ta shape memory alloys. Mater. Charact. 2015, 103, 65–74. [Google Scholar] [CrossRef]
- Hussein, A.H.; Gepreel, M.A.H.; Gouda, M.K.; Hefnawy, A.M.; Kandil, S.H. Biocompatibility of new Ti–Nb–Ta base alloys. Mater. Sci. Eng. C 2016, 61, 574–578. [Google Scholar] [CrossRef]
- Wang, J.C.; Liu, Y.J.; Qin, P.; Liang, S.X.; Sercombe, T.B.; Zhang, L.C. Selective laser melting of Ti–35Nb composite from elemental powder mixture: Microstructure, mechanical behavior and corrosion behavior. Mater. Sci. Eng. A 2019, 760, 214–224. [Google Scholar] [CrossRef]
- Karre, R.; Niranjan, M.K.; Dey, S.R. First principles theoretical investigations of low Young’s modulus beta Ti–Nb and Ti–Nb–Zr alloys compositions for biomedical applications. Mater. Sci. Eng. C 2015, 50, 52–58. [Google Scholar] [CrossRef]
- Veríssimo, N.C.; Figueiredo, R.S.; de Oliveira, H.G.; Rodrigues, C.A.; Caram, R.; Bertazzoli, R. Characterization of the photoactivity of nanotube layers grown on Ti–35Nb and Ti–35Nb–4Sn alloys. J. Mater. Sci. 2016, 51, 9384–9393. [Google Scholar] [CrossRef]
- Cardoso, G.C.; Kuroda, P.A.B.; Grandini, C.R. Influence of Nb addition on the structure, microstructure, Vickers microhardness, and Young’s modulus of new β Ti-xNb-5Mo alloys system. J. Mater. Res. Technol. 2023, 25, 3061–3070. [Google Scholar] [CrossRef]
- Cardoso, G.C.; de Almeida, G.S.; Corrêa, D.O.G.; Zambuzzi, W.F.; Buzalaf, M.A.R.; Correa, D.R.N.; Grandini, C.R. Preparation and characterization of novel as-cast Ti-Mo-Nb alloys for biomedical applications. Sci. Rep. 2022, 12, 11874. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.M.; Nagay, B.E.; Ribeiro, A.L.R.; da Cruz, N.C.; Rangel, E.C.; Fais, L.M.G.; Vaz, L.G.; Barão, V.A.R. Functionalization of an experimental Ti-Nb-Zr-Ta alloy with a biomimetic coating produced by plasma electrolytic oxidation. J. Alloys Compd. 2019, 770, 1038–1048. [Google Scholar] [CrossRef]
- Rabadia, C.D.; Liu, Y.J.; Cao, G.H.; Li, Y.H.; Zhang, C.W.; Sercombe, T.B.; Sun, H.; Zhang, L.C. High-strength β stabilized Ti-Nb-Fe-Cr alloys with large plasticity. Mater. Sci. Eng. A 2018, 732, 368–377. [Google Scholar] [CrossRef]
- Sobolev, A.; Kossenko, A.; Borodianskiy, K. Study of the Effect of Current Pulse Frequency on Ti-6Al-4V Alloy Coating Formation by Micro Arc Oxidation. Materials 2019, 12, 3983. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.-J.; Choi, H.-R.; Song, H.-J.; Park, Y.-J. Characterization and colorization of microarc-oxidized layers of binary titanium alloys. J. Alloys Compd. 2018, 732, 95–106. [Google Scholar] [CrossRef]
- Saeed, E.M.; Dawood, N.M.; Hasan, S.F. Improvement corrosion resistance of Ni-Ti alloy by TiO2 coating and hydroxyaptite/TiO2 composite coating using micro arc oxidation process. Mater. Today Proc. 2021, 42, 2789–2796. [Google Scholar] [CrossRef]
- Gabor, R.; Doubkova, M.; Gorosova, S.; Malanik, K.; Vandrovcova, M.; Cvrcek, L.; Drobikova, K.; Mamulova Kutlakova, K.; Bacakova, L. Preparation of highly wettable coatings on Ti–6Al–4V ELI alloy for traumatological implants using micro-arc oxidation in an alkaline electrolyte. Sci. Rep. 2020, 10, 19780. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Wang, X.; Mei, L.; Jiang, X.; Jin, M.; Xu, Y.; Li, J.; Li, X.; Meng, Z.; Zhu, J.; Wu, F. Hydroxyapatite-Coated Titanium by Micro-Arc Oxidation and Steam–Hydrothermal Treatment Promotes Osseointegration. Front. Bioeng. Biotechnol. 2021, 9, 625877. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Mohedano, M.; Martinez-Campos, E.; Arrabal, R.; Pardo, A.; Matykina, E. Bioactive multi-elemental PEO-coatings on titanium for dental implant applications. Mater. Sci. Eng. C 2019, 97, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Fosca, M.; Streza, A.; Antoniac, I.V.; Vadalà, G.; Rau, J.V. Ion-Doped Calcium Phosphate-Based Coatings with Antibacterial Properties. J. Funct. Biomater. 2023, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, X.; Wu, H.; Tian, L.; Ma, Y.; Tang, B. Microstructure and antibacterial properties of Cu-doped TiO2 coating on titanium by micro-arc oxidation. Appl. Surf. Sci. 2014, 292, 944–947. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Lazoryak, B.I.; Davidova, G.A.; Murzakhanov, F.F.; Gabbasov, B.F.; Petrakova, N.V.; Fosca, M.; Barinov, S.M.; Vadalà, G.; Uskoković, V.; et al. Antibacterial and cell-friendly copper-substituted tricalcium phosphate ceramics for biomedical implant applications. Mater. Sci. Eng. C 2021, 129, 112410. [Google Scholar] [CrossRef] [PubMed]
- Prosolov, K.A.; Khimich, M.A.; Rau, J.V.; Lychagin, D.V.; Sharkeev, Y.P. Influence of oblique angle deposition on Cu-substituted hydroxyapatite nano-roughness and morphology. Surf. Coat. Technol. 2020, 394, 125883. [Google Scholar] [CrossRef]
- Rau, J.V.; Curcio, M.; Raucci, M.G.; Barbaro, K.; Fasolino, I.; Teghil, R.; Ambrosio, L.; De Bonis, A.; Boccaccini, A.R. Cu-Releasing Bioactive Glass Coatings and Their in Vitro Properties. ACS Appl. Mater. Interfaces 2019, 11, 5812–5820. [Google Scholar] [CrossRef]
- Hang, R.; Gao, A.; Huang, X.; Wang, X.; Zhang, X.; Qin, L.; Tang, B. Antibacterial activity and cytocompatibility of Cu–Ti–O nanotubes. J. Biomed. Mater. Res. Part. A 2014, 102, 1850–1858. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Wang, X.; Wang, Y.; Hang, R.; Huang, X.; Tang, B.; Chu, P.K. Effects of copper nanoparticles in porous TiO2 coatings on bacterial resistance and cytocompatibility of osteoblasts and endothelial cells. Mater. Sci. Eng. C 2018, 82, 110–120. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Ma, Y.; Lin, N.; Fan, A.; Tang, B. Bactericidal behavior of Cu-containing stainless steel surfaces. Appl. Surf. Sci. 2012, 258, 10058–10063. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Matsumoto, N.; Sato, K.; Yoshida, K.; Hashimoto, K.; Toda, Y. Preparation and characterization of β-tricalcium phosphate co-doped with monovalent and divalent antibacterial metal ions. Acta Biomater. 2009, 5, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, P.A.B.; Montes, T.H.; Rossi, M.C.; Grandini, C.R.; Afonso, C.R.M. Unraveling crystalline phases, morphology, hardness, and adhesion of PEO anodic coatings on Ti-15Nb alloy surface. Mater. Lett. 2024, 356, 135607. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Grandini, C.R.; Afonso, C.R.M. Anodic MAO coating formation on Ti-25Ta alloy. Mater. Lett. 2024, 354, 135377. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Liu, J.; Wang, L.; Tang, Y.; Wang, K. A review on magnesium alloys for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 953344. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, G.C.; Buzalaf, M.A.R.; Correa, D.R.N.; Grandini, C.R. Effect of Thermomechanical Treatments on Microstructure, Phase Composition, Vickers Microhardness, and Young’s Modulus of Ti-xNb-5Mo Alloys for Biomedical Applications. Metals 2022, 12, 788. [Google Scholar]
- Ribeiro, A.R.; Oliveira, F.; Boldrini, L.C.; Leite, P.E.; Falagan-Lotsch, P.; Linhares, A.B.R.; Zambuzzi, W.F.; Fragneaud, B.; Campos, A.P.C.; Gouvêa, C.P.; et al. Micro-arc oxidation as a tool to develop multifunctional calcium-rich surfaces for dental implant applications. Mater. Sci. Eng. C 2015, 54, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.G.; Ribeiro, A.R.; Perez, G.; Archanjo, B.S.; Gouvea, C.P.; Araújo, J.R.; Campos, A.P.C.; Kuznetsov, A.; Almeida, C.M.; Maru, M.M.; et al. Understanding growth mechanisms and tribocorrosion behaviour of porous TiO2 anodic films containing calcium, phosphorous and magnesium. Appl. Surf. Sci. 2015, 341, 1–12. [Google Scholar] [CrossRef]
- Sousa, T.S.P.; Costa, N.d.A.d.; Correa, D.R.N.; Rocha, L.A.; Grandini, C.R. Morphology, Crystalline Structure and Chemical Composition Of MAO Treated Ti-15Zr-Mo Surfaces Enriched with Bioactive Ions. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Rossi, M.C.; dos Santos, R.F.; Kuroda, P.A.B.; Afonso, C.R.M. Characteristics of ceramic-like coatings obtained by plasma electrolyte oxidation on different Ti alloys. Boletín Soc. Española Cerámica Vidr. 2023. [Google Scholar] [CrossRef]
- Jönsson, B.; Hogmark, S. Hardness measurements of thin films. Thin Solid. Film. 1984, 114, 257–269. [Google Scholar] [CrossRef]
- Cardoso, G.C.; Kuroda, P.A.B.; Grandini, C.R. A detailed analysis of the MAO TiO2 coating hardness using the Jönsson and Hogmark “law-of-mixtures” model. Mater. Lett. 2023, 352, 135208. [Google Scholar] [CrossRef]
- Rau, J.V.; Latini, A.; Teghil, R.; De Bonis, A.; Fosca, M.; Caminiti, R.; Rossi Albertini, V. Superhard Tungsten Tetraboride Films Prepared by Pulsed Laser Deposition Method. ACS Appl. Mater. Interfaces 2011, 3, 3738–3743. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Barinov, S.M.; Rau, J.V.; Latini, A.; Scandurra, R.; Brunetti, B. Vickers and Knoop hardness of electron beam deposited ZrC and HfC thin films on titanium. Surf. Coat. Technol. 2006, 200, 4701–4707. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Grandini, C.R.; Afonso, C.R.M. Surface Characterization of New β Ti-25Ta-Zr-Nb Alloys Modified by Micro-Arc Oxidation. Materials 2023, 16, 2352. [Google Scholar] [CrossRef] [PubMed]
- Dziaduszewska, M.; Shimabukuro, M.; Seramak, T.; Zielinski, A.; Hanawa, T. Effects of Micro-Arc Oxidation Process Parameters on Characteristics of Calcium-Phosphate Containing Oxide Layers on the Selective Laser Melted Ti13Zr13Nb Alloy. Coatings 2020, 10, 745. [Google Scholar] [CrossRef]
- Molaeipour, P.; Allahkaram, S.R.; Akbarzadeh, S. Corrosion inhibition of Ti6Al4V alloy by a protective plasma electrolytic oxidation coating modified with boron carbide nanoparticles. Surf. Coat. Technol. 2022, 430, 127987. [Google Scholar] [CrossRef]
- Wang, D.-d.; Liu, X.-t.; Wang, Y.; Zhang, Q.; Li, D.-l.; Liu, X.; Su, H.; Zhang, Y.; Yu, S.-x.; Shen, D. Role of the electrolyte composition in establishing plasma discharges and coating growth process during a micro-arc oxidation. Surf. Coat. Technol. 2020, 402, 126349. [Google Scholar] [CrossRef]
- Halliday, D.; Resnick, R.; Walker, J. Fundamentos de Física, 10th ed.; Editora Livros Técnicos e Científicos (LTC): Rio de Janeiro, Brazil, 2016; Volume 3. [Google Scholar]
- Duarte, L.T.; Bolfarini, C.; Biaggio, S.R.; Rocha-Filho, R.C.; Nascente, P.A.P. Growth of aluminum-free porous oxide layers on titanium and its alloys Ti-6Al-4V and Ti-6Al-7Nb by micro-arc oxidation. Mater. Sci. Eng. C 2014, 41, 343–348. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Jiang, D.; Jiao, Y.; Wu, Y.; Peng, Z.; Zhou, J.; Wu, J.; Dong, Z. Synthesis and characterization of a bi-functional hydroxyapatite/Cu-doped TiO2 composite coating. Ceram. Int. 2019, 45, 6693–6701. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Huang, X.; Zhang, Y.; Han, Y. The dual function of Cu-doped TiO2 coatings on titanium for application in percutaneous implants. J. Mater. Chem. B 2016, 4, 3788–3800. [Google Scholar] [CrossRef]
- Çaha, I.; Alves, A.C.; Affonço, L.J.; Lisboa-Filho, P.N.; da Silva, J.H.D.; Rocha, L.A.; Pinto, A.M.P.; Toptan, F. Corrosion and tribocorrosion behaviour of titanium nitride thin films grown on titanium under different deposition times. Surf. Coat. Technol. 2019, 374, 878–888. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Z.; Gu, B.; Sun, J.; Zhu, L. Biological Activity and Antibacterial Property of Nano-structured TiO2 Coating Incorporated with Cu Prepared by Micro-arc Oxidation. J. Mater. Sci. Technol. 2013, 29, 237–244. [Google Scholar] [CrossRef]
- Wang, S.; Deng, L.; Lv, Y.; Zhang, T.; Zhang, X.; Dong, Z.; Cai, G. Construction of antifouling Cu-modified TiO2 coating via micro-arc oxidation: The influence of Cu content. Surf. Coat. Technol. 2023, 454, 129197. [Google Scholar] [CrossRef]
- Kumari, P.; Saha, R.; Saikia, G.; Bhujel, A.; Choudhury, M.G.; Jagdale, P.; Paul, S. Synthesis of Mixed-Phase TiO2–ZrO2 Nanocomposite for Photocatalytic Wastewater Treatment. Toxics 2023, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.A.; Correa, D.R.N.; Lisboa-Filho, P.N.; Sousa, T.S.P.; Grandini, C.R.; Rocha, L.A. Influence of the molybdenum on characteristics of oxide films produced by micro-arc oxidation on Ti-15Zr-based alloys. Surf. Coat. Technol. 2021, 408, 126856. [Google Scholar] [CrossRef]
- Leśniak-Ziółkowska, K.; Kazek-Kęsik, A.; Rokosz, K.; Raaen, S.; Stolarczyk, A.; Krok-Borkowicz, M.; Pamuła, E.; Gołda-Cępa, M.; Brzychczy-Włoch, M.; Simka, W. Electrochemical modification of the Ti-15Mo alloy surface in solutions containing ZnO and Zn3(PO4)2 particles. Mater. Sci. Eng. C 2020, 115, 111098. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Cai, R.; Zhao, C.; Sun, J.; Zhang, J.; Matthews, D.T.A. Advancement of plasma electrolytic oxidation towards non-valve metals. Surf. Coat. Technol. 2022, 442, 128403. [Google Scholar] [CrossRef]
- Cardoso, G.C.; Barbaro, K.; Kuroda, P.A.B.; Imperatori, L.; De Bonis, A.; Teghil, R.; Curcio, M.; Innocenzi, E.; Grigorieva, V.Y.; Vadalà, G.; et al. Incorporation of Ca, P, Mg, and Zn Elements in Ti-30Nb-5Mo Alloy by Micro-Arc Oxidation for Biomedical Implant Applications: Surface Characterization, Cellular Growth, and Microorganisms’ Activity. Coatings 2023, 13, 1577. [Google Scholar]
- Zhang, X.; Peng, Z.; Lu, X.; Lv, Y.; Cai, G.; Yang, L.; Dong, Z. Microstructural evolution and biological performance of Cu-incorporated TiO2 coating fabricated through one-step micro-arc oxidation. Appl. Surf. Sci. 2020, 508, 144766. [Google Scholar] [CrossRef]
- Yang, J.; Fang, K.; Xu, K.; Shen, X.; Xu, X. Effect of zinc or copper doping on corrosion resistance and anti-oxidative stress of strontium-based micro-arc oxidation coatings on titanium. Appl. Surf. Sci. 2023, 626, 157229. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; de Mattos, F.N.; Grandini, C.R.; Afonso, C.R.M. Influence of the heat treatment temperature on the MAO coating produced in the Ti-25Ta-25Zr alloy. J. Mater. Res. Technol. 2023, 26, 3881–3892. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Rossi, M.C.; Grandini, C.R.; Afonso, C.R.M. Assessment of applied voltage on the structure, pore size, hardness, elastic modulus, and adhesion of anodic coatings in Ca-, P-, and Mg-rich produced by MAO in Ti-25Ta-Zr alloys. J. Mater. Res. Technol. 2023, 26, 4656–4669. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Yu, F.; Wang, D.; Guan, S.; Li, Y.; Qi, M. Characterization and cytocompatibility of hierarchical porous TiO2 coatings incorporated with calcium and strontium by one-step micro-arc oxidation. Mater. Sci. Eng. C 2020, 109, 110610. [Google Scholar] [CrossRef] [PubMed]
- Karbowniczek, J.; Muhaffel, F.; Cempura, G.; Cimenoglu, H.; Czyrska-Filemonowicz, A. Influence of electrolyte composition on microstructure, adhesion and bioactivity of micro-arc oxidation coatings produced on biomedical Ti6Al7Nb alloy. Surf. Coat. Technol. 2017, 321, 97–107. [Google Scholar] [CrossRef]
- Yang, W.; Xu, D.; Guo, Q.; Chen, T.; Chen, J. Influence of electrolyte composition on microstructure and properties of coatings formed on pure Ti substrate by micro arc oxidation. Surf. Coat. Technol. 2018, 349, 522–528. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; de Mattos, F.N.; Grandini, C.R.; Afonso, C.R.M. Effect of heat treatment on the phases, pore size, roughness, wettability, hardness, adhesion, and wear of Ti-25Ta MAO coatings for use as biomaterials. J. Mater. Sci. 2023, 58, 15485–15498. [Google Scholar] [CrossRef]
- Li, G.; Ma, F.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Review of micro-arc oxidation of titanium alloys: Mechanism, properties and applications. J. Alloys Compd. 2023, 948, 169773. [Google Scholar] [CrossRef]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; ul Hasan, M.M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Cady, N.C.; Behnke, J.L.; Strickland, A.D. Copper-Based Nanostructured Coatings on Natural Cellulose: Nanocomposites Exhibiting Rapid and Efficient Inhibition of a Multi-Drug Resistant Wound Pathogen, A. baumannii, and Mammalian Cell Biocompatibility In Vitro. Adv. Funct. Mater. 2011, 21, 2506–2514. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tsutsumi, Y.; Nozaki, K.; Chen, P.; Yamada, R.; Ashida, M.; Doi, H.; Nagai, A.; Hanawa, T. Investigation of antibacterial effect of copper introduced titanium surface by electrochemical treatment against facultative anaerobic bacteria. Dent. Mater. J. 2020, 39, 639–647. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices. Part 5: Test for Cytotoxicity: In Vitro Methods. International Organization for Standardization: Genebra, Sweden, 2005.
- Chen, H.; Cheng, D.-H.; Huang, S.-C.; Lin, Y.-M. Comparison of flexural properties and cytotoxicity of interim materials printed from mono-LCD and DLP 3D printers. J. Prosthet. Dent. 2021, 126, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Jablonská, E.; Kubásek, J.; Vojtěch, D.; Ruml, T.; Lipov, J. Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials. Sci. Rep. 2021, 11, 6628. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.K.; Alonso-Alonso, M.L.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Rull, F.; Medina, J.; Coco, R.M.; Pastor, J.C. Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci. Rep. 2018, 8, 1425. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tong, X.; Lin, J.; Wei, A.; Li, Y.; Dargusch, M.; Wen, C. Binary Zn–Ti alloys for orthopedic applications: Corrosion and degradation behaviors, friction and wear performance, and cytotoxicity. J. Mater. Sci. Technol. 2021, 74, 216–229. [Google Scholar] [CrossRef]
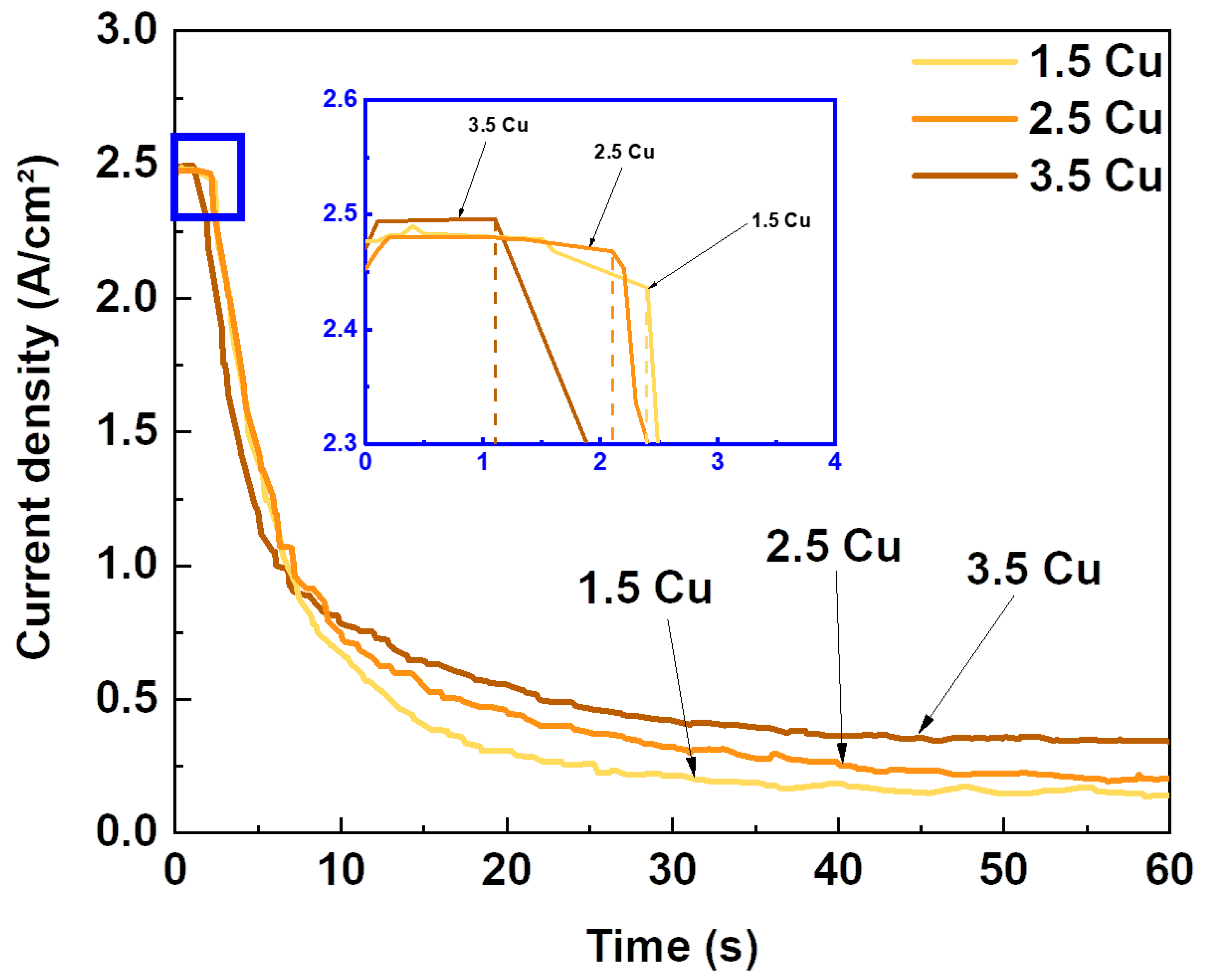
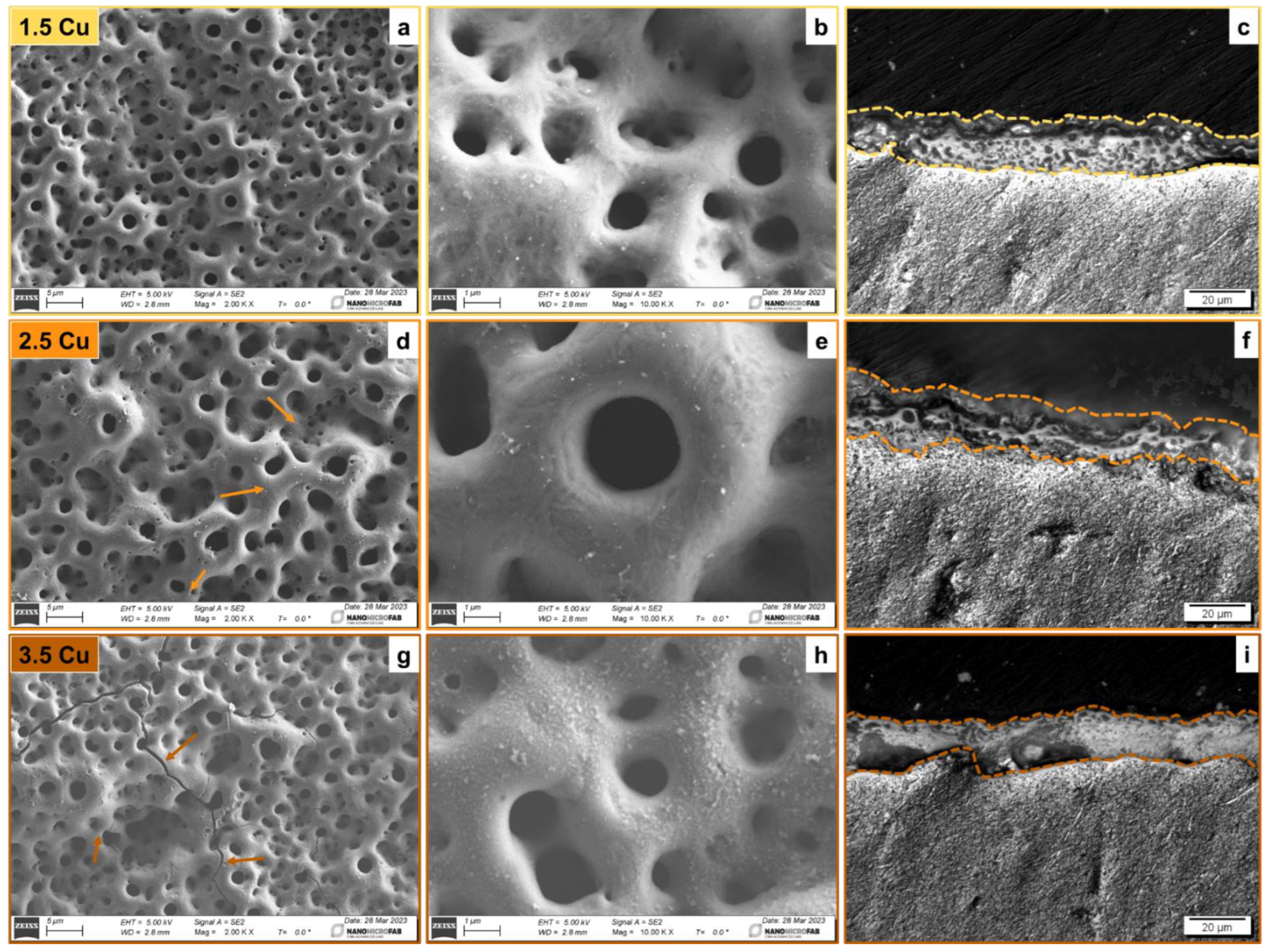
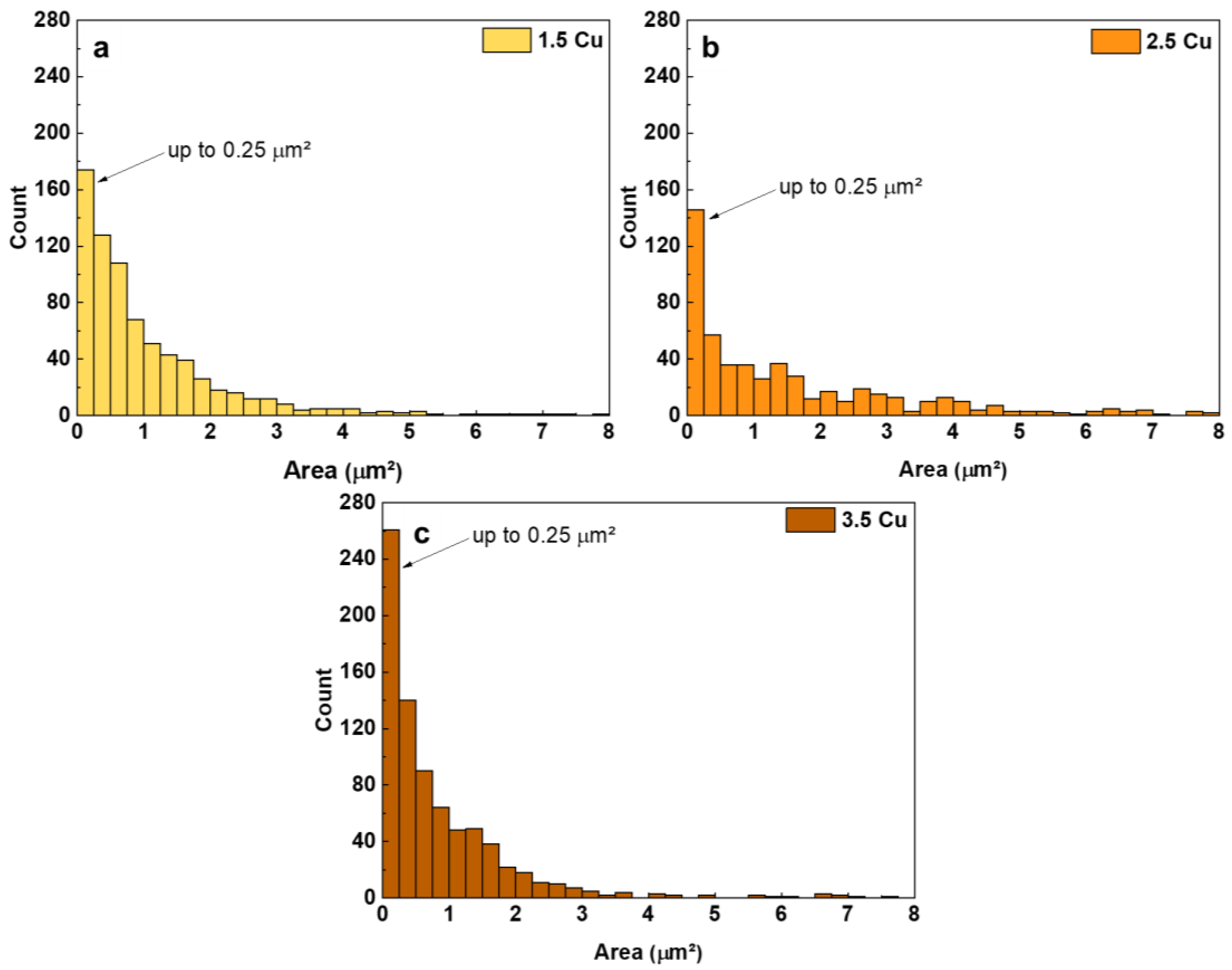
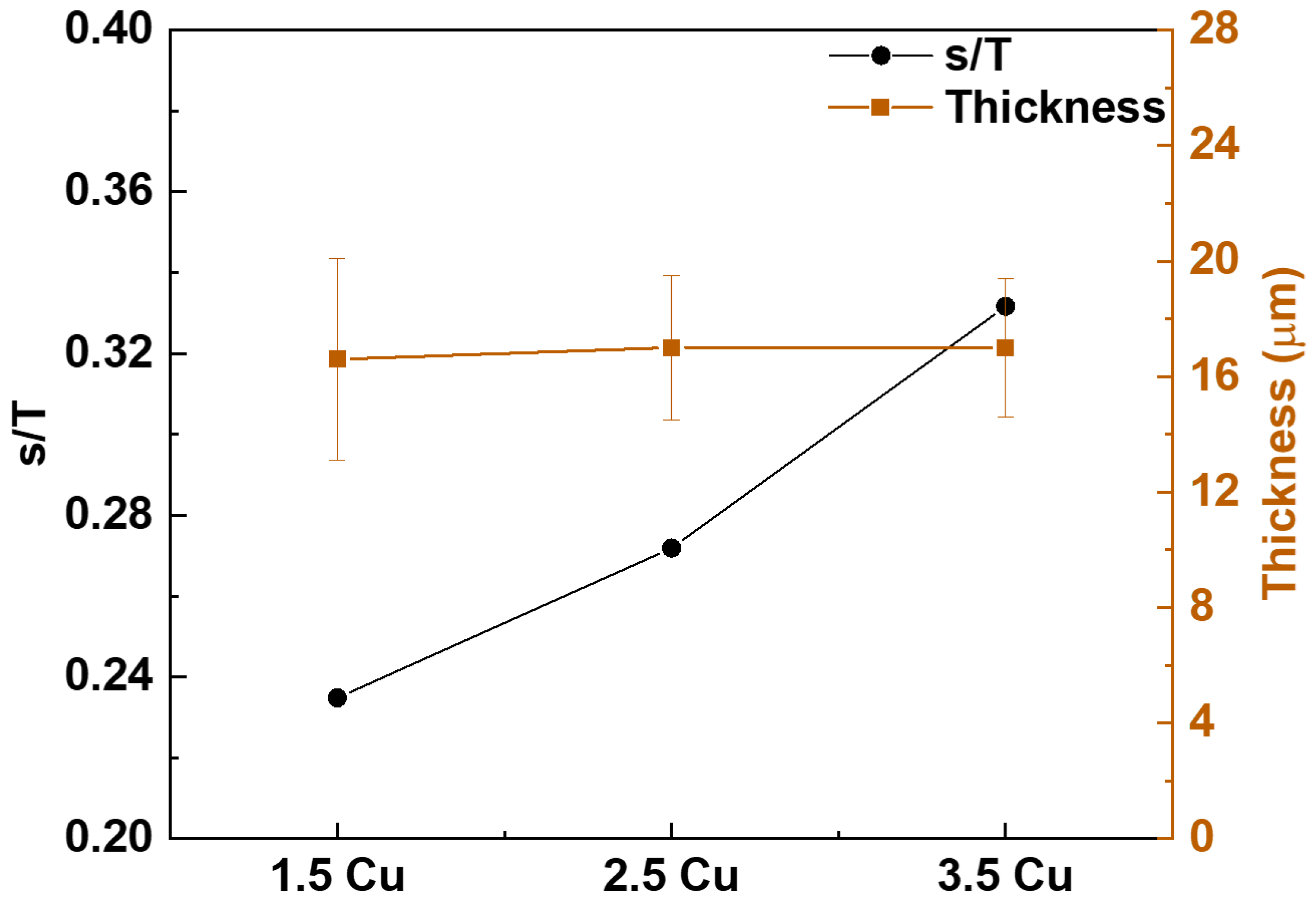




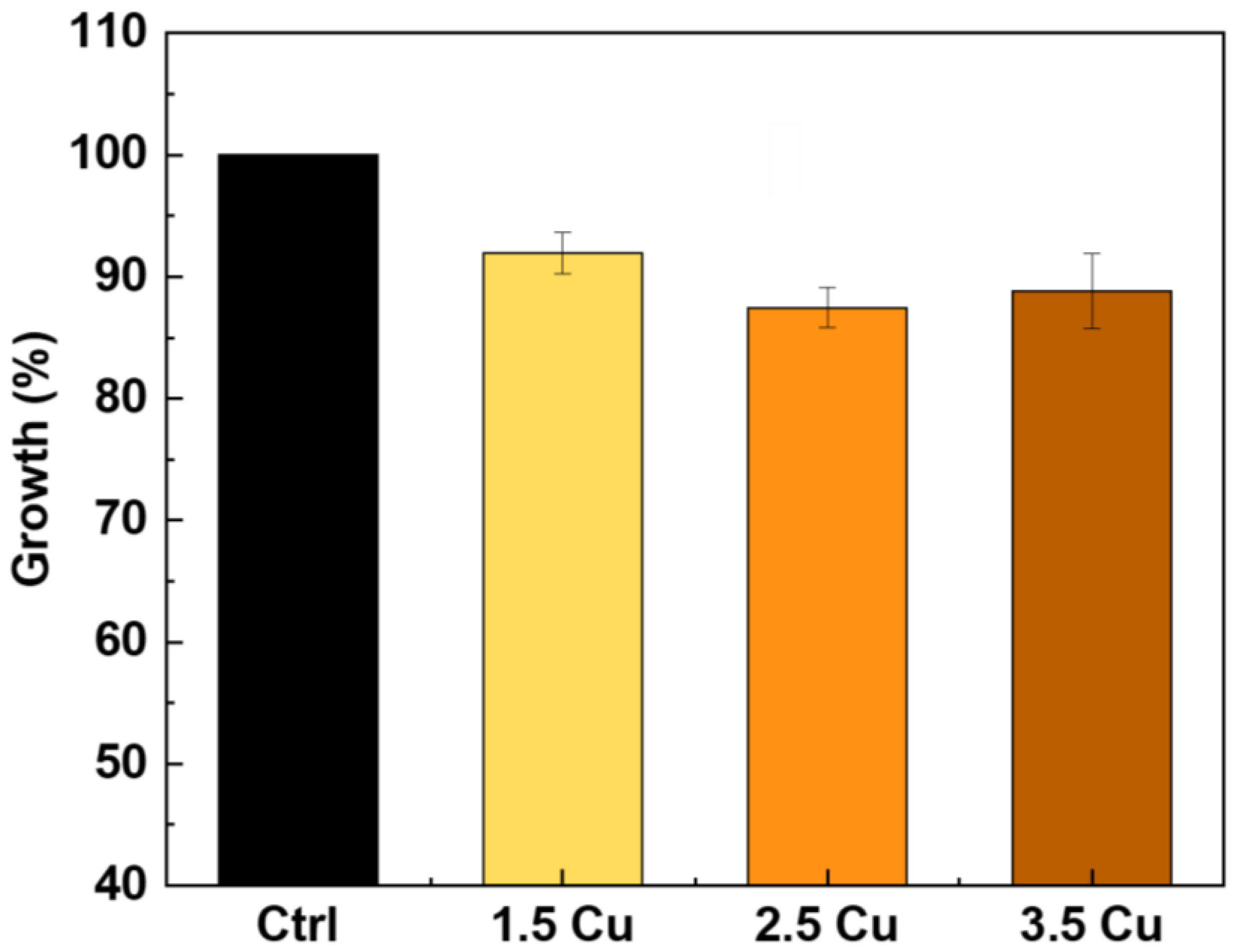
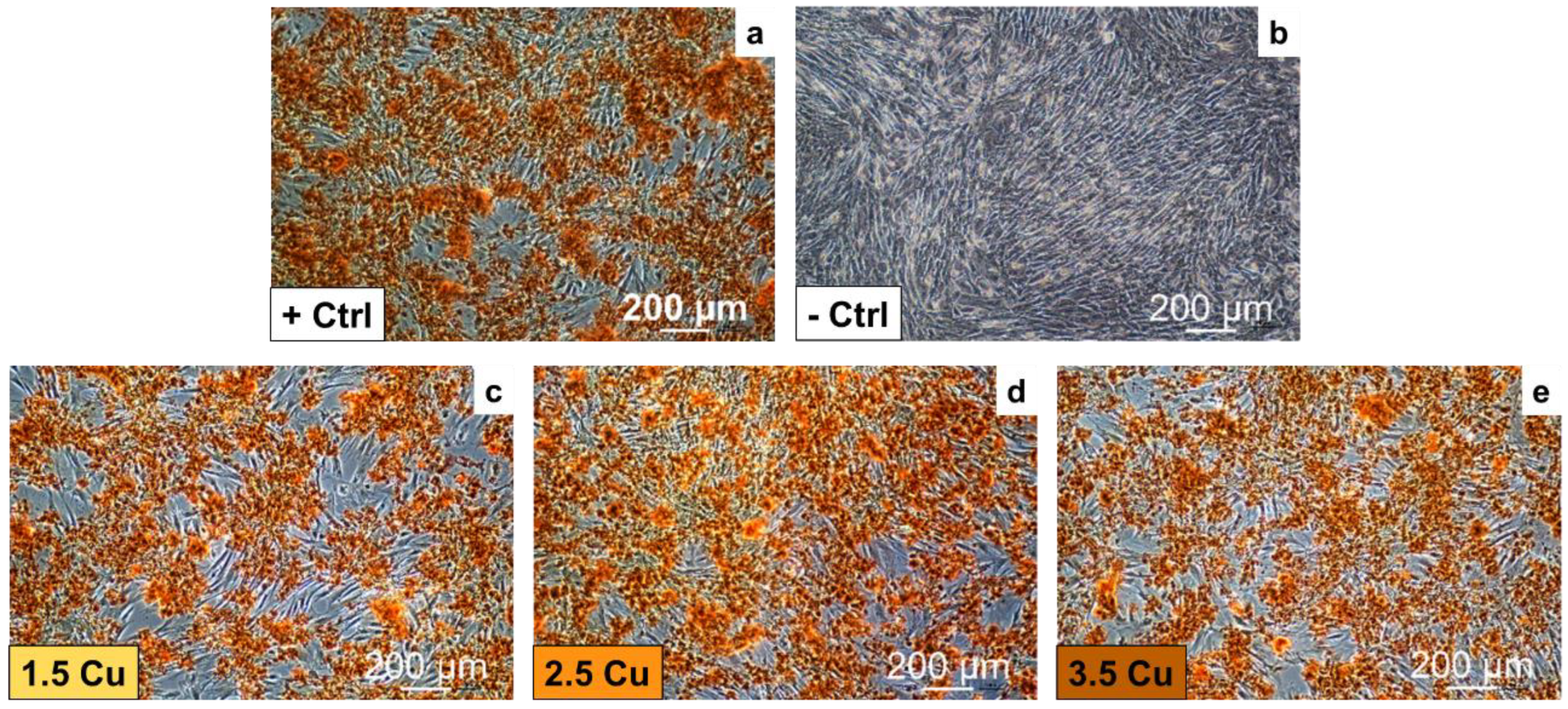
| Sample | Dielectric Breakdown (s) | Electric Charge Density of the Entire Process (C/cm²) |
|---|---|---|
| 1.5 Cu | 2.4 | 27.3 |
| 2.5 Cu | 2.1 | 32.6 |
| 3.5 Cu | 1.1 | 36.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, G.C.; Barbaro, K.; Kuroda, P.A.B.; De Bonis, A.; Teghil, R.; Krasnyuk, I.I., Jr.; Imperatori, L.; Grandini, C.R.; Rau, J.V. Antimicrobial Cu-Doped TiO2 Coatings on the β Ti-30Nb-5Mo Alloy by Micro-Arc Oxidation. Materials 2024, 17, 156. https://doi.org/10.3390/ma17010156
Cardoso GC, Barbaro K, Kuroda PAB, De Bonis A, Teghil R, Krasnyuk II Jr., Imperatori L, Grandini CR, Rau JV. Antimicrobial Cu-Doped TiO2 Coatings on the β Ti-30Nb-5Mo Alloy by Micro-Arc Oxidation. Materials. 2024; 17(1):156. https://doi.org/10.3390/ma17010156
Chicago/Turabian StyleCardoso, Giovana Collombaro, Katia Barbaro, Pedro Akira Bazaglia Kuroda, Angela De Bonis, Roberto Teghil, Ivan I. Krasnyuk, Jr., Luca Imperatori, Carlos Roberto Grandini, and Julietta V. Rau. 2024. "Antimicrobial Cu-Doped TiO2 Coatings on the β Ti-30Nb-5Mo Alloy by Micro-Arc Oxidation" Materials 17, no. 1: 156. https://doi.org/10.3390/ma17010156







