Possible Options for Utilization of EU Biomass Waste: Pyrolysis Char, Calorific Value and Ash Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Sample Preparation
2.3. Biochar Formation
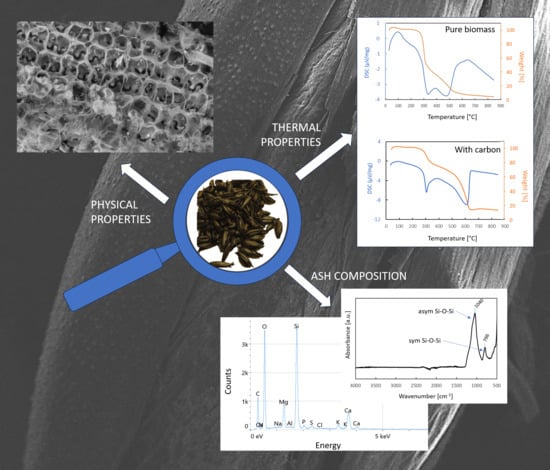
2.4. Sample Characterization
2.5. Single-Component Combustion and Co-Firing with Carbon
3. Results


4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Baum, R.; Wajszczuk, K.; Pepliński, B.; Wawrzynowicz, J. Potential for agricultural biomass production for Energy purposes in Poland: A review. Contemp. Econ. 2013, 7, 63–74. [Google Scholar] [CrossRef]
- Sahu, S.G.; Chakraborty, N.; Sarkar, P. Coal–biomass co-combustion: An overview. Renew. Sust. Energ. Rev. 2014, 39, 575–586. [Google Scholar] [CrossRef]
- Guo, H.; Cui, J.; Li, J. Biomass power generation in China: Status, policies and recommendations. Energy Reports 2022, 8, 687–696. [Google Scholar] [CrossRef]
- IDOM, 40 MW Biomass Power Plants in Fort St. James and Merritt, Canada. Available online: https://www.idom.com/en/project/40-mw-biomasa-power-plantas-in-fort-st-james-merrit-canada/ (accessed on 23 November 2023).
- Brown, M.A.; Favero, A.; Thomas, V.M.; Banboukian, A. The economic and environmental performance of biomass as an “intermediate” resource for power production. Utilities Policy 2019, 58, 52–62. [Google Scholar] [CrossRef]
- Agbor, E.; Zhang, X.; Kumar, A. A review of biomass co-firing in North America. Renew. Sust. Energ. Rev. 2014, 40, 930–943. [Google Scholar] [CrossRef]
- Chao, Y.H.; Kwong, P.C.W.; Wang, J.H.; Cheung, C.W.; Kendall, G. Co-firing coal with rice husk and bamboo and the impact on particulate matters and associated polycyclic aromatic hydrocarbon emissions. Bioresource Technol. 2008, 99, 83–93. [Google Scholar] [CrossRef]
- Yin, X.L.; Wu, C.Z.; Zheng, S.P.; Chen, Y. Design and operation of a CFB gasification and power generation system for rice husk. Biomass Bioener. 2002, 23, 181–187. [Google Scholar] [CrossRef]
- Baetge, S.; Kaltschmitt, M. Rice straw and rice husks as energy sources—Comparison of direct combustion and biogas production. Biomass Conver. Bioref. 2018, 8, 719–737. [Google Scholar] [CrossRef]
- Borkowska, H. Changes of dry matter content in Salix viminalis and Sida hermaphrodita yields of biomass depending on harvest date. Ann. UMCS 2005, 60, 155–161. [Google Scholar]
- Szczukowski, S.; Stolarski, M.; Tworkowski, J.; Rutkowski, P.; Goliński, P.; Mleczek, M.; Szentner, K. Yield and quality of biomass of selected willow species grown in a four-year harvest rotation. Fragm. Agron. 2014, 31, 107–114. [Google Scholar]
- Chew, K.W.; Chia, S.R.; Yen, H.-W.; Nomanbhay, S.; Ho, Y.C. Pau Loke Show Transformation of Biomass Waste into Sustainable Organic Fertilizers. Sustainability 2019, 11, 2266. [Google Scholar] [CrossRef]
- Santos, F.T.; Costa, M.S.S.M.; Costa, L.A.M.; Trindade, H.; Goufo, P. Application of Compost and Biochar Mixtures to Soils to Produce Parsley Plants Rich in Nutrients and Antioxidant Compounds. Chem. Proc. 2022, 10, 90. [Google Scholar]
- Gupta, S.; Kua, H. Factors determining the potential of biochar as a carbon capturing and sequestering construction material: Critical review. J. Mater. Civ. Eng. 2017, 29, 04017086. [Google Scholar] [CrossRef]
- Akhtar, A.; Sarmah, A.K. Novel biochar-concrete composites: Manufacturing, characterization and evaluation of the mechanical properties. Sci. Total Environ. 2018, 616, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedlacek, P.; Bielska, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Conver. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patra, B.R.; Podder, J.; Dalai, A.K. Synthesis of Biochar from Lignocellulosic Biomass for Diverse Industrial Applications and Energy Harvesting: Effects of Pyrolysis Conditions on the Physicochemical Properties of Biochar. Front. Mater. 2022, 9, 870184. [Google Scholar] [CrossRef]
- Maroušek, J.; Strunecký, O.; Stehel, V. Biochar farming: Defining economically perspective applications. Clean Technol. Environ. Policy 2019, 21, 1389–1395. [Google Scholar] [CrossRef]
- Meng, J.; He, T.; Sanganyado, E.; Lan, Y.; Zhang, W.; Han, X.; Chen, W. Development of the straw biochar returning concept in China. Biochar 2019, 1, 139–149. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Y.; Zhang, M.; Zhang, L.; Wu, W.; Huang, K. Magnetically-separable cobalt catalyst embedded in metal nitrate-promoted hierarchically porous N-doped carbon nanospheres for hydrodeoxygenation of lignin-derived species. Fuel 2023, 331, 125917. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Y.; Havener, K.; Zhang, M.; Zhang, L.; Wu, W.; Huang, K. Temperature-Controlled Selectivity of Hydrogenation and Hydrodeoxygenation of Biomass by Superhydrophilic Nitrogen/Oxygen Co-Doped Porous Carbon Nanosphere Supported Pd Nanoparticles. Small 2022, 18, 2106893. [Google Scholar] [CrossRef] [PubMed]
- Obernberger, I.; Dahl, J.; Brunner, T. Formation, Composition and Particle Size Distribution of Fly Ashes from biomass Combustion Plants. In Proceedings of the 4th Biomass Conference of the Americas, Oakland, CA, USA, 2 September 1999; Elsevier Science Ltd.: Amsterdam, The Netherlands; pp. 1377–1385. [Google Scholar]
- Commission Regulation (EU) No 742/2010 of 17 August 2010 Amending Regulation (EU) No 1272/2009 Laying down Common Detailed Rules the Implementation of Council Regulation (EC) No 1234/2007 as Regards Buying and Selling Agricultural Products under Public Intervention; Published by the Publication Office of the European Union, Aug. 17, 2010. Available online: https://op.europa.eu/en/publication-detail/-/publication/31e274f4-e43b-4fe6-9b0c-0387b7d36eb0/language-en (accessed on 29 November 2023).
- Tobiasz-Salach, R.; Stadnik, B.; Bajcar, M. Oat as a Potential Source of Energy. Energies 2023, 16, 6019. [Google Scholar] [CrossRef]
- González, M.E.; Osorio, L.R.-H.; González, A.; Hidalgo, P.; Meier, S.; Navia, R.; Cea, M. Oat hull biochar. BioResource 2017, 12, 2040–2057. [Google Scholar]
- Martínez-Toledo, C.; Valdés-Vidal, G.; Calabi-Floody, A.; González, M.E.; Reyes-Ortiz, O. Effect of Biochar from Oat Hulls on the Physical Properties of Asphalt Binder. Materials 2022, 15, 7000. [Google Scholar] [CrossRef]
- Tang, L.; Hamid, Y.; Zehra, A.; Sahito, Z.A.; He, Z.; Hussain, B.; Gurajala, H.K.; Yang, X. Characterization of fava bean (Vicia faba L.) genotypes for phytoremediation of cadmium and lead co-contaminated soils coupled with agro-production. Ecotoxic. Environ. Safety 2019, 171, 190–198. [Google Scholar] [CrossRef]
- Cazzato, E.; Tufarelli, V.; Ceci, E.; Stellacci, A.M.; Laudadio, V. Quality. yield and nitrogen fixation of faba bean seeds as affected by sulphur fertilization. Acta Agric. Scand. Sect. B Soil Plant Sci. 2012, 62, 732–738. [Google Scholar] [CrossRef]
- Maroušek, J.; Rowland, Z.; Valášková, K.; Král, P. Techno-economic assessment of potato waste management in developing economies. Clean Technol. Environ. Policy 2020, 22, 937–944. [Google Scholar] [CrossRef]
- Kot, A.M.; Pobiega, K.; Piwowarek, K.; Kieliszek, M.; Błażejak, S.; Gniewosz, M.; Lipińska, E. Biotechnological Methods of Management and Utilization of Potato Industry Waste—A Review. Potato Res. 2020, 63, 431–447. [Google Scholar] [CrossRef]
- Khanal, S.; Karimi, K.; Majumdar, S.; Kumar, V.; Verma, R.; Bhatia, S.K.; Kuca, K.; Esteban, J.; Kumar, D. Sustainable utilization and valorization of potato waste: State of the art, challenges, and perspectives. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- Garcia-Garcia, G.; Stone, J.; Rahimifard, S. Opportunities for waste valorisation in the food industry—A case study with four UK food manufacturers. J. Cleaner Produc. 2019, 211, 1339–1356. [Google Scholar] [CrossRef]
- Verma, M.; Loha, C.; Sinha, A.N.; Chatterjee, P.K. Drying of biomass for utilising in co-firing with coal and its impact on environment—A review. Renew. Sust. Energ. Rev. 2017, 71, 732–741. [Google Scholar] [CrossRef]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Biomass Proximate Analysis using Thermogravimetry. Biores. Technol. 2013, 139, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kazimierski, P.; Hercel, P.; Suchocki, T.; Smoliński, J.; Pladzyk, A.; Kardaś, D.; Łuczak, J.; Januszewicz, K. Pyrolysis of Pruning Residues from Various Types of Orchards and Pretreatment for Energetic Use of Biochar. Materials 2021, 14, 2969. [Google Scholar] [CrossRef]
- Manić, N.G.; Janković, B.Ž.; Stojiljković, D.D.; Jovanović, V.V.; Radojević, M.B. TGA-DSC-MS Analysis of Pyrolysis Process of Various Agricultural Residues. Therm. Sci. 2019, 23, 1457–1472. [Google Scholar] [CrossRef]
- Iwanek, E.; Glinski, M. Application of Thermal Analysis in Determining Properties of Herbaceous Materials. J. Chem. Educ. 2018, 95, 1359–1364. [Google Scholar] [CrossRef]
- Seletnik, B.; Puchalski, C. Suitability of Biochar and Biomass Ash in Basket Willow (Salix viminalis L.) Cultivation. Agronomy 2019, 9, 577. [Google Scholar] [CrossRef]
- Saletnik, B.; Zaguła, G.; Saletnik, A.; Bajcar, M.; Puchalski, C. Biochar and Ash Fertilization Alter the Chemical Properties of Basket Willow (Salix viminalis L.) and Giant Miscanthus (Miscanthus x giganteus). Agronomy 2020, 10, 660. [Google Scholar] [CrossRef]
- Iwanek, E.M.; Kirk, D.W. Application of Slow Pyrolysis to Convert Waste Plastics from a Compost-Reject Stream into Py-Char. Energies 2022, 15, 3072. [Google Scholar] [CrossRef]
- Odeh, A.O. Oualitative and quantitative ATR-FTIR analysis and its application to coal char of different ranks. J. Fuel Chem. Technol. 2015, 43, 129–137. [Google Scholar] [CrossRef]
- Abong, G.O.; Okoth, M.W.; Karuri, E.G.; Kabira, J.N.; Mathooko, F.M. Nutrient contents of raw and processed products from Kenyan potato cultivars. J. Appl. Biosci. 2009, 16, 877–886. [Google Scholar]
- Abbasi, K.S.; Qayyum, A.; Mehmood, A.; Mahmood, T.; Khan, S.U.; Liaquat, M.; Sohail, A.; Ahmad, A. Analysis of selective potato varieties and their functional assessment. Food Sci. Technol. Camp. 2019, 39, 308–314. [Google Scholar] [CrossRef]
- Betancur, Y.; Sánchez, A.; Bueno-López, A.; López, D. Potassium Catalytic Effect on Gasification Reactions of Coal and Coal/Biomass Blends under Oxy-combustion Conditions. An Isotopic Study Using 13C18O2. Energy Fuels 2018, 32, 2439–2449. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, B.; Zhu, J.; Wang, Z.; Ren, C.; Xie, H.; Guan, H.; Zhu, D. Research on Properties of Ash and Slag Composite Cementitious Materials for Biomass Power Plants. Processes 2023, 11, 1627. [Google Scholar] [CrossRef]
- Jaworska, B.; Stańczak, D.; Tarańska, J.; Jaworski, J. The Influence of Cement Substitution by Biomass Fly Ash on the Polymer–Cement Composites Properties. Materials 2021, 14, 3079. [Google Scholar] [CrossRef]








| Biomass | Char + Ash (Pyrolytic Reactor) | Char + Ash (Muffle Furnace) | Ash |
|---|---|---|---|
| Basket willow | 21.4 | 22.3 | 5.8 |
| Parsley root peels | 32.3 | 33.8 | 4.4 |
| Oats | 50.4 | 71.2 | 2.7 |
| Potato peels | 38.1 | 36.3 | 9.1 |
| Field beans | 35.4 | 40.1 | 3.6 |
| Hay | 33.7 | 33.4 | 7.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwanek, E.M.; Nietrzeba, U.; Pietras, M.; Marciniak, A.; Głuski, G.; Hupka, J.; Szymajda, M.; Kamiński, J.; Szerewicz, C.; Goździk, A.; et al. Possible Options for Utilization of EU Biomass Waste: Pyrolysis Char, Calorific Value and Ash Content. Materials 2024, 17, 226. https://doi.org/10.3390/ma17010226
Iwanek EM, Nietrzeba U, Pietras M, Marciniak A, Głuski G, Hupka J, Szymajda M, Kamiński J, Szerewicz C, Goździk A, et al. Possible Options for Utilization of EU Biomass Waste: Pyrolysis Char, Calorific Value and Ash Content. Materials. 2024; 17(1):226. https://doi.org/10.3390/ma17010226
Chicago/Turabian StyleIwanek (nee Wilczkowska), Ewa M., Urszula Nietrzeba, Marta Pietras, Aleksandra Marciniak, Gustaw Głuski, Jakub Hupka, Miłosz Szymajda, Jakub Kamiński, Cezary Szerewicz, Aleksandra Goździk, and et al. 2024. "Possible Options for Utilization of EU Biomass Waste: Pyrolysis Char, Calorific Value and Ash Content" Materials 17, no. 1: 226. https://doi.org/10.3390/ma17010226
APA StyleIwanek, E. M., Nietrzeba, U., Pietras, M., Marciniak, A., Głuski, G., Hupka, J., Szymajda, M., Kamiński, J., Szerewicz, C., Goździk, A., & Kirk, D. W. (2024). Possible Options for Utilization of EU Biomass Waste: Pyrolysis Char, Calorific Value and Ash Content. Materials, 17(1), 226. https://doi.org/10.3390/ma17010226