Microcrystallization Effects in Borosilicate Bioactive Glasses: Controllable Release of Bioactive Elements and In Vitro Degradation Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Microcrystallized Borosilicate Bioactive Glass
2.2. Sample Characterization
2.3. In Vitro Degradation and Mineralization
2.3.1. Degradation
2.3.2. Mineralization
2.4. Cell Proliferation and Cell Viability
2.4.1. Cell Culture
2.4.2. Material Extracts
2.4.3. Cell Proliferation and Viability Assay
2.4.4. Statistical Analysis
3. Results and Discussion
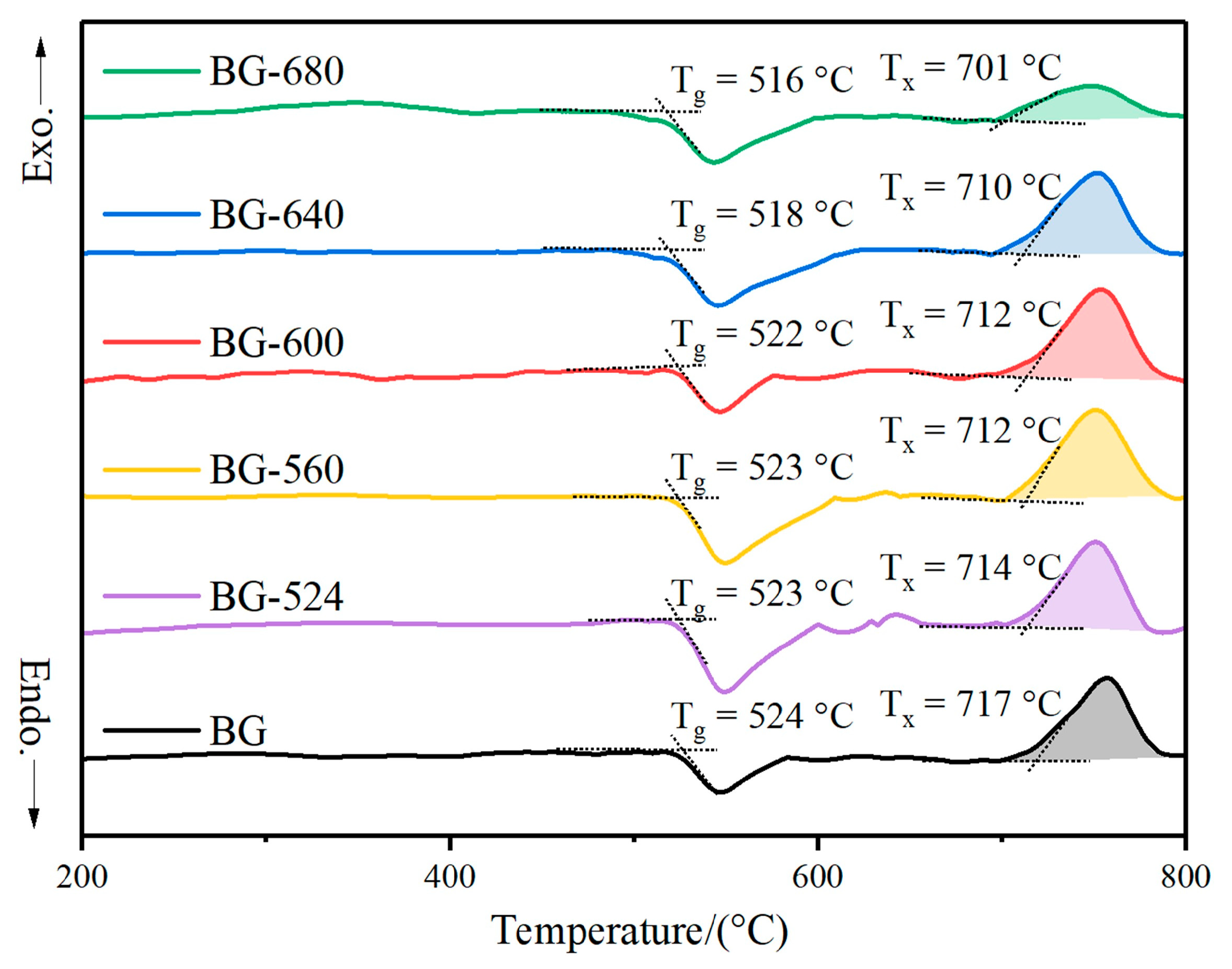
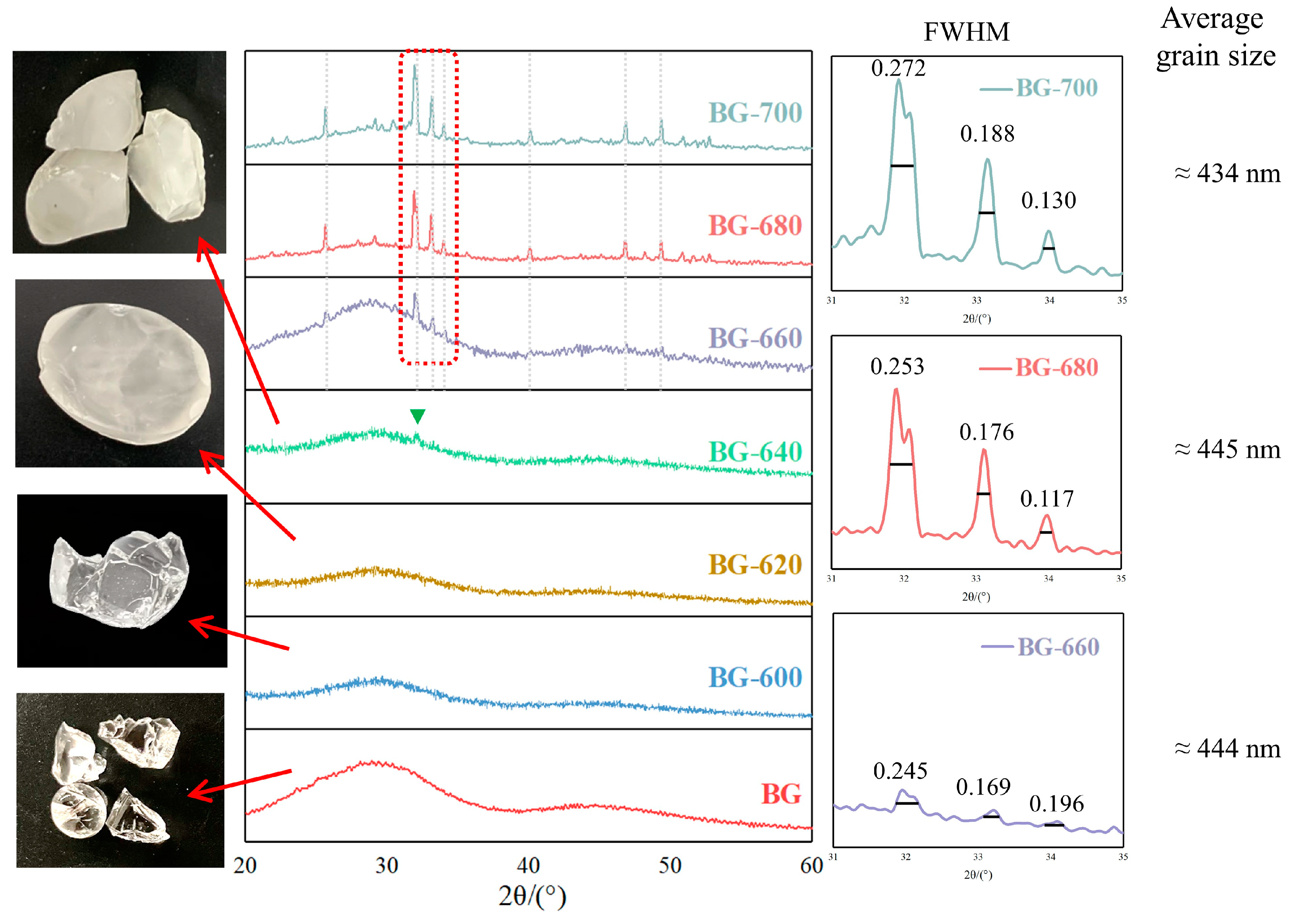
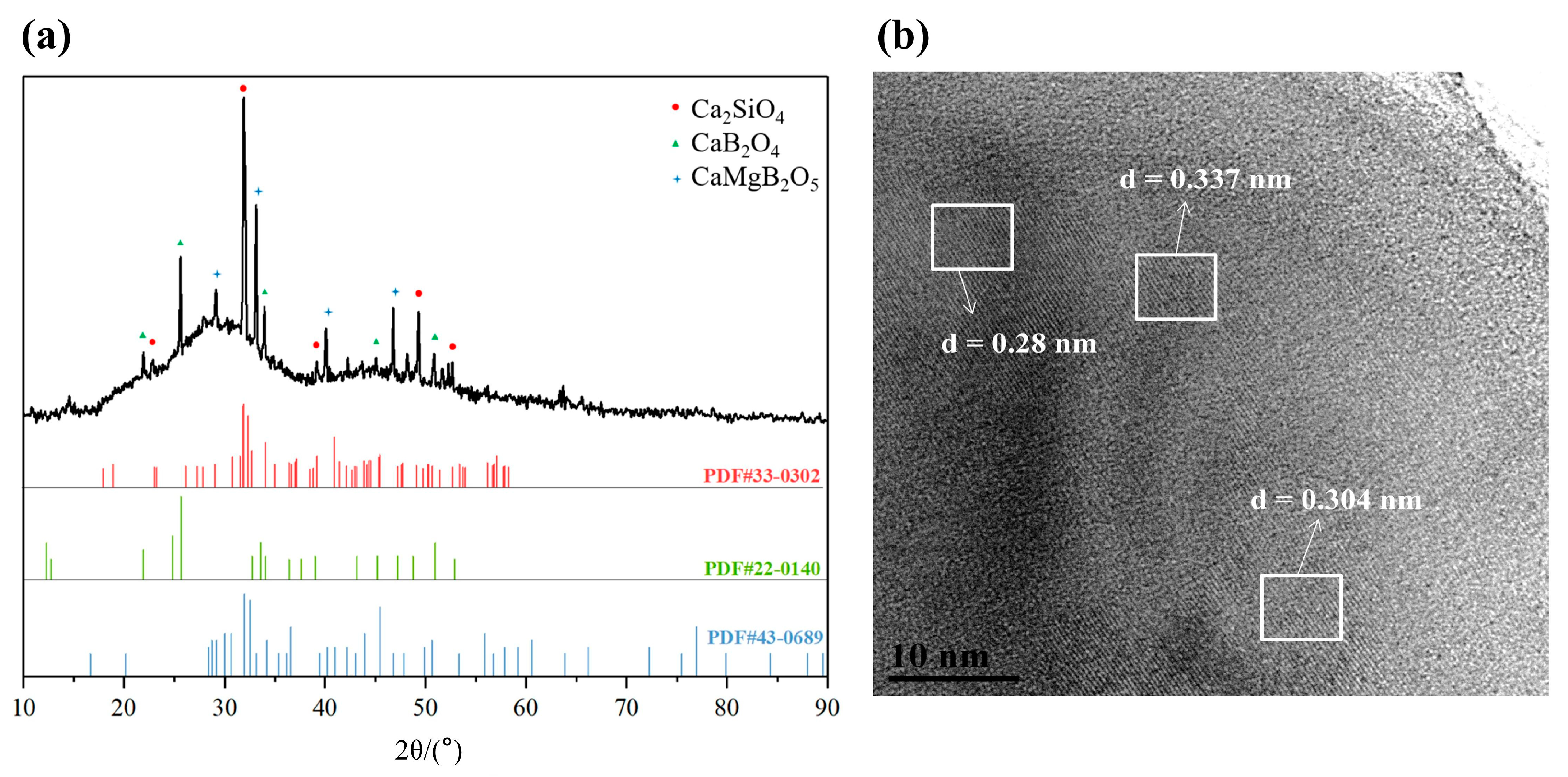
3.1. Microcrystallization
3.2. In Vitro Degradation
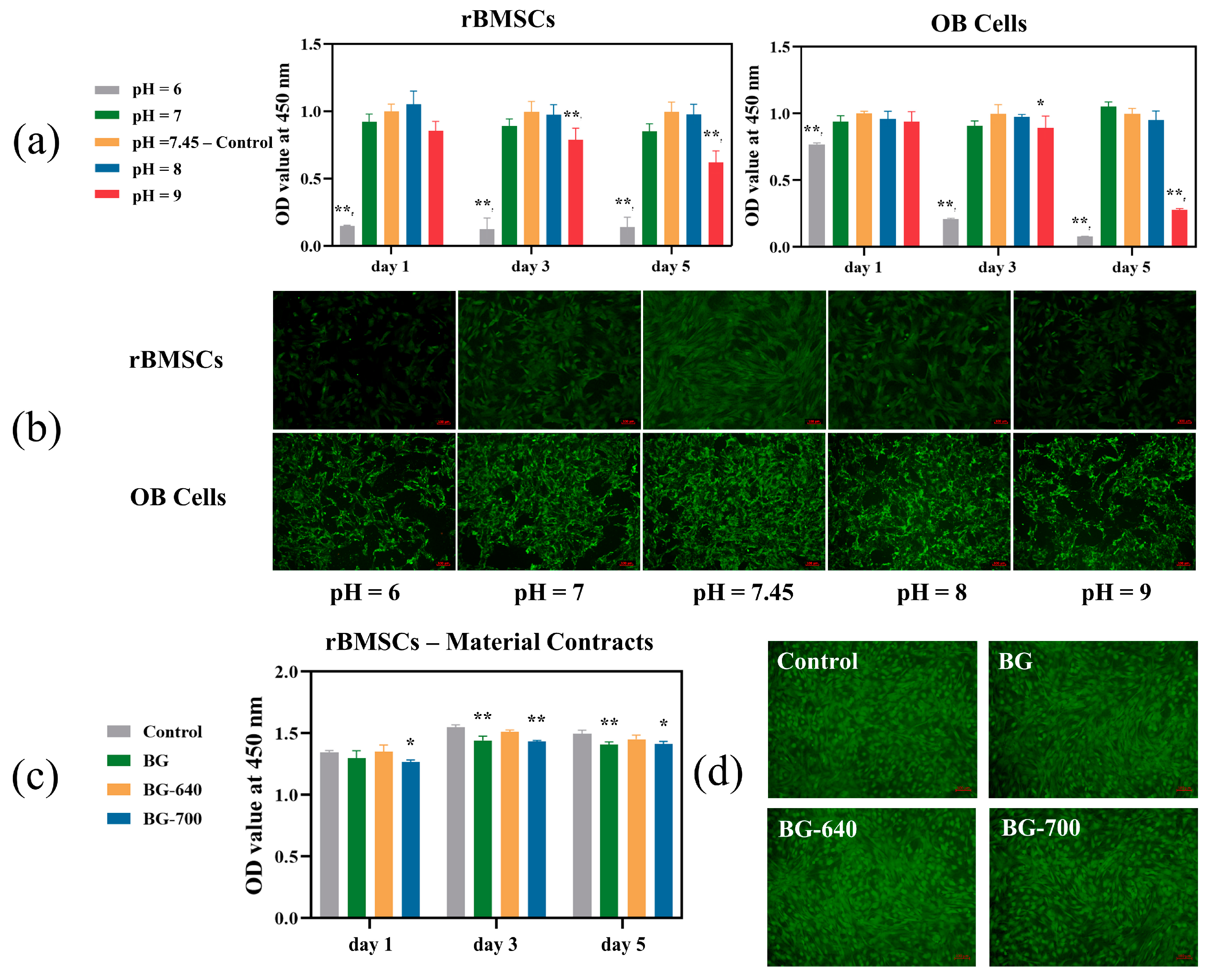
3.3. Cell Proliferation and Viability
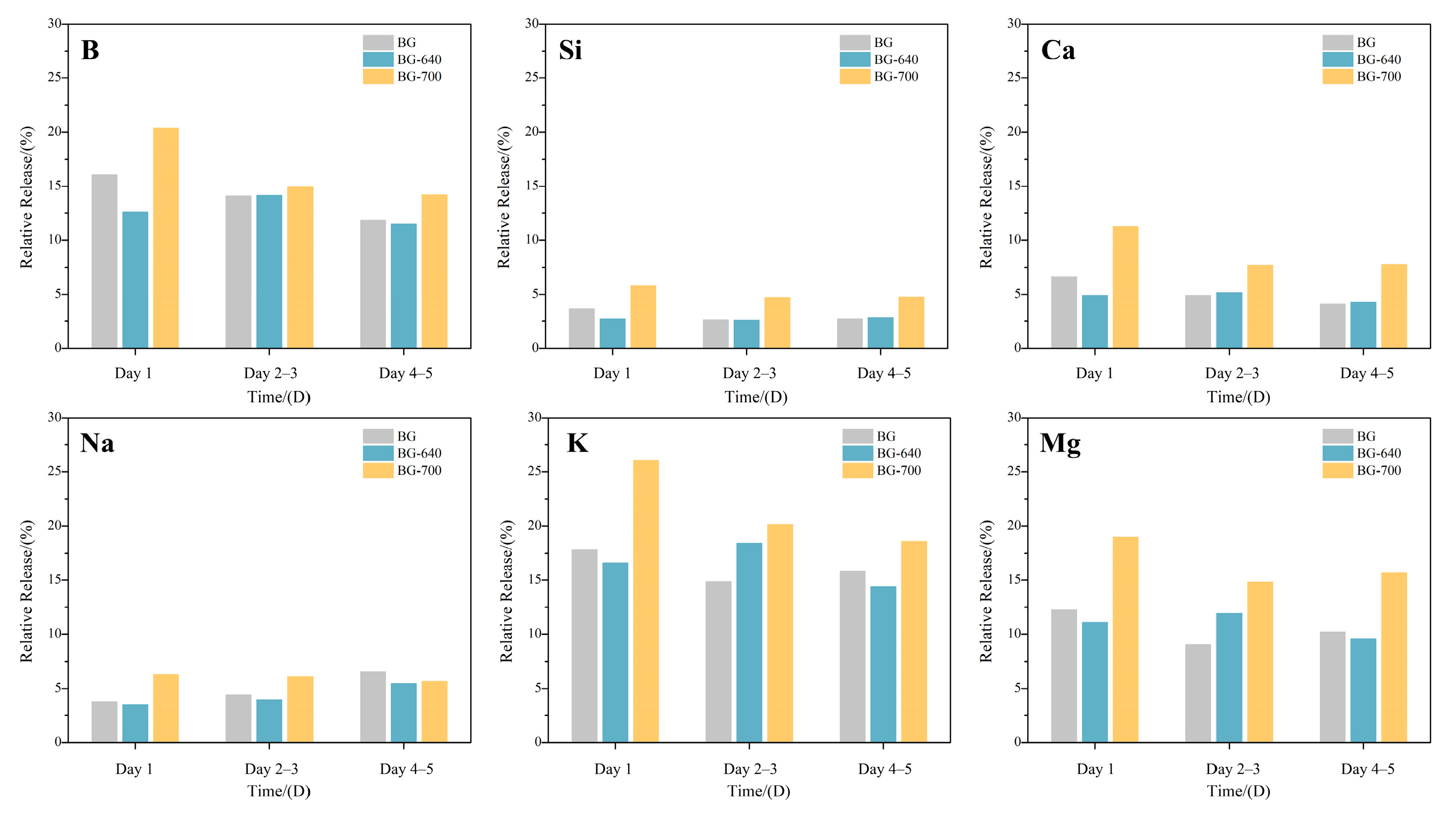
3.4. Controllable Release of Bioactive Elements
3.5. Mineralization Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dukle, A.; Murugan, D.; Nathanael, A.J.; Rangasamy, L.; Oh, T.H. Can 3D-Printed Bioactive Glasses Be the Future of Bone Tissue Engineering? Polymers 2022, 14, 1627. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.X.; Chen, X.; Geng, Z.; Su, J.C. Bone-targeted biomaterials: Strategies and applications. Chem. Eng. J. 2022, 446, 137133. [Google Scholar] [CrossRef]
- Xia, Y.L.; Wang, H.Y.; Li, Y.H.; Fu, C.F. Engineered bone cement trigger bone defect regeneration. Front. Mater. 2022, 9, 929618. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.H.; Wang, D.P.; Fu, Q.; Huang, W.H.; Rahaman, M.N. Preparation of bioactive glasses with controllable degradation behaviour and their bioactive characterization. Chin. Sci. Bull. 2007, 52, 272–276. [Google Scholar] [CrossRef]
- Schuhladen, K.; Pantulap, U.; Engel, K.; Jelen, P.; Olejniczak, Z.; Hupa, L.; Sitarz, M.; Boccaccini, A.R. Influence of the replacement of silica by boron trioxide on the properties of bioactive glass scaffolds. Int. J. Appl. Glass Sci. 2021, 12, 293–312. [Google Scholar] [CrossRef]
- Fu, Q.A.; Rahaman, M.N.; Bal, B.S.; Bonewald, L.F.; Kuroki, K.; Brown, R.F. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. II. In vitro and in vivo biological evaluation. J. Biomed. Mater. Res. A 2010, 95a, 172–179. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass (R). J. Mater. Sci.-Mater. M 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Hench, L.L. Bioactive materials: The potential for tissue regeneration. J. Biomed. Mater. Res. 1998, 41, 511–518. [Google Scholar] [CrossRef]
- Kaur, G.; Pandey, O.P.; Singh, K.; Homa, D.; Scott, B.; Pickrell, G. A review of bioactive glasses: Their structure, properties, fabrication, and apatite formation. J. Biomed. Mater. Res. A 2014, 102, 254–274. [Google Scholar] [CrossRef]
- Day, R.M. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng. 2005, 11, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.H.; Day, D.E.; Kittiratanapiboon, K.; Rahaman, M.N. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J. Mater. Sci.-Mater. M 2006, 17, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Uysal, T.; Ustdal, A.; Sonmez, M.F.; Ozturk, F. Stimulation of Bone Formation by Dietary Boron in an Orthopedically Expanded Suture in Rabbits. Angle Orthod. 2009, 79, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Vitale-Brovarone, C.; Miola, M.; Balagna, C.; Verne, E. 3D-glass-ceramic scaffolds with antibacterial properties for bone grafting. Chem. Eng. J. 2008, 137, 129–136. [Google Scholar] [CrossRef]
- Liu, X.; Huang, W.H.; Fu, H.L.; Yao, A.H.; Wang, D.P.; Pan, H.B.; Lu, W.W. Bioactive borosilicate glass scaffolds: Improvement on the strength of glass-based scaffolds for tissue engineering. J. Mater. Sci.-Mater. M 2009, 20, 365–372. [Google Scholar] [CrossRef]
- Shafaghi, R.; Rodriguez, O.; Wren, A.W.; Chiu, L.; Schemitsch, E.H.; Zalzal, P.; Waldman, S.D.; Papini, M.; Towler, M.R. In vitro evaluation of novel titania-containing borate bioactive glass scaffolds. J. Biomed. Mater. Res. A 2021, 109, 146–158. [Google Scholar] [CrossRef]
- Hamadouche, M.; Meunier, A.; Greenspan, D.C.; Blanchat, C.; Zhong, J.P.P.; La Torre, G.P.; Sedel, L. Long-term in vivo bioactivity and degradability of bulk sol-gel bioactive glasses. J. Biomed. Mater. Res. 2001, 54, 560–566. [Google Scholar] [CrossRef]
- Lin, S.; Van den Bergh, W.; Baker, S.; Jones, J.R. Protein interactions with nanoporous sol-gel derived bioactive glasses. Acta Biomater. 2011, 7, 3606–3615. [Google Scholar] [CrossRef]
- Gaddam, A.; Golebiewski, P.; Fernandes, H.R.; Pysz, D.; Neto, A.S.; Diduszko, R.; Malinowska, A.; Stepien, R.; Cimek, J.; Buczynski, R.; et al. Development of microfibers for bone regeneration based on alkali-free bioactive glasses doped with boron oxide. J. Am. Ceram. Soc. 2021, 104, 4492–4504. [Google Scholar] [CrossRef]
- Ning, J.; Yao, A.; Wang, D.P.; Huang, W.H.; Fu, H.L.; Liu, X.; Jiang, X.Q.; Zhang, X.L. Synthesis and in vitro bioactivity of a borate-based bioglass. Mater. Lett. 2007, 61, 5223–5226. [Google Scholar] [CrossRef]
- Fu, L.; Engqvist, H.; Xia, W. Glass-Ceramics in Dentistry: A Review. Materials 2020, 13, 1049. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Peng, W.; Deng, C. Preparation technology, properties and applications of microcrystalline ceramics. China Ceram. 2000, 36, 31. [Google Scholar]
- Chen, X.J.; Chen, X.H.; Brauer, D.S.; Wilson, R.M.; Hill, R.G.; Karpukhina, N. Bioactivity of Sodium Free Fluoride Containing Glasses and Glass-Ceramics. Materials 2014, 7, 5470–5487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Z.; Liang, S.L.; Wang, J.; Simon, G.P. Manipulation of mechanical compliance of elastomeric PGS by incorporation of halloysite nanotubes for soft tissue engineering applications. J. Mech. Behav. Biomed. 2011, 4, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Z.; Boccaccini, A.R. Coupling mechanical competence and bioresorbability in Bioglass (R)-derived tissue engineering scaffolds. Adv. Eng. Mater. 2006, 8, 285–289. [Google Scholar] [CrossRef]
- Chen, C.; Ding, J.X.; Wang, H.; Wang, D.P. Nd-doped Mesoporous Borosilicate Bioactive Glass-ceramic Bone Cement. J. Inorg. Mater. 2022, 37, 1245–1258. [Google Scholar] [CrossRef]
- Lu, L.N.; Lin, J.; Gu, Y.F.; Zhang, X.; Zhao, Y.S.; Huang, W.H. Effect of the crystallization treatment of bioactive borate-based glass containing strontium on its degradation and bio-activity in vitro. J. Funct. Mater. 2012, 43, 4. [Google Scholar]
- Madshal, M.A.; Abdelghany, A.M.; Abdelghany, M.I.; El-Damrawi, G. Biocompatible borate glasses doped with Gd2O3 for biomedical applications. Eur. Phys. J. Plus 2022, 137, 1014. [Google Scholar] [CrossRef]
- Yao, A.H.; Wang, D.P.; Huang, W.H.; Fu, Q.; Rahaman, M.N.; Day, D.E. In vitro bioactive characteristics of borate-based glasses with controllable degradation behavior. J. Am. Ceram. Soc. 2007, 90, 303–306. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproduce Invivo Surface-Structure Changes in Bioactive Glass-Ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Popa, A.C.; Stan, G.E.; Husanu, M.A.; Mercioniu, I.; Santos, L.F.; Fernandes, H.R.; Ferreira, J.M.F. Bioglass implant-coating interactions in synthetic physiological fluids with varying degrees of biomimicry. Int. J. Nanomed. 2017, 12, 683–707. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ge, W.; Gao, X.; Wang, S.; Jiang, S.; Hu, Y.; Yu, M.; Hu, S. Effect of SDT on the survival rate of endometrial cancer cells, assessed by the CCK-8 method. PLoS ONE 2015, 10, e0137980. [Google Scholar]
- Gao, J.; Bin, Y.E.; Wei-Hua, W.U.; Kong, J. Tachyzoites of Toxolasma gondii enhances the cytotoxicity of Etooside (V-16) to mouse colon cancer cell ct26 in vitro. Chin. J. Zoonoses 2010, 26, 720–724. [Google Scholar]
- Yao, A.H.; Lin, J.; Duan, X.; Huang, W.H.; Rahaman, M.N. Formation mechanism of multilayered structure on surface of bioactive borosilicate glass. Chin. J. Inorg. Chem. 2008, 24, 1132–1136. [Google Scholar]


| Glass | BG-524 | BG-560 | BG-600 | BG-620 | BG-640 | BG-660 | BG-680 | BG-700 |
|---|---|---|---|---|---|---|---|---|
| Melting (°C) | 1150 | |||||||
| Melting time (h) | 1 | |||||||
| Heating (°C) | 524 | 560 | 600 | 620 | 640 | 660 | 680 | 700 |
| Heating time (h) | 1 | |||||||
| Ion | Na+ | K+ | Mg2+ | Ca2+ | Cl− | HCO3− | HPO42− | SO42− | pH |
|---|---|---|---|---|---|---|---|---|---|
| Concentration (mmol·L−1) | 142 | 5 | 1.5 | 2.5 | 147.8 | 4.2 | 1 | 0.5 | 7.45–7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Zhang, M.; Lin, J. Microcrystallization Effects in Borosilicate Bioactive Glasses: Controllable Release of Bioactive Elements and In Vitro Degradation Properties. Materials 2024, 17, 32. https://doi.org/10.3390/ma17010032
Jin C, Zhang M, Lin J. Microcrystallization Effects in Borosilicate Bioactive Glasses: Controllable Release of Bioactive Elements and In Vitro Degradation Properties. Materials. 2024; 17(1):32. https://doi.org/10.3390/ma17010032
Chicago/Turabian StyleJin, Chengyun, Minhui Zhang, and Jian Lin. 2024. "Microcrystallization Effects in Borosilicate Bioactive Glasses: Controllable Release of Bioactive Elements and In Vitro Degradation Properties" Materials 17, no. 1: 32. https://doi.org/10.3390/ma17010032





