Hydrogels in Ophthalmology: Novel Strategies for Overcoming Therapeutic Challenges
Abstract
:1. Introduction
2. Overview of Hydrogels
2.1. Definition and Properties
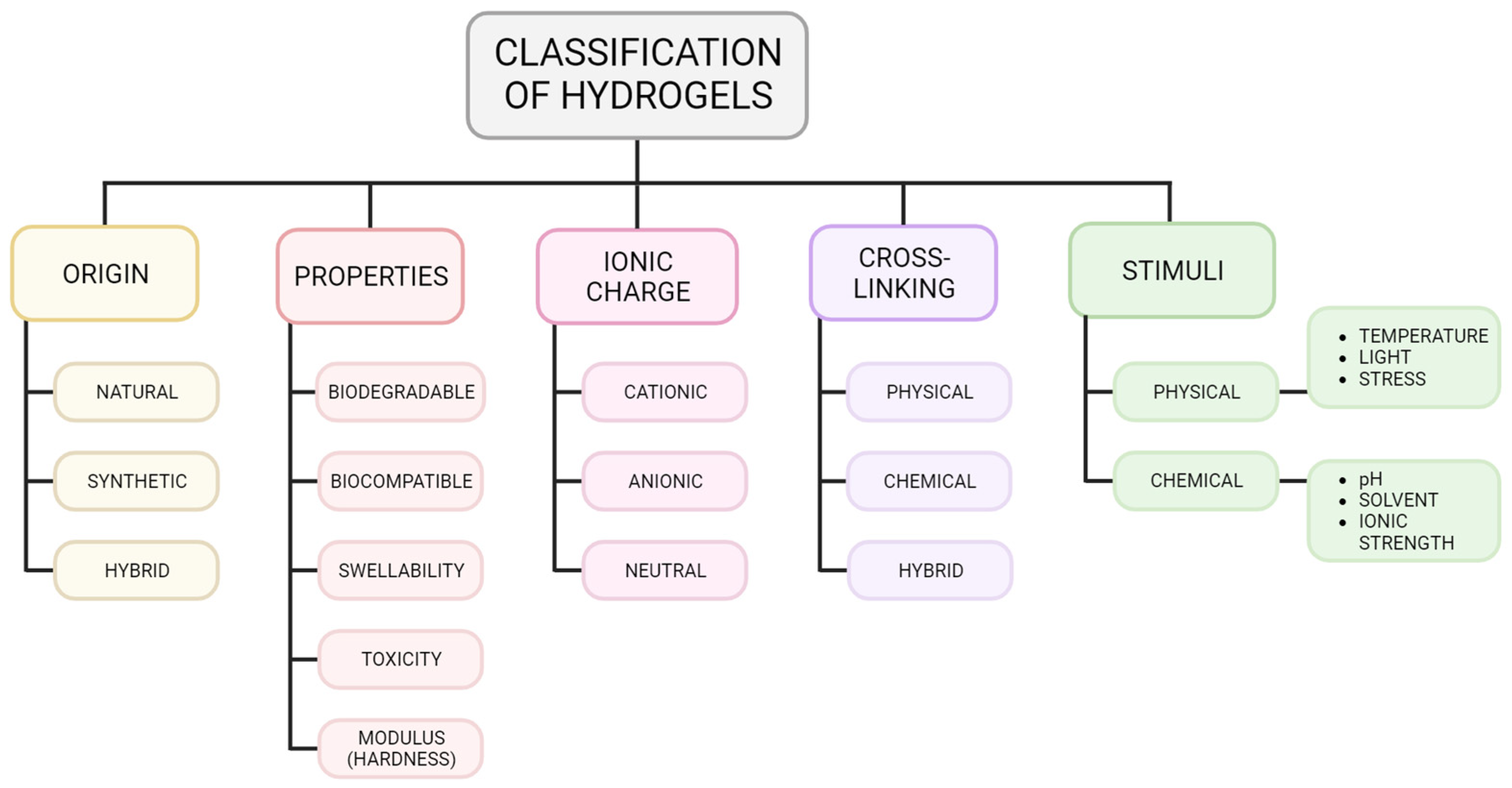
2.2. Classification of Hydrogels
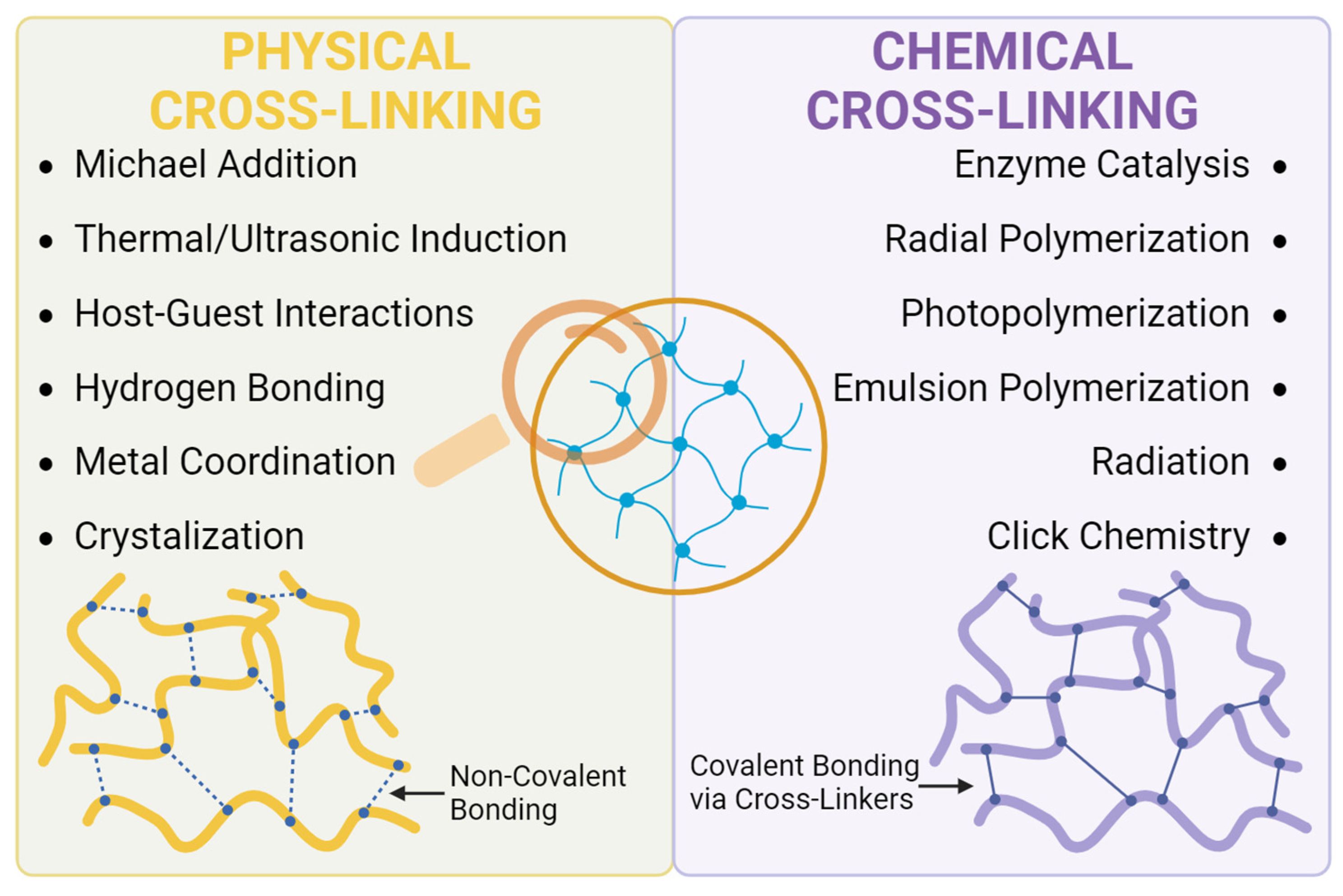
2.3. Hydrogel Synthesis and Challenges in Ophthalmology
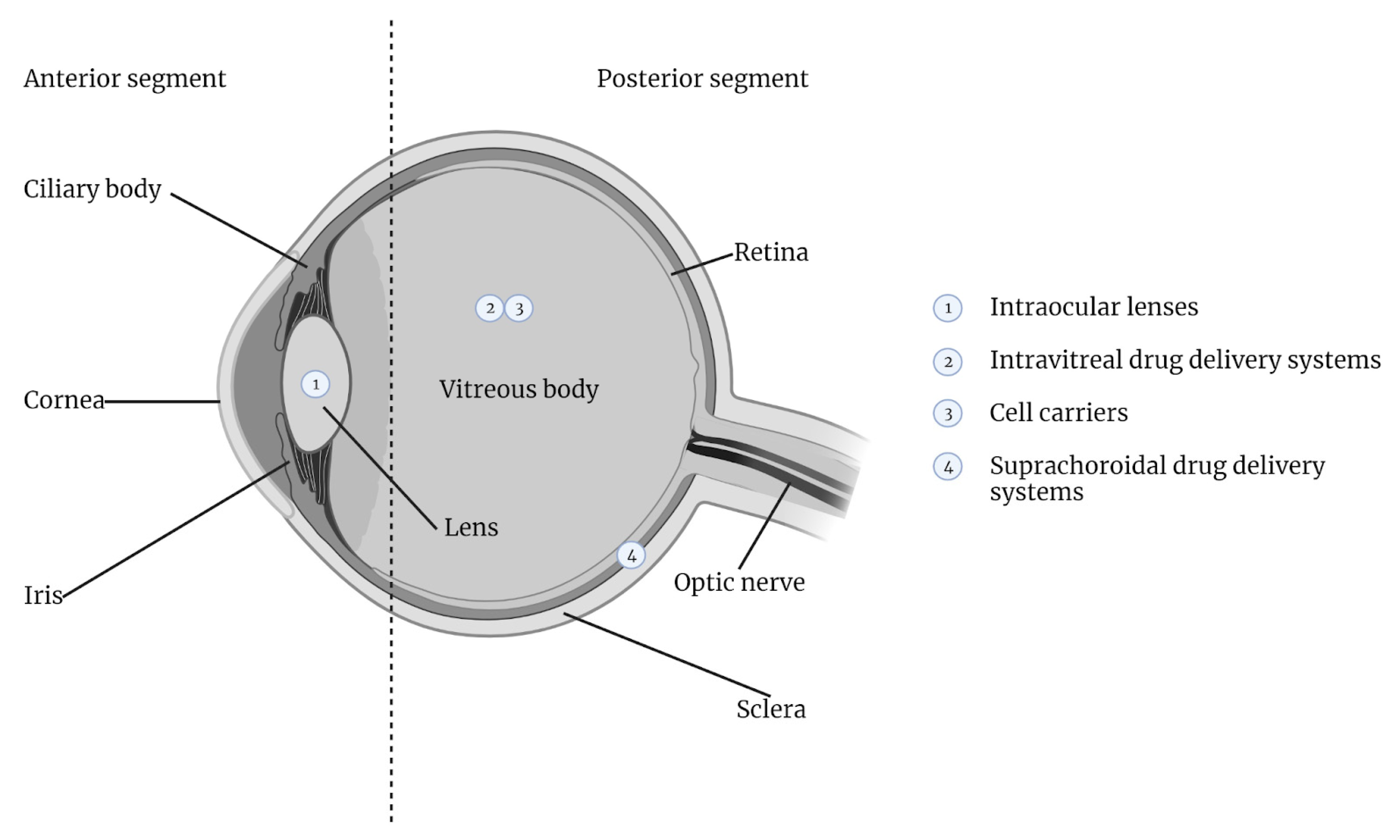
3. Ocular Anatomy and Physiology
3.1. Anatomical and Functional Complexity of the Posterior Segment of the Eye
- Inner Plexiform Layer: Facilitates synaptic interactions between bipolar and ganglion cells, crucial for signal integration.
- Inner Nuclear Layer: Hosts the nuclei of bipolar, horizontal, and amacrine cells, which are essential for visual signal processing.
- Outer Plexiform Layer: Features synapses between photoreceptors and bipolar or horizontal cells, vital for initial signal transduction.
- Outer Nuclear Layer: Comprises rod and cone photoreceptor cell bodies, responsible for capturing and translating light into neural impulses.
- Rod and Cone Segments: The functional parts of photoreceptor cells, where light absorption and phototransduction occur.
- Retinal Pigment Epithelium (RPE): This outermost layer absorbs stray light and nourishes the photoreceptors, among other functions.
3.2. Static and Dynamic Barriers of the Eye
3.2.1. Corneal Barrier
3.2.2. Vitreous Barrier
3.2.3. Aqueous Humor
3.2.4. Blood–Ocular Barrier (BOB)
4. Intravitreal Drug Delivery Systems
4.1. Synthetic and Semi-Synthetic Hydrogels for Intravitreal Ocular Drug Delivery
4.2. Natural Hydrogels for Intravitreal Ocular Drug Delivery
5. Suprachoroidal Drug Delivery Systems
6. Cell-Based Therapies for Ocular Delivery
6.1. Cell-Based Therapies for Diseases of Retinal Degeneration
6.2. Cell-Based Therapies for Corneal Damage
6.3. Limitations of Hydrogel in Cell-Based Therapies for Retinal Diseases
7. Utilization of Hydrogels in Intraocular Lens Technology
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, K.Y.; Joly-Chevrier, M.; Akbar, D.; Tran, S.D. Overcoming Treatment Challenges in Posterior Segment Diseases with Biodegradable Nano-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1094. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2022, 123, 834–873. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef]
- Kirchhof, S.; Goepferich, A.M.; Brandl, F.P. Hydrogels in Ophthalmic Applications. Eur. J. Pharm. Biopharm. 2015, 95, 227–238. [Google Scholar] [CrossRef]
- Almawash, S.; Osman, S.K.; Mustafa, G.; El Hamd, M.A. Current and Future Prospective of Injectable Hydrogels—Design Challenges and Limitations. Pharmaceuticals 2022, 15, 371. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable Thermoresponsive Hydrogels as Drug Delivery System for the Treatment of Central Nervous System Disorders: A Review. J. Control. Release 2021, 329, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cheng, Y.; Tong, J.; Zhang, L.; Wei, Y.; Tian, M. Recent Advances in Thermo-Sensitive Hydrogels for Drug Delivery. J. Mater. Chem. B 2021, 9, 2979–2992. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Han, Z. Injectable Hydrogels for Ophthalmic Applications. J. Control. Release 2017, 268, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.; Alunni-Cardinali, M.; Correa, N.; Caponi, S.; Holsgrove, T.; Barr, H.; Stone, N.; Winlove, C.P.; Fioretto, D.; Palombo, F. Viscoelastic Properties of Biopolymer Hydrogels Determined by Brillouin Spectroscopy: A Probe of Tissue Micromechanics. Sci. Adv. 2020, 6, eabc1937. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O. Viscoelastic Hydrogels for 3D Cell Culture. Biomater. Sci. 2017, 5, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- da Silva Fernandes, R.; Tanaka, F.N.; Angelotti, A.M.; Ferreira Júnior, C.R.; Yonezawa, U.G.; Watanuki Filho, A.; de Moura, M.R.; Aouada, F.A. 17—Properties, Synthesis, Characterization and Application of Hydrogel and Magnetic Hydrogels: A Concise Review. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Jogaiah, S., Singh, H.B., Fraceto, L.F., de Lima, R., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2021; pp. 437–457. ISBN 978-0-12-820092-6. [Google Scholar]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Taraban, M.; Yu, Y.B. The Effect of Ionic Strength on the Mechanical, Structural and Transport Properties of Peptide Hydrogels. Soft Matter 2012, 8, 11723–11731. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic Hydrogels: Synthesis, Novel Trends, and Applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Cai, M.-H.; Chen, X.-Y.; Fu, L.-Q.; Du, W.-L.; Yang, X.; Mou, X.-Z.; Hu, P.-Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhao, X.; Xu, C.; Wang, L.; Xia, Y. Progress in the Mechanical Enhancement of Hydrogels: Fabrication Strategies and Underlying Mechanisms. J. Polym. Sci. 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Vorov, O.K.; Livesay, D.R.; Jacobs, D.J. Conformational Entropy of an Ideal Cross-Linking Polymer Chain. Entropy 2008, 10, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Sakumichi, N.; Yoshikawa, Y.; Sakai, T. Linear Elasticity of Polymer Gels in Terms of Negative Energy Elasticity. Polym. J. 2021, 53, 1293–1303. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, Z.; Soontornworajit, B.; Zhou, J.; Wang, Y. Cell Adhesion on an Artificial Extracellular Matrix Using Aptamer-Functionalized PEG Hydrogels. Biomaterials 2012, 33, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Berkovitch, Y.; Seliktar, D. Semi-Synthetic Hydrogel Composition and Stiffness Regulate Neuronal Morphogenesis. Int. J. Pharm. 2017, 523, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in Crosslinking Strategies of Biomedical Hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Gorantla, S.; Waghule, T.; Rapalli, V.K.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Advanced Hydrogels Based Drug Delivery Systems for Ophthalmic Delivery. Recent. Pat. Drug Deliv. Formulation 2019, 13, 291–300. [Google Scholar] [CrossRef]
- Lin, K.T.; Wang, A.; Nguyen, A.B.; Iyer, J.; Tran, S.D. Recent Advances in Hydrogels: Ophthalmic Applications in Cell Delivery, Vitreous Substitutes, and Ocular Adhesives. Biomedicines 2021, 9, 1203. [Google Scholar] [CrossRef]
- Wu, K.Y.; Mina, M.; Sahyoun, J.-Y.; Kalevar, A.; Tran, S.D. Retinal Prostheses: Engineering and Clinical Perspectives for Vision Restoration. Sensors 2023, 23, 5782. [Google Scholar] [CrossRef]
- Mahabadi, N.; Al Khalili, Y. Neuroanatomy, Retina. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Margolis, R.; Spaide, R.F. A Pilot Study of Enhanced Depth Imaging Optical Coherence Tomography of the Choroid in Normal Eyes. Am. J. Ophthalmol. 2009, 147, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Emi, K.; Pederson, J.E.; Toris, C.B. Hydrostatic Pressure of the Suprachoroidal Space. Investig. Ophthalmol. Vis. Sci. 1989, 30, 233–238. [Google Scholar]
- Krohn, J.; Bertelsen, T. Light Microscopy of Uveoscleral Drainage Routes after Gelatine Injections into the Suprachoroidal Space. Acta Ophthalmol. Scand. 1998, 76, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Lampen, S.I.R.; Khurana, R.N.; Noronha, G.; Brown, D.M.; Wykoff, C.C. Suprachoroidal Space Alterations Following Delivery of Triamcinolone Acetonide: Post-Hoc Analysis of the Phase 1/2 HULK Study of Patients with Diabetic Macular Edema. Ophthalmic Surg. Lasers Imaging Retina 2018, 49, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Lin, A.S.P.; Edelhauser, H.F.; Prausnitz, M.R. Suprachoroidal Drug Delivery to the Back of the Eye Using Hollow Microneedles. Pharm. Res. 2011, 28, 166–176. [Google Scholar] [CrossRef]
- Patel, S.R.; Berezovsky, D.E.; McCarey, B.E.; Zarnitsyn, V.; Edelhauser, H.F.; Prausnitz, M.R. Targeted Administration into the Suprachoroidal Space Using a Microneedle for Drug Delivery to the Posterior Segment of the Eye. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4433–4441. [Google Scholar] [CrossRef]
- Krohn, J.; Bertelsen, T. Corrosion Casts of the Suprachoroidal Space and Uveoscleral Drainage Routes in the Human Eye. Acta Ophthalmol. Scand. 1997, 75, 32–35. [Google Scholar] [CrossRef]
- Chiang, B.; Kim, Y.C.; Edelhauser, H.F.; Prausnitz, M.R. Circumferential Flow of Particles in the Suprachoroidal Space Is Impeded by the Posterior Ciliary Arteries. Exp. Eye Res. 2016, 145, 424–431. [Google Scholar] [CrossRef]
- Akhter, M.H.; Ahmad, I.; Alshahrani, M.Y.; Al-Harbi, A.I.; Khalilullah, H.; Afzal, O.; Altamimi, A.S.A.; Najib Ullah, S.N.M.; Ojha, A.; Karim, S. Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System. Gels 2022, 8, 82. [Google Scholar] [CrossRef]
- Bachu, R.; Chowdhury, P.; Al-Saedi, Z.; Karla, P.; Boddu, S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and Obstacles of Ocular Pharmacokinetics and Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Ashkar, S.; Jain, S.; Marchand, M.; Tran, S.D. Breaking Barriers in Eye Treatment: Polymeric Nano-Based Drug-Delivery System for Anterior Segment Diseases and Glaucoma. Polymers 2023, 15, 1373. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Fujioka, J.K.; Gholamian, T.; Zaharia, M.; Tran, S.D. Suprachoroidal Injection: A Novel Approach for Targeted Drug Delivery. Pharmaceuticals 2023, 16, 1241. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-S.; Hou, P.-K.; Tai, T.-Y.; Lin, B.J. Blood-Ocular Barriers. Tzu Chi Med. J. 2008, 20, 25–34. [Google Scholar] [CrossRef]
- Sarkar, A.; Junnuthula, V.; Dyawanapelly, S. Ocular Therapeutics and Molecular Delivery Strategies for Neovascular Age-Related Macular Degeneration (nAMD). Int. J. Mol. Sci. 2021, 22, 10594. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, S.M.; Arepalli, S.; Ehlers, J.P. Current and Future Anti-VEGF Agents for Neovascular Age-Related Macular Degeneration. J. Exp. Pharmacol. 2021, 13, 905–912. [Google Scholar] [CrossRef]
- Schwartz, S.G.; Flynn, H.W.; Scott, I.U. Intravitreal Corticosteroids in the Management of Diabetic Macular Edema. Curr. Ophthalmol. Rep. 2013, 1, 144–149. [Google Scholar] [CrossRef]
- Ilochonwu, B.C.; Urtti, A.; Hennink, W.E.; Vermonden, T. Intravitreal Hydrogels for Sustained Release of Therapeutic Proteins. J. Control. Release 2020, 326, 419–441. [Google Scholar] [CrossRef]
- Annala, A.; Ilochonwu, B.C.; Wilbie, D.; Sadeghi, A.; Hennink, W.E.; Vermonden, T. Self-Healing Thermosensitive Hydrogel for Sustained Release of Dexamethasone for Ocular Therapy. ACS Polym. Au 2022, 3, 118–131. [Google Scholar] [CrossRef]
- Dosmar, E.; Vuotto, G.; Su, X.; Roberts, E.; Lannoy, A.; Bailey, G.J.; Mieler, W.F.; Kang-Mieler, J.J. Compartmental and COMSOL Multiphysics 3D Modeling of Drug Diffusion to the Vitreous Following the Administration of a Sustained-Release Drug Delivery System. Pharmaceutics 2021, 13, 1862. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Kim, S.; Wang, Z.; Liu, W.; Yiu, G.; Mieler, W.F.; Thomasy, S.M. Aflibercept-Loaded Microsphere-Hydrogel Drug Delivery System in a Nonhuman Primate Model. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3265. [Google Scholar]
- Liu, W.; Borrell, M.A.; Venerus, D.C.; Mieler, W.F.; Kang-Mieler, J.J. Characterization of Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Ranibizumab. Transl. Vis. Sci. Technol. 2019, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lee, B.-S.; Mieler, W.F.; Kang-Mieler, J.J. Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Bioactive Aflibercept In Vitro. Curr. Eye Res. 2019, 44, 264–274. [Google Scholar] [CrossRef]
- Liu, W.; Tawakol, A.P.; Rudeen, K.M.; Mieler, W.F.; Kang-Mieler, J.J. Treatment Efficacy and Biocompatibility of a Biodegradable Aflibercept-Loaded Microsphere-Hydrogel Drug Delivery System. Transl. Vis. Sci. Technol. 2020, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang-Mieler, J.J.; Liu, W.; Wang, Z.; Yiu, G.; Teixeira, L.B.C.; Mieler, W.F.; Thomasy, S.M. Safety and Biocompatibility of Aflibercept-Loaded Microsphere Thermo-Responsive Hydrogel Drug Delivery System in a Nonhuman Primate Model. Transl. Vis. Sci. Technol. 2020, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-C.; Chiu, Y.-C.; Chaw, J.-R.; Chen, C.-F.; Liu, H.-W. Thermo-Responsive Hydrogel as an Anti-VEGF Drug Delivery System to Inhibit Retinal Angiogenesis in Rex Rabbits. Technol. Health Care 2019, 27, 153–163. [Google Scholar] [CrossRef] [PubMed]
- López-Cano, J.J.; Sigen, A.; Andrés-Guerrero, V.; Tai, H.; Bravo-Osuna, I.; Molina-Martínez, I.T.; Wang, W.; Herrero-Vanrell, R. Thermo-Responsive PLGA-PEG-PLGA Hydrogels as Novel Injectable Platforms for Neuroprotective Combined Therapies in the Treatment of Retinal Degenerative Diseases. Pharmaceutics 2021, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, C.; Xu, B.; Tu, J.; Shen, Y. Synthesis, Physicochemical Properties and Ocular Pharmacokinetics of Thermosensitive in Situ Hydrogels for Ganciclovir in Cytomegalovirus Retinitis Treatment. Drug Deliv. 2018, 25, 59–69. [Google Scholar] [CrossRef]
- Xue, K.; Zhao, X.; Zhang, Z.; Qiu, B.; Tan, Q.S.W.; Ong, K.H.; Liu, Z.; Parikh, B.H.; Barathi, V.A.; Yu, W.; et al. Sustained Delivery of Anti-VEGFs from Thermogel Depots Inhibits Angiogenesis without the Need for Multiple Injections. Biomater. Sci. 2019, 7, 4603–4614. [Google Scholar] [CrossRef]
- Duan, N.; Mei, L.; Hu, L.; Yin, X.; Wei, X.; Li, Y.; Li, Q.; Zhao, G.; Zhou, Q.; Du, Z. Biomimetic, Injectable, and Self-Healing Hydrogels with Sustained Release of Ranibizumab to Treat Retinal Neovascularization. ACS Appl. Mater. Interfaces 2023, 15, 6371–6384. [Google Scholar] [CrossRef]
- Pachis, K.; Blazaki, S.; Tzatzarakis, M.; Klepetsanis, P.; Naoumidi, E.; Tsilimbaris, M.; Antimisiaris, S.G. Sustained Release of Intravitreal Flurbiprofen from a Novel Drug-in-Liposome-in-Hydrogel Formulation. Eur. J. Pharm. Sci. 2017, 109, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Ottonelli, I.; Bighinati, A.; Adani, E.; Loll, F.; Caraffi, R.; Vandelli, M.A.; Boury, F.; Tosi, G.; Duskey, J.T.; Marigo, V.; et al. Optimization of an Injectable Hydrogel Depot System for the Controlled Release of Retinal-Targeted Hybrid Nanoparticles. Pharmaceutics 2022, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.L.; Rezazadeh, M.; Hassanzadeh, F.; Akbari, V.; Dehghani, A.; Talebi, A.; Mostafavi, S.A. Preparation, Physicochemical, and Retinal Anti-Angiogenic Evaluation of Poloxamer Hydrogel Containing Dexamethasone/Avastin-Loaded Chitosan-N-Acetyl-L-Cysteine Nanoparticles. Int. J. Biol. Macromol. 2022, 220, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Meany, E.L.; Andaya, R.; Tang, S.; Kasse, C.M.; Fuji, R.N.; Grosskopf, A.K.; d’Aquino, A.L.; Bartoe, J.T.; Ybarra, R.; Shelton, A.; et al. Injectable Polymer-Nanoparticle Hydrogel for the Sustained Intravitreal Delivery of Bimatoprost. Adv. Ther. 2023, 6, 2200207. [Google Scholar] [CrossRef]
- Tibbitt, M.; Auerbach, A.; Jayagopal, A.; Langer, R. Sustained Intravitreal Delivery of Small and Large Molecules with Injectable Hydrogels. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2971. [Google Scholar]
- Jarrett, P.K.; Elhayek, R.F.; Jarrett, T.; Lattrell, Z.; McGrath, M.; Takach, S.; Talamo, J.H.; Sawhney, A. Tolerability of a 6 Month Sustained Hydrogel Delivery System for Tyrosine Kinase Inhibitors in Dutch Belted Rabbits. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1956. [Google Scholar]
- Jarrett, T.; Elhayek, R.F.; Lattrell, Z.; McGrath, M.; Takach, S.; Jarrett, P.K.; Talamo, J.H.; Sawhney, A. Pharmacokinetics of a 6 Month Sustained Hydrogel Delivery System for Tyrosine Kinase Inhibitors in Dutch Belted Rabbits. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1984. [Google Scholar]
- Elhayek, R.F.; Jarrett, T.; Lattrell, Z.; Kahn, E.; Takach, S.; Metzinger, J.L.; Goldstein, M.H.; Jarrett, P.K.; Sawhney, A. Effectiveness of Sustained Release TKI Hydrogel Combined with Bevacizumab in a VEGF Induced Retinal Leakage Model Through 12 Months. Investig. Ophthalmol. Vis. Sci. 2018, 59, 245. [Google Scholar]
- Patel, C.; Kahn, E.; Priem, M.; Iacona, J.; Vanslette, A.; Wong, E.; Blizzard, C.D.; Jarrett, P.K.; Goldstein, M.; Gurses-Ozden, R. A 6-Month GLP Toxicology Study of a Novel Hydrogel-Based, Axitinib Intravitreal Implant (OTX-TKI) in Non-Human Primates. Investig. Ophthalmol. Vis. Sci. 2022, 63, 298-F0101. [Google Scholar]
- Kahn, E.; Patel, C.; Priem, M.; Iacona, J.; Vanslette, A.; Wong, E.; Blizzard, C.D.; Jarrett, P.K.; Goldstein, M.; Gurses-Ozden, R. A Safety and Pharmacokinetic Study of a Novel Hydrogel-Based Axitinib Intravitreal Implant (OTX-TKI) in Non-Human Primates. Investig. Ophthalmol. Vis. Sci. 2022, 63, 297-F0100. [Google Scholar]
- Wong, J.G.; Chang, A.; Guymer, R.H.; Wickremasinghe, S.; Reilly, E.; Bell, N.; Vantipalli, S.; Moshfeghi, A.A.; Goldstein, M.H. Phase 1 Study of an Intravitreal Axitinib Hydrogel-Based Implant for the Treatment of Neovascular Age-Related Macular Degeneration (nAMD). Investig. Ophthalmol. Vis. Sci. 2021, 62, 218. [Google Scholar]
- Moshfeghi, A.A.; Khanani, A.M.; Eichenbaum, D.A.; Wykoff, C.C.; Couvillion, S.; Xavier, S.; Steinle, N.; Kaiser, P.; Gurses Ozden, R.U.S. Phase 1 Study of Intravitreal Axitinib Implant (OTX-TKI) for Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2023, 64, 936. [Google Scholar]
- Zhang, T.; Han, X.; Zhong, Y.; Kam, H.T.; Qiao, D.; Chen, Z.; Chan, K.W.Y.; Chong, W.P.; Chen, J. Regulatory T Cell Intravitreal Delivery Using Hyaluronan Methylcellulose Hydrogel Improves Therapeutic Efficacy in Experimental Autoimmune Uveitis. Biomater. Adv. 2023, 151, 213496. [Google Scholar] [CrossRef] [PubMed]
- Delplace, V.; Ortin-Martinez, A.; Tsai, E.L.S.; Amin, A.N.; Wallace, V.; Shoichet, M.S. Controlled Release Strategy Designed for Intravitreal Protein Delivery to the Retina. J. Control. Release 2019, 293, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, D.; Zhang, Y.; Li, W.; Li, F.; Feng, H.; Ma, L.; Yang, C.; Peng, Z.; Song, G.; et al. An Injectable Hydrogel Based on Hyaluronic Acid Prepared by Schiff Base for Long-Term Controlled Drug Release. Int. J. Biol. Macromol. 2023, 245, 125341. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lin, X.; Wang, Q.; He, M.; Chau, Y. Long-Term Therapeutic Effect in Nonhuman Primate Eye from a Single Injection of Anti-VEGF Controlled Release Hydrogel. Bioeng. Transl. Med. 2019, 4, e10128. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.S.Y.; Tsang, K.K.; Chu, A.M.W.; Chan, B.P.; Yao, K.M.; Lo, A.C.Y. Injectable Cell-Encapsulating Composite Alginate-Collagen Platform with Inducible Termination Switch for Safer Ocular Drug Delivery. Biomaterials 2019, 201, 53–67. [Google Scholar] [CrossRef]
- Wong, F.S.Y.; Tsang, K.K.; Chan, B.P.; Lo, A.C.Y. Both Non-Coated and Polyelectrolytically-Coated Intraocular Collagen-Alginate Composite Gels Enhanced Photoreceptor Survival in Retinal Degeneration. Biomaterials 2023, 293, 121948. [Google Scholar] [CrossRef]
- Gao, H.; Chen, M.; Liu, Y.; Zhang, D.; Shen, J.; Ni, N.; Tang, Z.; Ju, Y.; Dai, X.; Zhuang, A.; et al. Injectable Anti-Inflammatory Supramolecular Nanofiber Hydrogel to Promote Anti-VEGF Therapy in Age-Related Macular Degeneration Treatment. Adv. Mater. 2023, 35, e2204994. [Google Scholar] [CrossRef]
- Lin, C.-C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef]
- Murakami, T.; Hoshi, S.; Okamoto, F.; Sakai, T.; Katashima, T.; Naito, M.; Oshika, T. Analysis of the Sustained Release Ability of Bevacizumab-Loaded Tetra-PEG Gel. Exp. Eye Res. 2022, 223, 109206. [Google Scholar] [CrossRef]
- Guragain, S.; Bastakoti, B.P.; Malgras, V.; Nakashima, K.; Yamauchi, Y. Multi-Stimuli-Responsive Polymeric Materials. Chemistry 2015, 21, 13164–13174. [Google Scholar] [CrossRef]
- Ilochonwu, B.C.; van der Lugt, S.A.; Annala, A.; Di Marco, G.; Sampon, T.; Siepmann, J.; Siepmann, F.; Hennink, W.E.; Vermonden, T. Thermo-Responsive Diels-Alder Stabilized Hydrogels for Ocular Drug Delivery of a Corticosteroid and an Anti-VEGF Fab Fragment. J. Control. Release 2023, 361, 334–349. [Google Scholar] [CrossRef]
- Dosmar, E.; Liu, W.; Patel, G.; Rogozinski, A.; Mieler, W.F.; Kang-Mieler, J.J. Controlled Release of Vancomycin from a Thermoresponsive Hydrogel System for the Prophylactic Treatment of Postoperative Acute Endophthalmitis. Transl. Vis. Sci. Technol. 2019, 8, 53. [Google Scholar] [CrossRef]
- Liu, W.; Puskar, A.; Mieler, W.F.; Kang-Mieler, J.J. Efficacy of a Biodegradable Aflibercept-Loaded Microsphere-Hydrogel Drug Delivery System in a Laser-Induced Choroidal Neovascularization Rat Model. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3262. [Google Scholar]
- Hu, C.-C.; Chaw, J.-R.; Chen, C.-F.; Liu, H.-W. Controlled Release Bevacizumab in Thermoresponsive Hydrogel Found to Inhibit Angiogenesis. Biomed. Mater. Eng. 2014, 24, 1941–1950. [Google Scholar] [CrossRef]
- Ilochonwu, B.C.; Mihajlovic, M.; Maas-Bakker, R.F.; Rousou, C.; Tang, M.; Chen, M.; Hennink, W.E.; Vermonden, T. Hyaluronic Acid-PEG-Based Diels-Alder In Situ Forming Hydrogels for Sustained Intraocular Delivery of Bevacizumab. Biomacromolecules 2022, 23, 2914–2929. [Google Scholar] [CrossRef]
- Pirmardvand Chegini, S.; Varshosaz, J.; Dehghani, A.; Minaiyan, M.; Mirmohammad Sadeghi, H. Ocular Delivery of Sunitinib-Loaded Nanoparticles Doped in Tragacanthic Acid Hydrogel in Treatment of Diabetic Retinopathy in Rats. Drug Dev. Ind. Pharm. 2022, 48, 29–39. [Google Scholar] [CrossRef]
- Akulo, K.A.; Adali, T.; Moyo, M.T.G.; Bodamyali, T. Intravitreal Injectable Hydrogels for Sustained Drug Delivery in Glaucoma Treatment and Therapy. Polymers 2022, 14, 2359. [Google Scholar] [CrossRef]
- Nunes, D.; Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings. Polymers 2022, 14, 1010. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Wan, C.; Muya, L.; Kansara, V.; Ciulla, T.A. Suprachoroidal Delivery of Small Molecules, Nanoparticles, Gene and Cell Therapies for Ocular Diseases. Pharmaceutics 2021, 13, 288. [Google Scholar] [CrossRef]
- Ham, Y.; Mehta, H.; Kang-Mieler, J.; Mieler, W.F.; Chang, A. Novel Drug Delivery Methods and Approaches for the Treatment of Retinal Diseases. Asia Pac. J. Ophthalmol. 2023, 12, 402. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Rudeen, K.M.; Liu, W.; Mieler, W.F. Advances in Ocular Drug Delivery Systems. Eye 2020, 34, 1371–1379. [Google Scholar] [CrossRef]
- Jung, J.H.; Chae, J.J.; Prausnitz, M.R. Targeting Drug Delivery within the Suprachoroidal Space. Drug Discov. Today 2019, 24, 1654–1659. [Google Scholar] [CrossRef]
- Naftali Ben Haim, L.; Moisseiev, E. Drug Delivery via the Suprachoroidal Space for the Treatment of Retinal Diseases. Pharmaceutics 2021, 13, 967. [Google Scholar] [CrossRef]
- Dietze, J.; Blair, K.; Havens, S.J. Glaucoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hao, H.; He, B.; Yu, B.; Yang, J.; Xing, X.; Liu, W. Suprachoroidal Injection of Polyzwitterion Hydrogel for Treating Glaucoma. Biomater. Adv. 2022, 142, 213162. [Google Scholar] [CrossRef]
- Chae, J.J.; Jung, J.H.; Zhu, W.; Gerberich, B.G.; Bahrani Fard, M.R.; Grossniklaus, H.E.; Ethier, C.R.; Prausnitz, M.R. Drug-Free, Nonsurgical Reduction of Intraocular Pressure for Four Months after Suprachoroidal Injection of Hyaluronic Acid Hydrogel. Adv. Sci. 2020, 8, 2001908. [Google Scholar] [CrossRef]
- Chung, Y.G.; Toris, C.B.; Gulati, V.; Fan, S.; Prausnitz, M.; Ethier, C.R. Hydrogel Expansion of the Suprachoroidal Space Lowers IOP in Rabbit and Monkey Eyes. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3475. [Google Scholar]
- Liu, S.; Tang, J.; Ji, F.; Lin, W.; Chen, S. Recent Advances in Zwitterionic Hydrogels: Preparation, Property, and Biomedical Application. Gels 2022, 8, 46. [Google Scholar] [CrossRef]
- Bevilacqua, P.; Nuzzo, S.; Torino, E.; Condorelli, G.; Salvatore, M.; Grimaldi, A.M. Antifouling Strategies of Nanoparticles for Diagnostic and Therapeutic Application: A Systematic Review of the Literature. Nanomaterials 2021, 11, 780. [Google Scholar] [CrossRef]
- Kohli, P.; Tripathy, K. Scleral Buckling. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Szurman, P.; Boden, K.; Januschowski, K. Suprachoroidal Hydrogel Buckling as a Surgical Treatment of Retinal Detachment: Biocompatibility and First Experiences. Retina 2016, 36, 1786–1790. [Google Scholar] [CrossRef]
- Boden, K.T.; Januschowski, K.; Szurman, P. Suprachoroidal Hydrogel Buckle—A New Minimal-Invasive Technique in Treatment of Rhegmatogenous Retinal Detachment. Klin. Monbl Augenheilkd. 2019, 236, 308–312. [Google Scholar] [CrossRef]
- Jung, J.H.; Kim, S.S.; Chung, H.; Hejri, A.; Prausnitz, M.R. Six-Month Sustained Delivery of Anti-VEGF from in-Situ Forming Hydrogel in the Suprachoroidal Space. J. Control. Release 2022, 352, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Desit, P.; Prausnitz, M.R. Targeted Drug Delivery in the Suprachoroidal Space by Swollen Hydrogel Pushing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Pandala, N.; LaScola, M.; Mulfaul, K.; Stone, E.M.; Mullins, R.F.; Tucker, B.A.; Lavik, E. Development of Chemically Crosslinked PEG-PAA Hydrogels Suitable for Engineering of the Vascularized Outer Retina. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3735-F0341. [Google Scholar]
- Pandala, N.; Han, I.; Meyering, E.; Lang, M.; Mullins, R.; Tucker, B.A. Autologous Choroidal Endothelial Cell Replacement Using Laminin Based Hydrogels for the Treatment of AMD. Investig. Ophthalmol. Vis. Sci. 2023, 64, 1241. [Google Scholar]
- Wu, K.Y.; Kulbay, M.; Toameh, D.; Xu, A.Q.; Kalevar, A.; Tran, S.D. Retinitis Pigmentosa: Novel Therapeutic Targets and Drug Development. Pharmaceutics 2023, 15, 685. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, S.; Galloway, C.A.; Singh, R. Pluripotent Stem Cells to Model Degenerative Retinal Diseases: The RPE Perspective. Adv. Exp. Med. Biol. 2019, 1186, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, X.; Zhu, W.; Zhong, Z.; Moran, A.; Wang, W.; Zhang, K.; Chen, S. Null 3D Bioprinting of Hydrogels for Retina Cell Culturing. Bioprinting 2018, 11, e00029. [Google Scholar] [CrossRef]
- Mitrousis, N.; Hacibekiroglu, S.; Ho, M.T.; Sauvé, Y.; Nagy, A.; van der Kooy, D.; Shoichet, M.S. Hydrogel-Mediated Co-Transplantation of Retinal Pigmented Epithelium and Photoreceptors Restores Vision in an Animal Model of Advanced Retinal Degeneration. Biomaterials 2020, 257, 120233. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.; Choi, J.H.; Lee, S.; Lee, W.; Lee, S.W.; Kim, W.; Song, Y.; Tumursukh, N.-E.; Song, J.E.; Khang, G. Fabrication and Evaluation of Gellan Gum/Hyaluronic Acid Hydrogel for Retinal Tissue Engineering Biomaterial and the Influence of Substrate Stress Relaxation on Retinal Pigment Epithelial Cells. Molecules 2022, 27, 5512. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, D.; Jeong, Y.W.; Choi, M.J.; Lee, G.W.; Thangavelu, M.; Song, J.E.; Khang, G. Engineering Retinal Pigment Epithelial Cells Regeneration for Transplantation in Regenerative Medicine Using PEG/Gellan Gum Hydrogels. Int. J. Biol. Macromol. 2019, 130, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Choi, J.H.; Lee, J.; Youn, J.; Kim, W.; Jeon, G.; Lee, S.W.; Song, J.E.; Khang, G. Dopamine-Functionalized Gellan Gum Hydrogel as a Candidate Biomaterial for a Retinal Pigment Epithelium Cell Delivery System. ACS Appl. Bio Mater. 2021, 4, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Rim, M.A.; Choi, J.H.; Park, A.; Youn, J.; Lee, S.; Kim, N.E.; Song, J.E.; Khang, G. Characterization of Gelatin/Gellan Gum/Glycol Chitosan Ternary Hydrogel for Retinal Pigment Epithelial Tissue Reconstruction Materials. ACS Appl. Bio Mater. 2020, 3, 6079–6087. [Google Scholar] [CrossRef]
- Seo, J.S.; Tumursukh, N.-E.; Choi, J.H.; Song, Y.; Jeon, G.; Kim, N.E.; Kim, S.J.; Kim, N.; Song, J.E.; Khang, G. Modified Gellan Gum-Based Hydrogel with Enhanced Mechanical Properties for Application as a Cell Carrier for Cornea Endothelial Cells. Int. J. Biol. Macromol. 2023, 236, 123878. [Google Scholar] [CrossRef] [PubMed]
- Dromel, P.C.; Singh, D.; Andres, E.; Likes, M.; Kurisawa, M.; Alexander-Katz, A.; Spector, M.; Young, M. A Bioinspired Gelatin-Hyaluronic Acid-Based Hybrid Interpenetrating Network for the Enhancement of Retinal Ganglion Cells Replacement Therapy. NPJ Regen. Med. 2021, 6, 85. [Google Scholar] [CrossRef]
- Park, J.; Baranov, P.; Aydin, A.; Abdelgawad, H.; Singh, D.; Niu, W.; Kurisawa, M.; Spector, M.; Young, M.J. In Situ Cross-Linking Hydrogel as a Vehicle for Retinal Progenitor Cell Transplantation. Cell Transpl. 2019, 28, 596–606. [Google Scholar] [CrossRef]
- COLOMBE, P.; Singh, D.; Spector, M.; Young, M.J. 3D Hydrogels Protect Human Retinal Progenitor Cells from Stress Exerted during Transplantation. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3328. [Google Scholar]
- Tang, Z.; Jiang, F.; Zhang, Y.; Zhang, Y.; YuanYang; Huang, X.; Wang, Y.; Zhang, D.; Ni, N.; Liu, F.; et al. Mussel-Inspired Injectable Hydrogel and Its Counterpart for Actuating Proliferation and Neuronal Differentiation of Retinal Progenitor Cells. Biomaterials 2019, 194, 57–72. [Google Scholar] [CrossRef]
- Tang, Q.; Lu, B.; He, J.; Chen, X.; Fu, Q.; Han, H.; Luo, C.; Yin, H.; Qin, Z.; Lyu, D.; et al. Exosomes-Loaded Thermosensitive Hydrogels for Corneal Epithelium and Stroma Regeneration. Biomaterials 2022, 280, 121320. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Sharifi, H.; Akbari, A.; Koza, D.; Dohlman, C.H.; Paschalis, E.I.; Chodosh, J. Photo-Cross-Linked Gelatin Glycidyl Methacrylate/N-Vinylpyrrolidone Copolymeric Hydrogel with Tunable Mechanical Properties for Ocular Tissue Engineering Applications. ACS Appl. Bio Mater. 2021, 4, 7682–7691. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Mashayekhan, S.; Baradaran-Rafii, A.; Kim, K. Development and in Vitro Evaluation of Photocurable GelMA/PEGDA Hybrid Hydrogel for Corneal Stromal Cells Delivery. Mater. Today Commun. 2021, 27, 102459. [Google Scholar] [CrossRef]
- Haghighat, M.; Iranbakhsh, A.; Baharara, J.; Ebadi, M.; Sotoodehnejadnematalahi, F. Effect of β-Carotene on the Differentiation Potential of Ciliary Epithelium-Derived MSCs Isolated from Mouse Eyes on Alginate-Based Scaffolds. Exp. Eye Res. 2021, 202, 108346. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Shin, E.Y.; Shin, M.E.; Choi, M.J.; Carlomagno, C.; Song, J.E.; Khang, G. Enhanced Retinal Pigment Epithelium (RPE) Regeneration Using Curcumin/Alginate Hydrogels: In Vitro Evaluation. Int. J. Biol. Macromol. 2018, 117, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.C.; Hallam, D.; Karimi, A.; Mellough, C.B.; Chen, J.; Steel, D.H.W.; Lako, M. 3D Culture of Human Pluripotent Stem Cells in RGD-Alginate Hydrogel Improves Retinal Tissue Development. Acta Biomater. 2017, 49, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Soroushzadeh, S.; Karamali, F.; Masaeli, E.; Atefi, A.; Nasr Esfahani, M.H. Scaffold Free Retinal Pigment Epithelium Sheet Engineering Using Modified Alginate-RGD Hydrogel. J. Biosci. Bioeng. 2022, 133, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.K.; Manzar, Z.; Bachman, L.A.; Andrews-Pfannkoch, C.; Knudsen, T.; Hill, M.; Schmidt, H.; Iezzi, R.; Pulido, J.S.; Marmorstein, A.D. Fibrin Hydrogels as a Xenofree and Rapidly Degradable Support for Transplantation of Retinal Pigment Epithelium Monolayers. Acta Biomater. 2018, 67, 134–146. [Google Scholar] [CrossRef]
- García Delgado, A.B.; de la Cerda, B.; Alba Amador, J.; Valdés Sánchez, M.L.; Fernández-Muñoz, B.; Relimpio López, I.; Rodríguez de la Rúa, E.; Díez Lloret, A.; Calado, S.M.; Sánchez Pernaute, R.; et al. Subretinal Transplant of Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium on Nanostructured Fibrin-Agarose. Tissue Eng. Part A 2019, 25, 799–808. [Google Scholar] [CrossRef]
- Soleimannejad, M.; Ebrahimi-Barough, S.; Soleimani, M.; Nadri, S.; Tavangar, S.M.; Roohipoor, R.; Yazdankhah, M.; Bayat, N.; Riazi-Esfahani, M.; Ai, J. Fibrin Gel as a Scaffold for Photoreceptor Cells Differentiation from Conjunctiva Mesenchymal Stem Cells in Retina Tissue Engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 805–814. [Google Scholar] [CrossRef]
- Wei, Y.; Alexandre, U.; Ma, X. Hydrogels to Support Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells. Brain Sci. 2022, 12, 1620. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yin, P.; Li, T.; Luo, L.; Yang, Y.; Wang, L.; Su, W.; Wang, Y.; Li, Y.; Wang, Y.; et al. Injectable Composite Hydrogels Encapsulating Gelatin Methacryloyl/Chitosan Microspheres as ARPE-19 Cell Transplantation Carriers. Biomater. Sci. 2022, 11, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Tang, Z.; Zhang, Y.; Ju, Y.; Gao, H.; Sun, N.; Liu, F.; Gu, P.; Zhang, W. Enhanced Proliferation and Differentiation of Retinal Progenitor Cells through a Self-Healing Injectable Hydrogel. Biomater. Sci. 2019, 7, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Ahearne, M. Silk Fibroin Based Interpenetrating Network Hydrogel for Corneal Stromal Regeneration. Int. J. Biol. Macromol. 2022, 223, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Hu, Y.; Shi, H.; Bao, Z.; Wu, Y.; Jiang, J.; Li, X. Biofunctional Peptide-Click PEG-Based Hydrogels as 3D Cell Scaffolds for Corneal Epithelial Regeneration. J. Mater. Chem. B 2022, 10, 5938–5945. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Lace, R.; Carserides, C.; Gallagher, A.G.; Wellings, D.A.; Williams, R.L.; Levis, H.J. Poly-ε-Lysine Based Hydrogels as Synthetic Substrates for the Expansion of Corneal Endothelial Cells for Transplantation. J. Mater. Sci. Mater. Med. 2019, 30, 102. [Google Scholar] [CrossRef] [PubMed]
- Lace, R.; Duffy, G.L.; Gallagher, A.G.; Doherty, K.G.; Maklad, O.; Wellings, D.A.; Williams, R.L. Characterization of Tunable Poly-ε-Lysine-Based Hydrogels for Corneal Tissue Engineering. Macromol. Biosci. 2021, 21, e2100036. [Google Scholar] [CrossRef]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Bongiovì, F.; Giammona, G. Hyaluronic Acid and Beta Cyclodextrins Films for the Release of Corneal Epithelial Cells and Dexamethasone. Carbohydr. Polym. 2017, 166, 281–290. [Google Scholar] [CrossRef]
- Na, K.-S.; Fernandes-Cunha, G.M.; Varela, I.B.; Lee, H.J.; Seo, Y.A.; Myung, D. Effect of Mesenchymal Stromal Cells Encapsulated within Polyethylene Glycol-Collagen Hydrogels Formed in Situ on Alkali-Burned Corneas in an Ex Vivo Organ Culture Model. Cytotherapy 2021, 23, 500–509. [Google Scholar] [CrossRef]
- Zakaria, N.; Haagdorens, M.; Liszka, A.; Ulcinas, A.; Cepla, V.; Valiokas, R.; Kozak Ljunggren, M.; Samanta, A.; Tassignon, M.-J.B.R.; Tal, Y.; et al. Recombinant Human Collagen Type I Hydrogels as Superior Cell Carriers for Corneal Epithelial Stem Cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2280. [Google Scholar]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in Vitro Characterization of Cross-Linked Collagen-Gelatin Hydrogel Using EDC/NHS for Corneal Tissue Engineering Applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, F.S.; Federico, S.; Pitarresi, G.; Fiorica, C.; Giammona, G. Gellan Gum-Based Delivery Systems of Therapeutic Agents and Cells. Carbohydr. Polym. 2020, 229, 115430. [Google Scholar] [CrossRef] [PubMed]
- Juriga, D.; Kalman, E.E.; Toth, K.; Barczikai, D.; Szöllősi, D.; Földes, A.; Varga, G.; Zrinyi, M.; Jedlovszky-Hajdu, A.; Nagy, K.S. Analysis of Three-Dimensional Cell Migration in Dopamine-Modified Poly(Aspartic Acid)-Based Hydrogels. Gels 2022, 8, 65. [Google Scholar] [CrossRef]
- Amaral, N.M.; Dodd, M.; Rambarran, T.; Sheardown, H. N-Isopropylacrylamide-Based Cellular Scaffold for Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1922. [Google Scholar]
- Lowe, T.; Damera, S.C. Injectable Hydrogels for Stem Cell Based Regenerative Treatment of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4567. [Google Scholar]
- Sohn, E.; Worthington, K.S.; Jiao, C.; Kaalberg, E.E.; Russell, S.R.; Gibson-Corley, K.N.; Mullins, R.F.; Stone, E.M.; Tucker, B. Compatibility of a Biodegradable Retinal Cell Graft for the Treatment of Retinal Degenerative Blindness. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4585. [Google Scholar]
- Gomes, J.Á.P.; Geraldes Monteiro, B.; Melo, G.B.; Smith, R.L.; Cavenaghi Pereira da Silva, M.; Lizier, N.F.; Kerkis, A.; Cerruti, H.; Kerkis, I. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Human Immature Dental Pulp Stem Cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1408–1414. [Google Scholar] [CrossRef]
- Kumar, A.; Yun, H.; Funderburgh, M.L.; Du, Y. Regenerative Therapy for the Cornea. Prog. Retin. Eye Res. 2022, 87, 101011. [Google Scholar] [CrossRef]
- Blenkinsop, T.A.; Corneo, B.; Temple, S.; Stern, J.H. Ophthalmologic Stem Cell Transplantation Therapies. Regen. Med. 2012, 7, 32–39. [Google Scholar] [CrossRef]
- Nair, D.S.R.; Seiler, M.J.; Patel, K.H.; Thomas, V.; Camarillo, J.C.M.; Humayun, M.S.; Thomas, B.B. Tissue Engineering Strategies for Retina Regeneration. Appl. Sci. 2021, 11, 2154. [Google Scholar] [CrossRef] [PubMed]
- Nazeer, N.; Ahmed, M. Chapter 13—Polymers in Medicine. In Polymer Science and Nanotechnology; Narain, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 281–323. ISBN 978-0-12-816806-6. [Google Scholar]
- Vacalebre, M.; Frison, R.; Corsaro, C.; Neri, F.; Santoro, A.; Conoci, S.; Anastasi, E.; Curatolo, M.C.; Fazio, E. Current State of the Art and Next Generation of Materials for a Customized IntraOcular Lens According to a Patient-Specific Eye Power. Polymers 2023, 15, 1590. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Tang, J.; Han, Y.; Xu, X.; Hao, X.; Chen, H. Hydrophilic Modification of Intraocular Lens via Surface Initiated Reversible Addition-Fragmentation Chain Transfer Polymerization for Reduced Posterior Capsular Opacification. Colloids Surf. B Biointerfaces 2017, 151, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Karahan, E.; Er, D.; Kaynak, S. An Overview of Nd:YAG Laser Capsulotomy. Med. Hypothesis Discov. Innov. Ophthalmol. 2014, 3, 45. [Google Scholar] [PubMed]
- Li, X.; Zhao, Y.; Wang, K.; Wang, L.; Yang, X.; Zhu, S. Cyclodextrin-Containing Hydrogels as an Intraocular Lens for Sustained Drug Release. PLoS ONE 2017, 12, e0189778. [Google Scholar] [CrossRef] [PubMed]
- Toffoletto, N.; Salema-Oom, M.; Anguiano Igea, S.; Alvarez-Lorenzo, C.; Saramago, B.; Serro, A.P. Drug-Loaded Hydrogels for Intraocular Lenses with Prophylactic Action against Pseudophakic Cystoid Macular Edema. Pharmaceutics 2021, 13, 976. [Google Scholar] [CrossRef]
- Artigas, J.M.; García-Domene, M.C.; Navea, A.; Botella, P.; Fernández, E. Intra-Ocular Lens Optical Changes Resulting from the Loading of Dexamethasone. Biomed. Opt. Express 2017, 8, 4621. [Google Scholar] [CrossRef]
- Shin, M.-K.; Ji, Y.W.; Moon, C.-E.; Lee, H.; Kang, B.; Jinn, W.-S.; Ki, J.; Mun, B.; Kim, M.-H.; Lee, H.K.; et al. Matrix Metalloproteinase 9-Activatable Peptide-Conjugated Hydrogel-Based Fluorogenic Intraocular-Lens Sensor. Biosens. Bioelectron. 2020, 162, 112254. [Google Scholar] [CrossRef]




| Examples of Types of Polymers | Advantages | Disadvantages | References |
|---|---|---|---|
| Natural source polymers for hydrogels | |||
| Collagen, chitosan, gelatin, alginate, hyaluronic acid |
|
| [7,8,20,21] |
| Synthetic source polymers for hydrogels | |||
| Poly(ethylene glycol) (PEG), poly(acrylic acid) (PAA), N–isopropyl acrylamide |
|
| [9,20,21,26] |
| Semi-synthetic source polymers for hydrogels: synthetic polymers conjugated to ECM a components | |||
| PEG + albumin, PEG + gelatin, PEG + fibronectin |
|
| [21,27] |
| Material Type b | Indication b | Drug Delivered | Key Features | Challenges and Considerations | Current Usage | References |
|---|---|---|---|---|---|---|
| NIPAAm-PEG-NIPAAm triblock polymers | PDR; DME; RVO | DEX |
|
| Pre-clinical: in vitro | [51] |
| PEG + NIPAAm-based hydrogel composites | Glaucoma; wAMD with CNV; PDR; DME; RVO | Vancomycin, aflibercept, ranibizumab |
|
| Pre-clinical: in vitro (computational models) and in vivo | [52,53,54,55,56,57] |
| mPEG-PLGA-BOX | wAMD | Bevacizumab |
|
| Pre-clinical: in vitro and in vivo | [58] |
| PLGA-PEG-PLGA triblock copolymer | Neurodegeneration of the retina | DEX |
|
| Pre-clinical: in vitro | [59] |
| PBLA-PEG-PBLA triblock copolymer | Retinitis caused by cytomegalovirus | Ganciclovir |
|
| Pre-clinical: In vitro | [60] |
| Poly (PEG/PPG/PCL) urethane | wAMD; PDR | Bevacizumab, aflibercept |
|
| Pre-clinical: in vitro and in vivo | [61] |
| Poloxamer-based hydrogels | Non-specific neovascularization | Ranibizumab, flurbiprofen, bevacizumab |
|
| Pre-clinical: in vitro and in vivo | [62,63,64,65] |
| Polymer nanoparticle hydrogels | Glaucoma | Bimatoprost |
|
| Pre-clinical: in vitro and in vivo | [66,67] |
| PEG-based OTX-TKI hydrogels | wAMD | Bevacizumab, ataxinib |
|
| Pre-clinical: in vitro and in vivo Clinical Phase I trial | [68,69,70,71,72,73,74] |
| HAMC-based hydrogels | wAMD; RP | Tregs, CNTF |
|
| Pre-clinical: in vivo | [75,76] |
| HA-based hydrogels | wAMD; endophthalmitis | Bevacizumab, voriconazole |
|
| Pre-clinical: in vitro and in vivo | [77,78] |
| Composite alginate–collagen gels | wAMD; RP | GDNF as a sample delivery molecule |
|
| Pre-clinical: in vitro and in vivo | [79,80] |
| Drug-loaded nanofiber hydrogel + CaCl2 | wAMD | BetP |
|
| Pre-clinical: in vitro and in vivo | [81] |
| Material Type b | Indication | Cell Type/Drug | Key Features b | Challenges and Considerations | Current Usage | References |
|---|---|---|---|---|---|---|
| HA-based 3D bioprinting | Retinal cell regeneration | RPCs |
|
| Pre-clinical: in vitro | [114] |
| HAMC hydrogel | Retinal degenerative diseases | RPEs + photoreceptor cells |
|
| Pre-clinical: in vitro and in vivo | [115] |
| Gellan gum-based hydrogels | RPE cell regeneration for RP, AMD, and hereditary retinal dystrophies | RPEs |
|
| Pre-clinical: in vitro | [116,117,118,119,120] |
| Gelatin-based hydrogels | Cell replacement for retinal diseases, corneal damage | RPCs, RPEs, MSCs, CSCs |
|
| Pre-clinical: in vitro and in vivo | [121,122,123,124,125,126,127] |
| Alginate hydrogels | Retinal degenerative diseases | MSCs, RPEs |
|
| Pre-clinical: in vitro | [128,129,130,131] |
| Fibrin-based hydrogels | AMD and retinal degeneration | MSCs, RPEs |
|
| Pre-clinical: in vitro and in vivo | [132,133,134,135] |
| GelMA hydrogel + chitosan microspheres | AMD | RPEs |
|
| Pre-clinical: in vitro | [136] |
| Chitosan hydrochloride with oxidized dextran | RPE cell regeneration for AMD and retinal degeneration | RPEs |
|
| Pre-clinical: in vitro and in vivo | [137] |
| SF and PAA hydrogel | Corneal stromal tissue regeneration | CSCs |
|
| Pre-clinical: in vitro | [138] |
| Peptide-based hydrogels | Cell replacement for corneal and retinal diseases | HCECs |
|
| Pre-clinical: in vitro and in vivo | [139,140,141] |
| HA and beta-cyclodextrin hydrogel | Non-specified corneal damage | HCECs + DEX |
|
| Pre-clinical: in vitro | [142] |
| Collagen-based modified hydrogels | Corneal alkali burns | MSCs, LESCs |
|
| Pre-clinical: in vitro | [143,144,145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Akbar, D.; Giunta, M.; Kalevar, A.; Tran, S.D. Hydrogels in Ophthalmology: Novel Strategies for Overcoming Therapeutic Challenges. Materials 2024, 17, 86. https://doi.org/10.3390/ma17010086
Wu KY, Akbar D, Giunta M, Kalevar A, Tran SD. Hydrogels in Ophthalmology: Novel Strategies for Overcoming Therapeutic Challenges. Materials. 2024; 17(1):86. https://doi.org/10.3390/ma17010086
Chicago/Turabian StyleWu, Kevin Y., Dania Akbar, Michel Giunta, Ananda Kalevar, and Simon D. Tran. 2024. "Hydrogels in Ophthalmology: Novel Strategies for Overcoming Therapeutic Challenges" Materials 17, no. 1: 86. https://doi.org/10.3390/ma17010086
APA StyleWu, K. Y., Akbar, D., Giunta, M., Kalevar, A., & Tran, S. D. (2024). Hydrogels in Ophthalmology: Novel Strategies for Overcoming Therapeutic Challenges. Materials, 17(1), 86. https://doi.org/10.3390/ma17010086






