Abstract
Nowadays, we have observed the dynamic development of bio-heating plants that use wood biomass for heating or energy purposes. The result of this process is a reduction in carbon dioxide emissions as well as in the production of biomass ash (BA). Despite the waste nature of BA, it should be carefully analyzed and assessed for various applications, including environmental ones. Due to the features attributed to BA, including its alkaline reaction, the high capacity of its sorption complex, relatively low salinity, and significant content of macro- and microelements, a hypothesis was put forward in this work undertaken about the positive role of BA as an immobilizing factor for Cd-, Pb-, and Zn-contaminated soils. This research was based on a pot experiment in which four series were considered: (1) BA; (2) BA + Cd; (3) BA + Pb; and (4) BA + Zn. BA was used at doses of 30, 60, and 90 mg pot−1, and metals at doses of 2 mg Cd, 100 mg Pb, and 300 mg Zn kg−1 of soil. The test plant was corn grown for green mass. The study took into account the influence of BA on the content of the total forms of heavy metals (Metot) and their available forms (Meav). In the soil without the addition of metals, a significant increase in the content of Cdtot and Cdav, and a decrease in the content of Zntot were observed due to the application of BA. The addition of metals against the background of the BA used resulted in a significant increase in Cdtot, Pbtot, and Zntot, as well as an increase in the available forms of Pbav but a decrease in Znav. However, there was no significant increase in the Cdav content. The obtained results may indicate the potentially immobilizing role of BA only in the case of zinc. They may constitute the basis for further, more detailed research aimed at determining the role of BA in the immobilization of various metals in soil.
1. Introduction
In the search for effective materials capable of absorbing heavy metals from the environment, including the soil environment, special attention should be paid to waste materials or byproducts of industrial processes. Such processes among others include biomass incineration for heating or energy purposes. The use of biomass in energy production is aimed at reducing the emission of carbon oxides, sulfur dioxide, nitrogen oxides, and other toxic substances into the environment, compared to classic solid and liquid fossil energy sources [1,2], which is one of the basic assumptions of the “Polish Energy Policy until 2030” program [2]. It is estimated that by 2050, 33% to 50% of global energy resources could be covered by biomass burning [3]. The biomass combustion process on a global scale reduces exhaust gas emissions into the environment; however, at the same time it generates a byproduct in the form of biomass ash (BA) [3]. Among the types of biomasses, wood biomass in the form of wood chips is most often used for heating purposes. From the emission point of view, the combustion of wood chips is not included in the carbon dioxide (CO2) emission limits. The use of biomass in the energy sector reduces the accumulative carbon footprint [4]. However, the rational use of produced BA turns out to be problematic. This is because biomass ash (BA) has a variable composition, which is influenced not only by the type of biomass burned [1,2,3,5,6] and its storage time [6], but also by the temperature during the combustion process [2,3] and the possible treatment of the biomass before combustion [1]. According to Zając et al. [5], an increase in the temperature of the wood biomass combustion process reduces the contents of Zn, Pb, Cd, and Cu in BA and increases the content of Cr, Ni, and Fe. Nzihou and Stanmore [7] claim that all metals except Hg are retained in BA. According to the authors, at temperatures below 1000 °C, metals are evenly distributed in each BA particle. However, at temperatures higher than 1000 °C, the external shell of dust molecules is decomposed and smaller particles containing higher concentrations of trace elements are formed. Higher metal concentrations in the finer BA particles result from the devolatilization of metals and their re-condensation. According to Munawar et al. [4], BA storage and transportation also promote the transformation of compounds contained in BA, which react with each other through oxidation and dehydration, creating new, complex compounds.
If BA comes from a reliable source that guarantees a pure and homogeneous raw material, then it contains specific, stable amounts of minerals and trace elements [4]. Due to the abundance of BA in nutrients, including macroelements (Ca, K, Mg, P, Na) and microelements (Zn, Fe, Mn, Cu, B), the application of this material to the soil should be considered first, as it has been proven to have a positive effect on soil properties [4,8]. BA, characterized by a negligible level of xenobiotics, can be used as a substitute for lime in agriculture or for the reclamation of degraded land to improve soil properties. Some authors propose the use of BA as a binding material in the process of fertilizer production from other waste, including sewage sludge [3]. The environmentally friendly management of BA would classify them as raw materials rather than waste and would also promote sustainable development [4] in line with the assumptions of the circular economy. However, the long-term application of BA to soil may become a source of pollution or even contamination of soils due to the presence of heavy metals and other inorganic compounds [1,5] as well as polycyclic aromatic hydrocarbons (PAHs) [3]. Moreover, the process of the compounds leaching from BA after their application to the soil still needs to be clarified [3].
This present work draws attention to the aspect related to the possibility of using BA in soils with increased Cd, Pb, and Zn content. The focus was on the above-mentioned elements because, for example, Cd is characterized by a significant degree of dispersion in bedrocks, high mobility in the environment, and easy movement in the food chain [9,10]. The contamination of soil resources with Cd is constantly increasing, and the contribution of anthropogenic activities in this aspect is very high [10]. In turn, Pb, although considered a less mobile element, is also closely related to the mineralogical and granulometric composition of soils and the parent rock. Pb migrates equally easily in the food chain, especially in soil-contaminated conditions, and, like Cd, it is a toxic element to living organisms. However, Zn is characterized by high solubility in soil solution. Zn has a double meaning: on the one hand, it is a very important microelement in plant nutrition and an essential supplement to the human diet. In plant cultivation, both deficiency and excess of this element may be harmful [9]. The mentioned metals are constantly emitted into the environment as a result of anthropogenic activities such as the rapidly developing metallurgical industry, improper waste disposal, the use of fertilizers and plant protection products, and the use of municipal sewage sludge in agriculture. Their harmfulness and health effects have been widely described in the literature [11]. Moreover, a common feature of these elements is that the acidic reaction of soils favors their mobility in the environment [9]. This knowledge enables the search for appropriate methods of their immobilization in the soil, based on the use of sorbent materials with similar properties. Ultimately, this facilitates the selection of immobilizing additives used for soils contaminated with various metals without causing contradictory effects on their mobility, bioavailability, and, importantly, toxicity [12]. There is relatively little information in the literature on the immobilization role of BA that these materials can play in relation to toxic substances contained in the soil. However, the potential of BA in improving soil properties associated with an increase in pH is quite well known [4,8], as it is able to increase the sorption complex [8] and increase retention in relation to nutrients [8,13,14] and water [4]. The above information provides the basis for indicating the possibility of immobilizing heavy metals in soils as a result of the soil application of BA. However, it should be admitted that most of the research results published so far have not taken into account the properties of BA in relation to the immobilization of heavy metals in the soil. Rather, BA is considered to be a significant source of heavy metals which it can release into the soil during its application [1]. Additionally, the storage of BA can in turn cause the migration of metals and other pollutants not only to soils but also to waters [6]. Due to the few mentions in the literature about the potential role of BA in Cd immobilization [8], the purpose of the research undertaken was to verify these few reports.
In line with these considerations, a research hypothesis was formulated in this study stating that BA may be a factor in immobilizing Cd, Pb, and Zn in soil with increased content of these metals, compared to the null hypothesis stating that there is no effect of BA on the content of the total and available forms of heavy metals in the soil. The primary aim of this work was to demonstrate the possibility of the immobilization of Cd, Pb, and Zn in soil as a result of BA application. The specific objective was to verify whether the adopted doses of BA in conditions of increased metal content would prove effective in immobilizing these elements.
2. Materials and Methods
2.1. Experiment Description
This research was based on a pot experiment, which was carried out in a vegetation hall located on the campus of the University of Warmia and Mazury in Olsztyn (Poland). The experiment included four series: (1) BA, (2) BA + Cd, (3) BA + Pb, and (4) BA + Zn. In all series, ash from wood biomass combustion (BA) was used as a potential Cd-, Pb-, and Zn-immobilizing additive at doses of 30, 60, and 90 g pot−1. The simulated soil pollution with metals was 2 mg Cd, 100 mg Pb, and 300 mg Zn kg−1 soil. Each series included a control subject without the addition of BA. The mass of soil used in the experiment was 9 kg pot−1.
Metals were introduced in the form of aqueous solutions of cadmium acetate (Cd(CH3COO)2 2H2O) (POCh Gliwice, Poland), lead acetate (Pb(CH3COO)2 3H2O) (POCh Gliwice, Poland), and zinc acetate (Zn(CH3COO)2 2H2O) (Chempur, Piekary Śląskie, Poland). Mineral fertilization was applied to each pot, regardless of the series: 0.111 g N, 0.113 g K, and 0.067 g P kg−1 soil. N was applied in the urea form (CH4N2O), P in the form of ammonium phosphate (NH4)2HPO4), and K as a potassium sulfate (K2SO4).
The metal levels used in the experiment (Cd, Pb, and Zn), according to the Regulation of the Minister of the Environment of 1 September 2016 [15], were the maximum allowable contents of these elements in agricultural soils in Poland. According to the mentioned act, these are the maximum permissible concentrations, which according to the Institute of Soil Cultivation and Fertilization in Puławy [16] are referred to as “increased contents”.
In total, the experiment included 16 treatments. Each treatment was considered in three replications. In the experiment, corn (Zea mays L.) was grown for green mass for a period of 51 days (from June 14 to August 4). After harvesting the plants, soil samples were taken from each pot, then dried in the open air and sieved through a sieve with a mesh diameter of 2 mm. Samples prepared in this way were stored in paper packages until chemical analyses were performed.
2.2. Soil Properties
The soil used in the research had a low pH = 4.2 (measured in 1 mol KCl) and the specific electric conductivity (EC) of 0.019 dS m−1. According to its mineralogical composition, the soil was classified as proper brown soil [17], which is typical for most areas of the Warmian–Masurian province (Poland). The soil was characterized by a poor sorption complex (SC). The sum of base cations (SBC), hydrolytic acidity (HAC), and cation exchange capacity (CEC) were 34.5, 22.0, and 56.50 mmol(+) kg−1 of soil, respectively. The base saturation (BS) was only 38.73%. The total carbon content (Ctot) and total nitrogen content (Ntot) were 4.377 g and 0.562 g kg−1, respectively, and the C/N ratio was 7.79. The content of available forms of phosphorus (Pav), potassium (Kav), and magnesium (Mgav) were 79.03; 123.5, and 21.13 mg kg−1 of soil, respectively. The content of the total forms of heavy metals Cdtot, Pbtot, and Zntot were 0.687; 13.22, and 18.57 mg kg−1 of the soil, and the available forms of Cdav, Pbav, and Znav were 0.130; 6.276, and 9.902 mg kg−1 of the soil, respectively.
2.3. Biomass Ash (BA) Properties
The biomass ash (BA) used in the experiment (Figure 1a) originated from Municipal Heating Energy Company in Olsztyn (MPEC Olsztyn, Olsztyn, Poland) and was a by-product of the combustion process of wood chips (Figure 1b), which in Poland are obtained mainly from the processing of coniferous trees, among others that are dominated by species of the genus Pinus L. (Figure 1c). These wood chips are burned in one of the boilers located at MPEC Olsztyn—the Kortowo-Bio heating plant (Figure 1d). During the heating season, the Kortowo-Bio Heating Plant burns approximately 50,000 Mg wood chips [18].

Figure 1.
Kortowo-Bio Heating Plant of Municipal Heating Energy Company in Olsztyn (MPEC Olsztyn). (a) Biomass ash (BA), (b) wood chips, (c) forest waste, and (d) view of the Kortowo-Bio Heating Plant [18].
Biomass ash (BA), according to the legal acts [19] currently applicable in Poland, is considered to be waste and is classified under the code 10 01 03 [19].
According to the performed analyses, BA was classified as an alkaline material (pHKCl = 10.31). The high BA reaction resulted from the level of saturation of the sorption complex (SC) with basic cations (BS), which was 82.38%. The individual SC components were: SBC—3501; HAC—750.0, and CEC—4257 mmol(+) kg−1 BA. Moreover, quite high Ctot content was recorded as Ctot—208.0 g kg−1. The Ntot content was 4.29 g kg−1, the C/N ratio—48.48, and EC—4.51 dS m−1. The dry matter content (DM) in BA was 80.4%. Among the determined total forms of macronutrients in BA content, Ktot (17.18 g kg−1) and Catot (43.97 g kg−1) dominated. Significantly lower levels were found for Ptot (6.36 g kg−1) and Mgtot (6.20 g kg−1), and the lowest content was 1.36 g kg−1, which was recorded for Natot. The largest amount of available forms of macronutrient contents in BA was Kav, followed by Pav, and the smallest amount was Mgav, the contents of which were 410.0, 110.0, and 57.00 mg kg−1 of BA, respectively. The contents of Cdtot, Pbtot, and Zntot (the elements considered in this work) were 1.50; 74.53, and 448.5 mg kg−1 of BA, respectively. In turn, the content of the total forms of other heavy metals in BA: Fe, Mn, Cu, Ni, Co, and Cr, were, respectively, 5684.3, 2377.3, 26.54, 49.95, 4.808, and 30.41 mg kg−1 of BA d.m.
2.4. List of Chemical Analyses
2.4.1. Biomass Ash (BA)
The ash samples were placed on metal trays and dried in the open air until a constant mass was obtained. Then, in order to obtain a homogeneous structure, the material was crushed in a mortar. The analyses performed included the following: pH, EC, SBC, and HAC; the content of DM; Ctot; the total forms of macronutrients (Ntot, Ptot, Ktot, Mgtot, Catot and Natot); and the available forms of Pav, Kav, and Mgav, as well as close to the total content of trace elements (Cdtot, Pbtot, Zntot, Fetot, Mntot, Cutot, Nitot, Crtot and Cotot).
2.4.2. Soil
The soil was collected before setting up the experiment (initial soil for testing) and separately from each pot after the experiment. All samples were dried in the open air, sieved through a sieve with a mesh diameter of 2 mm, and additionally ground in a mortar. Chemical analyses of the initial soil also included pH; EC; sorption properties (SBC, HAC); the content of Ctot; available forms of Pav, Kav, and Mgav; as well as the contents of trace elements (Cdtot, Pbtot, Zntot) and their available forms.
2.5. Methodology of Chemical Analyses
Chemical analyses were performed using the following methods (Figure 2).

Figure 2.
Chemical analyses methods [20,21,22,23,24,25].
The results were validated on the basis of the reference material CRM0120-50G (TraceMetals/Sandy Loam 2, SIGMA-ALDRICH Chemie GmbH, Schnelldorf, Germany) and CRM012-100G (TraceMetals/Fly Ash 2, SIGMA-ALDRICH Chemie GmbH, Schnelldorf, Germany). Moreover, the content of heavy metals (total and available forms) was determined using Fluka standard materials (Charlotte, NC, USA, Cd 51994, Co 05202, Cr 02733, Cu 38996, Fe 16596, Mn 63534, Ni 42242, Pb 16595, and Zn 188227).
Based on the determinations of hydrolytic acidity (HAC) and the sum of the bases (SBC) of the analyzed ash and soil samples, the capacity of the sorption complex (CEC) and the degree of saturation of these materials with basic cations (BS) were calculated. However, based on the determination of the content of total forms and available trace elements, the share of available forms in the total content was calculated [26].
2.6. Statistical Analysis
The statistical analysis included the LSD test (two-way ANOVA), standard deviation (SD), and Pearson’s simple correlation coefficient (r). The LSD test allowed us to assess the impacts of individual BA doses on the possible immobilization of Cd, Pb, and Zn in soil with increased levels of heavy metals. In the LSD analysis, significance and homogeneous groups were determined at the significance level of p < 0.05 using the Duncan test. The simple Pearson coefficient (r) was used to determine the relationship between the tested features. The estimated coefficient allowed us to determine the direction of the influence of increasing ash doses on the tested features. The significance of the obtained values of the correlation coefficient (r) was determined based on statistical tables [27]. Microsoft Excel® for Microsoft 365 MSO, v. 2206 (Microsoft, Redmond, WA, USA) was used for statistical calculations [28] and Statistica v. 13.3 PL (TIBCO Software Inc., Palo Alto, CA, USA) [29].
3. Results
3.1. Contents of the Total Forms of Heavy Metals
The mean Cdtot content in the soil from the series, depending on the series, ranged from 0.588 mg (BA) to 1.910 mg kg−1 (BA + Cd) (Table 1).

Table 1.
Cd content in the soil (mg kg−1)—total forms.
The selected series in the experiment indicated that the soil application of BA increased the Cdtot content in the soil. However, a linear and highly significant increase in Cdtot content (r = 0.887 **) was observed only in series 1 (with BA alone), in which the highest BA dose (90 g pot−1) had a statistically significant impact on the Cdtot content. Simulated soil contamination in series 2 (BA + Cd), 3 (BA + Pb) and 4 (BA + Zn) additionally increased the Cdtot contents in the soil. However, by far the highest Cdtot content in the soil was recorded in series 2, in which a significant increase in Cdtot content was observed in the treatment with the second dose of BA (60 g pot−1).
The mean Pbtot content in the series ranged from 12.88 mg in series 1 (BA) to 81.83 mg kg−1 of soil in series 3 (BA + Pb) simulated with Pb pollution (Table 2). In series 1 (with only BA), the BA doses used resulted in a slight increase in the Pbtot content. A significant increase in Pbtot in relation to BA doses was noted only in series 3 (BA + Pb), but only up to the second BA dose (60 g pot−1). The highest Pbtot content (94.33 mg kg−1 soil) in this series was recorded after the application of the first dose of BA. However, at the highest BA dose, the Pbtot content in the soil was lower than the content in the other objects in this series.

Table 2.
Pb content in soil (mg kg−1)—total forms.
Similar to the soil contents of Cdtot and Pbtot, the content of Zntot was also closely correlated with the experimental series (Table 3).

Table 3.
Zn content in the soil (mg kg−1)—total forms.
A similar Zntot content was recorded in the first three series (BA; BA + Cd; BA + Pb), with a mean ranging from 18.42 to 19.50 mg kg−1 of soil. However, in the series with soil simulated as being polluted with Zn (BA + Zn), the Zntot content in the soil was 10 times higher than the content in the other series and amounted to 195.0 mg kg−1 of soil. In turn, the application of BA without the use of a polluting substance resulted in a decrease in the Zntot content, which can be observed in series 1 (BA). However, in the remaining series, against the background of increasing BA doses, an increase in Zntot content was noted, as indicated by the correlation coefficient (r). In these series, the highest Zntot content was recorded at the highest BA dose.
3.2. Contents of the Available Forms of Heavy Metals
The Cdav content in the soil was strongly correlated with the experimental series (Table 4). The highest content was recorded in series 2 with cadmium acetate (mean 1.538 mg kg−1), and in the remaining series, the content was seven to 11 times lower (0.126–0.146 mg Cdav kg−1 of soil). The lowest Cdav content was recorded in series 1 with BA alone. The obtained value of the correlation coefficient (r) indicated an increase in the Cdav content in the soil as a result of BA application in series 1 (BA) and in series 3 (BA + Pb). However, these differences did not show statistical significance in the LSD test.

Table 4.
Cd content in soil (mg kg−1)—available forms.
The mean content of the available forms of Pbav was at a similar level in series 1 (BA), 2 (BA + Cd), and 4 (BA + Zn), from 6.190 mg to 6.899 mg kg−1 (Table 5). A significantly higher mean Pbav content (58.06 mg kg−1 soil) was recorded in series 3 with lead acetate. The application of BA-only to the soil increased the Pbav content, but this increase was not statistically significant. Only in the series with lead acetate was a significantly higher Pbav content found in the treatments with BA rather than in the control. The difference in the content between the control and the treatments with BA ranged from 29% to 36%.

Table 5.
Pb content in the soil (mg kg−1)—available forms.
Slightly different relationships were observed in the Znav content in the soil (Table 6). Among the series considered, a significantly higher mean Znav content was recorded in series 1 (with only BA) and in series 4 (BA + Zn). This content was as follows: 10.70 and 26.95 mg Znav kg−1 soil, respectively. In the remaining series, the Znav content was much lower; in series 2 (BA + Cd) it was 9.223, and in series 3 (BA + Pb) 8.759 mg kg−1 of soil. In the objects with the addition of BA, a higher Znav content was usually recorded than in the control objects of individual series. These were statistically significant differences. The exception was series 4 (BA + Zn), in which the content of available Zn in the treatments with BA was significantly lower than in the control treatment (without BA).

Table 6.
Zn content in the soil (mg kg−1)—available forms.
3.3. Correlations—Total Forms vs. Available Forms of Heavy Metals
Pearson’s simple coefficient (r) indicated a positive and highly significant correlation between the available forms and the total forms of the considered metal content in the soil (Table 7). The Cdav content was highly significantly correlated to the Cdtot content (r = 0.988 **), the Pbav content to Pbtot (r = 0.986 **), and the Znav content to Zntot (r = 0.982 **). In the remaining relations, significant relationships were observed but were of a different negative nature. However, no significant correlation was found in the Cdtot and Zntot systems.

Table 7.
Correlation between the contents of the total and available forms of heavy metals.
3.4. The Share of the Content of Available Forms of Heavy Metals in Their Total Forms
The share of available forms in the total content of the considered metals was strongly related to the experimental series (Figure 3).
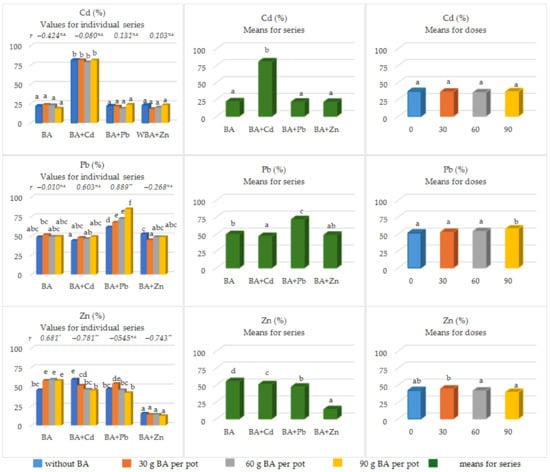
Figure 3.
The share of the available forms of heavy metals (Cd, Pb, Zn) in the total pool of the analyzed soils. As part of the LSDp ≤ 0.05 analysis, different letters (a, b, c, d, abc, etc.) next to the given values indicate the significant impact of individual BA doses on the tested feature; *—significant at p ≤ 0.05; **—highly significant p ≤ 0.01; n.s.—not significant).
In the case of Cd, these values ranged from 21.01% to 80.65%. The lowest share of the available forms of Cd was observed in series 4 (BA + Zn) and the highest was observed in series 2 (BA + Cd). The two remaining series were characterized by the share of available forms of Cd in the range of 21.35–21.78%. The BA doses used did not significantly alter the amount of Cdav in Cdtot.
In turn, the average share of Pbav content in Pbtot in the individual experimental series ranged from 46.62% in series 2 (BA + Cd) to 71.40% in series 3 with lead acetate (BA + Pb). In series 1 (BA) and 2 (BA + Cd), increasing doses of BA did not significantly alter the indicated mean values. However, in series 3 (BA + Pb), the share of Pbav in Pbtot increased significantly at each BA dose. In series 4 (BA + Zn), the share of Pbav in Pbtot was significantly lower only after the first dose of BA (30 mg pot−1). The remaining BA doses had no significant effect on this feature.
In the case of Zn, the results obtained were different. The highest share of Znav in Zntot was observed in series 1 (only with BA), which amounted to 55.27%. However, in series 2 (BA + Cd) and 3 (BA + Pb), this share was significantly lower and amounted to 50.72% and 47.19%, respectively. The lowest share of Znav in Zntot was recorded in the series with simulated Zn pollution (13.89%) and was significantly lower in relation to the remaining series. In series 1 (only with BA), the share of Znav in Zntot increased significantly with increasing doses of BA (r = 0.681 **), and the LSD test indicated a significant effect of the first dose of BA (30 mg pot−1). However, in the series with the addition of Cd and Zn, the share of Znav in Zntot decreased significantly against the background of increasing doses of BA (−0.781 ** < r < −0.743 **).
4. Discussion
The BA used in the presented investigation was characterized by an alkaline reaction and a favorable sorption complex, as well as a relatively low salinity. Moreover, it contained significant amounts of Ctot, K, Ca, Fe, Mn, and Zn. The mentioned properties contributed to the attempt to evaluate this waste in terms of its natural use as a soil improver. Currently, special attention is paid to the fertilizing potential of BA due to its abundance of nutrients such as Ca, K, and a number of microelements [3,4]. Currently, some researchers have determined the target dose of this material for cultivation purposes based on the K content in BA [14].
However, as the literature data indicate [2,4] the composition of BA can be very diverse, not only in terms of the content of macro- and micronutrients, such as Zn, but also in terms of the content of toxic elements, including: Cd and Pb. According to Szostek et al. [30], the composition of ashes after combustion of a mixture of wood and agricultural biomass, among those considered in the presented work, is dominated by Zn (470 mg kg−1). A much lower content level is found for Pb (37.63 mg kg−1), and the lowest is found for Cd (3.69 mg kg−1). Similar changes in the contents of these metals were also observed by Pazalja et al. [1] in pellet ashes. Importantly, according to Szostek et al. [30], these metals occur in the smallest amounts in the exchangeable fraction, which is the most mobile and the most available to plants. In the cases of Pb and Zn, the residual fraction has the largest share of their total contents, and, in the case of Cd, the reducible fraction.
Looking a bit more widely, we can assume that a high pH value and a sorption complex rich in base cations will enable the use of BA as a sorbent of heavy metals in soil with an increased content of heavy metals. This was the assumption behind our research, in which we observed a significant increase in the contents of Cdtot and Cdav in the soil and a significant decrease in the content of Zntot as a result of BA application (Table 1, Table 3 and Table 4). Importantly, this significant increase in the contents of Cdtot and Cdav correlated with BA doses (Table 1 and Table 4) did not correspond to an increase in the proportion of Cdav in Cdtot (Figure 3). In turn, the share of Pbav in Pbtot occurred after the introduction of the highest BA dose (90 g pot−1), and the share of Znav in Zntot decreased significantly at the BA doses of 60 and 90 g pot−1. The obtained results can be explained by the fact that BA application increased the soil reaction [4,8], and this results in the transformation of heavy metals, including Cd, Pb [31,32], and Zn [33] into unavailable (insoluble) forms. Moreover, the addition of BA increases soil porosity and aeration and thus improves the ability to conduct cation exchange and retain nutrients and water in soil [4]. BA application increases the Ctot content in the soil and increases CEC [8], which also promotes the immobilization of heavy metals in the soil [32,33]. Amendments that immobilize pollutants in the soil reduce the leaching of trace elements and, at the same time, their bioavailability [33,34]. This happens by inducing various sorption processes, including adsorption on mineral surfaces, surface precipitation, ion exchange, and the formation of stable complexes with organic ligands [33]. The retention of heavy metals by organic matter depends on many factors, among which soil pH plays an important role [33,34]. This indicator can be regulated by the use of BA [4,8]. The immobilization of Pb and Zn in soil is closely correlated with pH. The lowest mobility of Pb and Zn is observed under neutral to slightly alkaline reaction conditions [33]. According to Hamid et al. [13] alkaline additives increase the soil pH and the negative charge of the surface, thereby reducing the activity of heavy metals. As a result of these changes, obtained after the use of BA, an increase in the content of nutrients in the soil is observed [13], including available forms of P, K, and Mg [8,14].
When applying BA to the soil, attention should be paid to the balance of heavy metals in the soil, because the cultivation of edible plants in soil contaminated with toxic elements is not allowed [31]. The contamination of soil, especially agricultural soils, with heavy metals poses a risk not only to the environment but also to human health [11,13,35,36]. Soils contaminated with heavy metals should first be subjected to remediation processes, of which BA is a promising material [35]. A 3-year study by Szostek et al. [30] showed that the use of BA as a fertilizer significantly increased the Zn content in the soil but did not increase the concentrations of Cd and Pb. In turn, in the research of Wierzbowska et al. [14], only a slight increase in the content of Pb total form was observed. However, in the research by Rolka et al. [8] an increase in the contents of total Pb and Cd, an increase in the available forms of Zn and Pb, and a decrease in the content of available forms of Cd were observed. The current presented studies clearly demonstrate a close relationship between the contents of available forms of metals and their total content in the soil (Table 4). These relations were closely related to simulated soil contamination with the tested metals.
The high share of available forms of Cd, Pb, and Zn and their total forms in the presented research (Figure 2) should also be inextricably linked to the type of extractant used, which in this study was 1 mol dm−3 HCl. HCl is a very strong extractant because it releases metals associated not only with the exchangeable fraction but also with the carbonates and the organic and oxide fractions [34]. HCl was chosen deliberately to indicate the share of metal forms in the soil that may be potentially available to plants, i.e., forms that can be included in the biological cycle [34].
Although there is little data in the literature on the immobilization role of BA, nevertheless, when comparing the composition of BA with the composition of biochar or fly ash from hard coal, one can assume the high effectiveness of BA in this aspect. Hard coal fly ash like BA is characterized by an alkaline reaction, is rich in nutrients, and has a higher water retention capacity. The application of fly ash to soil usually improves the physical, chemical, and biological properties of soils [37,38,39]. Like the use of BA, the application of fly ash may also result in the release of trace elements into the environment or their immobilization. In the research by Ram and Masto [40], the observed process of the immobilization of heavy metals as a result of the use of fly ash was intensified by the addition of other mineral or organic substances. A good result in terms of Pb immobilization in the soil was caused by the use of fly ash together with peat, which, according to Kumpiene et al. [41] results in a significant reduction in the bioavailability of this metal. Importantly, such treatments can be used in situ to remediate Pb-contaminated soils [41].
In recent years, the available literature has paid more and more attention to the immobilization roles of a mixture of fly ash and biochar [42], biochar itself [43,44,45,46,47], and biochar with various minerals [36] or charcoal [48,49]. The mixture of coal ash and biochar showed an effective immobilization of Pb and Cd, which was mainly related to the increased pH value [42]. Biochar obtained from agricultural waste or plant residues produced in the pyrolysis process can act as an effective surface sorbent for Zn [44,47,50], Pb [46,47], and Cd, indicating the permanent retention of these metals [47,50]. Biochar significantly increases soil pH [10,35,43,47,51], increases CEC [43,51], and reduces the mobility of Cd and Zn [12,35]. However, the use of biochar may also have negative environmental effects, including the penetration of pollutants from biochar into the soil. Moreover, the economic benefits of using biochar for recultivation purposes are still not satisfactory [51]. A composite of biochar with minerals, which has a large specific surface area, porous structures, and various surface functional groups, has turned out to be effective in immobilizing metals [36]. According to Cui et al. [48], the addition of charcoal increases the contents of organic matter and total forms of Cd and reduces the amount of its CaCl2-extractable. However, the authors draw attention to the fact that when immobilizing heavy metals, it is very important to ensure the stability of soil aggregates, especially when the reclaimed area is used for agriculture. Interesting results can also be observed in the work of Ðurić et al. [52], in which the effective immobilization of Cd, Pb, and Zn in the soil was noted after the use of paper-ash. The immobilization of these metals occurred through the precipitation of insoluble hydroxides and carbonates. Creating permanent bonds of metals with paper-ash ingredients protects the environment against the possibility of further migration of these elements. Ashes from sewage sludge combustion activated with oxalic acid play a significant role as a material for immobilizing Pb in the soil [53], which can effectively reduce the leaching of Pb from the soil, precipitating it in the stable form of lead phosphate minerals. However, in the case of an excess of oxalic acid, phosphates and Zn may be washed out.
Similarly, in order to improve the immobilization properties of BA, combining this material with other byproducts that have stable compositions and are free from undesirable impurities can be considered. Such a mixture could include materials that will be additional sources of nitrogen, of which BA contains negligible amounts. There are also studies in which potential sorption materials are subjected to various modifications in order to improve their properties and thus improve immobilization effects. An example here is the research of Xu et al. [32], who subjected fly ash to various modifications in order to improve the immobilization effects concerning Pb and Cd. They achieved positive results after subjecting the ashes to low temperatures and hydrothermal treatments. Also, in the case of BA, an aspect worth considering seems to be the selection of the biomass combustion temperature, which may contribute to better quality in terms of heavy metal immobilization in the soil.
5. Conclusions
The application of the adopted BA doses to the soil with natural Cd, Pb, and Zn content (series 1) resulted in an increase in the Cdtot and Cdav contents and a decrease in Zntot. However, a statistically significant increase was recorded for the content of Cdtot when applying 90 g of BA pot−1. Despite these observations, the share of available forms of Cd and Pb in their total content did not change with respect to different doses of BA. The exception was the content of Znav, the share of which in the total content increased as a result of the BA application. Simulated soil pollution with heavy metals resulted in a multiple increase in their total and available content, which was observed in all objects. As a result of these changes, an additional increase in the content of the available forms of Pbav and a decrease in the content of Znav after BA application were observed. However, no significant increase in Cdav content in the soil was detected. Similarly, the share of the total contents of the available forms of the above-mentioned metals was much higher in the soil with simulated pollution with these elements. However, in the context of increasing BA doses, no increase in the share of Cdav in Cdtot was observed. In the case of Pb, the increase in the share of Pbav in Pbtot occurred after the introduction of the highest BA dose (90 g pot−1), and the share of Znav in Zntot decreased at the BA doses of 60 and 90 g pot−1.
The statistical analysis of the results proves a strong correlation between the total content of the metals in question and their available forms. This fact was significantly influenced by the simulated soil contamination.
The obtained results indicate the immobilization effect of BA only with respect to zinc. The analyzed ashes were used on soils with increased metal content. However, the promising trends observed in the formation of Znav in the Zntot pool should be compared in the future with the possibility of using BA in attempts at the remediation of soils with higher levels of contamination and also in relation to other heavy metals.
Author Contributions
Conceptualization, E.R., M.W., A.C.Ż. and A.S.-N.; methodology, E.R., M.W., A.C.Ż. and A.S.-N.; software, E.R., M.W., A.C.Ż. and A.S.-N.; validation, E.R.; formal analysis, E.R.; investigation, E.R. and A.S.-N.; data curation, E.R.; writing—original draft preparation, E.R. and A.S.-N.; writing—review and editing, E.R., M.W., A.C.Ż. and A.S.-N.; visualization, E.R., M.W., A.C.Ż. and A.S.-N.; supervision, E.R., M.W. and A.C.Ż.; project administration, E.R.; funding acquisition, M.W.; corresponding author, M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Warmia and Mazury in Olsztyn, Faculty of Agriculture and Forestry, Department of Agricultural and Environmental Chemistry (grant No. 30.610.004-110).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pazalja, M.; Salihović, M.; Sulejmanović, J.; Smajović, A.; Begić, S.; Špirtović-Halilović, S.; Sher, F. Heavy metals content in ashes of wood pellets and the health risk assessment related to their presence in the environment. Sci. Rep. 2021, 11, 17952. [Google Scholar] [CrossRef] [PubMed]
- Diatta, J.; Kowalski, M. Ashes from Combustion of Plant Biomass (Phytoashes)—Recyckling and Agro-Chemical Potential. In Proceedings of the Materials of XXIV International Conference on Ashes from the Energy Industry, Zakopane, Poland, 26–28 September 2017; pp. 1–15. Available online: http://unia-ups.pl/wp-content/uploads/2016/03/Diatta.pdf (accessed on 10 February 2024).
- Odzijewicz, J.I.; Wołejko, E.; Wydro, U.; Wasil, M.; Jabłońska-Trypuć, A. Utilization of ashes from biomass combustion. Energies 2022, 15, 9653. [Google Scholar] [CrossRef]
- Munawar, M.A.; Khoja, A.H.; Naqvi, S.R.; Mehran, M.T.; Hassan, M.; Liaquat, R.; Dawood, U.F. Challenges and opportunities in biomass ash management and its utilization in novel applications. Renew. Sustain. Energy Rev. 2021, 150, 111451. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Szczepaniak, M. Influence of biomass incineration temperature on the content of selected heavy metals in the ash used for fertilizing purposes. Appl. Sci. 2019, 9, 1790. [Google Scholar] [CrossRef]
- Smołka-Danielowska, D.; Jabłońska, M. Chemical and mineral composition of ashes from wood biomass combustion in domestic wood-fired furnaces. Int. J. Environ. Sci. Technol. 2022, 19, 5359–5372. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B. The fate of heavy metals during combustion and gasification of contaminated biomass—A brief review. J. Hazard. Mater. 2013, 256–257, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Rolka, E.; Żołnowski, A.C.; Wyszkowski, M.; Zych, W.; Skorwider-Namiotko, A. Wood Biomass ash (WBA) from the heat production process as a mineral amendment for improving selected soil properties. Energies 2023, 16, 5110. [Google Scholar] [CrossRef]
- Terelak, H.; Piotrowska, M.; Motowicka-Terelak, T.; Stuczyński, T.; Budzyńska, K. The Content of Heavy Metals and Sulphur in Soils of Agricultural Land of Poland and the Degree of Their Pollution with These Elements. Probl. Notebook. Progress Agric. Sci. 1995, 418, 45–60. Available online: https://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-article-70433682-9b9d-21.463f-8d0c-171dd982101d (accessed on 12 February 2024).
- Ahmed, N.; Shah, A.R.; Danish, S.; Fahad, S.; Ali, M.A.; Zarei, T.; Vranová, V.; Datta, R. Immobilization of Cd in soil by biochar and new emerging chemically produced carbon. J. King Saud Univ. Agric. Sci. 2021, 33, 101472. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Wang, X.; Hussain, B.; Yaseen, M.; Aziz, M.Z.; Yang, X. Immobilization of cadmium and lead in contaminated paddy field using inorganic and organic additives. Sci. Rep. 2018, 8, 17839. [Google Scholar] [CrossRef] [PubMed]
- Wierzbowska, J.; Sienkiewicz, S.; Żarczyński, P.; Krzebietke, S. Environmental application of ash from incinerated biomass. Agronomy 2020, 10, 482. [Google Scholar] [CrossRef]
- Ministry of the Environment. Regulation of the Minister of the Environment of September 1, 2016 on the Method of Assessing Pollution of the Earth’s Surface. J. Laws Repub. Policy 2016, 1395, 1–86. [Google Scholar]
- Terelak, H.; Piotrowska, M.; Motowicka-Terelak, T.; Stuczyński, T.; Budzyńska, K.; Pietruch, C.; Sroczyński, W. Chemical properties of soils and the content of heavy metals and sulfur in soils and plants. In Expertise Prepared for the Ministry of Agriculture and Food; IUNG Publishing House: Puławy, Poland, 1994; pp. 1–96. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014; World Soil Resources Report. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Update 2015; World Soil Resources; Reports No. 106; FAO: Rome, Italy, 2015; p. 192. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 18 November 2023).
- MPEC Olsztyn. Available online: https://sway.cloud.microsoft/MCrZLaAJFsDYw0bg?ref=email (accessed on 15 March 2024).
- Ministry of Climate. Regulation of the Minister of Climate of 3 January 2020 on the waste catalog. J. Laws Repub. Policy 2020, 10, 1–48. [Google Scholar]
- Karczewska, A.; Kabała, C. Methodology of Laboratory Analyzes of Soils and Plants; University of Life Sciences: Wrocław, Poland, 2008. [Google Scholar]
- ISO 11261; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995.
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Assessment of Soil and Plants Properties, 1st ed.; Institute of Environmental Protection: Warsaw, Poland, 1991. [Google Scholar]
- US Environmental Protection Agency. Method 3051. Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils; US Environmental Protection Agency: Washington, DC, USA, 2007; Available online: https://settek.com/documents/EPA-Methods/PDF/EPA-Method-3051.pdf (accessed on 10 February 2023).
- Egner, H.; Riehm, H.; Domingo, W.R. Untersuchungen uber die chemische bodenanalyse als grundlage fur die beurteilung des nahrstoffzustandes der boden. II. Chemische extraktionsmethoden zur phosphorund kaliumbestimmung. K. Lantbrukshögskolans Ann. 1960, 26, 199–215. [Google Scholar]
- CEM Corporation. CEM Mars 6 Operation Manual; CEM Corporation: Matthews, NC, USA, 2017. [Google Scholar]
- Rolka, E.; Wyszkowski, M. Availability of trace elements in soil with simulated cadmium, lead and zinc pollution. Minerals 2021, 11, 879. [Google Scholar] [CrossRef]
- Burdzy, J. Statistical Tables; Lodz University of Technology Publishing House: Lublin, Poland, 1999. [Google Scholar]
- Microsoft. MS Excel® for Microsoft 365 MSO; Microsoft Corporation: Albuquerque, NM, USA, 2021. [Google Scholar]
- Tibco. Statistica Data Analysis Software System; Tibco Software Inc.: Palo Alto, CA, USA, 2021. [Google Scholar]
- Szostek, M.; Szpunar-Krok, E.; Ilek, A. Chemical speciation of trace elements in soil fertilized with biomass combustion ash and their accumulation in winter oilseed rape plants. Agronomy 2023, 13, 942. [Google Scholar] [CrossRef]
- Wang, W.; Song, W.; Zhou, T.; Wang, Z.; Christie, P.; Wu, L. Soil Metal Immobilization in Agricultural Land Contaminated with Cadmium and Lead: A Case Study of Effectiveness Evaluation in Lanping, Southwest China. Bull. Environ. Contam. Toxicol. 2021, 107, 1227–1235. Available online: https://link.springer.com/article/10.1007/s00128-021-03267-8 (accessed on 12 March 2024). [CrossRef]
- Xu, D.; Ji, P.; Wang, L.; Zhao, X.; Hu, X.; Huang, X.; Zhao, H.; Liu, F. Effect of modified fly ash on environmental safety of two soils contaminated with cadmium and lead. Ecotoxicol. Environ. Saf. 2021, 215, 112175. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Ociepa, E.; Ociepa-Kubicka, A.; Okoniewska, E.; Lach, J. The immobilization of zinc and cadmium in the soil as a result of the use of waste substrates. Ann. Set Environ. Prot. 2013, 15, 1772–1786. [Google Scholar]
- Wang, R.; Mohammad Shafi, M.; Ma, J.; Zhong, B.; Guo, J.; Hu, X.; Xu, W.; Yang, Y.; Ruan, Z.; Wang, Y.; et al. Effect of amendments on contaminated soil of multiple heavy metals and accumulation of heavy metals in plants. Environ. Sci. Pollut. Res. 2018, 25, 28695–28704. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; You, L.C.; Lyu, H.H.; Liu, Y.X.; He, L.L.; Hu, Y.D.; Luo, F.C.; Yang, S.M. Role of biochar–mineral composite amendment on the immobilization of heavy metals for Brassica chinensis from naturally contaminated soil. Environ. Technol. Innov. 2022, 28, 102622. [Google Scholar] [CrossRef]
- Ciećko, Z.; Żołnowski, A.C.; Chełstowski, A. Long-term effect of coal fly ash application on soil total nitrogen and organic carbon concentrations. In Application of Phytotechnologies for Cleanup of Industrial, Agricultural, and Wastewater Contamination; Kulakow, P.A., Pidlisnyuk, V.V., Eds.; Springer: Dodrecht, The Netherlands, 2010; pp. 147–158. [Google Scholar]
- Ciećko, Z.; Żołnowski, A.C.; Madej, M.; Wasiak, G.; Lisowski, J. Long-term effects of hard coal fly ash on selected soil 666 properties. Pol. J. Environ. Stud. 2015, 24, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Ciećko, Z.; Żołnowski, A.C.; Madej, M.; Wasiak, G.; Lisowski, J.; Rolka, E. The long-term impact of ameliorating doses of hard coal fly ash on shaping the content of selected microelements in agricultural soil. Pol. J. Soil Sci. 2015, 48, 671. [Google Scholar] [CrossRef]
- Ram, L.C.; Masto, R.E. Fly ash for soil amelioration: A review on the influence of ash blending with inorganic and organic amendments. Earth Sci. Rev. 2014, 128, 52–74. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of Pb- and Cu-contaminated soil using coal fly ash and peat. Environ. Pollut. 2007, 145, 365–373. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Y.; Sun, Y.; Miao, W.; Liu, Z. Co-pyrolysis of corn stover with industrial coal ash for in situ efficient remediation of heavy metals in multi-polluted soil. Environ. Pollut. 2021, 289, 117840. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Eyles, J.L.; Sizmur, T.; Collins, C.D.; Hodson, M.E. Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements. Environ. Pollut. 2011, 159, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhao, J.; Yang, W.; He, L.; Wei, W.; Tan, X.; Wang, J.; Lin, A. Evaluation of biochar pyrolyzed from kitchen waste, corn straw, and peanut hulls on immobilization of Pb and Cd in contaminated soil. Environ. Pollut. 2020, 261, 114133. [Google Scholar] [CrossRef] [PubMed]
- Barčauskaitė, K.; Anne, O.; Mockevičienė, I.; Repšienė, R.; Šiaudinis, G.; Karčauskienė, D. Determination of heavy metals immobilization by chemical fractions in contaminated soil amended with biochar. Sustainability 2023, 15, 8677. [Google Scholar] [CrossRef]
- Cui, L.; Li, L.; Zhang, A.; Pan, G.; Bao, D.; Chang, A.; Cui, L.; Li, L.; Zhang, A.; Pan, G.; et al. Biochar Amendment Greatly Reduces Rice Cd Uptake in a Contaminated Paddy Soil: A Two-Year Field Experiment. Bioresources 2011, 6, 2605–2618. Available online: https://bioresources.cnr.ncsu.edu/wp-content/uploads/2016/06/BioRes_06_3_2605_Cui_LZPB_Biochar_Amend_Rice_CD_Soil_Exper_1651.pdf (accessed on 26 February 2024). [CrossRef]
- Żolnowski, A.; Ciecko, Z.; Najmowicz, T. Arsenic content in and uptake by plants from arsenic-contaminated soil. In Application of Phytotechnologies for Cleanup of Industrial, Agricultural, and Wastewater Contamination; Kulakow, P.A., Pidlisnyuk, V.V., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 135–145. [Google Scholar]
- Beesley, L.; Marmiroli, M. The immobilization and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollut. 2011, 159, 474–480. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Hu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Ðurić, M.; Oprčkal, P.; Serjun, V.Z.; Pranjić, A.M.; Ščančar, J.; Milačič, R.; Mladenovič, A. Environmental impacts and immobilization mechanisms of cadmium, lead and zinc in geotechnical composites made from contaminated soil and paper-ash. Appl. Sci. 2021, 11, 11822. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Chen, Z.; Xue, Q.; Chen, X.; Mu, Y.; Poon, C.S. Immobilization of high-Pb contaminated soil by oxalic acid activated incinerated sewage sludge ash. Environ. Pollut. 2021, 284, 117720. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).