Photodegradation of Microplastics through Nanomaterials: Insights into Photocatalysts Modification and Detailed Mechanisms
Abstract
:1. Introduction
2. Enhancement of Photocatalytic Efficiency
2.1. Element Doping
2.2. Heterojunction Construction

2.3. Other Improvement Strategies
2.3.1. Sensitizers
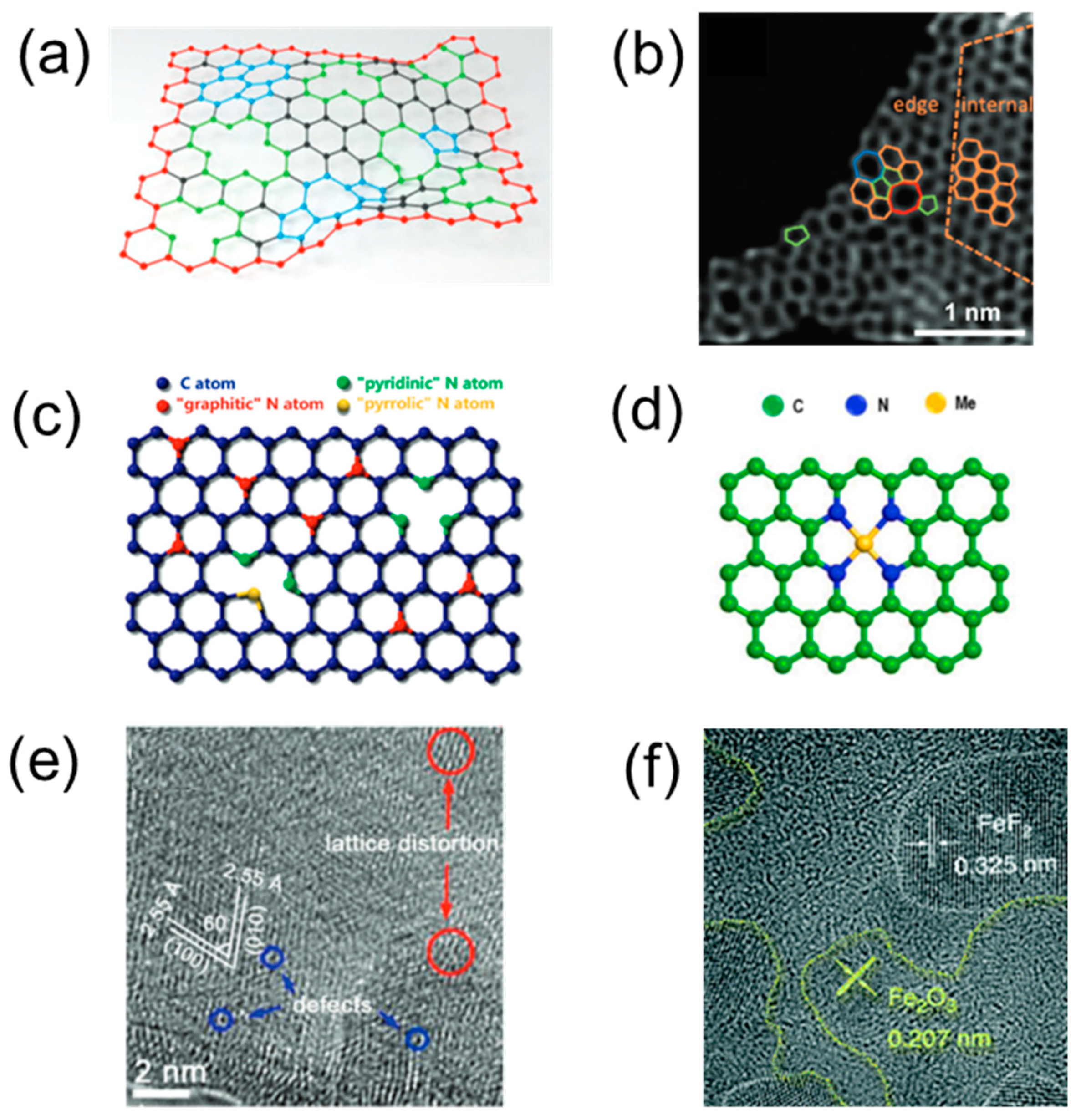
2.3.2. Defect Engineering
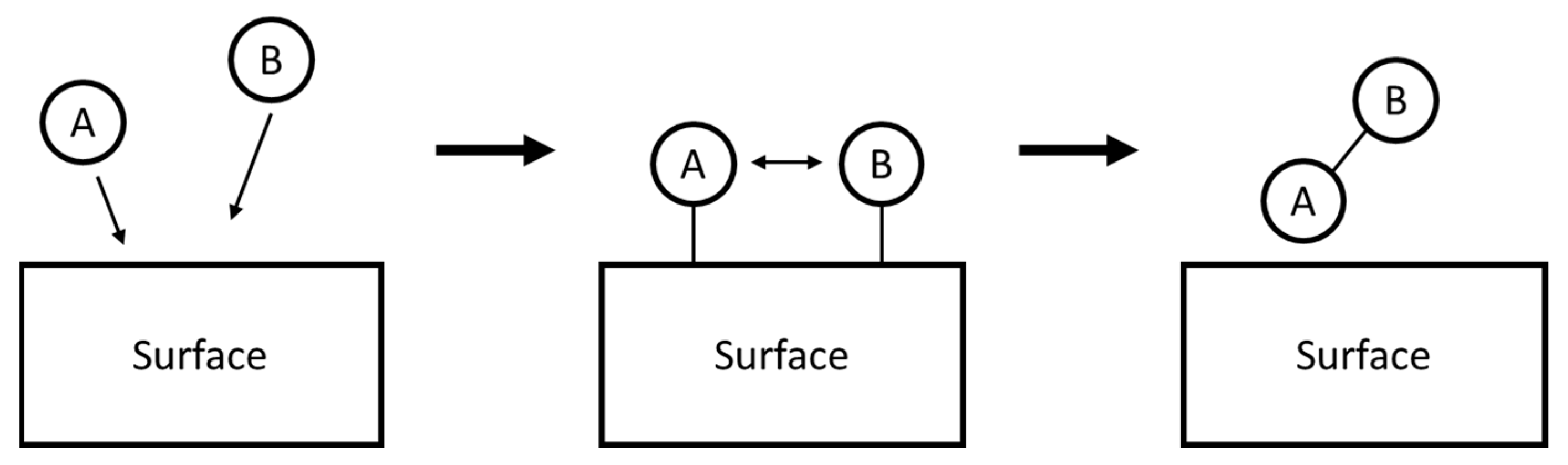
2.3.3. Surface Modification and Morphology Control
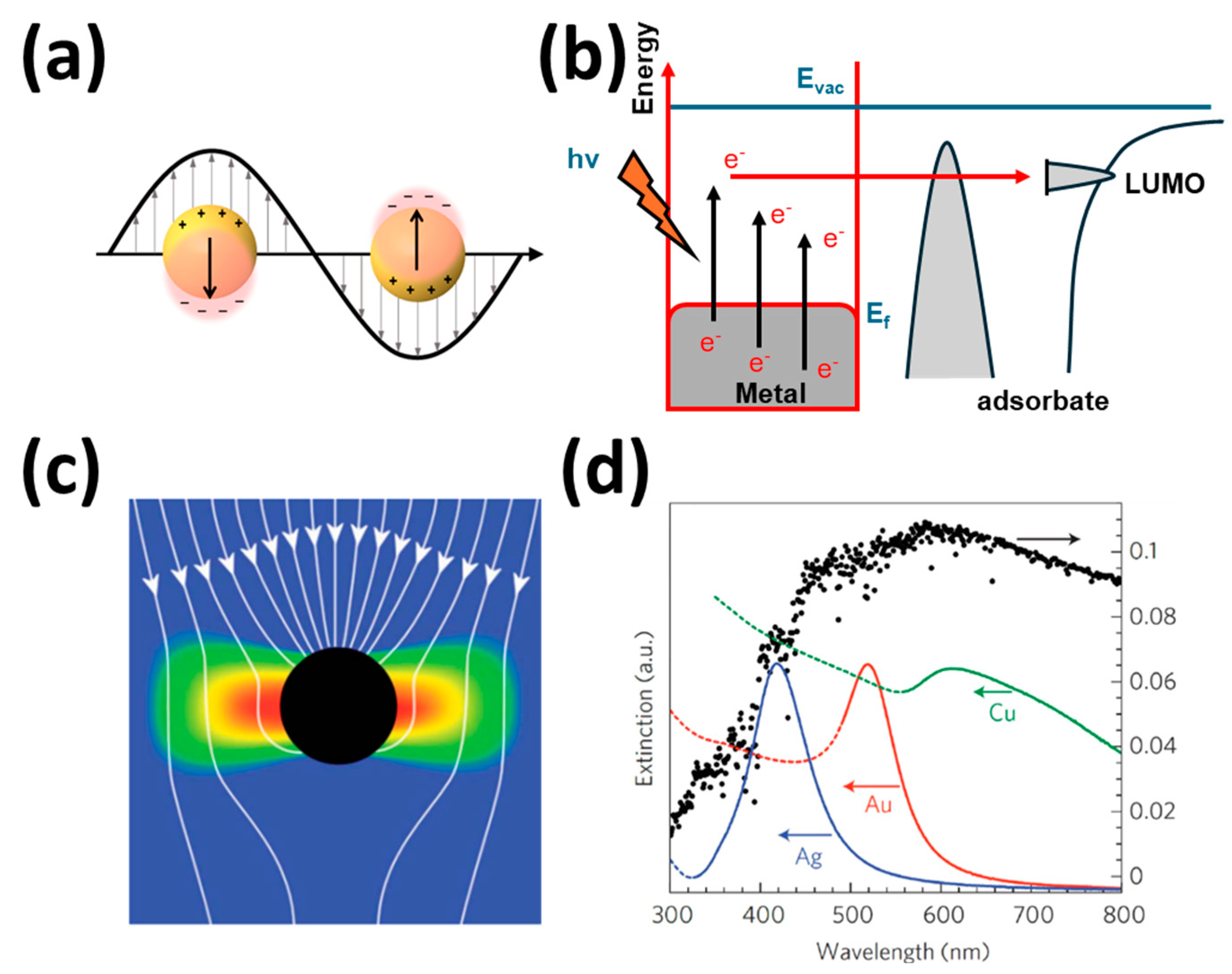
2.3.4. Nobel Metal Deposition (Plasmonic Photocatalysts)
2.4. Photocatalysts with Support Materials
2.5. Factors Influencing Photocatalytic Degradation of Microplastics
3. Photocatalytic Mechanisms in Nanomaterial-Mediated Plastic Degradation
3.1. Techniques Used to Investigate Mechanisms
3.2. Mechanism of Photocatalysis
3.2.1. Free Radical Mechanism



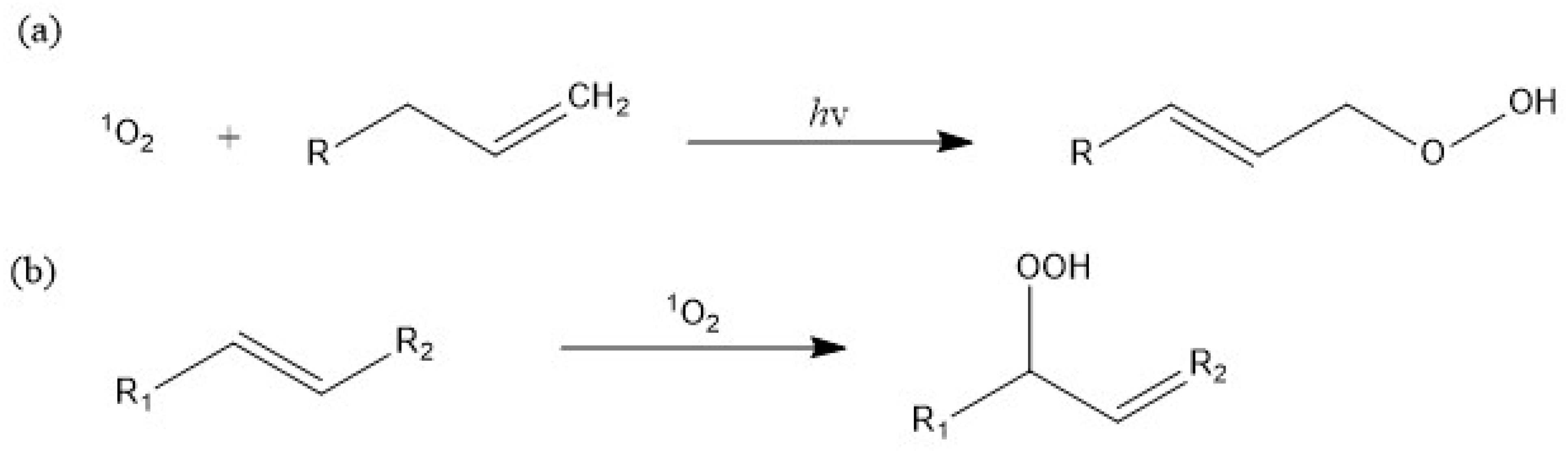
3.2.2. Singlet Oxygen Oxidation Mechanism
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- OECD iLibrary Plastics Use in 2019. Available online: https://www.oecd-ilibrary.org/environment/data/global-plastic-outlook/plastics-use-in-2019_efff24eb-en (accessed on 13 February 2023).
- Häder, D.-P.; Banaszak, A.T.; Villafañe, V.E.; Narvarte, M.A.; González, R.A.; Helbling, E.W. Anthropogenic Pollution of Aquatic Ecosystems: Emerging Problems with Global Implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef] [PubMed]
- Erren, T.; Zeuß, D.; Steffany, F.; Meyer-Rochow, B. Increase of Wildlife Cancer: An Echo of Plastic Pollution? Nat. Rev. Cancer 2009, 9, 842. [Google Scholar] [CrossRef]
- Machovsky-Capuska, G.E.; Amiot, C.; Denuncio, P.; Grainger, R.; Raubenheimer, D. A Nutritional Perspective on Plastic Ingestion in Wildlife. Sci. Total Environ. 2019, 656, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Bauer, F.; Nielsen, T.D.; Nilsson, L.J.; Palm, E.; Ericsson, K.; Fråne, A.; Cullen, J. Plastics and Climate Change—Breaking Carbon Lock-Ins through Three Mitigation Pathways. One Earth 2022, 5, 361–376. [Google Scholar] [CrossRef]
- Pelegrini, K.; Maraschin, T.G.; Brandalise, R.N.; Piazza, D. Study of the Degradation and Recyclability of Polyethylene and Polypropylene Present in the Marine Environment. J. Appl. Polym. Sci. 2019, 136, 48215. [Google Scholar] [CrossRef]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics Removal in Wastewater Treatment Plants: A Critical Review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- D’Avignon, G.; Gregory-Eaves, I.; Ricciardi, A. Microplastics in Lakes and Rivers: An Issue of Emerging Significance to Limnology. Environ. Rev. 2022, 30, 228–244. [Google Scholar] [CrossRef]
- Lechner, A.; Keckeis, H.; Lumesberger-Loisl, F.; Zens, B.; Krusch, R.; Tritthart, M.; Glas, M.; Schludermann, E. The Danube so Colourful: A Potpourri of Plastic Litter Outnumbers Fish Larvae in Europe’s Second Largest River. Environ. Pollut. 2014, 188, 177–181. [Google Scholar] [CrossRef]
- Elkhatib, D.; Oyanedel-Craver, V. A Critical Review of Extraction and Identification Methods of Microplastics in Wastewater and Drinking Water. Environ. Sci. Technol. 2020, 54, 7037–7049. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The Physical Impacts of Microplastics on Marine Organisms: A Review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and Effects of Plastic Additives on Marine Environments and Organisms: A Review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Zazouli, M.; Nejati, H.; Hashempour, Y.; Dehbandi, R.; Nam, V.T.; Fakhri, Y. Occurrence of Microplastics (MPs) in the Gastrointestinal Tract of Fishes: A Global Systematic Review and Meta-Analysis and Meta-Regression. Sci. Total Environ. 2022, 815, 152743. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Pedriza, A.; Jaumot, J. Interaction of Environmental Pollutants with Microplastics: A Critical Review of Sorption Factors, Bioaccumulation and Ecotoxicological Effects. Toxics 2020, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Bacha, A.-U.-R.; Nabi, I.; Zhang, L. Mechanisms and the Engineering Approaches for the Degradation of Microplastics. ACS EST Eng. 2021, 1, 1481–1501. [Google Scholar] [CrossRef]
- Chu, S.; Zhang, B.; Zhao, X.; Soo, H.S.; Wang, F.; Xiao, R.; Zhang, H. Photocatalytic Conversion of Plastic Waste: From Photodegradation to Photosynthesis. Adv. Energy Mater. 2022, 12, 2200435. [Google Scholar] [CrossRef]
- Santos, R.G.; Capuska, E.; Andrades, R. Plastic Ingestion as an Evolutionary Trap: Toward a Holistic Understanding. Science 2021, 373, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Microbead-Free Waters Act of 2015. 2015; p. Public Law No. 114-114 Stat. 3129. Available online: https://www.congress.gov/bill/114th-congress/house-bill/1321/text (accessed on 15 March 2023).
- International Union for Conservation of Nature. IUCN 2017: International Union for Conservation of Nature Annual Report 2017; IUCN: Gland, Switzerland, 2018. [Google Scholar]
- Rajala, K.; Grönfors, O.; Hesampour, M.; Mikola, A. Removal of Microplastics from Secondary Wastewater Treatment Plant Effluent by Coagulation/Flocculation with Iron, Aluminum and Polyamine-Based Chemicals. Water Res. 2020, 183, 116045. [Google Scholar] [CrossRef] [PubMed]
- Sarno, A.; Olafsen, K.; Kubowicz, S.; Karimov, F.; Sait, S.T.L.; Sørensen, L.; Booth, A.M. Accelerated Hydrolysis Method for Producing Partially Degraded Polyester Microplastic Fiber Reference Materials. Environ. Sci. Technol. Lett. 2021, 8, 250–255. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M. The Effect of Water Ozonation in the Presence of Microplastics on Water Quality and Microplastics Degradation. Sci. Total Environ. 2024, 929, 172595. [Google Scholar] [CrossRef]
- Kelkar, V.P.; Rolsky, C.B.; Pant, A.; Green, M.D.; Tongay, S.; Halden, R.U. Chemical and Physical Changes of Microplastics during Sterilization by Chlorination. Water Res. 2019, 163, 114871. [Google Scholar] [CrossRef] [PubMed]
- Malankowska, M.; Echaide-Gorriz, C.; Coronas, J. Microplastics in Marine Environment: A Review on Sources, Classification, and Potential Remediation by Membrane Technology. Environ. Sci. Water Res. Technol. 2021, 7, 243–258. [Google Scholar] [CrossRef]
- Cunha, C.; Faria, M.; Nogueira, N.; Ferreira, A.; Cordeiro, N. Marine vs Freshwater Microalgae Exopolymers as Biosolutions to Microplastics Pollution. Environ. Pollut. 2019, 249, 372–380. [Google Scholar] [CrossRef]
- Torena, P.; Alvarez-Cuenca, M.; Reza, M. Biodegradation of Polyethylene Terephthalate Microplastics by Bacterial Communities from Activated Sludge. Can. J. Chem. Eng. 2021, 99, S69–S82. [Google Scholar] [CrossRef]
- Lin, S.; Huang, H.; Ma, T.; Zhang, Y. Photocatalytic Oxygen Evolution from Water Splitting. Adv. Sci. 2021, 8, 2002458. [Google Scholar] [CrossRef] [PubMed]
- Tofa, T.S.; Kunjali, K.L.; Paul, S.; Dutta, J. Visible Light Photocatalytic Degradation of Microplastic Residues with Zinc Oxide Nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef]
- Lagarde, F.; Olivier, O.; Zanella, M.; Daniel, P.; Hiard, S.; Caruso, A. Microplastic Interactions with Freshwater Microalgae: Hetero-Aggregation and Changes in Plastic Density Appear Strongly Dependent on Polymer Type. Environ. Pollut. 2016, 215, 331–339. [Google Scholar] [CrossRef]
- Li, L.; Xu, G.; Yu, H.; Xing, J. Dynamic Membrane for Micro-Particle Removal in Wastewater Treatment: Performance and Influencing Factors. Sci. Total Environ. 2018, 627, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, Identification and Removal of Microplastic Particles and Fibers in Conventional Activated Sludge Process and Advanced MBR Technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, G.; Yue, J.; Xing, X.; Yang, Z.; Wang, X.; Wang, Q.; Zhang, J. Enhanced Removal of Polyethylene Terephthalate Microplastics through Polyaluminum Chloride Coagulation with Three Typical Coagulant Aids. Sci. Total Environ. 2021, 800, 149589. [Google Scholar] [CrossRef]
- Sturm, M.T.; Horn, H.; Schuhen, K. Removal of Microplastics from Waters through Agglomeration-Fixation Using Organosilanes—Effects of Polymer Types, Water Composition and Temperature. Water 2021, 13, 675. [Google Scholar] [CrossRef]
- Zafar, R.; Park, S.Y.; Kim, C.G. Surface Modification of Polyethylene Microplastic Particles during the Aqueous-Phase Ozonation Process. Environ. Eng. Res. 2020, 26, 200412. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Ding, J.; Song, Z.; Yang, B.; Zhang, C.; Guan, B. Degradation of Nano-Sized Polystyrene Plastics by Ozonation or Chlorination in Drinking Water Disinfection Processes. Chem. Eng. J. 2022, 427, 131690. [Google Scholar] [CrossRef]
- Lin, J.; Yan, D.; Fu, J.; Chen, Y.; Ou, H. Ultraviolet-C and Vacuum Ultraviolet Inducing Surface Degradation of Microplastics. Water Res. 2020, 186, 116360. [Google Scholar] [CrossRef]
- Arossa, S.; Martin, C.; Rossbach, S.; Duarte, C.M. Microplastic Removal by Red Sea Giant Clam (Tridacna Maxima). Environ. Pollut. 2019, 252, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, R.; Nithya, P.M.; Girish Kumar, S. Noble Metal Deposited Graphitic Carbon Nitride Based Heterojunction Photocatalysts. Appl. Surf. Sci. 2020, 508, 145142. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A Review on Non Metal Ion Doped Titania for the Photocatalytic Degradation of Organic Pollutants under UV/Solar Light: Role of Photogenerated Charge Carrier Dynamics in Enhancing the Activity. Appl. Catal. B Environ. 2013, 140–141, 559–587. [Google Scholar] [CrossRef]
- Rehman, S.; Ullah, R.; Butt, A.M.; Gohar, N.D. Strategies of Making TiO2 and ZnO Visible Light Active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Ng, A.M.C. Strategies for Improving the Efficiency of Semiconductor Metal Oxide Photocatalysis. Mater. Horiz. 2014, 1, 400–410. [Google Scholar] [CrossRef]
- Sudha, D.; Sivakumar, P. Review on the Photocatalytic Activity of Various Composite Catalysts. Chem. Eng. Process. Process Intensif. 2015, 97, 112–133. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic Reduction of Carbon Dioxide in Aqueous Suspensions of Semiconductor Powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Tian, Y.; Zhan, Y.; Zhu, J. Degradation of organic pollutants in flocculated liquid digestate using photocatalytic titanate nanofibers: Mechanism and response surface optimization. Front. Agric. Sci. Eng. 2023, 10, 492–502. [Google Scholar] [CrossRef]
- Ye, X.; Tian, Y.; Gao, M.; Cheng, F.; Lan, J.; Chen, H.; Lanoue, M.; Huang, S.; Tian, R. Efficient Photocatalytic Core-Shell of Titanate Nanowire/rGO. Catalysts 2024, 14, 218. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Tu, W.; Chen, G.; Xu, R. Metal-Free Photocatalysts for Various Applications in Energy Conversion and Environmental Purification. Green Chem. 2017, 19, 882–899. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Liu, H.; Huang, J. Design, Modification and Application of Semiconductor Photocatalysts. J. Taiwan Inst. Chem. Eng. 2018, 93, 590–602. [Google Scholar] [CrossRef]
- Shao, W.; Wang, H.; Zhang, X. Elemental Doping for Optimizing Photocatalysis in Semiconductors. Dalton Trans. 2018, 47, 12642–12646. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, L.; Xiao, Y.; Collins, T.; Akhter, A.; Huang, Y.; Tian, Z.R. Mo-Doped Titanate Nanofibers from Hydrothermal Syntheses for Improving Bone Scaffold. Charact. Appl. Nanomater. 2024, 7, 3587. [Google Scholar] [CrossRef]
- Schöttner, L.; Erker, S.; Schlesinger, R.; Koch, N.; Nefedov, A.; Hofmann, O.T.; Wöll, C. Doping-Induced Electron Transfer at Organic/Oxide Interfaces: Direct Evidence from Infrared Spectroscopy. J. Phys. Chem. C 2020, 124, 4511–4516. [Google Scholar] [CrossRef] [PubMed]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of Doped Photocatalysts for Organic Pollutant Degradation—A Review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Matsuo, H.; Noguchi, Y.; Miyayama, M. Gap-State Engineering of Visible-Light-Active Ferroelectrics for Photovoltaic Applications. Nat. Commun. 2017, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Cole, P.; Tian, Y.; Thornburgh, S.; Malloy, M.; Roeder, L.; Zhang, L.; Patel, M.; Xiao, Y.; Huang, Y.; Tian, Z.R. Hydrothermal Synthesis of Valve Metal Ta-Doped Titanate Nanofibers for Potentially Engineering Bone Tissue. Charact. Appl. Nanomater. 2024, 6, 3606. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Mathpal, M.C.; Kumar, P.; Singh, M.K.; Soler, M.A.G.; Agarwal, A. Structural, Optical and Photoconductivity of Sn and Mn Doped TiO2 Nanoparticles. J. Alloys Compd. 2015, 622, 37–47. [Google Scholar] [CrossRef]
- Yao, B.; Peng, C.; Zhang, W.; Zhang, Q.; Niu, J.; Zhao, J. A Novel Fe(III) Porphyrin-Conjugated TiO2 Visible-Light Photocatalyst. Appl. Catal. B Environ. 2015, 174–175, 77–84. [Google Scholar] [CrossRef]
- Viswanathan, B.; Krishanmurthy, K.R. Nitrogen Incorporation in TiO2: Does It Make a Visible Light Photo-Active Material? Int. J. Photoenergy 2012, 2012, e269654. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Yang, S.; Lee, H. Surface Analysis of N-Doped TiO2 Nanorods and Their Enhanced Photocatalytic Oxidation Activity. Appl. Catal. B Environ. 2017, 204, 209–215. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Ao, Z.; Wang, S. Degradation of Cosmetic Microplastics via Functionalized Carbon Nanosprings. Matter 2019, 1, 745–758. [Google Scholar] [CrossRef]
- Zhou, D.; Luo, H.; Zhang, F.; Wu, J.; Yang, J.; Wang, H. Efficient Photocatalytic Degradation of the Persistent PET Fiber-Based Microplastics over Pt Nanoparticles Decorated N-Doped TiO2 Nanoflowers. Adv. Fiber Mater. 2022, 4, 1094–1107. [Google Scholar] [CrossRef]
- Giampietri, A.; Drera, G.; Sangaletti, L. Band Alignment at Heteroepitaxial Perovskite Oxide Interfaces. Experiments, Methods, and Perspectives. Adv. Mater. Interfaces 2017, 4, 1700144. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Qin, J.; Dou, Y.; Wu, F.; Yao, Y.; Andersen, H.R.; Hélix-Nielsen, C.; Lim, S.Y.; Zhang, W. In-Situ Formation of Ag2O in Metal-Organic Framework for Light-Driven Upcycling of Microplastics Coupled with Hydrogen Production. Appl. Catal. B Environ. 2022, 319, 121940. [Google Scholar] [CrossRef]
- Feng, B.; Wu, Z.; Liu, J.; Zhu, K.; Li, Z.; Jin, X.; Hou, Y.; Xi, Q.; Cong, M.; Liu, P.; et al. Combination of Ultrafast Dye-Sensitized-Assisted Electron Transfer Process and Novel Z-Scheme System: AgBr Nanoparticles Interspersed MoO3 Nanobelts for Enhancing Photocatalytic Performance of RhB. Appl. Catal. B Environ. 2017, 206, 242–251. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and Heterogeneous Photo-Catalysis at Semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, L.; Zhang, F.; Wu, J.; Wang, H.; Yang, J. Feasible Degradation of Polyethylene Terephthalate Fiber-Based Microplastics in Alkaline Media with Bi2O3@N-TiO2 Z-Scheme Photocatalytic System. Adv. Sustain. Syst. 2022, 6, 2100516. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-Scheme Photocatalysts: Principles, Synthesis, and Applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Zhang, W.; Mohamed, A.R.; Ong, W.-J. Z-Scheme Photocatalytic Systems for Carbon Dioxide Reduction: Where Are We Now? Angew. Chem. Int. Ed. 2020, 59, 22894–22915. [Google Scholar] [CrossRef]
- Ruales-Lonfat, C.; Barona, J.F.; Sienkiewicz, A.; Bensimon, M.; Vélez-Colmenares, J.; Benítez, N.; Pulgarín, C. Iron Oxides Semiconductors Are Efficients for Solar Water Disinfection: A Comparison with Photo-Fenton Processes at Neutral pH. Appl. Catal. B Environ. 2015, 166–167, 497–508. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 Step-Scheme H2-Production Photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Jin, Z. In Situ XPS Proved Graphdiyne (CnH2n-2)-Based CoFe LDH/CuI/GD Double S-Scheme Heterojunction Photocatalyst for Hydrogen Evolution. Sep. Purif. Technol. 2023, 311, 123229. [Google Scholar] [CrossRef]
- Li, Z.; Ai, W.; Zhang, Y.; Zhang, J.; Liu, W.; Zhong, D.; Cai, Y.; Johansson, E.; Boschloo, G.; Jin, W.; et al. Magnetic Carbon Nanotube Modified S-Scheme TiO2-x/g-C3N4/CNFe Heterojunction Coupled with Peroxymonosulfate for Effective Visible-Light-Driven Photodegradation via Enhanced Interfacial Charge Separation. Sep. Purif. Technol. 2023, 308, 122897. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Michelin, C.; Hoffmann, N. Photosensitization and Photocatalysis—Perspectives in Organic Synthesis. ACS Catal. 2018, 8, 12046–12055. [Google Scholar] [CrossRef]
- Narayan, M.R. Review: Dye Sensitized Solar Cells Based on Natural Photosensitizers. Renew. Sustain. Energy Rev. 2012, 16, 208–215. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Chen, Y.; Shi, L.; Zhu, Y. Enhancement of Photocatalytic Degradation of Polyethylene Plastic with CuPc Modified TiO2 Photocatalyst under Solar Light Irradiation. Appl. Surf. Sci. 2008, 254, 1825–1829. [Google Scholar] [CrossRef]
- Hou, S.; Gao, X.; Lv, X.; Zhao, Y.; Yin, X.; Liu, Y.; Fang, J.; Yu, X.; Ma, X.; Ma, T.; et al. Decade Milestone Advancement of Defect-Engineered g-C3N4 for Solar Catalytic Applications. Nano-Micro Lett. 2024, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lei, Y.; Wang, D.; Li, Y. Defect Engineering in Earth-Abundant Electrocatalysts for CO2 and N2 Reduction. Energy Environ. Sci. 2019, 12, 1730–1750. [Google Scholar] [CrossRef]
- Zhang, S.; Si, Y.; Li, B.; Yang, L.; Dai, W.; Luo, S. Atomic-Level and Modulated Interfaces of Photocatalyst Heterostructure Constructed by External Defect-Induced Strategy: A Critical Review. Small 2021, 17, 2004980. [Google Scholar] [CrossRef]
- Ai, M.; Zhang, J.-W.; Wu, Y.-W.; Pan, L.; Shi, C.; Zou, J.-J. Role of Vacancies in Photocatalysis: A Review of Recent Progress. Chem. Asian J. 2020, 15, 3599–3619. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ni, K.; Ye, J.; Xie, J.; Zhu, Y.; Song, L. Tailoring the Structure of Carbon Nanomaterials toward High-End Energy Applications. Adv. Mater. 2018, 30, 1802104. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, L.; Du, A.; Gao, G.; Chen, J.; Yan, X.; Brown, C.L.; Yao, X. Defect Graphene as a Trifunctional Catalyst for Electrochemical Reactions. Adv. Mater. 2016, 28, 9532–9538. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Guo, C.; Zheng, Y.; Qiao, S.-Z. Surface and Interface Engineering of Noble-Metal-Free Electrocatalysts for Efficient Energy Conversion Processes. Acc. Chem. Res. 2017, 50, 915–923. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Peng, H.-Q.; Yang, R.; Jiang, Z.; Zhou, X.; Lee, C.-S.; Zhao, H.; Zhang, W. Iron Vacancies Induced Bifunctionality in Ultrathin Feroxyhyte Nanosheets for Overall Water Splitting. Adv. Mater. 2018, 30, 1803144. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F.; et al. Activating and Optimizing MoS2 Basal Planes for Hydrogen Evolution through the Formation of Strained Sulphur Vacancies. Nat. Mater. 2016, 15, 48–53. [Google Scholar] [CrossRef]
- Wang, G.; Lv, S.; Shen, Y.; Li, W.; Lin, L.; Li, Z. Advancements in Heterojunction, Cocatalyst, Defect and Morphology Engineering of Semiconductor Oxide Photocatalysts. J. Mater. 2024, 10, 315–338. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Langmuir–Hinshelwood and Light-Intensity Dependence Analyses of Photocatalytic Oxidation Rates by Two-Dimensional-Ladder Kinetic Simulation. J. Phys. Chem. C 2018, 122, 28748–28756. [Google Scholar] [CrossRef]
- Mathew, R.A.; Mowla, M.; Shakiba, S.; Berté, T.B.; Louie, S.M. Prediction of Nanoparticle Photoreactivity in Mixtures of Surface Foulants Requires Kinetic (Non-Equilibrium) Adsorption Considerations. Environ. Sci. Technol. 2024, 58, 8542–8553. [Google Scholar] [CrossRef]
- Batzill, M. Fundamental Aspects of Surface Engineering of Transition Metal Oxide Photocatalysts. Energy Environ. Sci. 2011, 4, 3275–3286. [Google Scholar] [CrossRef]
- Jiang, R.; Lu, G.; Yan, Z.; Liu, J.; Wu, D.; Wang, Y. Microplastic Degradation by Hydroxy-Rich Bismuth Oxychloride. J. Hazard. Mater. 2021, 405, 124247. [Google Scholar] [CrossRef] [PubMed]
- Abouelela, M.M.; Kawamura, G.; Matsuda, A. A Review on Plasmonic Nanoparticle-Semiconductor Photocatalysts for Water Splitting. J. Clean. Prod. 2021, 294, 126200. [Google Scholar] [CrossRef]
- Valenti, M.; Jonsson, M.P.; Biskos, G.; Schmidt-Ott, A.; Smith, W.A. Plasmonic Nanoparticle-Semiconductor Composites for Efficient Solar Water Splitting. J. Mater. Chem. A 2016, 4, 17891–17912. [Google Scholar] [CrossRef]
- Knight, M.W.; Sobhani, H.; Nordlander, P.; Halas, N.J. Photodetection with Active Optical Antennas. Science 2011, 332, 702–704. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.-S.; Tsai, D.P. Plasmonic Photocatalysis. Rep. Prog. Phys. 2013, 76, 046401. [Google Scholar] [CrossRef] [PubMed]
- Maulana, D.A.; Ibadurrohman, M. Slamet Synthesis of Nano-Composite Ag/TiO2 for Polyethylene Microplastic Degradation Applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012054. [Google Scholar] [CrossRef]
- Fadli, M.H.; Ibadurrohman, M.; Slamet, S. Microplastic Pollutant Degradation in Water Using Modified TiO2 Photocatalyst Under UV-Irradiation. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012055. [Google Scholar] [CrossRef]
- Warren, S.C.; Thimsen, E. Plasmonic Solar Water Splitting. Energy Environ. Sci. 2012, 5, 5133–5146. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-Metal Nanostructures for Efficient Conversion of Solar to Chemical Energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Jiang, R.; Wang, F.; Fang, C.; Wang, J.; Yu, J.C. Plasmon-Enhanced Chemical Reactions. J. Mater. Chem. A 2013, 1, 5790–5805. [Google Scholar] [CrossRef]
- Gordon, T.R.; Paik, T.; Klein, D.R.; Naik, G.V.; Caglayan, H.; Boltasseva, A.; Murray, C.B. Shape-Dependent Plasmonic Response and Directed Self-Assembly in a New Semiconductor Building Block, Indium-Doped Cadmium Oxide (ICO). Nano Lett. 2013, 13, 2857–2863. [Google Scholar] [CrossRef]
- Agrawal, A.; Kriegel, I.; Milliron, D.J. Shape-Dependent Field Enhancement and Plasmon Resonance of Oxide Nanocrystals. J. Phys. Chem. C 2015, 119, 6227–6238. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Manchala, S.; Elayappan, V.; Lee, H.-G.; Shanker, V. Chapter 7—Plasmonic Photocatalysis: An Extraordinary Way to Harvest Visible Light. In Photocatalytic Systems by Design; Sakar, M., Balakrishna, R.G., Do, T.-O., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 187–216. ISBN 978-0-12-820532-7. [Google Scholar]
- M’Bra, I.C.; García-Muñoz, P.; Drogui, P.; Keller, N.; Trokourey, A.; Robert, D. Heterogeneous Photodegradation of Pyrimethanil and Its Commercial Formulation with TiO2 Immobilized on SiC Foams. J. Photochem. Photobiol. A Chem. 2019, 368, 1–6. [Google Scholar] [CrossRef]
- Allé, P.H.; Garcia-Muñoz, P.; Adouby, K.; Keller, N.; Robert, D. Efficient Photocatalytic Mineralization of Polymethylmethacrylate and Polystyrene Nanoplastics by TiO2/β-SiC Alveolar Foams. Environ. Chem. Lett. 2021, 19, 1803–1808. [Google Scholar] [CrossRef]
- Nabi, I.; Bacha, A.-U.-R.; Li, K.; Cheng, H.; Wang, T.; Liu, Y.; Ajmal, S.; Yang, Y.; Feng, Y.; Zhang, L. Complete Photocatalytic Mineralization of Microplastic on TiO2 Nanoparticle Film. iScience 2020, 23, 101326. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Peng, X.; Xue, J.; Wei, Y.; Sun, Y.; Dai, Y. A Biomass-Derived, All-Day-Round Solar Evaporation Platform for Harvesting Clean Water from Microplastic Pollution. J. Mater. Chem. A 2021, 9, 11013–11024. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Vieira, Y.; Lima, E.C.; Foletto, E.L.; Dotto, G.L. Microplastics Physicochemical Properties, Specific Adsorption Modeling and Their Interaction with Pharmaceuticals and Other Emerging Contaminants. Sci. Total Environ. 2021, 753, 141981. [Google Scholar] [CrossRef]
- Fernández, J.; Kiwi, J.; Baeza, J.; Freer, J.; Lizama, C.; Mansilla, H.D. Orange II Photocatalysis on Immobilised TiO2. Appl. Catal. B Environ. 2004, 48, 205–211. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Hernández-López, J.M.; Rivera De la Rosa, J.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. Microplastic Pollution Reduction by a Carbon and Nitrogen-Doped TiO2: Effect of pH and Temperature in the Photocatalytic Degradation Process. J. Hazard. Mater. 2020, 395, 122632. [Google Scholar] [CrossRef]
- Llorente-García, B.E.; Hernández-López, J.M.; Zaldívar-Cadena, A.A.; Siligardi, C.; Cedillo-González, E.I. First Insights into Photocatalytic Degradation of HDPE and LDPE Microplastics by a Mesoporous N–TiO2 Coating: Effect of Size and Shape of Microplastics. Coatings 2020, 10, 658. [Google Scholar] [CrossRef]
- Finčur, N.; Šojić Merkulov, D.; Putnik, P.; Despotović, V.; Banić, N.; Lazarević, M.; Četojević-Simin, D.; Agbaba, J.; Abramović, B. Environmental Photocatalytic Degradation of Antidepressants with Solar Radiation: Kinetics, Mineralization, and Toxicity. Nanomaterials 2021, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Suyama, Y.; Otsuki, M.; Ogisu, S.; Kishikawa, R.; Tagami, J.; Ikeda, M.; Kurata, H.; Cho, T. Effects of Light Sources and Visible Light-Activated Titanium Dioxide Photocatalyst on Bleaching. Dent. Mater. J. 2009, 28, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, H.; Wu, J.; Wu, X.; Shi, Y.; Yang, Z.; Huang, K.; Guo, X.; Gao, S. Polystyrene Microplastics Accelerated Photodegradation of Co-Existed Polypropylene via Photosensitization of Polymer Itself and Released Organic Compounds. Water Res. 2022, 214, 118209. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New Strategy for Microplastic Degradation: Green Photocatalysis Using a Protein-Based Porous N-TiO2 Semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Wang, L.; Kaeppler, A.; Fischer, D.; Simmchen, J. Photocatalytic TiO2 Micromotors for Removal of Microplastics and Suspended Matter. ACS Appl. Mater. Interfaces 2019, 11, 32937–32944. [Google Scholar] [CrossRef] [PubMed]
- Uheida, A.; Mejía, H.G.; Abdel-Rehim, M.; Hamd, W.; Dutta, J. Visible Light Photocatalytic Degradation of Polypropylene Microplastics in a Continuous Water Flow System. J. Hazard. Mater. 2021, 406, 124299. [Google Scholar] [CrossRef]
- Lee, J.-M.; Busquets, R.; Choi, I.-C.; Lee, S.-H.; Kim, J.-K.; Campos, L.C. Photocatalytic Degradation of Polyamide 66; Evaluating the Feasibility of Photocatalysis as a Microfibre-Targeting Technology. Water 2020, 12, 3551. [Google Scholar] [CrossRef]
- Jiao, X.; Zheng, K.; Chen, Q.; Li, X.; Li, Y.; Shao, W.; Xu, J.; Zhu, J.; Pan, Y.; Sun, Y.; et al. Photocatalytic Conversion of Waste Plastics into C2 Fuels under Simulated Natural Environment Conditions. Angew. Chem. Int. Ed. 2020, 59, 15497–15501. [Google Scholar] [CrossRef]
- Sacco, N.A.; Zoppas, F.M.; Devard, A.; González Muñoz, M.D.P.; García, G.; Marchesini, F.A. Recent Advances in Microplastics Removal from Water with Special Attention Given to Photocatalytic Degradation: Review of Scientific Research. Microplastics 2023, 2, 278–303. [Google Scholar] [CrossRef]
- Ricardo, I.A.; Alberto, E.A.; Silva Júnior, A.H.; Macuvele, D.L.P.; Padoin, N.; Soares, C.; Gracher Riella, H.; Starling, M.C.V.M.; Trovó, A.G. A Critical Review on Microplastics, Interaction with Organic and Inorganic Pollutants, Impacts and Effectiveness of Advanced Oxidation Processes Applied for Their Removal from Aqueous Matrices. Chem. Eng. J. 2021, 424, 130282. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, W.; Yang, H.; Fan, Q.; Xiong, F.; Liu, S.; Li, D.-S.; Liu, B. Halide Perovskite Composites for Photocatalysis: A Mini Review. EcoMat 2021, 3, e12079. [Google Scholar] [CrossRef]
- Beard, M.C.; Ellingson, R.J. Multiple Exciton Generation in Semiconductor Nanocrystals: Toward Efficient Solar Energy Conversion. Laser Photonics Rev. 2008, 2, 377–399. [Google Scholar] [CrossRef]
- Kumar, S.; Ojha, K.; Ganguli, A.K. Interfacial Charge Transfer in Photoelectrochemical Processes. Adv. Mater. Interfaces 2017, 4, 1600981. [Google Scholar] [CrossRef]
- Cho, S.; Choi, W. Solid-Phase Photocatalytic Degradation of PVC–TiO2 Polymer Composites. J. Photochem. Photobiol. A Chem. 2001, 143, 221–228. [Google Scholar] [CrossRef]
- Luo, H.; Xiang, Y.; Li, Y.; Zhao, Y.; Pan, X. Photocatalytic Aging Process of Nano-TiO2 Coated Polypropylene Microplastics: Combining Atomic Force Microscopy and Infrared Spectroscopy (AFM-IR) for Nanoscale Chemical Characterization. J. Hazard. Mater. 2021, 404, 124159. [Google Scholar] [CrossRef]
- Tian, L.; Chen, Q.; Jiang, W.; Wang, L.; Xie, H.; Kalogerakis, N.; Ma, Y.; Ji, R. A Carbon-14 Radiotracer-Based Study on the Phototransformation of Polystyrene Nanoplastics in Water versus in Air. Environ. Sci. Nano 2019, 6, 2907–2917. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band Gap Engineered TiO2 Nanoparticles for Visible Light Induced Photoelectrochemical and Photocatalytic Studies. J. Mater. Chem. A 2013, 2, 637–644. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Shang, J.; Chai, M.; Zhu, Y. Solid-Phase Photocatalytic Degradation of Polystyrene Plastic with TiO2 as Photocatalyst. J. Solid State Chem. 2003, 174, 104–110. [Google Scholar] [CrossRef]
- Shang, J.; Chai, M.; Zhu, Y. Photocatalytic Degradation of Polystyrene Plastic under Fluorescent Light. Environ. Sci. Technol. 2003, 37, 4494–4499. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, P.; Wu, X.; Shi, H.; Huang, H.; Wang, H.; Gao, S. Insight into Chain Scission and Release Profiles from Photodegradation of Polycarbonate Microplastics. Water Res. 2021, 195, 116980. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A.; Mohammed, A.A. Green Synthesis of Palladium Nanoparticles Using Ananas Comosus Leaf Extract for Solid-Phase Photocatalytic Degradation of Low Density Polyethylene Film. J. Environ. Chem. Eng. 2019, 7, 103270. [Google Scholar] [CrossRef]
- Hageman, H.J. Photoinitiators for Free Radical Polymerization. Prog. Org. Coat. 1985, 13, 123–150. [Google Scholar] [CrossRef]
- Hawkins, W.L. Polymer Degradation. In Polymer Degradation and Stabilization; Hawkins, W.L., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 3–34. ISBN 978-3-642-69376-2. [Google Scholar]
- Venkataramana, C.; Botsa, S.M.; Shyamala, P.; Muralikrishna, R. Photocatalytic Degradation of Polyethylene Plastics by NiAl2O4 Spinels-Synthesis and Characterization. Chemosphere 2021, 265, 129021. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Surekha, P.; Rajagopal, C.; Chatterjee, S.N.; Choudhary, V. Studies on the Photo-Oxidative Degradation of LDPE Films in the Presence of Oxidised Polyethylene. Polym. Degrad. Stab. 2007, 92, 1151–1160. [Google Scholar] [CrossRef]
- Lee, Q.Y.; Li, H. Photocatalytic Degradation of Plastic Waste: A Mini Review. Micromachines 2021, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Hrycay, E.G.; Bandiera, S.M. Chapter Two—Involvement of Cytochrome P450 in Reactive Oxygen Species Formation and Cancer. In Advances in Pharmacology; Hardwick, J.P., Ed.; Cytochrome P450 Function and Pharmacological Roles in Inflammation and Cancer; ; Academic Press: Cambridge, MA, USA, 2015; Volume 74, pp. 35–84. [Google Scholar]
- Rabek, J.F.; Rånby, B. The Role of Singlet Oxygen in the Photooxidation of Polymers. Photochem. Photobiol. 1978, 28, 557–569. [Google Scholar] [CrossRef]
- Carlsson, D.J.; Wiles, D.M. Importance of Singlet Oxygen in the Degradation of Rubbers and Plastics. Rubber Chem. Technol. 1974, 47, 991–1004. [Google Scholar] [CrossRef]
- Schmidt, R. Photosensitized Generation of Singlet Oxygen. Photochem. Photobiol. 2006, 82, 1161–1177. [Google Scholar] [CrossRef]
- Przygoda, M.; Bartusik-Aebisher, D.; Dynarowicz, K.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Cellular Mechanisms of Singlet Oxygen in Photodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 16890. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhu, B.; Cheng, B.; Macyk, W.; Wang, L.; Yu, J. Catalytic Conversion of Styrene to Benzaldehyde over S-Scheme Photocatalysts by Singlet Oxygen. ACS Catal. 2023, 13, 459–468. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. Springerplus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, N. Mechanistic Implications of Plastic Degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
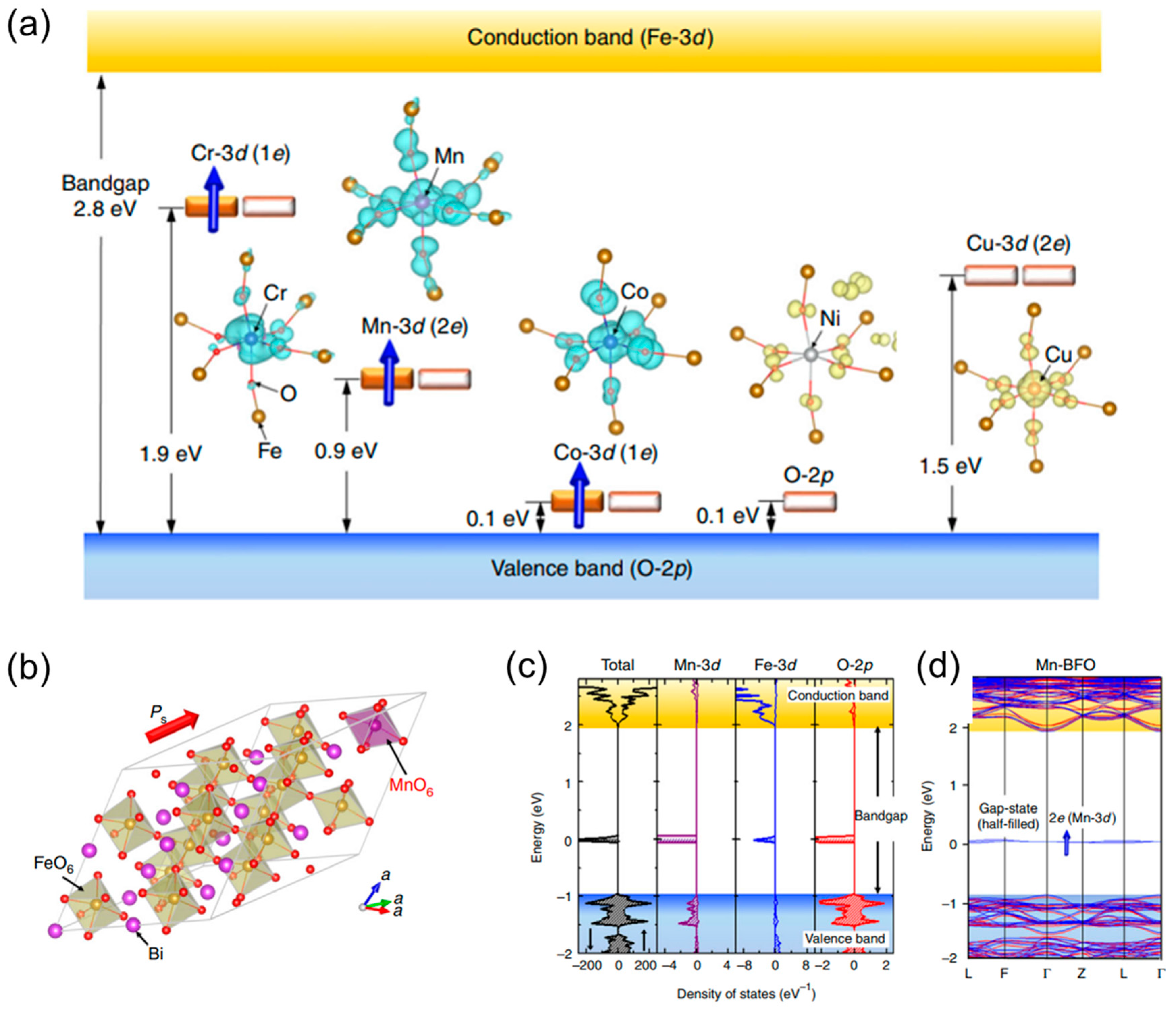
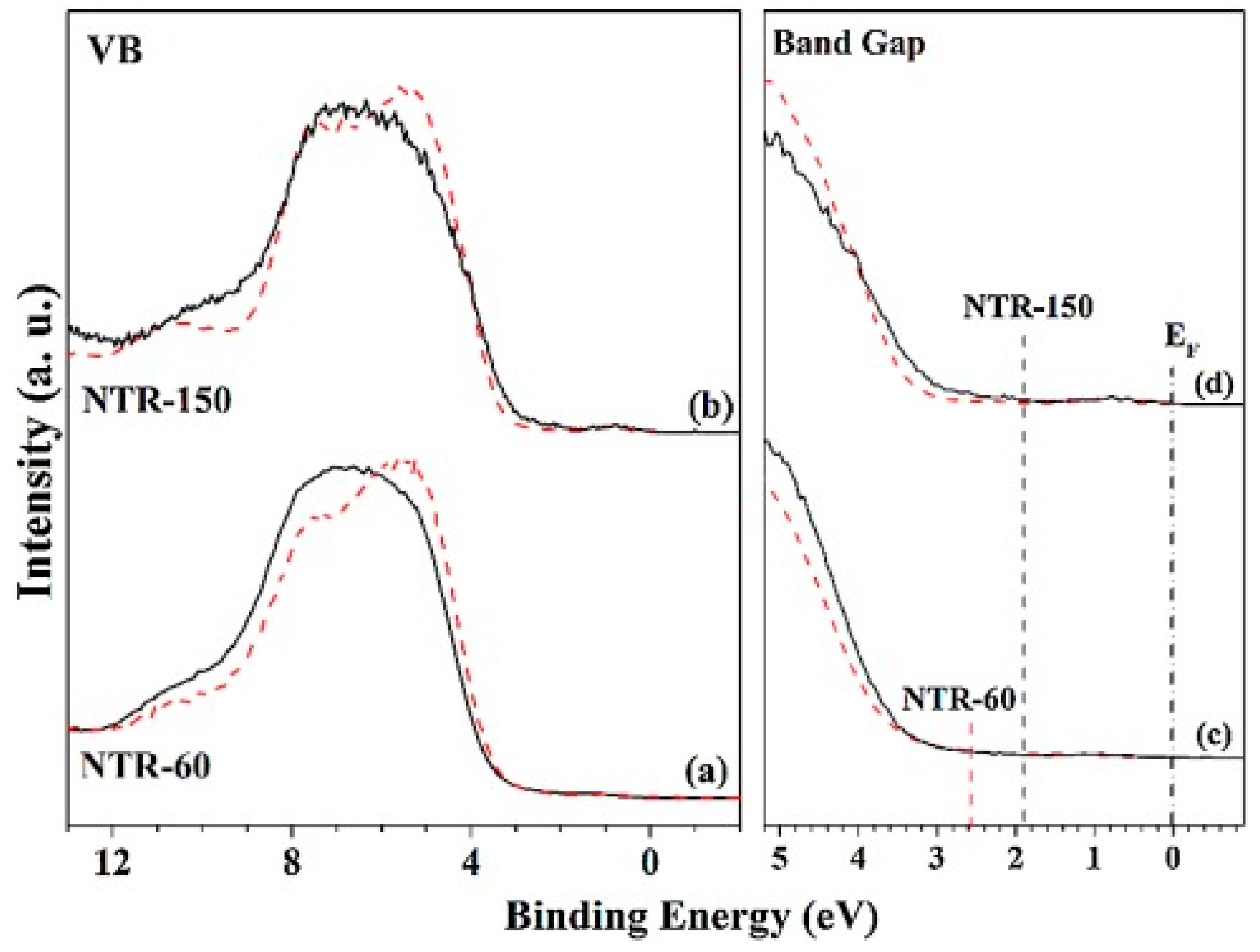
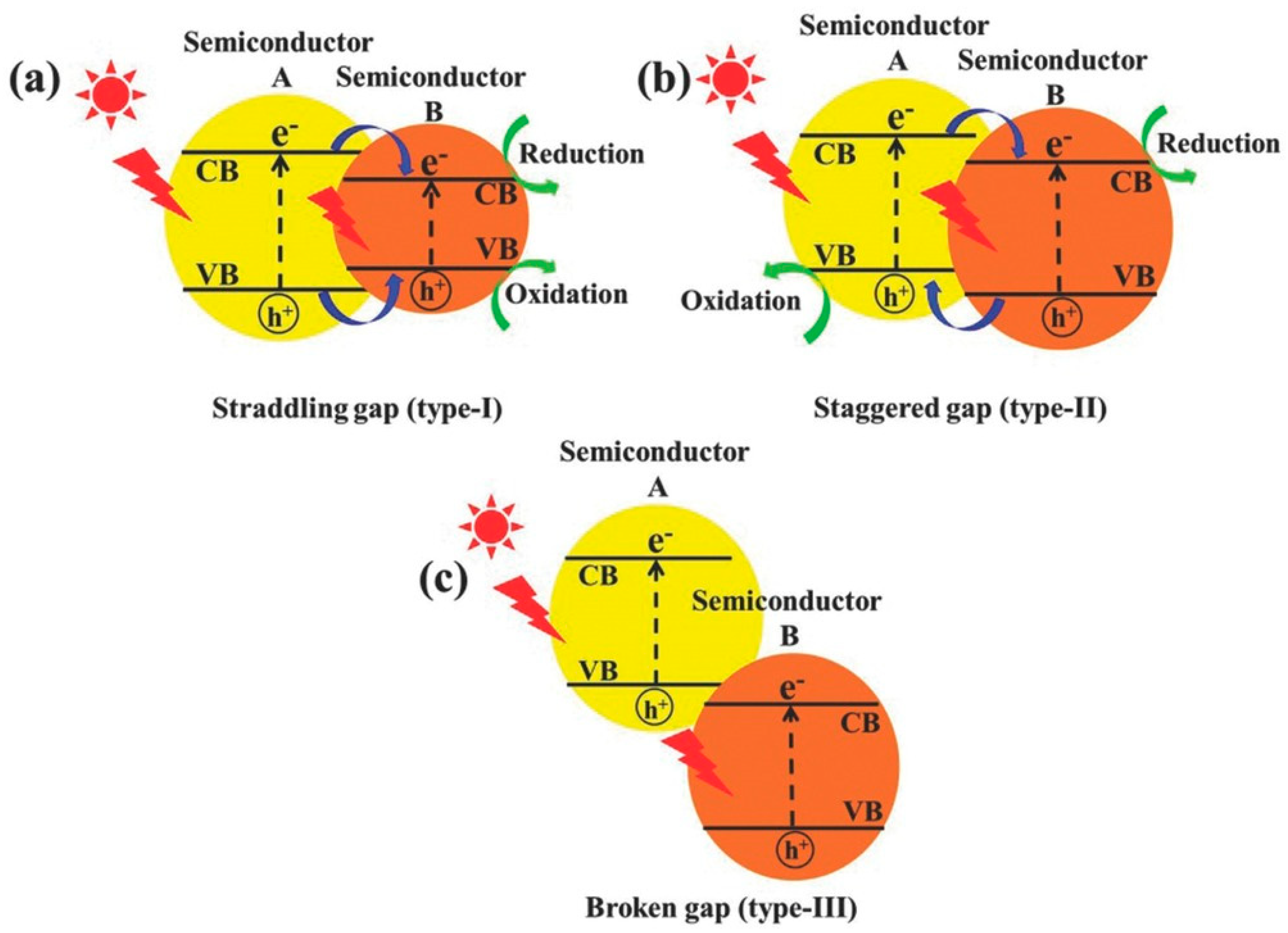
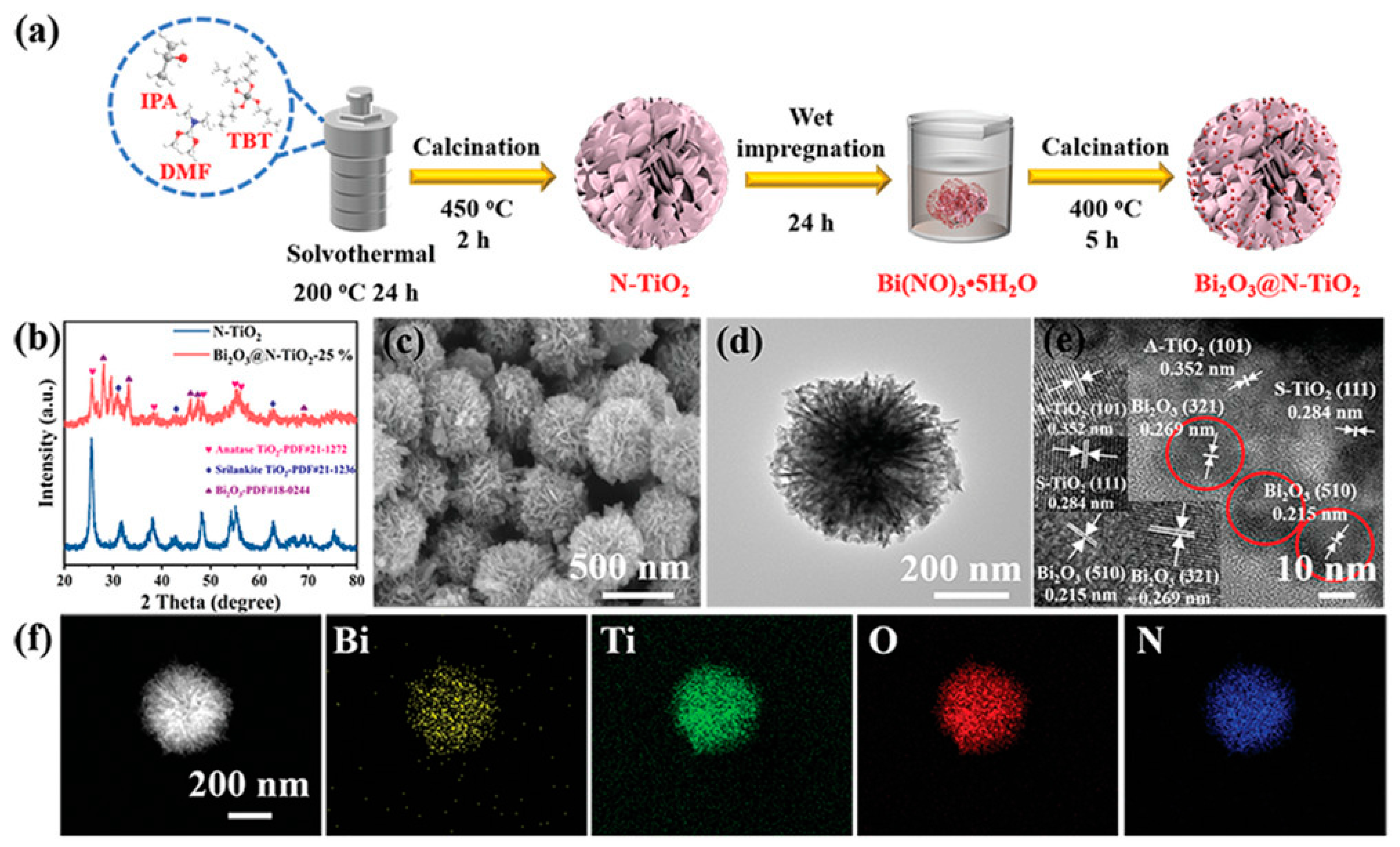

| Method | Efficiency (%) | Advantages | Drawbacks | Targeted MPs | Size | References |
|---|---|---|---|---|---|---|
| Adsorption on Green Microalgae | 94.5 (recovery rate) | High affinity, selective based on surface charge | Nonrecyclable, chemical adherence, surface poisoning | PP | >400 μm | [30] |
| Dynamic Membranes | >80 (NTU) | Low resistance, easy operation, nonchemical treatment | Frequent cleaning, energy demand, sludge accumulation | N/A | 1.65–516 μm (>90 μm) | [31] |
| Advanced Membrane Bioreactor | Usually > 98% | Combined with other advanced treatment | Shape dependency, membrane fouling | fibers (82%) and particles (18%) | mostly < 1 mm | [32] |
| Coagulation and Agglomeration | 91.45 | Removes small microparticles, controllable conditions, simple devices, low cost | Chemical addition, not suitable for large MPs | PET | <500 μm | [33] |
| 97–98.3 | PE/PP | 318 ± 258 μm | [34] | |||
| Hydrolysis | Complete degradation | Fast and cheap | High energy demand, strongly alkaline | Partially degraded polyester MP fibers | 20–25 μm | [22] |
| Ozonation | 44 | no chemicals added, effective than chlorination, irrelated to pH | Low solubility, higher expenses than chlorination, leave residuals and byproducts | PE | Various sizes | [35] |
| Chlorination | 7.1 | Cheap, and chlorine residuals still work | Poor treatment effect, leaves a chlorinated taste, may cause skin irritation | nano-sized PS | <1 μm–5 mm | [36] |
| UV-induced Oxidation | N/A | No chemicals needed | Require extreme dose irradiation, low efficiency | PS, PE, PVC, PET | 150–300 nm | [37] |
| Biological Ingestion | 66.03 | Simple, low operating costs, wide applicability, flexible | uncontrollable environmental conditions, difficulty analyzing products, reproducibility | PE | 53–500 μm | [38] |
| MP Type | Size | Photocatalyst (w or w/o Carrier) | Size | Irradiation | pH | Temperature (°C) | Exposure Time (h) | Results (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| PE | 700–1000 µm | MP/N-TiO2 film composite | 220 to 920 nm | 27 W fluorescent lamp (400–800 nm) | - | room temperature | 18 | 0.064 | [119] |
| 725 ± 108 µm | C,N-TiO2 particles | - | 50 W LED light (400–800 nm) | 3 | 0 | 50 | 0.7177 | [60] | |
| 382 µm | N-TiO2 on Pluronic® F127 membrane | 12 ± 3 nm | 50 W Visible LED Lamp (400–800 nm) | 3 | - | 50 | 0.0465 | [115] | |
| 200–250 µm | hydroxy-rich ultrathin BiOCl | 10–40 nm | 250 W Xe lamp with UV cut-off filter (λ > 420 nm) | - | 10 | 5.38 | [93] | ||
| PS | 400-nm | TiO2 on Triton X-100 membrane | - | UV | - | room temperature | 12 | 98.4 | [110] |
| 3 µm | Au@Ni@TiO2 micromotors | 700 nm + 10 nm Ni + 30 nm Au | UV | - | - | - | 71 | [120] | |
| PP | 154.8 ± 1.4 µm | ZnO nanorods | ~1.6 µm long and ~200 nm wide | visible light | - | - | 456 | 65 | [121] |
| PMMA | 105 nm | β-SiC foam-supported TiO2–P25 | - | UVA (354 nm) | 6.3 | - | 7 | 50 | [109] |
| Polyamide 66 fiber | 10 μm in diameter, 1.0 mm in length | TiO2 P25 powder | 21 nm | UVC (254 nm) | - | 25–38 | 48 | 97 | [122] |
| PET fiber | ~25 μm in diameter, 5 mm in length | Pt@N-TiO2-1.5% | 500 nm | 300 W Xe lamp with AM 1.5 filter | - | - | 48 | 29 | [62] |
| PVC | - | Nb2O5 atomic layers | - | 300 W Xe lamp with AM 1.5 G filter | - | ~25 | 90 | 90 | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Tian, Y.; Xu, W.; Zhu, J. Photodegradation of Microplastics through Nanomaterials: Insights into Photocatalysts Modification and Detailed Mechanisms. Materials 2024, 17, 2755. https://doi.org/10.3390/ma17112755
Xiao Y, Tian Y, Xu W, Zhu J. Photodegradation of Microplastics through Nanomaterials: Insights into Photocatalysts Modification and Detailed Mechanisms. Materials. 2024; 17(11):2755. https://doi.org/10.3390/ma17112755
Chicago/Turabian StyleXiao, Yiting, Yang Tian, Wenbo Xu, and Jun Zhu. 2024. "Photodegradation of Microplastics through Nanomaterials: Insights into Photocatalysts Modification and Detailed Mechanisms" Materials 17, no. 11: 2755. https://doi.org/10.3390/ma17112755






