Dry Electrode Processing Technology and Binders
Abstract
1. Introduction
2. Dry Processing Methods for Electrodes
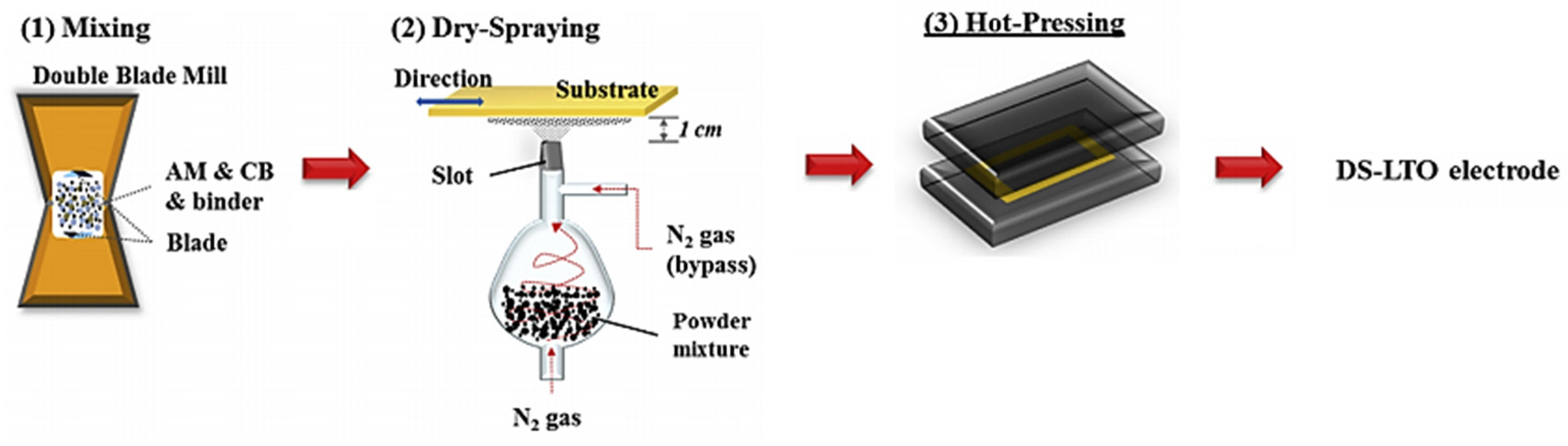
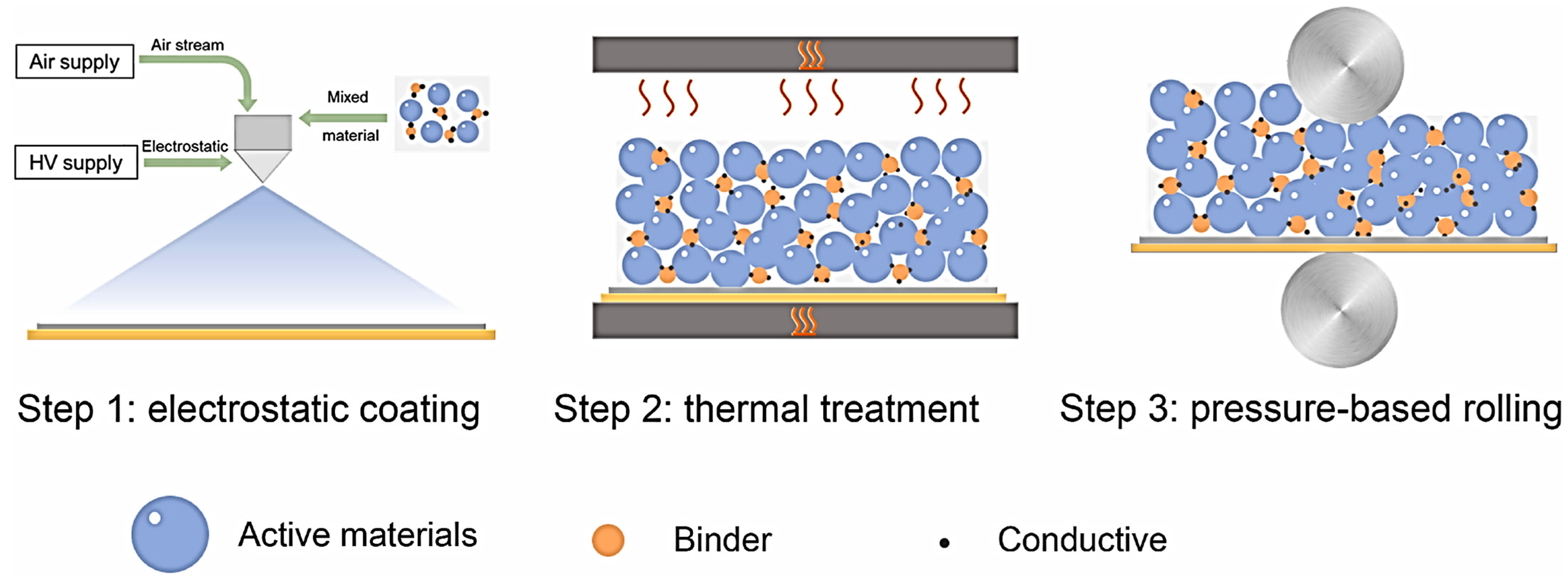
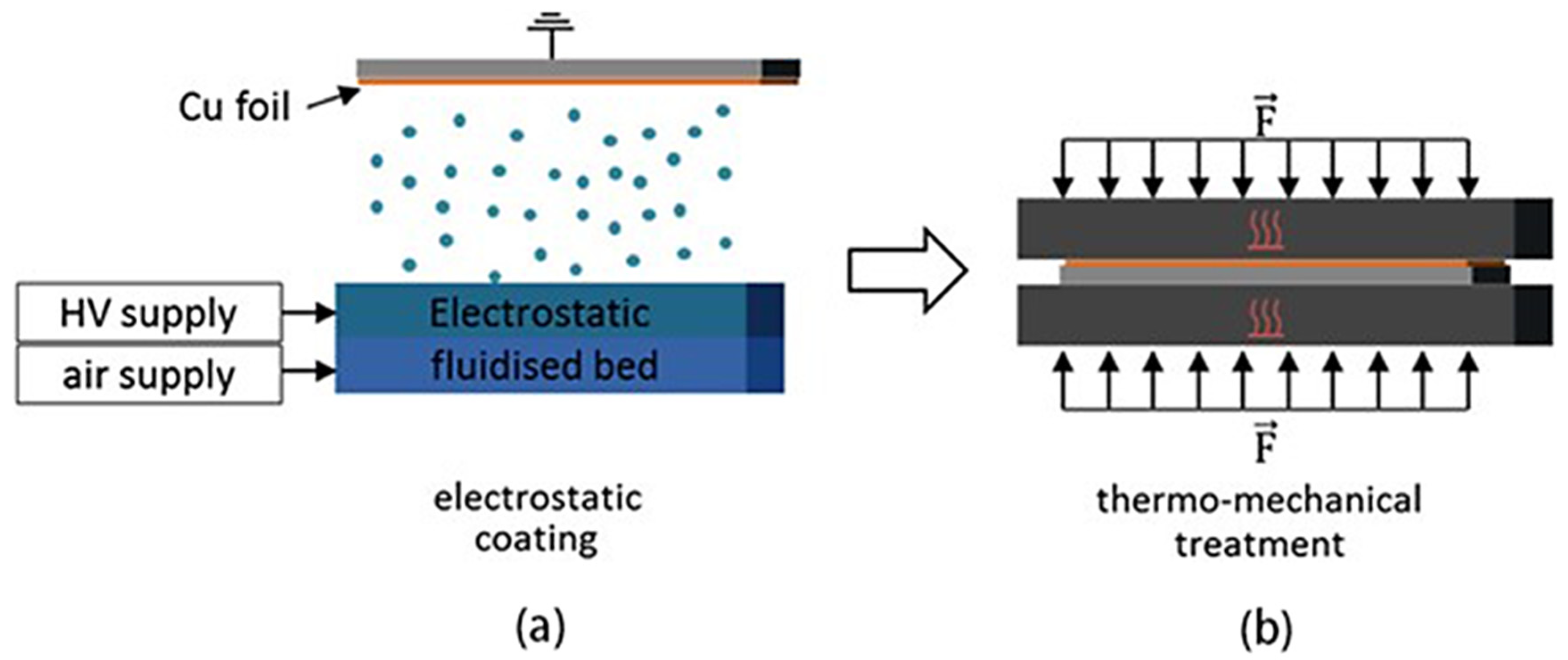
2.1. Dry Spraying Deposition
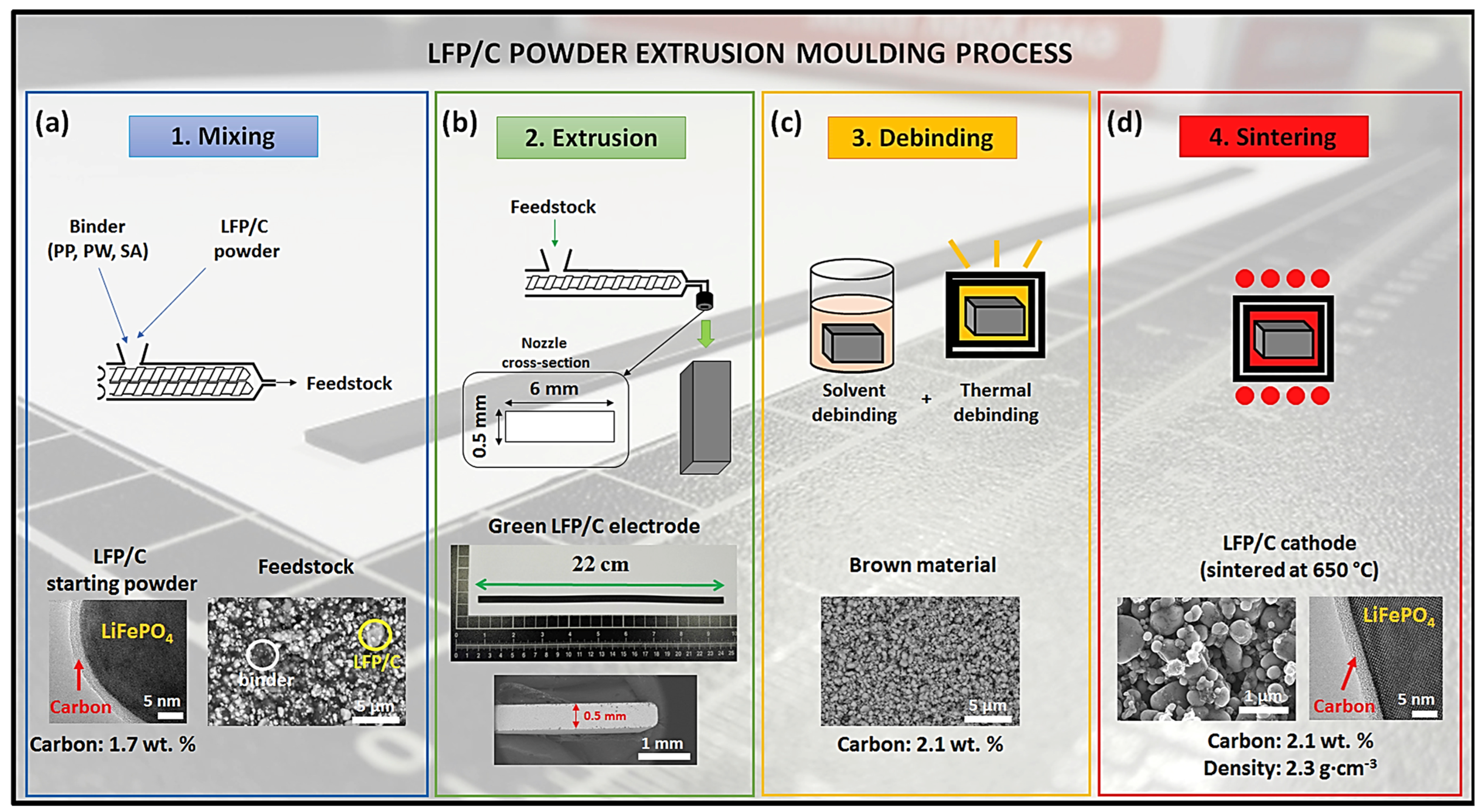
2.2. Melt Extrusion
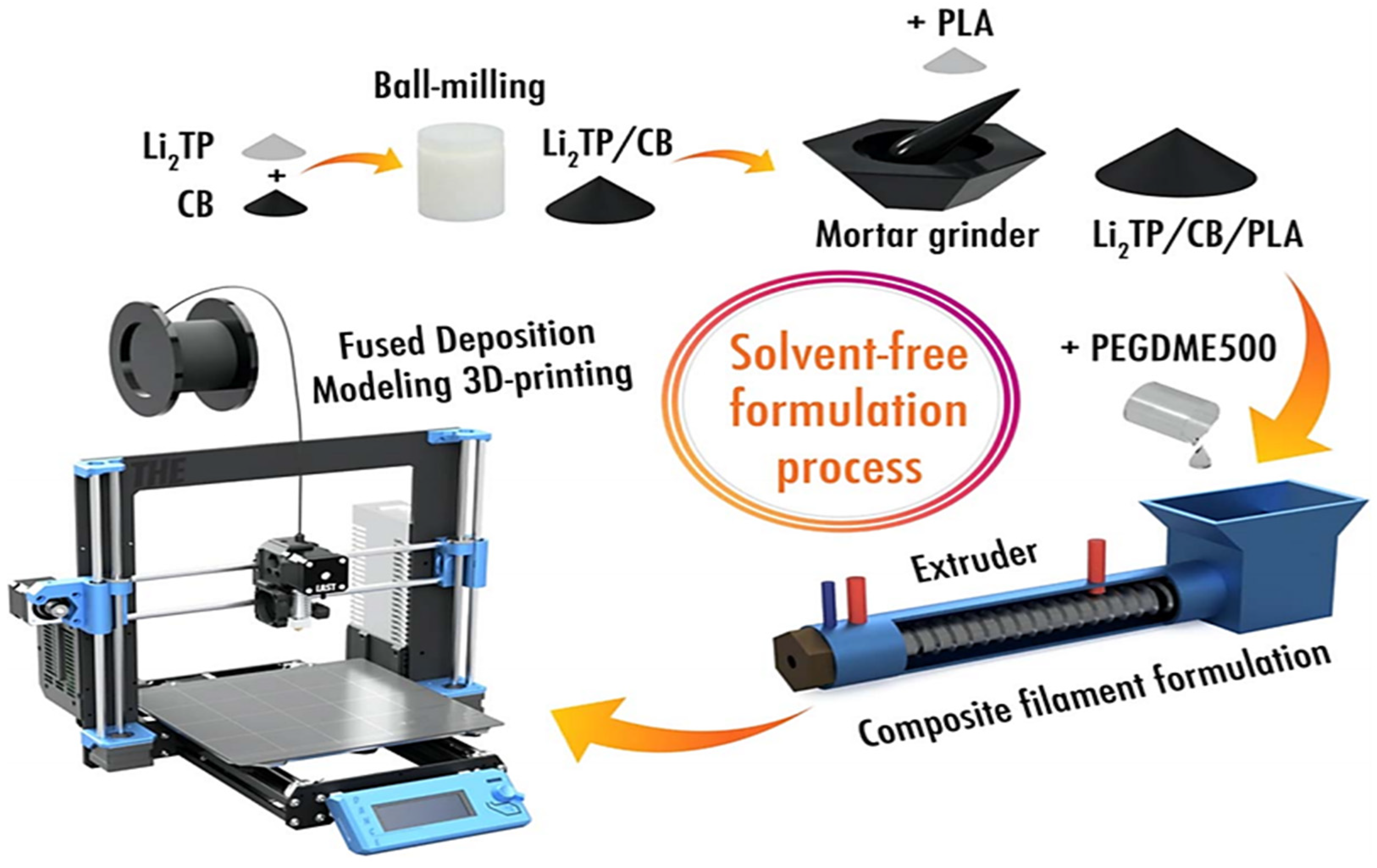
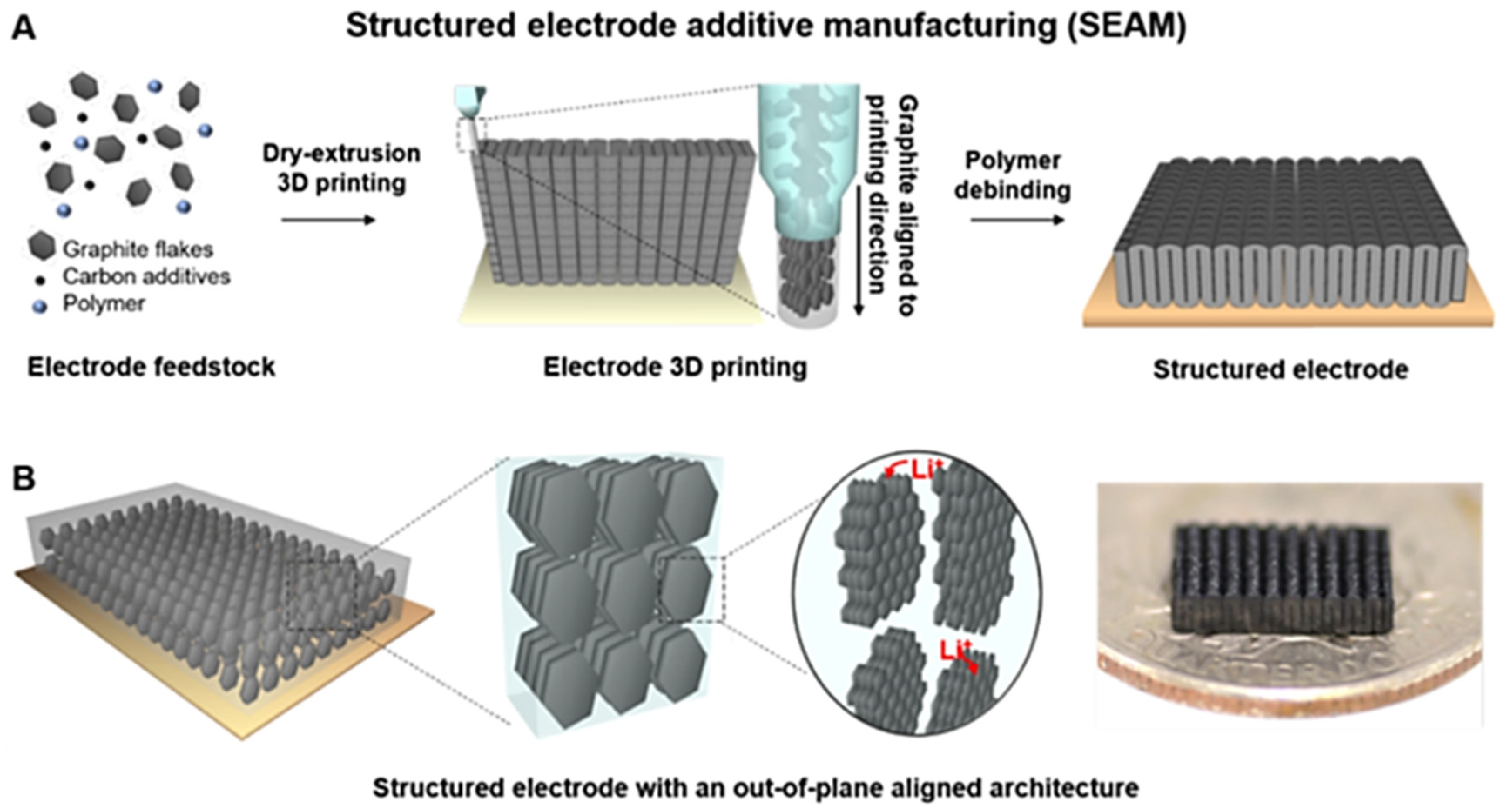
2.3. 3D Printing
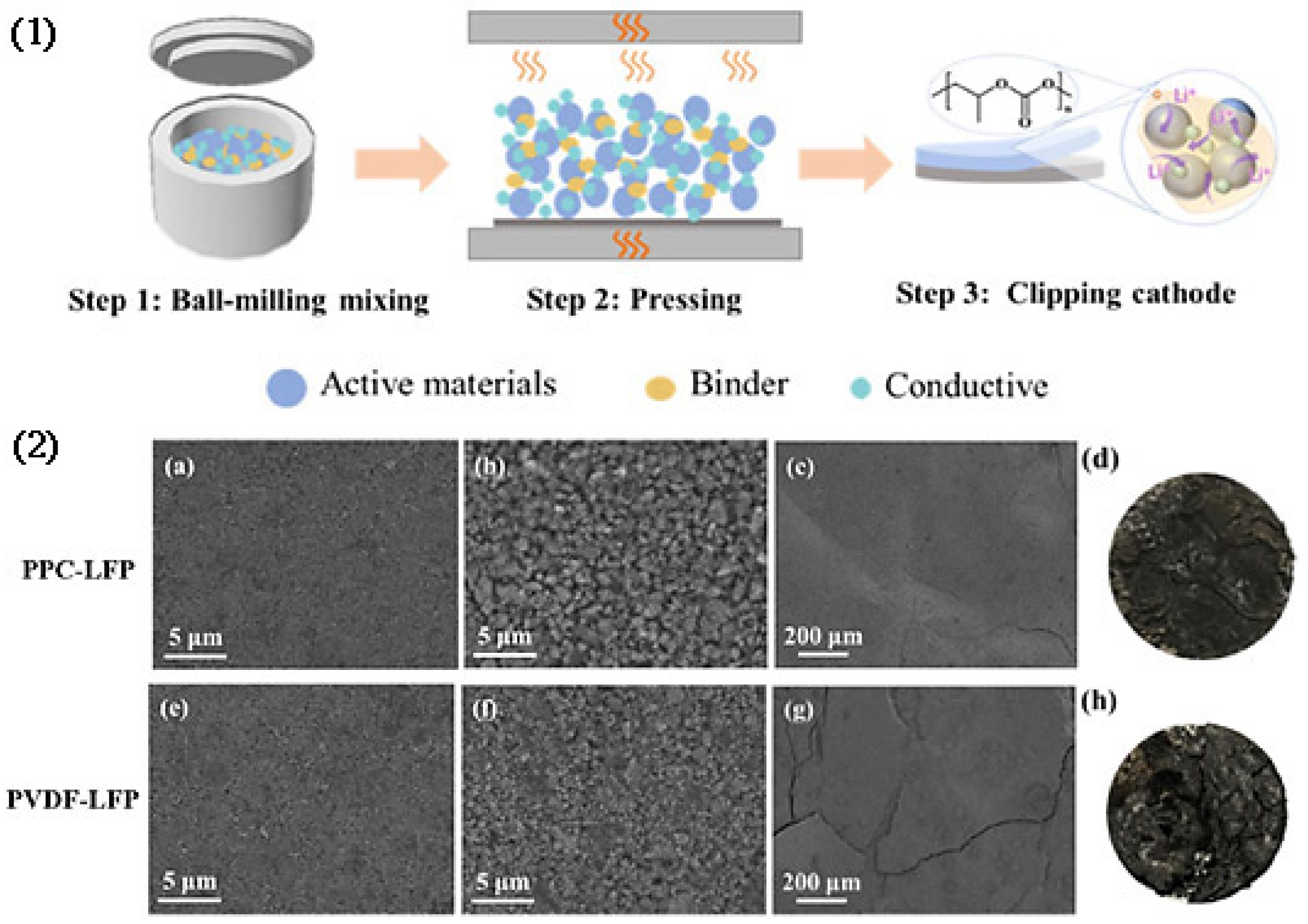
2.4. Powder Compression
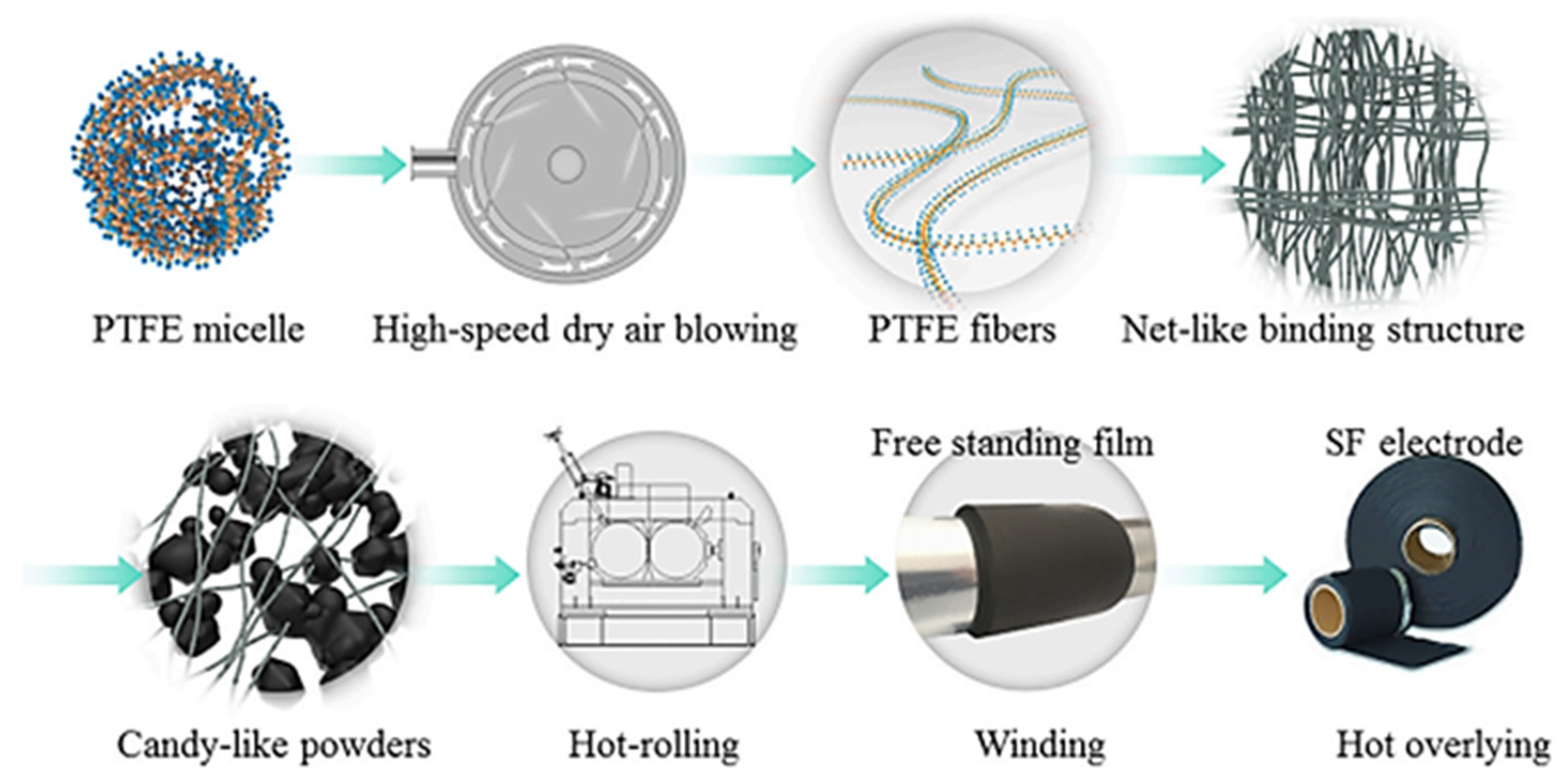
2.5. Polymer Fibrillation
2.5.1. The Application of PTFE in Cathodes
2.5.2. The Application of PTFE in Anodes
2.5.3. Other Binders with the Ability to Be Fibrillated
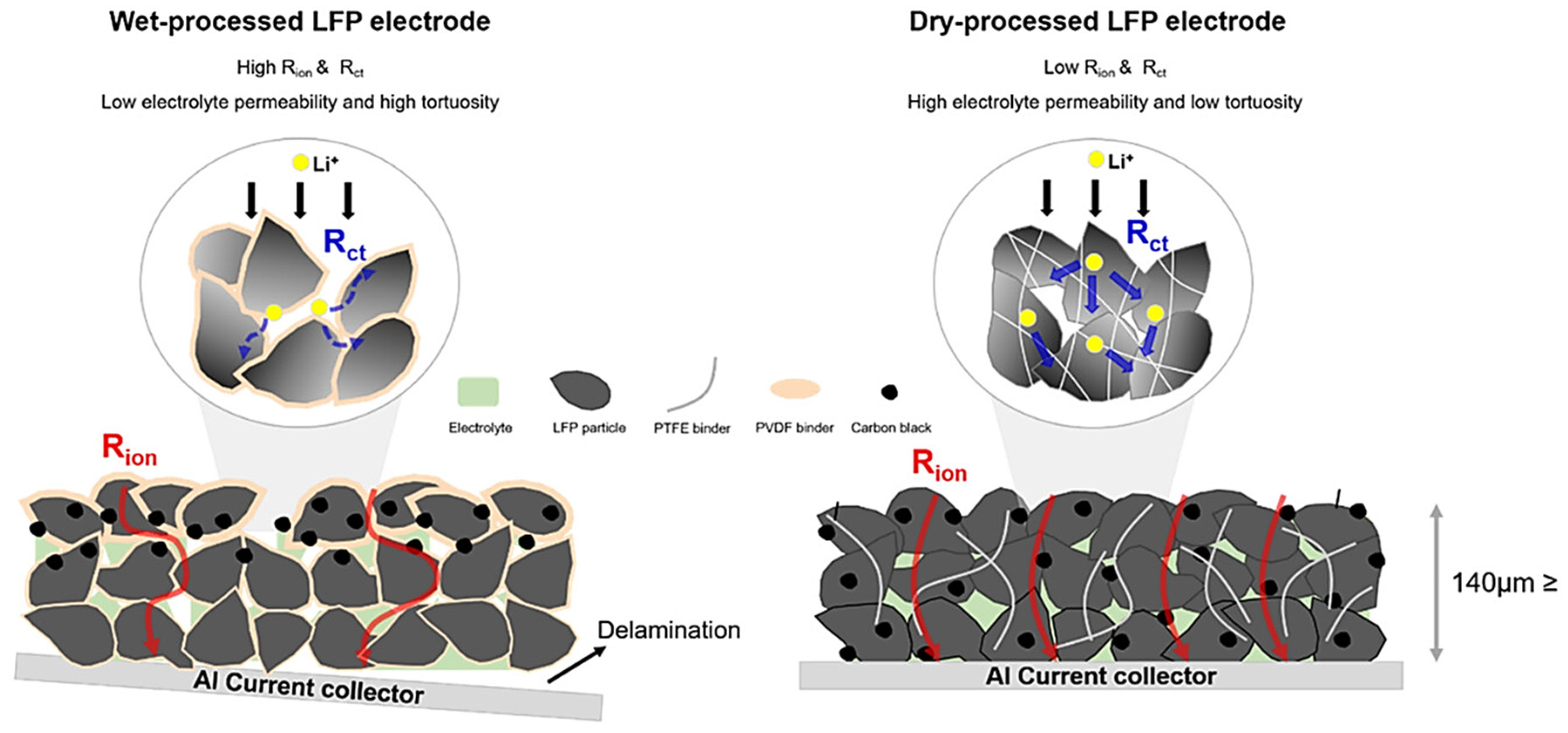
3. Advantages of the Dry Process
3.1. Improve Cell Performance
3.1.1. Higher Compaction Density
3.1.2. Better Rate Performance
3.1.3. Larger Area Capacity
3.1.4. Better Mechanical Properties
3.1.5. More Ion Channels
3.1.6. Fewer Residues
3.2. Reduce Production Costs
3.2.1. Energy Savings
3.2.2. Low Cost of Materials and Equipment
3.3. Protection of Environmental Resources
3.4. Broadening the Range of Applications
4. Challenges of the Dry Electrode Technology
4.1. Study of Dry Mixing Systems
4.2. Determination of Electrode Parameters
4.3. Selection and Optimization of the Binder
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Han, X.; Hu, Z.; Zhang, X.; Tao, Z.; Chen, J. Nanostructured Mn-based Oxides for Electrochemical Energy Storage and Conversion. Chem. Soc. Rev. 2015, 44, 699–728. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, J.; Bucur, C.B.; Gregory, T. Quest for Nonaqueous Multivalent Secondary Batteries: Magnesium and Beyond. Chem. Rev. 2014, 114, 11683–11720. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical Energy Storage for Transportation—Approaching the Limits of, and Going Beyond, Lithium-Ion Batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The Lithium-Ion Battery: State of the Art and Future Perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Shen, H.; Wang, Q.; Chen, Z.; Rong, C.; Chao, D. Application and Development of Silicon Anode Binders for Lithium-Ion Batteries. Materials 2023, 16, 4266. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High-Energy Lithium-Ion Batteries: Recent Progress and a Promising Future in Applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- Zhan, C.; Cai, F.; Amine, K.; Lu, J. Advanced Lithium Batteries for Automobile Applications at ABAA-9. ACS Energy Lett. 2017, 2, 1628–1631. [Google Scholar] [CrossRef]
- Wood, D.L.; Wood, M.; Li, J.; Du, Z.; Ruther, R.E.; Hays, K.A.; Muralidharan, N.; Geng, L.; Mao, C.; Belharouak, I. Perspectives on the Relationship Between Materials Chemistry and Roll-to-Roll Electrode Manufacturing for High-Energy Lithium-Ion Batteries. Energy Storage Mater. 2020, 29, 254–265. [Google Scholar] [CrossRef]
- Schnell, J.; Günther, T.; Knoche, T.; Vieider, C.; Köhler, L.; Just, A.; Keller, M.; Passerini, S.; Reinhart, G. All-Solid-State Lithium-Ion and Lithium Metal Batteries—Paving the Way to Large-Scale Production. J. Power Source 2018, 382, 160–175. [Google Scholar] [CrossRef]
- Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; Vanhaverbeke, L. Cost Projection of State of the Art Lithium-Ion Batteries for Electric Vehicles Up to 2030. Energies 2017, 10, 1314. [Google Scholar] [CrossRef]
- Schmitt, M.; Baunach, M.; Wengeler, L.; Peters, K.; Junges, P.; Scharfer, P.; Schabel, W. Slot-Die Processing of Lithium-Ion Battery Electrodes—Coating Window Characterization. Chem. Eng. Process. Process Intensif. 2013, 68, 32–37. [Google Scholar] [CrossRef]
- Schmitt, M.; Scharfer, P.; Schabel, W. Slot Die Coating of Lithium-Ion Battery Electrodes: Investigations on Edge Effect Issues for Stripe and Pattern Coatings. J. Coat. Technol. Res. 2013, 11, 57–63. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Conveying Advanced Li-Ion Battery Materials into Practice The Impact of Electrode Slurry Preparation Skills. Adv. Energy Mater. 2016, 6, 1600655. [Google Scholar] [CrossRef]
- Haufroid, V.; Jaeger, V.K.; Jeggli, S.; Eisenegger, R.; Bernard, A.; Friedli, D.; Lison, D.; Hotz, P. Biological Monitoring and Health Effects of Low-Level Exposure to N-Methyl-2-Pyrrolidone: A Cross-Sectional Study. Int. Arch. Occup. Environ. Health 2014, 87, 663–674. [Google Scholar] [CrossRef]
- Wang, M.; Dong, X.; Escobar, I.C.; Cheng, Y.-T. Lithium Ion Battery Electrodes Made Using Dimethyl Sulfoxide (DMSO)—A Green Solvent. ACS Sustain. Chem. Eng. 2020, 8, 11046–11051. [Google Scholar] [CrossRef]
- Wood, D.L.; Quass, J.D.; Li, J.; Ahmed, S.; Ventola, D.; Daniel, C. Technical and Economic Analysis of Solvent-Based Lithium-Ion Electrode Drying with Water and NMP. Dry. Technol. 2018, 36, 234–244. [Google Scholar] [CrossRef]
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life Cycle Assessment of Lithium-Ion Batteries for Plug-in Hybrid Electric Vehicles—Critical Issues. J. Clean. Prod. 2010, 18, 1519–1529. [Google Scholar] [CrossRef]
- Klemens, J.; Schneider, L.; Herbst, E.C.; Bohn, N.; Müller, M.; Bauer, W.; Scharfer, P.; Schabel, W. Drying of NCM Cathode Electrodes with Porous, Nanostructured Particles Versus Compact Solid Particles: Comparative Study of Binder Migration as a Function of Drying Conditions. Energy Technol. 2022, 10, 2100985. [Google Scholar] [CrossRef]
- von Horstig, M.-W.; Schoo, A.; Loellhoeffel, T.; Mayer, J.K.; Kwade, A. A Perspective on Innovative Drying Methods for Energy-Efficient Solvent-Based Production of Lithium-Ion Battery Electrodes. Energy Technol. 2022, 10, 2200689. [Google Scholar] [CrossRef]
- Ahmed, S.; Nelson, P.A.; Gallagher, K.G.; Dees, D.W. Energy Impact of Cathode Drying and Solvent Recovery During Lithium-Ion Battery Manufacturing. J. Power Source 2016, 322, 169–178. [Google Scholar] [CrossRef]
- Susarla, N.; Ahmed, S.; Dees, D.W. Modeling and Analysis of Solvent Removal During Li-Ion Battery Electrode Drying. J. Power Source 2018, 378, 660–670. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Yang, T.; Ge, D.; Wood, D.L., 3rd; Li, Z. Water-Based Electrode Manufacturing and Direct Recycling of Lithium-Ion Battery Electrodes-A Green and Sustainable Manufacturing System. iScience 2020, 23, 101081. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Evaluation on a Water-Based Binder for the Graphite Anode of Li-Ion Batteries. J. Power Source 2004, 138, 226–231. [Google Scholar] [CrossRef]
- Ryu, M.; Hong, Y.K.; Lee, S.Y.; Park, J.H. Ultrahigh Loading Dry-Process for Solvent-Free Lithium-Ion Battery Electrode Fabrication. Nat. Commun. 2023, 14, 1316. [Google Scholar] [CrossRef]
- Verdier, N.; Foran, G.; Lepage, D.; Prebe, A.; Ayme-Perrot, D.; Dolle, M. Challenges in Solvent-Free Methods for Manufacturing Electrodes and Electrolytes for Lithium-Based Batteries. Polymers 2021, 13, 323. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Wang, Z.; Volkov, V.; Lou, F.; Yu, Z. Recent Technology Development in Solvent-Free Electrode Fabrication for Lithium-Ion Batteries. Renew. Sustain. Energy Rev. 2023, 183, 113515. [Google Scholar] [CrossRef]
- Bouguern, M.D.; Madikere Raghunatha Reddy, A.K.; Li, X.; Deng, S.; Laryea, H.; Zaghib, K. Engineering Dry Electrode Manufacturing for Sustainable Lithium-Ion Batteries. Batteries 2024, 10, 39. [Google Scholar] [CrossRef]
- Al-Shroofy, M.; Zhang, Q.; Xu, J.; Chen, T.; Kaur, A.P.; Cheng, Y.-T. Solvent-Free Dry Powder Coating Process for Low-Cost Manufacturing of LiNi1/3Mn1/3Co1/3O2 Cathodes in Lithium-Ion Batteries. J. Power Source 2017, 352, 187–193. [Google Scholar] [CrossRef]
- Park, D.-W.; Cañas, N.A.; Wagner, N.; Friedrich, K.A. Novel Solvent-Free Direct Coating Process for Battery Electrodes and Their Electrochemical Performance. J. Power Source 2016, 306, 758–763. [Google Scholar] [CrossRef]
- Ludwig, B.; Zheng, Z.; Shou, W.; Wang, Y.; Pan, H. Solvent-Free Manufacturing of Electrodes for Lithium-ion Batteries. Sci. Rep. 2016, 6, 23150. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.; Liu, J.; Chen, I.M.; Liu, Y.; Shou, W.; Wang, Y.; Pan, H. Understanding Interfacial-Energy-Driven Dry Powder Mixing for Solvent-Free Additive Manufacturing of Li-Ion Battery Electrodes. Adv. Mater. Interfaces 2017, 4, 1700570. [Google Scholar] [CrossRef]
- Schälicke, G.; Landwehr, I.; Dinter, A.; Pettinger, K.-H.; Haselrieder, W.; Kwade, A. Solvent-Free Manufacturing of Electrodes for Lithium-Ion Batteries via Electrostatic Coating. Energy Technol. 2019, 8, 1900309. [Google Scholar] [CrossRef]
- Lee, S.H.; Huang, C.; Johnston, C.; Grant, P.S. Spray Printing and Optimization of Anodes and Cathodes for High Performance Li-Ion Batteries. Electrochim. Acta 2018, 292, 546–557. [Google Scholar] [CrossRef]
- Liu, J.; Ludwig, B.; Liu, Y.; Pan, H.; Wang, Y. Strengthening the Electrodes for Li-Ion Batteries with a Porous Adhesive Interlayer through Dry-Spraying Manufacturing. ACS Appl. Mater. Interfaces 2019, 11, 25081–25089. [Google Scholar] [CrossRef]
- Liu, J.; Ludwig, B.; Liu, Y.; Zheng, Z.; Wang, F.; Tang, M.; Wang, J.; Wang, J.; Pan, H.; Wang, Y. Scalable Dry Printing Manufacturing to Enable Long-Life and High Energy Lithium-Ion Batteries. Adv. Mater. Technol. 2017, 2, 1700106. [Google Scholar] [CrossRef]
- Zhen, E.; Jiang, J.; Lv, C.; Huang, X.; Xu, H.; Dou, H.; Zhang, X. Effects of Binder Content on Low-Cost Solvent-Free Electrodes Made by Dry-Spraying Manufacturing for Lithium-Ion Batteries. J. Power Source 2021, 515, 230644. [Google Scholar] [CrossRef]
- Yonaga, A.; Kawauchi, S.; Mori, Y.; Xuanchen, L.; Ishikawa, S.; Nunoshita, K.; Inoue, G.; Matsunaga, T. Effects of Dry Powder Mixing on Electrochemical Performance of Lithium-Ion Battery Electrode Using Solvent-Free Dry Forming Process. J. Power Source 2023, 581, 233466. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.; Kim, M.; Lee, H.; Choi, J.; Park, H.B.; Kim, H.; Jang, J.; Kim, Y.H.; Song, T.; et al. 10 mAh cm−2 Cathode by Roll-to-Roll Process for Low Cost and High Energy Density Li-Ion Batteries. Adv. Energy Mater. 2024, 14, 2303455. [Google Scholar] [CrossRef]
- Huang, P.; Xu, S.; Zhong, W.; Fu, H.; Luo, Y.; Xiao, Z.; Zhang, M. Carbon Quantum Dots Inducing Formation of β Phase in PVDF-HFP to Improve the Piezoelectric Performance. Sens. Actuators A Phys. 2021, 330, 112880. [Google Scholar] [CrossRef]
- Oliveira, F.; Leterrier, Y.; Månson, J.A.; Sereda, O.; Neels, A.; Dommann, A.; Damjanovic, D. Process Influences on the Structure, Piezoelectric, and Gas-Barrier Properties of PVDF-TrFE Copolymer. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 496–506. [Google Scholar] [CrossRef]
- Keller, D.G.; Giovannoni, R.T.; MacFadden, K.O. Extrusion of Electrode Material by Liquid Injection into Extruder Barrel. U.S. Patent 5,725,822, 10 March 1998. [Google Scholar]
- Lavoie, P.-A.; Laliberté, R.; Besner, S.; Gagnon, Y.; Simoneau, M.; Vallée, A. Positive Electrode Films for Alkali Metal Polymer Batteries and Method for Making Same. U.S. Patent 7,700,018, 20 April 2010. [Google Scholar]
- Dreger, H.; Haselrieder, W.; Kwade, A. Influence of Dispersing by Extrusion and Calendering on the Performance of Lithium-Ion Battery Electrodes. J. Energy Storage 2019, 21, 231–240. [Google Scholar] [CrossRef]
- Astafyeva, K.; Dousset, C.; Bureau, Y.; Stalmach, S.L.; Dufour, B. High Energy Li-Ion Electrodes Prepared via a Solventless Melt Process. Batter. Supercaps 2020, 3, 341–343. [Google Scholar] [CrossRef]
- Haarmann, M.; Haselrieder, W.; Kwade, A. Extrusion-Based Processing of Cathodes: Influence of Solid Content on Suspension and Electrode Properties. Energy Technol. 2019, 8, 1801169. [Google Scholar] [CrossRef]
- El Khakani, S.; Verdier, N.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Rochefort, D.; Dollé, M. Melt-Processed Electrode for Lithium Ion Battery. J. Power Source 2020, 454, 227884. [Google Scholar] [CrossRef]
- Sotomayor, M.E.; de la Torre-Gamarra, C.; Levenfeld, B.; Sanchez, J.-Y.; Varez, A.; Kim, G.-T.; Varzi, A.; Passerini, S. Ultra-Thick Battery Electrodes for High Gravimetric and Volumetric Energy Density Li-Ion Batteries. J. Power Source 2019, 437, 226923. [Google Scholar] [CrossRef]
- de la Torre-Gamarra, C.; Sotomayor, M.E.; Sanchez, J.-Y.; Levenfeld, B.; Várez, A.; Laïk, B.; Pereira-Ramos, J.-P. High Mass Loading Additive-Free LiFePO4 Cathodes with 500 μm Thickness for High Areal Capacity Li-Ion Batteries. J. Power Source 2020, 458, 228033. [Google Scholar] [CrossRef]
- Sun, K.; Wei, T.S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3D Printing of Interdigitated Li-Ion Microbattery Architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Wang, Y.; Yan, C.; Yao, Y.; Chen, Y.; Dai, J.; Lacey, S.; Wang, Y.; Wan, J.; Li, T.; et al. Graphene Oxide-Based Electrode Inks for 3D-Printed Lithium-Ion Batteries. Adv. Mater. 2016, 28, 2587–2594. [Google Scholar] [CrossRef] [PubMed]
- Maurel, A.; Grugeon, S.; Armand, M.; Fleutot, B.; Courty, M.; Prashantha, K.; Davoisne, C.; Tortajada, H.; Panier, S.; Dupont, L. Overview on Lithium-Ion Battery 3D-Printing by Means of Material Extrusion. ECS Trans. 2020, 98, 3. [Google Scholar] [CrossRef]
- Osswald, T.A.; Puentes, J.; Kattinger, J. Fused Filament Fabrication Melting Model. Addit. Manuf. 2018, 22, 51–59. [Google Scholar] [CrossRef]
- Carneiro, O.S.; Silva, A.F.; Gomes, R. Fused Deposition Modeling with Polypropylene. Mater. Des. 2015, 83, 768–776. [Google Scholar] [CrossRef]
- Too, M.H.; Leong, K.F.; Chua, C.K.; Du, Z.H.; Yang, S.F.; Cheah, C.M.; Ho, S.L. Investigation of 3D Non-Random Porous Structures by Fused Deposition Modelling. Int. J. Adv. Manuf. Technol. 2002, 19, 217–223. [Google Scholar] [CrossRef]
- Maurel, A.; Russo, R.; Grugeon, S.; Panier, S.; Dupont, L. Environmentally Friendly Lithium-Terephthalate/Polylactic Acid Composite Filament Formulation for Lithium-Ion Battery 3D-Printing via Fused Deposition Modeling. ECS J. Solid State Sci. Technol. 2021, 10, 037004. [Google Scholar] [CrossRef]
- Park, S.; Shi, B.; Shang, Y.; Deng, K.; Fu, K. Structured Electrode Additive Manufacturing for Lithium-Ion Batteries. Nano Lett. 2022, 22, 9462–9469. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.Y.; Lim, H.N.; Mahdi, M.A.; Wahid, M.H.; Huang, N.M. Three-Dimensional Printed Electrode and Its Novel Applications in Electronic Devices. Sci. Rep. 2018, 8, 7399. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Xie, H.; Gao, T.; Yao, Y.; Pastel, G.; Han, X.; Li, Y.; Zhao, J.; Fu, K.K.; et al. 3D-Printed All-Fiber Li-Ion Battery toward Wearable Energy Storage. Adv. Funct. Mater. 2017, 27, 1703140. [Google Scholar] [CrossRef]
- Kirsch, D.J.; Lacey, S.D.; Kuang, Y.; Pastel, G.; Xie, H.; Connell, J.W.; Lin, Y.; Hu, L. Scalable Dry Processing of Binder-Free Lithium-Ion Battery Electrodes Enabled by Holey Graphene. ACS Appl. Energy Mater. 2019, 2, 2990–2997. [Google Scholar] [CrossRef]
- Han, X.; Yang, Z.; Zhao, B.; Zhu, S.; Zhou, L.; Dai, J.; Kim, J.W.; Liu, B.; Connell, J.W.; Li, T.; et al. Compressible, Dense, Three-Dimensional Holey Graphene Monolithic Architecture. ACS Nano 2017, 11, 3189–3197. [Google Scholar] [CrossRef]
- Walker, B.A.; Plaza-Rivera, C.O.; Sun, S.-S.; Lu, W.; Connell, J.W.; Lin, Y. Dry-Pressed Lithium Nickel Cobalt Manganese Oxide (NCM) Cathodes Enabled by Holey Graphene Host. Electrochim. Acta 2020, 362, 137129. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, D.; Xiao, M.; Wang, S.; Feng, Y.; Huang, S.; Meng, Y. New Potential Substitute of PVDF Binder: Poly(Propylene Carbonate) for Solvent-Free Manufacturing High-Loading Cathodes of LiFePO4|Li Batteries. Ionics 2023, 29, 3895–3906. [Google Scholar] [CrossRef]
- Mitchell, P.; Zhong, L.; Xi, X. Recyclable Dry Particle Based Adhesive Electrode and Methods of Making Same. U.S. Patent 7,342,770, 11 March 2008. [Google Scholar]
- Mitchell, P.; Xi, X.; Zhong, L.; Zou, B. Particle Packaging Systems and Methods. U.S. Patent Application 11/116,882, 10 November 2005. [Google Scholar]
- Lu, Y.; Zhao, C.-Z.; Yuan, H.; Hu, J.-K.; Huang, J.-Q.; Zhang, Q. Dry Electrode Technology, the Rising Star in Solid-State Battery Industrialization. Matter 2022, 5, 876–898. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Zhang, K.; Huang, L.; Shen, H.; Chen, Z.; Rong, C.; Wang, G.; Jiang, Z. A Polytetrafluoroethylene-Based Solvent-Free Procedure for the Manufacturing of Lithium-Ion Batteries. Materials 2023, 16, 7232. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Kirchhoff, S.; Jagle, K.; De, A.; Ehrling, S.; Hartel, P.; Dorfler, S.; Abendroth, T.; Schumm, B.; Althues, H.; et al. Sustainable Protein-Based Binder for Lithium-Sulfur Cathodes Processed by a Solvent-Free Dry-Coating Method. ChemSusChem 2022, 15, e202201320. [Google Scholar] [CrossRef]
- Zhang, G.; Fu, Y.; Zhang, Q.; Xie, Z. Benzo[1,2-b:4,5-b’]dithiophene-Dioxopyrrolothiophen Copolymers for High Performance Solar Cells. Chem. Commun. 2010, 46, 4997–4999. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.; Koo, J.K.; Im, H.-J.; Kim, Y.-J. Astonishing Performance Improvements of Dry-Film Graphite Anode for Reliable Lithium-Ion Batteries. Chem. Eng. J. 2023, 476, 146299. [Google Scholar] [CrossRef]
- Hippauf, F.; Schumm, B.; Doerfler, S.; Althues, H.; Fujiki, S.; Shiratsuchi, T.; Tsujimura, T.; Aihara, Y.; Kaskel, S. Overcoming Binder Limitations of Sheet-Type Solid-State Cathodes Using a Solvent-Free Dry-Film Approach. Energy Storage Mater. 2019, 21, 390–398. [Google Scholar] [CrossRef]
- Matthews, G.; Wheeler, S.; Ramírez-González, J.; Grant, P. Solvent-Free NMC Electrodes for Li-Ion Batteries: Unravelling the Microstructure and Formation of the PTFE Nano-Fibril Network. Front. Energy Res. 2023, 11, 1336344. [Google Scholar] [CrossRef]
- Rayavarapu, P.R.; Sharma, N.; Peterson, V.K.; Adams, S. Variation in Structure and Li+-Ion Migration in Argyrodite-Type Li6PS5X (X = Cl, Br, I) Solid Electrolytes. J. Solid State Electrochem. 2011, 16, 1807–1813. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, M.; Gao, H.; Hou, D.; Yu, C.; Liu, C.; Zhang, D.; Wu, J.-C.; Yang, J.; Chen, D. Dense Integration of Solvent-Free Electrodes for Li-Ion Supercabattery with Boosted Low Temperature Performance. J. Power Source 2020, 473, 228553. [Google Scholar] [CrossRef]
- Yao, W.; Chouchane, M.; Li, W.; Bai, S.; Liu, Z.; Li, L.; Chen, A.X.; Sayahpour, B.; Shimizu, R.; Raghavendran, G.; et al. A 5 V-Class Cobalt-Free Battery Cathode with High Loading Enabled by Dry Coating. Energy Environ. Sci. 2023, 16, 1620–1630. [Google Scholar] [CrossRef]
- Duong, H.; Suszko, A.; Feigenbaum, H. (Invited) Dry Electrode Process Technology. ECS Meet. Abstr. 2016, MA2016–01, 475. [Google Scholar]
- Duong, H.; Shin, J.; Yudi, Y. Dry electrode coating technology. Power Sources Conf. 2018, 3, 34–37. [Google Scholar]
- Li, G.; Xue, R.; Chen, L. The Influence of Polytetrafluorethylene Reduction on the Capacity Loss of the Carbon Anode for Lithium Ion Batteries. Solid State Ion. 1996, 90, 221–225. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Lou, F.; Yu, Z. Leveraging Synergies by Combining Polytetrafluorethylene with Polyvinylidene Fluoride for Solvent-Free Graphite Anode Fabrication. Energy Technol. 2022, 10, 2200732. [Google Scholar] [CrossRef]
- Zhang, Y.; Huld, F.; Lu, S.; Jektvik, C.; Lou, F.; Yu, Z. Revisiting Polytetrafluorethylene Binder for Solvent-Free Lithium-Ion Battery Anode Fabrication. Batteries 2022, 8, 57. [Google Scholar] [CrossRef]
- Nagai, A. Applications of Polyvinylidene Fluoride-Related Materials for Lithium-Ion Batteries. In Lithium-Ion Batteries: Science and Technologies; Yoshio, M., Brodd, R.J., Kozawa, A., Eds.; Springer: New York, NY, USA, 2009; pp. 155–161. [Google Scholar]
- Lee, T.; An, J.; Chung, W.J.; Kim, H.; Cho, Y.; Song, H.; Lee, H.; Kang, J.H.; Choi, J.W. Non-Electroconductive Polymer Coating on Graphite Mitigating Electrochemical Degradation of PTFE for a Dry-Processed Lithium-Ion Battery Anode. ACS Appl. Mater. Interfaces 2024, 16, 8930–8938. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Kong, D.; Quan, L.; He, J.; Liu, J.; Tang, Z.; Chen, S.; Cai, Q.; Zhang, R.; Liu, H.; et al. Removing Electrochemical Constraints on Polytetrafluoroethylene as Dry-Process Binder for High-Loading Graphite Anodes. Joule 2024. [Google Scholar] [CrossRef]
- Magoshi, J.; Magoshi, Y.; Nakamura, S. Mechanism of Fiber Formation of Silkworm. In Silk Polymers; American Chemical Society: Washington, DC, USA, 1993; Volume 544, pp. 292–310. [Google Scholar]
- Boudeville, V.; Grugeon, S.; Maurel, A.; Lesieur, R.; Louati, M.; Cayla, A.; Ursescu, S.; Campagne, C.; Panier, S.; Dupont, L. Solvent-Free Extrusion of a LiFePO4-Based Monofilament for Three-Dimensional Printing of a Lithium-Ion Battery Positive Electrode. J. Power Source 2024, 593, 233973. [Google Scholar] [CrossRef]
- Lee, D.; Manthiram, A. Stable Cycling with Intimate Contacts Enabled by Crystallinity-Controlled PTFE-Based Solvent-Free Cathodes in All-Solid-State Batteries. Small Methods 2023, 7, e2201680. [Google Scholar] [CrossRef]
- Tao, R.; Steinhoff, B.; Uzun, K.; La Riviere, B.; Sardo, K.; Skelly, B.; Hill, R.; Cheng, Y.-T.; Li, J. Correlation Among Porosity, Mechanical Properties, Morphology, Electronic Conductivity and Electrochemical Kinetics of Dry-Processed Electrodes. J. Power Source 2023, 581, 233481. [Google Scholar] [CrossRef]
- Shearing, P.R.; Brandon, N.P.; Gelb, J.; Bradley, R.; Withers, P.J.; Marquis, A.J.; Cooper, S.; Harris, S.J. Multi Length Scale Microstructural Investigations of a Commercially Available Li-Ion Battery Electrode. J. Electrochem. Soc. 2012, 159, A1023–A1027. [Google Scholar] [CrossRef]
- Kwon, K.; Kim, J.; Han, S.; Lee, J.; Lee, H.; Kwon, J.; Lee, J.; Seo, J.; Kim, P.J.; Song, T.; et al. Low-Resistance LiFePO4 Thick Film Electrode Processed with Dry Electrode Technology for High-Energy-Density Lithium-Ion Batteries. Small Sci. 2024. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Z.; Ludwig, B.; Pan, H. Dry Powder Based Electrode Additive Manufacturing. U.S. Patent 10,547,044, 28 January 2020. [Google Scholar]
- Jeschull, F.; Brandell, D.; Wohlfahrt-Mehrens, M.; Memm, M. Water-Soluble Binders for Lithium-Ion Battery Graphite Electrodes: Slurry Rheology, Coating Adhesion, and Electrochemical Performance. Energy Technol. 2017, 5, 2108–2118. [Google Scholar] [CrossRef]
- Bryntesen, S.N.; Strømman, A.H.; Tolstorebrov, I.; Shearing, P.R.; Lamb, J.J.; Stokke Burheim, O. Opportunities for the State-of-the-Art Production of LIB Electrodes—A Review. Energies 2021, 14, 1406. [Google Scholar] [CrossRef]
- Zhou, C.; Bag, S.; Lv, B.; Thangadurai, V. Understanding the Role of Solvents on the Morphological Structure and Li-Ion Conductivity of Poly(vinylidene fluoride)-Based Polymer Electrolytes. J. Electrochem. Soc. 2020, 167, 070552. [Google Scholar] [CrossRef]
- Li, J.; Daniel, C.; An, S.J.; Wood, D. Evaluation Residual Moisture in Lithium-Ion Battery Electrodes and Its Effect on Electrode Performance. MRS Adv. 2016, 1, 1029–1035. [Google Scholar] [CrossRef]
- Aranmala, K.; Chanhaew, A.; Rahmawati, M.; Ikhsanudin, M.N.; Meethong, N. Effects of Conductive Agents on Electrochemical Performance of Water-Based LiNi0.6Mn0.2Co0.2O2 Cathodes for Cylindrical Cell Production of Lithium-Ion Batteries. Defect. Diffus. Forum 2022, 417, 169–176. [Google Scholar] [CrossRef]
- Yuan, C.; Deng, Y.; Li, T.; Yang, F. Manufacturing Energy Analysis of Lithium Ion Battery Pack for Electric Vehicles. CIRP Ann. 2017, 66, 53–56. [Google Scholar] [CrossRef]
- Balandier, J.-Y.; Quist, F.; Amato, C.; Bouzakraoui, S.; Cornil, J.; Sergeyev, S.; Geerts, Y. Synthesis of Soluble Oligothiophenes Bearing Cyano Groups, Their Optical and Electrochemical Properties. Tetrahedron 2010, 66, 9560–9572. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and Future Lithium-Ion Battery Manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.L.; Li, J.; Daniel, C. Prospects for Reducing the Processing Cost of Lithium Ion Batteries. J. Power Source 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Liu, N.; Lee, H.W.; McDowell, M.T.; Cui, Y. Dry-Air-Stable Lithium Silicide-Lithium Oxide Core-Shell Nanoparticles as High-Capacity Prelithiation Reagents. Nat. Commun. 2014, 5, 5088. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, Z.; Wang, H.; Liu, W.; Lee, H.W.; Yan, K.; Zhuo, D.; Lin, D.; Liu, N.; Cui, Y. Artificial Solid Electrolyte Interphase-Protected LixSi Nanoparticles: An Efficient and Stable Prelithiation Reagent for Lithium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 8372–8375. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Huang, T.; Wan, L.; Yu, A. Pre-Lithiating SiO Anodes for Lithium-Ion Batteries by a Simple, Effective, and Controllable Strategy Using Stabilized Lithium Metal Powder. ACS Sustain. Chem. Eng. 2021, 9, 648–657. [Google Scholar] [CrossRef]
- Forney, M.W.; Ganter, M.J.; Staub, J.W.; Ridgley, R.D.; Landi, B.J. Prelithiation of Silicon-Carbon Nanotube Anodes for Lithium Ion Batteries by Stabilized Lithium Metal Powder (SLMP). Nano Lett. 2013, 13, 4158–4163. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Feng, B.; Li, Y.; Wu, S.; Yin, Q.; Yu, Z.; Huang, J. The Effect of Temperature-Induced Phase Transition of PTFE on the Dynamic Mechanical Behavior and Impact-Induced Initiation Characteristics of Al/PTFE. Polym. Test. 2020, 91, 106835. [Google Scholar] [CrossRef]




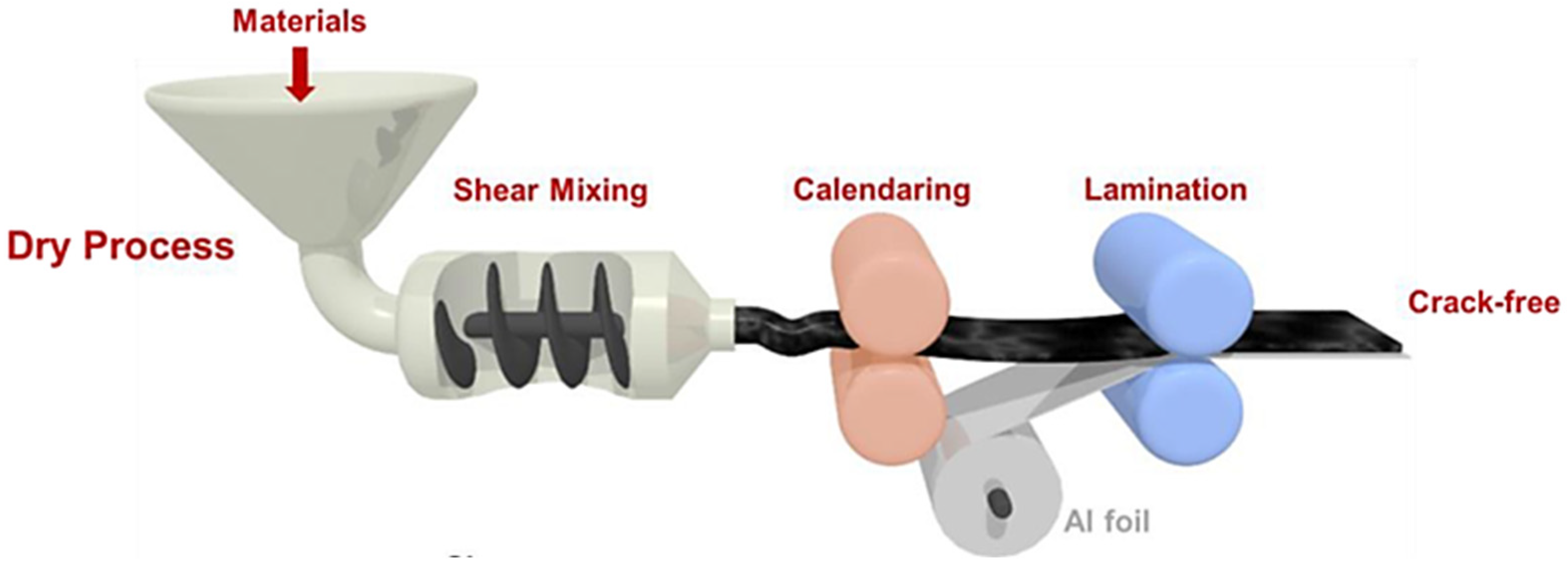

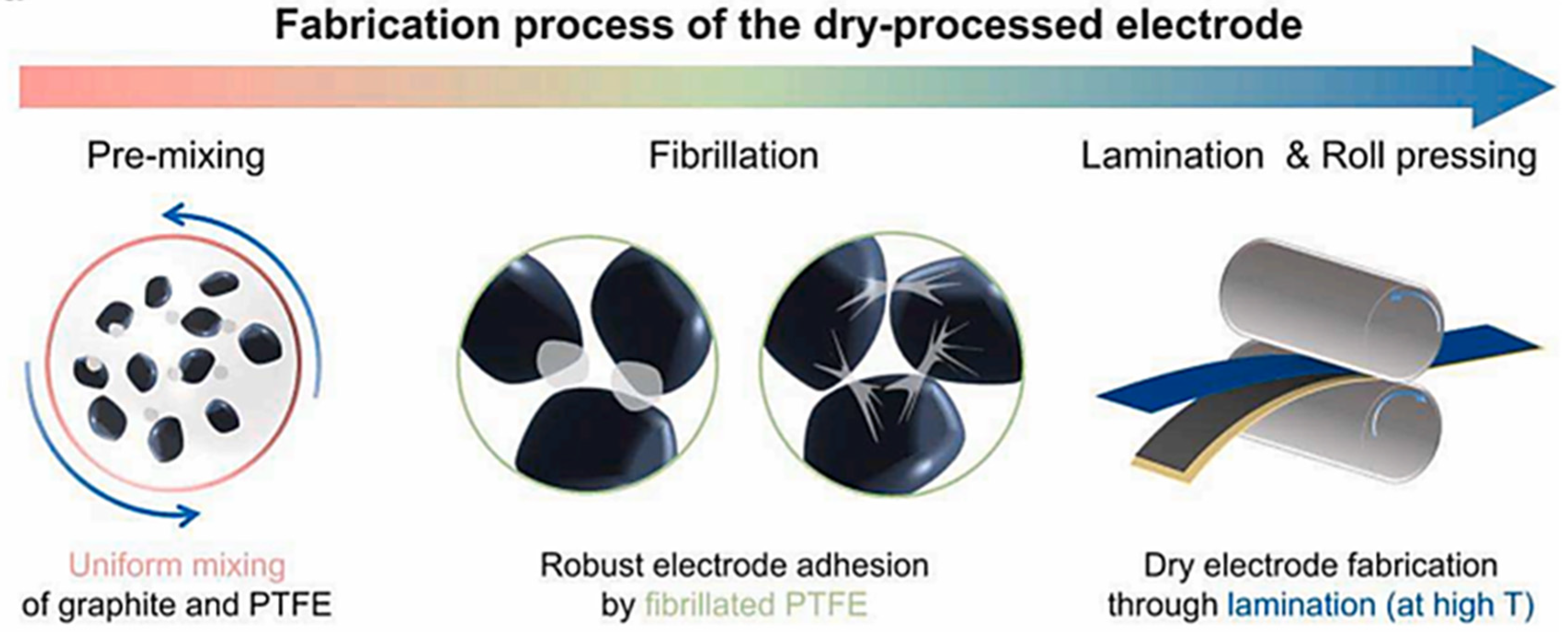
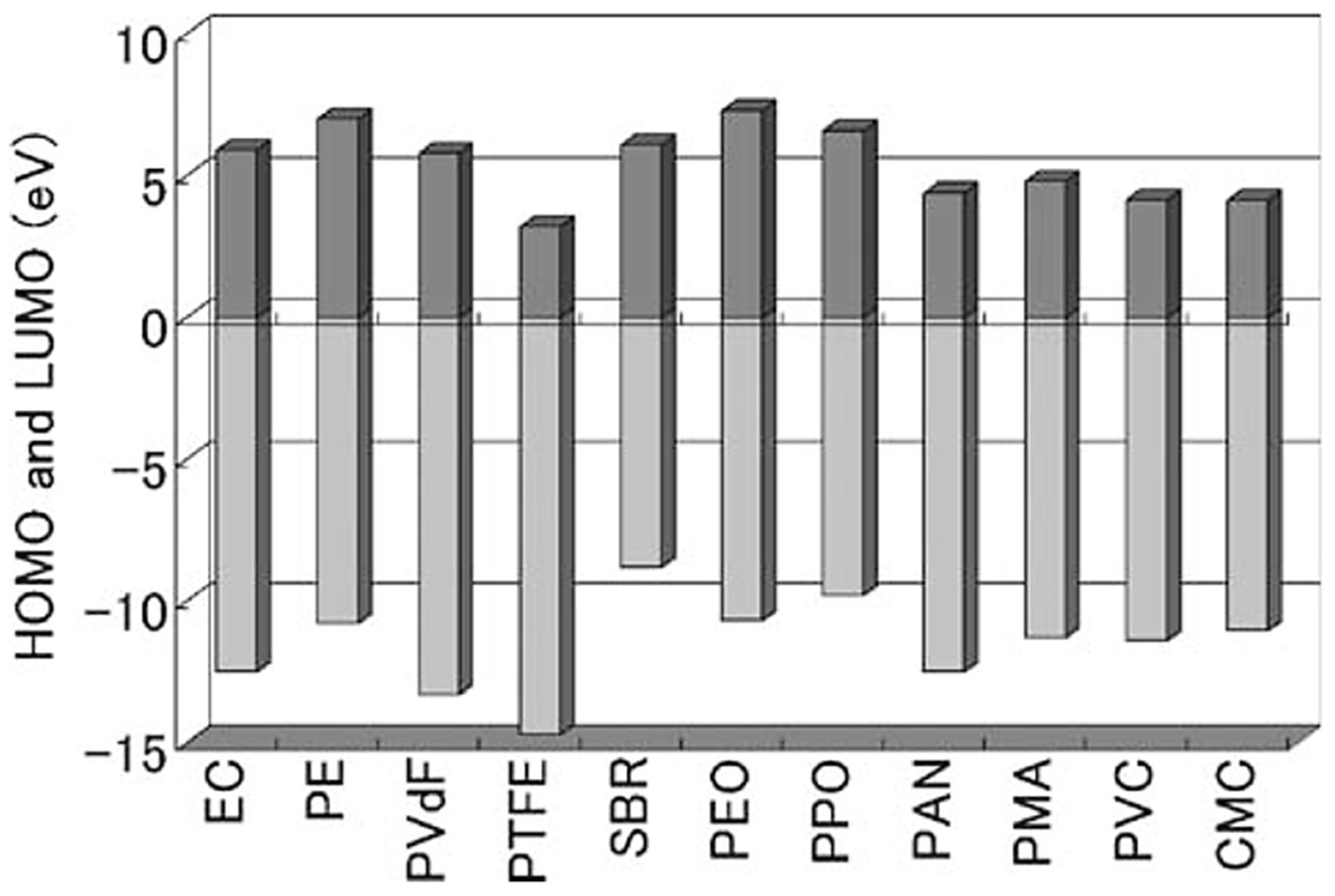

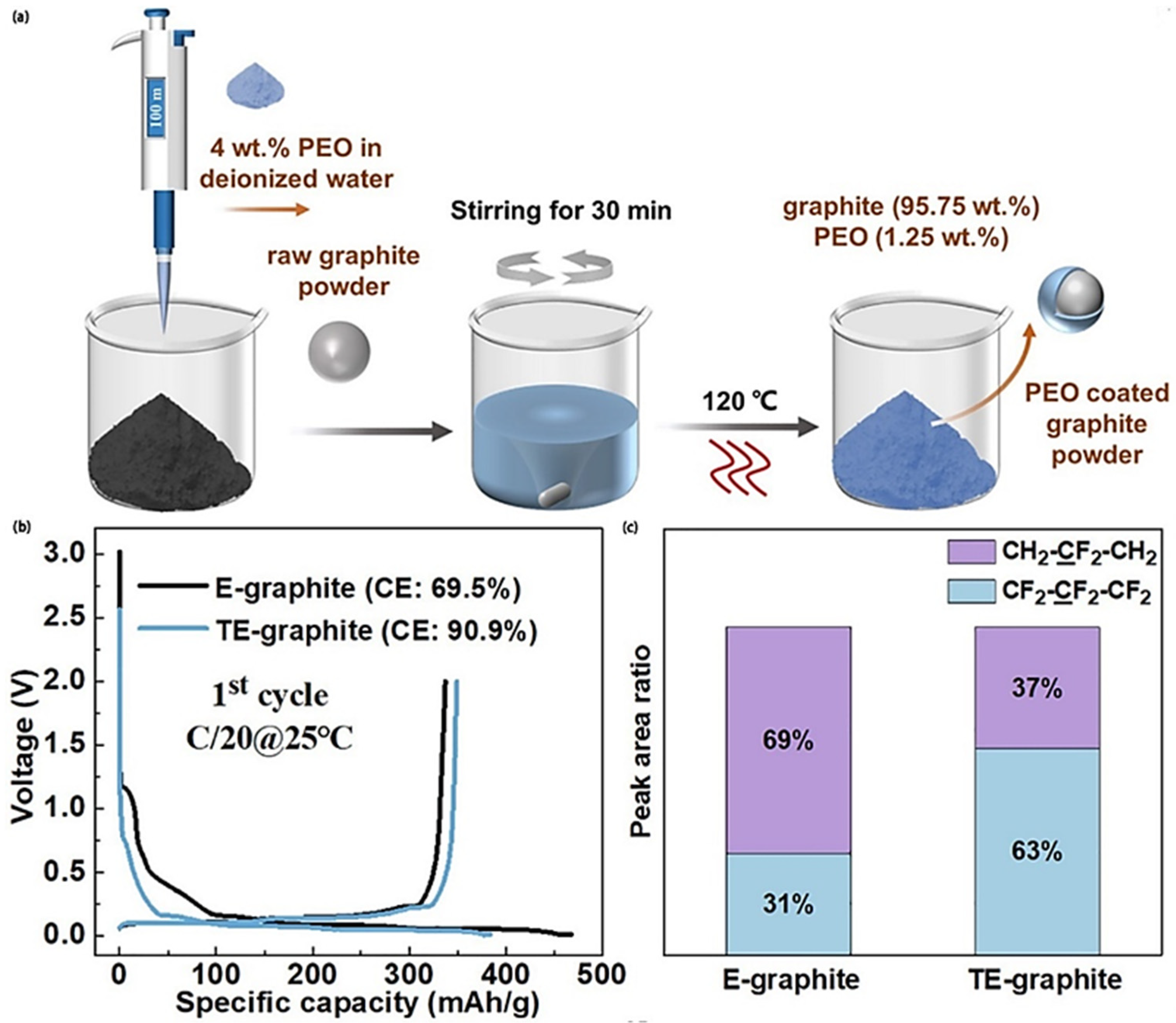

| Porosity | Bonding Strength | Capacity | Retention (0.5C, 50 Cycles) | |
|---|---|---|---|---|
| Dry painted electrode | ~30% | 148.8 kPa | 121 mAhg−1 | 70% |
| Slurry-cast electrode | ~50% | 84.3 kPa | <121 mAhg−1 | 58% |
| Active Material (wt.%) | Carbon Black (wt.%) | Carbon Nanofiber (wt.%) | Binder (wt.%) | PPC Liq (wt.%) | PPC Sol (wt.%) | |
|---|---|---|---|---|---|---|
| NCA compound | 64.0 | 3.9 | 1.9 | 1.3 | 10.1 | 18.8 |
| NCA electrode | 90.0 | 5.5 | 2.7 | 1.8 | - | - |
| Graphite compound | 61.6 | 2.7 | 0.7 | 2.7 | 21.0 | 11.3 |
| Graphite electrode | 91.0 | 4.0 | 1.0 | 4.0 | - | - |
| Procedure | Binders | Operating Temperature | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|---|
| Dry Spraying Deposition | Mostly PVDF. | 20–120 °C | Can be used in almost all types of particle-based fabrications. | Hard to control mass loading, thickness, and homogeneity. | [30,31,32,33,34,35,36,37,38,39] |
| Hot Melting and Extrusion | PP, PW, SA, etc. | >300 °C | The process is simple and the equipment is inexpensive. | A large amount of polymer is required; high operating temperature. | [46,48,49,50] |
| 3D printing | PVDF, PLA and other polymers. | 180–230 °C | Strong designability. The shape of electrode is controllable. | Not suitable for mass production. | [57,58,86] |
| Powder compression | Holey graphite and PPC. | Around 120 °C | Easy to operate, and no binder is usually required. | The electrode performance is relatively poor. | [61,62,64] |
| Polymer Fibrillation | PTFE and sericin. | 20–100 °C | Low binder content; suitable for current “roll to roll” production lines. | Unstable at anode. | [69,72,75,76,80,81,87] |
| Lithium-Ion Battery Production | ||||||
|---|---|---|---|---|---|---|
| Direct Labor (Hours/Year) | Capital Equipment (Millions) | Plant Area (Square Meters) | Direct Labor (Hours/Year) | Capital Equipment (Millions) | Plant Area (Square Meters) | |
| Wet processing lines | 511,871 | 109.85 | 12,569 | 595,918 | 139.10 | 15,958 |
| Dry processing lines | 441,021 | 94.28 | 10,918 | 499,600 | 112.61 | 13,326 |
| Saving percentage | 21.6% | 14.2% | 13.1% | 16.2% | 19.0% | 16.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Li, D.; Wang, X.; Gao, J.; Shen, H.; Zhang, H.; Rong, C.; Chen, Z. Dry Electrode Processing Technology and Binders. Materials 2024, 17, 2349. https://doi.org/10.3390/ma17102349
Zhang K, Li D, Wang X, Gao J, Shen H, Zhang H, Rong C, Chen Z. Dry Electrode Processing Technology and Binders. Materials. 2024; 17(10):2349. https://doi.org/10.3390/ma17102349
Chicago/Turabian StyleZhang, Kaiqi, Dan Li, Xuehan Wang, Jingwan Gao, Huilin Shen, Hao Zhang, Changru Rong, and Zheng Chen. 2024. "Dry Electrode Processing Technology and Binders" Materials 17, no. 10: 2349. https://doi.org/10.3390/ma17102349
APA StyleZhang, K., Li, D., Wang, X., Gao, J., Shen, H., Zhang, H., Rong, C., & Chen, Z. (2024). Dry Electrode Processing Technology and Binders. Materials, 17(10), 2349. https://doi.org/10.3390/ma17102349






