The Influence of the Alkylamino Group on the Solvatochromic Behavior of 5-(4-substituted-arylidene)-1,3-dimethylpyrimidine-2,4,6-triones: Synthesis, Spectroscopic and Computational Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Details
2.2. Synthesis Route and Basic Characterization
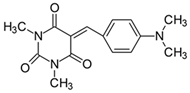
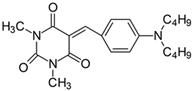
2.2.1. 5-[4-(N,N-Dimethylamino)benzylidene]-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trion (1)
- 1H NMR (200 MHz, DMSO-d6) σ (ppm): 3.126 (s, 6H, NCH3), 3.214 (s, 6H, N(CH3)2), 6.776–6.823 (d, J = 9.4 Hz, 2H, Ar), 8.222 (s, 1H, -CH=), 8.387–8.433 (d, J = 9.2 Hz, 2H, Ar).
- 13C NMR (50 MHz, DMSO-d6) δ (ppm): 27.903, 28.531 (NCH3 in barbituric acid) 39.691 (CH3, NCH3), 111.128, 139.000 (CH, Ar), 156.195 (-CH=), 109.253, 119.939, 145.900, 154.129, 161.065, 163.113 (C).
- IR (KBr): 2922 (-CH), 1713, 1660, 1608 (C=O), 1534, 1506 (C=C), 1196, 1141, 1085 (C-N), 831, 786, 752 (=CH).
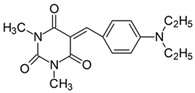
2.2.2. 5-[4-(N,N-Diethylamino)benzylidene]-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trion (2)
- The dye was prepared using 1,3-dimethylbarbituric acid (1.56 g, 0.01 mol), 4-(N,N-diethylamino)enzaldehyde (1.77 g, 0.01 mol), and acetic anhydride (5 mL). Dark-brown crystals were obtained; yield 81.2%, mp 190 °C, Rf 0.64.
- 1H NMR (200 MHz, DMSO-d6) σ (ppm): 1.112–1.181 (t, 6H, -CH3), 3.200 (s, 6H, NCH3), 3.450–3.555 (m, 4H, NCH2-), 6.758–6.803 (d, J = 9 Hz, 2H, Ar), 8.184 (s, 1H, -CH=), 8.367–8.413 (d, J = 9.2 Hz, 2H, Ar).
- 13C NMR (50 MHz, DMSO-d6) δ (ppm): 12.529 (CH3), 27.867, 28.504 (NCH3 in barbituric acid), 44.270 (NCH2-), 110.846, 139.473 (CH, Ar), 155.986 (-CH=), 108.652, 119.612, 151.125, 152.217, 161.056, 163.140 (C).
- IR (KBr): 2970 (-CH), 1711, 1655, 1607 (C=O), 1536, 1501 (C=C), 1207, 1159, 1078 (C-N), 820, 787, 756 (=CH).
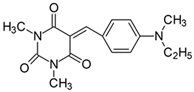
2.2.3. 5-[4-(Ethylmethylamino)benzylidene]-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trion (3)
- The dye was prepared using 1,3-dimethylbarbituric acid (1.56 g, 0.01 mol), 4-[N,N-ethyl(methyl)amino]benzaldehyde (1.63 g, 0.01 mol), and acetic anhydride (5 mL). Bright orange needles were obtained; yield 91.6%, mp 168 °C, Rf 0.45.
- 1H NMR (200 MHz, DMSO-d6) σ (ppm): 1.099–1.169 (t, 3H, -CH3), 3.092 (s, 3H, NCH3), 3.350 (s, 6H, NCH3) 3.516–3.621 (m, 2H, NCH2-), 6.793–6.838 (d, J = 9 Hz, 2H, Ar), 8.215 (s, 1H, -CH=) 8.389–8.435 (d, J = 9.2 Hz, 2H, Ar).
- 13C NMR (50 MHz, DMSO-d6) δ (ppm): 11.664 (CH3), 27.894, 28.522 (NCH3 in barbituric acid), 37.397 (NCH3) 46.145 (NCH2-) 111.028, 139.264 (CH, Ar), 156.104 (-CH=), 108.962, 119.839, 151.125, 153.164, 161.047, 163.122 (C).
- IR (KBr): 2977 (-CH), 1712, 1654, 1608 (C=O), 1536, 1506 (C=C), 1198, 1162, 1083 (C-N), 818, 793, 786, 756 (=CH).
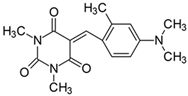
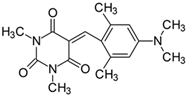
2.2.4. 5-[4-(N,N-Dimethylamino)-2-methylbenzylidene]-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trion (4)
- The dye was prepared using 1,3-dimethylbarbituric acid (1.56 g, 0.01 mol), 4-(N,N-dimethylamino-2-methyl)benzaldehyde (1.63 g, 0.01 mol), and acetic anhydride (5 mL). Orange-red petals were obtained; yield 93.1%, mp 210 °C, Rf 0.59.
- 1H NMR (200 MHz, DMSO-d6) σ (ppm): 2.425 (s, 3H, -CH3), 3.104 (s, 6H, NCH3), 3.192 (s, 3H, NCH3), 3.215 (s, 3H, NCH3), 6.594–6.667 (m, 2H, Ar), 8.577 (s, 1H, -CH=), 8.677–8.722 (d, J = 9 Hz, 1H, Ar).
- 13C NMR (50 MHz, DMSO-d6) δ (ppm): 20.867 (CH3), 27.912, 28.467 (NCH3 in barbituric acid), 39.636 (NCH3), 108.707, 112.612, 136.206 (CH, Ar), 152.936 (-CH=), 109.289, 119.065, 145.772, 151.161, 154.101, 160.783, 163.204 (C).
- IR (KBr): 2947 (-CH), 1707, 1652, 1611 (C=O), 1533, 1507 (C=C), 1303, 1212, 1077 (C-N), 837, 783, 755 (=CH).
2.2.5. 5-[4-(N,N-Dibutylamino)benzylidene]-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trion (5)
- The dye was prepared using 1,3-dimethylbarbituric acid (1.56 g, 0.01 mol), 4-(N,N-dibutylamino)benzaldehyde (2.32 g, 0.01 mol), and acetic anhydride (5 mL). Long, thin light-orange needles were obtained; yield 88.2%, mp 142.8 °C, Rf 0.76.
- 1H NMR (200 MHz, DMSO-d6) σ (ppm): 0.892–0.964 (t, 6H, -CH3), 1.293–1.366 (m, 4H, -CH2-), 1.526–1.561 (m, 4H, -CH2-), 3.218 (s, 6H, NCH3), 3.449–3.483 (t, 4H, NCH2-), 6.754–6.800 (d, J = 9.2 Hz, 2H, Ar), 8.197 (s, 1H, -CH=), 8.371–8.415 (d, J = 8.8 Hz, 2H, Ar).
- 13C NMR (50 MHz, DMSO-d6) δ (ppm): 13.849 (-CH3), 27.885, 28.522 (NCH3 in barbituric acid), 19.583, 29.132 (-CH2-), 50.050 (NCH2-), 111.010, 139.355, (CH, Ar) 155.958 (-CH=), 108.698, 119.630, 151.152, 152.645, 161.092, 163.167 (C).
- IR (KBr): 2955 (-CH), 1717, 1660, 1607 (C=O), 1540, 1505 (C=C), 1202, 1163, 1086 (C-N), 817, 787, 757 (=CH).
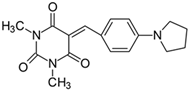
2.2.6. 5-[4-(N-Pyrrolidino)benzylidene]-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trion (6)
- The dye was prepared using 1,3-dimethylbarbituric acid (1.56 g, 0.01 mol), 4-(1-pyrrolidino)benzaldehyde (1.75 g, 0.01 mol), and acetic anhydride (5 mL). Dark-red flakes were obtained; yield 66.8%, mp 230 °C, Rf 0.56.
- 1H NMR (200 MHz, DMSO-d6) σ (ppm): 1.998–2.029 (m, 4H, -CH2-), 3.222 (s, 6H, NCH3), 3.419–3.450 (m, 4H, NCH2-), 6.651–6.695 (d, J = 8.8 Hz, 2H, Ar), 8.226 (s, 1H, -CH=), 8.410–8.445 (d, J = 7 Hz, 2H, Ar).
- 13C NMR (50 MHz, DMSO-d6) δ (ppm): 27.912, 28.531 (CH3 in barbituric acid), 24.872 (-CH2-), 47.692 (NCH2-), 111.711, 139.319 (CH, Ar), 156.277 (-CH=), 108.597, 119.894, 150.900, 151.689, 161.110, 163.177 (C).
- IR (KBr): 2958 (-CH), 1714, 1654, 1608 (C=O), 1533, 1506 (C=C), 1192, 1156, 1078 (C-N), 799, 784, 754 (=CH).
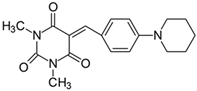
2.2.7. 5-[4-(N-Piperidinyl)benzylidene]-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trion (7)
- The dye was prepared using 1,3-dimethylbarbituric acid (1.56 g, 0.01 mol), 4-(1-piperidinyl)benzaldehyde (1.85 g, 0.01 mol), and acetic anhydride (5 mL). Long, irregular, brown needles were obtained; yield 35.1%, mp 197.4 °C, Rf 0.8.
- 1H NMR (200 MHz, DMSO-d6) σ (ppm): 1.616 (m, 6H, -CH2-), 3.211 (s, 6H, NCH3), 3.553 (m, 4H, NCH2-), 6.966–7.011 (d, J = 9 Hz, 2H, Ar), 8.169 (s, 1H, -CH=), 8.350–8.394 (d, J = 8.8 Hz, 2H, Ar).
- 13C NMR (50 MHz, DMSO-d6) δ (ppm): 27.921, 28.549 (CH3 in barbituric acid), 24.007, 25.236 (-CH2-), 47.374 (NCH2-), 112.157, 139.173 (CH, Ar), 155.813 (-CH=), 109.617, 120.376, 151.125, 153.865, 161.019, 163.076 (C).
- IR (KBr): 2942 (-CH), 1710, 1653, 1607 (C=O), 1540, 1506 (C=C), 1202, 1165, 1084 (C-N), 814, 788, 755 (=CH).
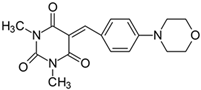
2.2.8. 5-[4-(4-Morpholinyl)benzylidene]-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trion (8)
- The dye was prepared using 1,3-dimethylbarbituric acid (1.56 g, 0.01 mol), 4-(4-morpholinyl)benzaldehyde (1.91 g, 0.01 mol), and acetic anhydride (5 mL). Dark-red needles were obtained; yield 75.2%, mp 265 °C, Rf 0.58.
- 1H NMR (200 MHz, DMSO-d6) σ (ppm): 3.223 (s, 6H, NCH3), 3.449–3.498 (t, 4H, -CH2-), 3.713–3.759 (t, 4H, NCH2-), 7.005–7.049 (d, J = 8.8 Hz, 2H, Ar), 8.248 (s, 1H, -CH=), 8.366–8.411 (d, J = 9 Hz, 2H, Ar).
- 13C NMR (100 MHz, DMSO-d6) δ (ppm): 27.971, 28.593 (CH3 in barbituric acid), 46.207 (NCH2-), 65.807 (OCH2-), 112.426, 138.600 (CH, Ar), 156.070 (-CH=), 111.305, 121.686, 151.238, 154.292, 161.130, 163.098 (C).
- IR (KBr): 2970 (-CH), 1711, 1653, 1609 (C=O), 1545, 1514 (C=C), 1242, 1208, 1123, 1084 (C-N), 836, 787, 754 (=CH).
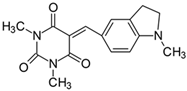
2.2.9. 1,3-Dimethyl-5-[(1-methyl-2,3-dihydro-1H-indolyl)methylidene]pyrimidine-2,4,6(1H,3H,5H)-trion (9)
- The dye was prepared using 1,3-dimethylbarbituric acid (1.56 g, 0.01 mol), 4-(1-methyl-2,3-dihydro-1H-indolyl)benzaldehyde (1.61 g, 0.01 mol), and acetic anhydride (5 mL). Dark-red crystals were obtained; yield 80.1%, mp 226 °C, Rf 0.56.
- 1H NMR (200 MHz, DMSO-d6) σ (ppm): 2.991 (s, 3H, NCH3), 3.045–3.086 (t, 2H, -CH2-), 3.215 (s, 6H, NCH3), 3.662–3.745 (t, 2H, NCH2-), 6.551–6.594 (d, J = 8.6 Hz, 1H, Ar), 8.079–8.124 (d, J = 9 Hz, 1H, Ar), 8.167 (s, 1H, -CH=), 8.480 (s, 1H, Ar).
- 13C NMR (50 MHz, DMSO-d6) δ (ppm): 27.894, 28.504 (CH3 in barbituric acid), 33.064 (NCH3), 26.392 (-CH2-), 53.991 (NCH2), 104.838, 131.090, 142.486 (CH, Ar) 156.031 (-CH=), 121.177, 127.595, 130.007, 158.015, 163.277, 165.534, 177.204 (C).
- IR (KBr): 2952 (-CH), 1708, 1652, 1616 (C=O), 1506, 1462 (C=C), 1294, 1160, 1071 (C-N), 802, 787, 757 (=CH).
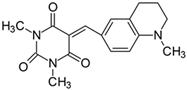
2.2.10. 1,3-Dimethyl-5-[(1-methyl-1,2,3,4-tetrahydroquinolinyl)methylidene]pyrimidine-2,4,6(1H,3H,5H)-trion (10)
- The dye was prepared using 1,3-dimethylbarbituric acid (1.56 g, 0.01 mol), 4-(1-methyl-1,2,3,4-tetrahydroquinoline)benzaldehyde (1.75 g, 0.01 mol), and acetic anhydride (5 mL). Dark-red crystals were obtained; yield 73.5%, mp 228.4 °C, Rf 0.41.
- 1H NMR (200 MHz, DMSO-d6) σ (ppm): 1.894 (m, 2H, -CH2-), 2.717 (t, 2H, -CH2-), 3.459 (t, 2H, NCH2-), 3.079 (s, 3H, NCH3), 3.216 (s, 6H, NCH3), 6.677–6.721 (d, J = 8.8 Hz, 1H, Ar), 8.165 (s, 1H, -CH=), 8.205 (s, 1H, Ar), 8.259–8.304 (d, J = 9 Hz, 1H, Ar).
- 13C NMR (50 MHz, DMSO-d6) δ (ppm): 27.906, 28.515 (CH3 in barbituric acid), 38.597 (NCH3), 20.893, 27.043 (-CH2-), 50.880 (NCH2), 110.034, 137.091, 151.722 (CH, Ar) 156.196 (-CH=), 108.179, 119.996, 121.438, 138.814, 151.302, 161.221, 163.363 (C).
- IR (KBr): 2942 (-CH), 1709, 1652, 1609 (C=O), 1503, 1477 (C=C), 1205, 1160, 1076 (C-N), 803, 786, 756 (=CH).
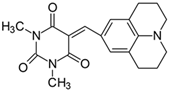
2.2.11. 1,3-Dimethyl-5-[(juloidine)methylidene]pyrimidine-2,4,6(1H,3H,5H)-trion (11)
- The dye was prepared using 1,3-dimethylbarbituric acid (1.56 g, 0.01 mol), 2,3,6,7-tetrahydro-1H,5H-pyrido [3,2,1-ij]quinoline-9-carbaldehyde (2.01 g, 0.01 mol), and acetic anhydride (5 mL). Bright maroon needles were obtained; yield 76.1%, mp 223.6 °C (lit. 197–198 °C [54]), Rf 0.32.
- 1H NMR (200 MHz, DMSO-d6) σ (ppm): 1.883 (m, 4H, -CH2-), 2.700 (t, 4H, -CH2-), 3.394 (t, 4H, NCH2-), 3.210 (s, 6H, NCH3), 8.079 (s, 3H, Ar, -CH=).
- 13C NMR (50 MHz, DMSO-d6) δ (ppm): 27.888, 28.480 (CH3 in barbituric acid), 20.550, 26.976 (-CH2-), 49.829 (NCH2), 120.223, 136.8464 (CH, Ar) 155.866 (-CH=), 106.965, 119.3338, 149.066, 151.333, 161.221, 163.457 (C).
- IR (KBr): 2950 (-CH), 1708, 1653 (C=O), 1499 (C=C), 1215, 1185, 1161, 1099 (C-N), 785, 756 (=CH).
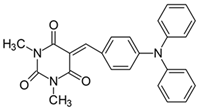
2.2.12. 1,3-Dimethyl-5-[4-(N,N-diphenylamino)benzylidene]pyrimidine-2,4,6(1H,3H,5H)-trion (12)
- The dye was prepared using 1,3-dimethylbarbituric acid (1.56 g, 0.01 mol), 4-(N,N-diphenylamino)benzaldehyde (2.73 g, 0.01 mol), and acetic anhydride (5 mL). Spherical, dark brown grains were obtained; yield 46.1%, mp 193 °C (lit. 190 °C [55]), Rf 0.55.
- 1H NMR (200 MHz, DMSO-d6) σ (ppm): 3.192 (s, 3H, NCH3) 3.218 (s, 3H, NCH3), 6.758–6.802 (d, J = 8.8 Hz, 2H, Ar), 7.270 (m, 5H, Ar), 7.448 (m, 5H, Ar), 8.230 (s, 1H, -CH=), 8.238–8.285 (d, J = 9.4 Hz, 2H, Ar).
- 13C NMR (50 MHz, DMSO-d6) δ (ppm): 28.012, 28.640 (CH3 in barbituric acid), 113.276, 116.744, 123.853, 137.471, 144.899 (CH, Ar) 155.394 (-CH=), 125.938, 126.348, 126.630, 130.025, 151.034, 152.117, 160.764, 162.703 (C).
- IR (KBr): 2953 (-CH), 1729, 1662, (C=O), 1589 (C=C), 1193, 1156, 1086 (C-N), 792, 755, 700 (=CH).
2.2.13. 5-[4-(N,N-Dimethylamino)-2,6-dimethylbenzylidene]-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trion (13)
- The dye was prepared using 1,3-dimethylbarbituric acid (1.56 g, 0.01 mol), 4-(N,N-dimethylamino-2,6-dimethyl)benzaldehyde (1.77 g, 0.01 mol), and acetic anhydride (5 mL). Orange-red petals were obtained; yield 64.7%, mp 192 °C, Rf 0.59.
- 1H NMR (200 MHz, DMSO-d6) σ (ppm): 2.134 (s, 6H, CH3), 2.972 (s, 6H, NCH3), 3.128 (s, 3H, NCH3), 3.231 (s, 3H, NCH3), 6.446 (s, 2H, Ar), 8.431 (s, 1H, -CH=).
- 13C NMR (50 MHz, DMSO-d6) δ (ppm): 20.940 (CH3), 27.858, 28.367 (CH3 in barbituric acid), 39.691 (NCH3), 110.755 (CH, Ar), 155.330 (-CH=), 117.727, 122.042, 139.410, 151.107, 151.207, 159.663, 161.911 (C).
- IR (KBr): 2923, (-CH), 1724, 1667, 1611 (C=O), 1545, 1512 (C=C), 1156, 1090 (C-N), 784, 756 (=CH).
3. Results
3.1. Synthesis and Design Strategy
3.2. Structure Effect on Spectroscopic Properties
3.3. Solvent Effect on Spectroscopic Properties
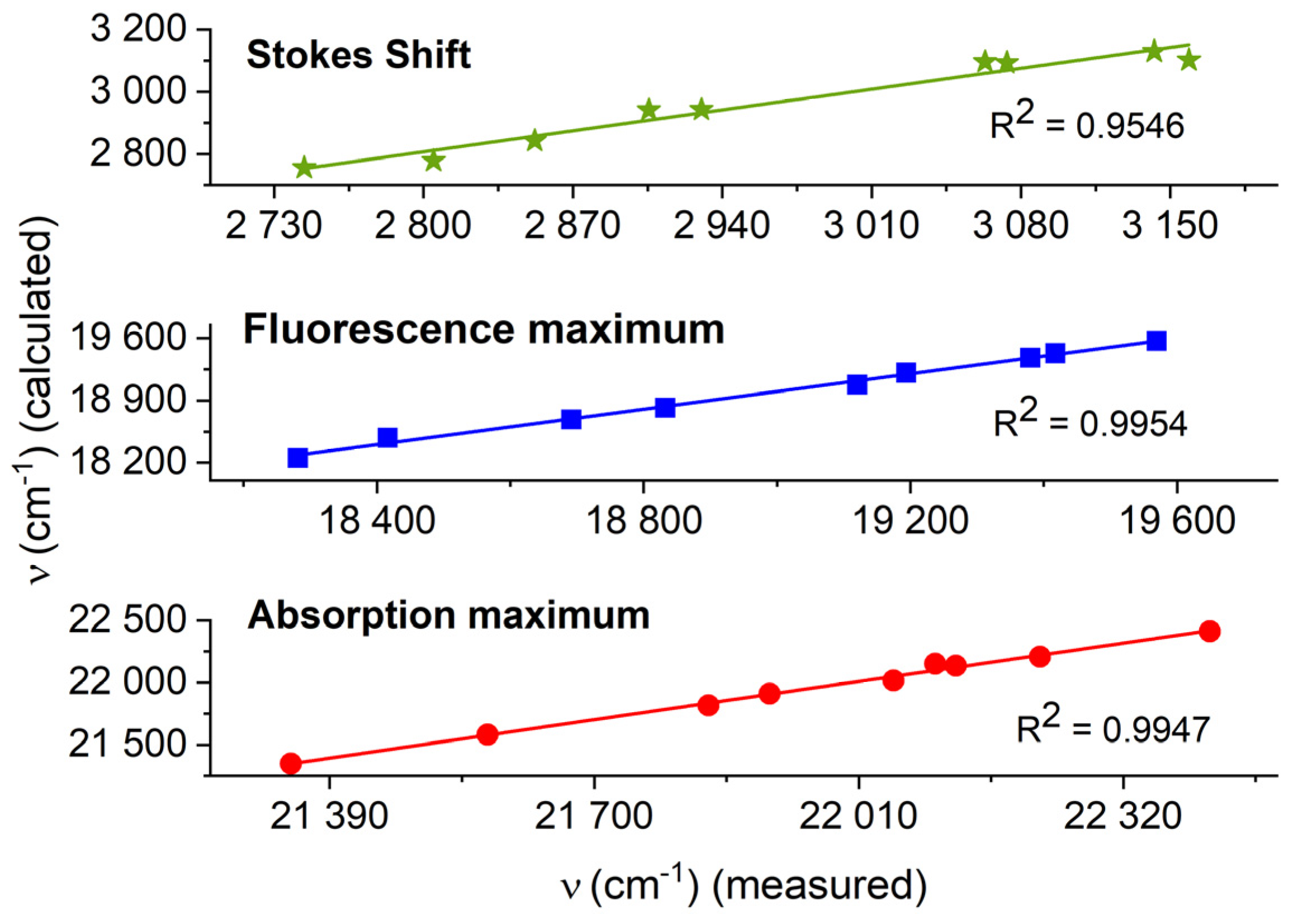
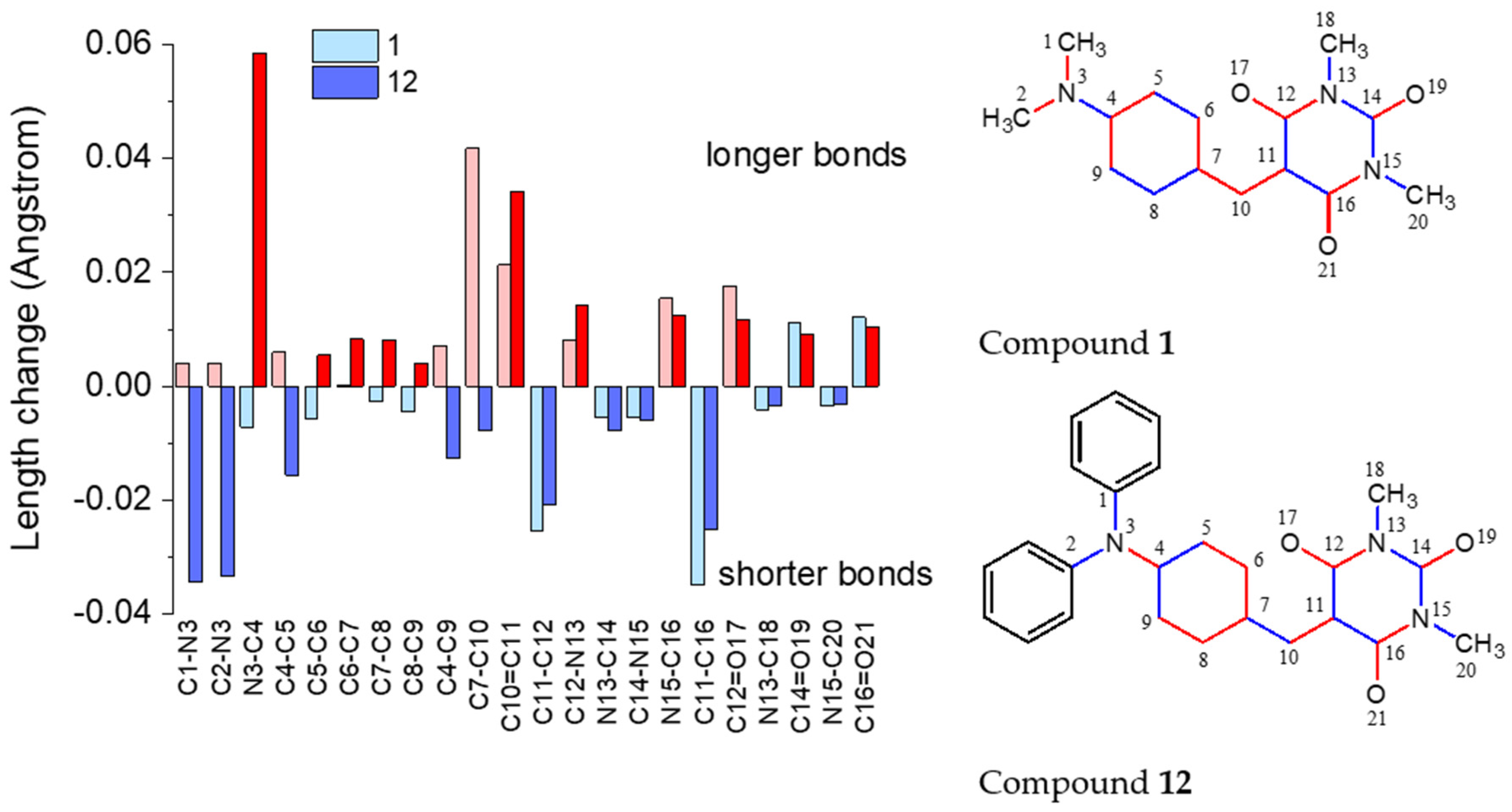
3.4. Theoretical Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishchenko, A.A.; Kulinich, A.V.; Bondarev, S.L.; Knyukshto, V.N. Electronic structure and fluorescent properties of malononitrile-based merocyanines with positive and negative solvatochromism. Opt. Spectrosc. 2008, 104, 57–68. [Google Scholar] [CrossRef]
- Hamidian, H.; Zahedian, N.; Ghazanfari, D.; Fozooni, S. Synthesis and Evaluation of Changes Induced by Solvent and Substituent in Electronic Absorption Spectra of New Azo Disperse Dyes Containig Barbiturate Ring. J. Spectrosc. 2013, 2013, 276413. [Google Scholar] [CrossRef]
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A Review. Chem. Rev. 2000, 100, 1973–2012. [Google Scholar] [CrossRef]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent Development of Chemosensors Based on Cyanine Platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef]
- Kulinich, A.V.; Ishchenko, A.A. Merocyanine dyes: Synthesis, structure, properties and applications. Russ. Chem. Rev. 2009, 78, 141–164. [Google Scholar] [CrossRef]
- Shindy, H.A.; El-Maghraby, M.A.; Eissa, F.M. Synthesis and colour spectrophotometric measurements of some novel merocyanine dyes. Dyes Pigm. 2012, 92, 929–935. [Google Scholar] [CrossRef]
- Kulinich, A.V.; Derevyanko, N.A.; Ishchenko, A.A.; Bondarev, S.L.; Knyukshto, V.N. Structure and fluorescent properties of merocyanines based on N,N-diethylthiobarbituric acid. J. Photochem. Photobiol. A Chem. 2008, 197, 40–49. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Wejnerowska, G. Highly Effective Sensitizers Based on Merocyanine Dyes for Visible Light Initiated Radical Polymerization. Polymers 2020, 12, 1242. [Google Scholar] [CrossRef] [PubMed]
- Madadi, N.R.; Penthala, N.R.; Janganati, V.; Crooks, P.A. Synthesis and anti-proliferative activity of aromatic substituted 5-((1-benzyl-1H-indol-3-yl)methylene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione analogs against human tumor cell lines. Bioorg. Med. Chem. Lett. 2014, 24, 601–603. [Google Scholar] [CrossRef]
- Dastan, D.; Ebadi, A. Effect of substitution on the binding affinity of 5-bezylidenebarbituric acid derivatives to ctDNA: In silico and in vitro studies. J. Chem. Sci. 2022, 134, 20. [Google Scholar] [CrossRef]
- Luzhnova, S.A.; Tyrkov, A.G.; Gabitova, N.M.; Yurtaeva, E.A. Synthesis and Antimicrobial Activity of 5-(Arylmethylidene)-2,4,6-Pyrimidine-2,4,6(1H,3H,5H)-Triones. Pharm. Chem. J. 2018, 52, 506–509. [Google Scholar] [CrossRef]
- Shestakov, A.N.; Pankova, A.S.; Golubev, P.; Khlebnikov, A.F.; Kuznetsov, M.A. Brønsted acid mediated cyclizations of ortho-aryl(ethynyl)pyrimidines. Tetrahedron 2017, 73, 3939–3948. [Google Scholar] [CrossRef]
- Edwards, A.A.; Alexander, B.D. UV-Visible Absorption Spectroscopy, Organic Applications. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Academic Press: Oxford, UK, 2017; pp. 511–519. [Google Scholar] [CrossRef]
- Lippert, E. Dipolmoment und elektronenstruktur von angeregten molekülen. Z. Naturforsch. 1955, 10a, 541–545. [Google Scholar] [CrossRef]
- Lippert, E. Spektroskopische bestimmung des dipolmomentes aromatischer verbindungen im ersten angeregten singulettzustand. Ber. Bunsenges. Phys. Chem. 1957, 61, 962–975. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent Effects upon Fluorescence Spectra and the Dipolemoments of Excited Molecules. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef]
- McRae, E.G. Theory of Solvent Effects on Molecular Electronic Spectra. Frequency Shifts. J. Phys. Chem. 1957, 61, 562–572. [Google Scholar] [CrossRef]
- Bakhshiev, N.G. Universal molecular interactions and their effect on the position of the electronic spectra of molecules in two-component solutions. Opt. Spectrosc. (USSR) 1961, 10, 379–384. [Google Scholar]
- Bilot, L.; Kawski, A. Zur Theorie des Einflusses von Lösungsmitteln auf die Elektronenspektren der Moleküle. Z. Naturforsch. 1962, 17A, 621–627. [Google Scholar] [CrossRef]
- Bilot, L.; Kawski, A. Dipolmomente einiger Phthalimid-Derivate im ersten angeregten Singulettzustand. Z. Naturforsch. 1963, 18A, 256. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Pietrzak, M. Dipole moment determination of 4-[N-(5,6,7,8-tetrahydroisoquinolinium-5-ylidene)methyl]-N,N-dialkylaniline iodides in solution. Spectrochim. Acta Part A 2011, 79, 985–992. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- Guzow, K.; Ceszlak, A.; Kozarzewska, M.; Wiczk, W. Influence of substituents on the nitrogen atom of 3-[2-(4-aminophenyl)benzoxazol-5-yl]alanine derivatives on their photophysical properties-solvatochromic studies. Photochem. Photobiol. Sci. 2011, 10, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Samanta, A.; Radhakrishnan, T.P. Excited State Dipole Moments from an Efficient Analysis of Solvatochromic Stokes Shift Data. J. Phys. Chem. 1994, 98, 9133–9136. [Google Scholar] [CrossRef]
- Kumar, S.; Rao, V.C.; Rastogi, R.C. Excited-state dipole moments of some hydroxycoumarin dyes using an efficient solvatochromic method based on the solvent polarity parameter, ETN. Spectrochim. Acta Part A 2001, 57, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Suppan, P.; Ghoneim, N. Solvatochromism; Royal Society of Chemistry: London, UK, 1997. [Google Scholar]
- Katritzky, A.R.; Fara, D.C.; Yang, H.; Tämm, K.; Tamm, T.; Karelson, M. Quantitative measures of solvent polarity. Chem. Rev. 2004, 104, 175–198. [Google Scholar] [CrossRef] [PubMed]
- Catalán, J.; Hopf, H. Empirical treatment of the inductive and dispersive components of solute−solvent interactions: The solvent polarizability (SP) scale. Eur. J. Org. Chem. 2004, 2004, 4694–4702. [Google Scholar] [CrossRef]
- Catalán, J.; Díaz, C.; López, V.; Pérez, P.; De Paz, J.L.G.; Rodríguez, J.G. A Generalized Solvent Basicity Scale: The Solvatochromism of 5-Nitroindoline and Its Homomorph 1-Methyl-5-nitroindoline. Liebigs Ann. 1996, 1996, 1785–1794. [Google Scholar] [CrossRef]
- Catalán, J. Toward a Generalized Treatment of the Solvent Effect Based on Four Empirical Scales: Dipolarity (SdP, a New Scale), Polarizability (SP), Acidity (SA), and Basicity (SB) of the Medium. J. Phys. Chem. B 2009, 113, 5951–5960. [Google Scholar] [CrossRef] [PubMed]
- Kamlet, M.J.; Taft, R.W. The solvatochromic comparison method. I. The.beta.-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.; Taft, R. An examination of linear solvation energy relationships. Prog. Phys. Org. Chem. 1981, 13, 485–630. [Google Scholar]
- Taft, R.W.; Kamlet, M.J. The solvatochromic comparison method. 2. The. alpha.-scale of solvent hydrogen-bond donor (HBD) acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Gawinecki, R.; Andrzejak, S.; Puchala, A. Efficiency of the Vilsmeier-Haack method in the synthesis of p-aminobenzaldehydes. Org. Prep. Proced. Int. 1998, 30, 455–460. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Gordel, M.; Szeremeta, J.; Grela, I.; Samoć, M. Photostability of push-pull phenanthroimidazole derivative upon one- and two-photon excitation. Dyes Pigm. 2017, 136, 150–160. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Scuseria, G.E.; Barone, V. Accurate excitation energies from time-dependent density functional theory: Assessing the PBE0 model. J. Chem. Phys. 1999, 111, 2889–2899. [Google Scholar] [CrossRef]
- Guido, C.; Caprasecca, S. Corrected Linear Response. State-Specific Correction to Solvent Polarization Response; Molecolab: Pisa, Italy, 2016. [Google Scholar] [CrossRef]
- Krawczyk, P.; Wybranowski, T.; Kaźmierski, Ł.; Hołyńska-Iwan, I.; Bratkowska, M.; Cysewski, P.; Jędrzejewska, B. 2′-(1H-phenanthro [9,10-d]imidazol-2-yl)-phenyl-4-carboxylic acid N-hydroxysuccinimide ester: A new phenanthroimidazole derivative as a fluorescent probe for medical imaging applications. Spectrochim. Acta Part A 2020, 228, 117757. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, P. 4-(4-Chloro-2-oxo-3(1H-phenanthro[9,10-d] imidazol-2-yl)-2H-chromen-6-yl)benzaldehyde as a fluorescent probe for medical imaging: Linear and nonlinear optical properties. Photochem. Photobiol. Sci. 2020, 19, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, P.; Jędrzejewska, B.; Seklecka, K.; Cytarska, J.; Łączkowski, K.Z. Effect of the Chloro-Substitution on Electrochemical and Optical Properties of New Carbazole Dyes. Materials 2021, 14, 3091. [Google Scholar] [CrossRef] [PubMed]
- Kula, S.; Krawczyk, P.; Filapek, M.; Maroń, A.M. Influence of N-donor substituents on physicochemical properties of phenanthro[9,10-d]imidazole derivatives. J. Lumin. 2021, 233, 117910. [Google Scholar] [CrossRef]
- Szukalski, A.; Krawczyk, P.; Sahraoui, B.; Jędrzejewska, B. Multifunctional Oxazolone Derivative as an Optical Amplifier, Generator, and Modulator. J. Phys. Chem. B 2022, 126, 1742–1757. [Google Scholar] [CrossRef]
- Szukalski, A.; Krawczyk, P.; Sahraoui, B.; Rosińska, F.; Jędrzejewska, B. A Modified Oxazolone Dye Dedicated to Spectroscopy and Optoelectronics. J. Org. Chem. 2022, 87, 7319–7332. [Google Scholar] [CrossRef] [PubMed]
- Le Bahers, T.; Adamo, C.; Ciofini, I. A Qualitative Index of Spatial Extent in Charge-Transfer Excitations. J. Chem. Theory Comput. 2011, 7, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Arivazhagan, M.; Muniappan, P.; Meenakshi, R.; Rajavel, G. PCM/TD-DFT analysis of 1-bromo-2,3-dichlorobenzene-A combined study of experimental (FT-IR and FT-Raman) and theoretical calculations. Spectrochim. Acta Part A 2013, 105, 497–508. [Google Scholar] [CrossRef]
- Würthner, F.; Yao, S. Merocyanine Dyes Containing Imide Functional Groups: Synthesis and Studies on Hydrogen Bonding to Melamine Receptors. J. Org. Chem. 2003, 68, 8943–8949. [Google Scholar] [CrossRef]
- Ding, S.; Yao, B.; Schobben, L.; Hong, Y. Barbituric Acid Based Fluorogens: Synthesis, Aggregation-Induced Emission, and Protein Fibril Detection. Molecules 2020, 25, 32. [Google Scholar] [CrossRef]
- Rezende, M.C.; Campodonico, P.; Abuin, E.; Kossanyi, J. Merocyanine-type dyes from barbituric acid derivatives. Spectrochim. Acta Part A 2001, 57, 1183–1190. [Google Scholar] [CrossRef]
- Haldar, M.K.; Scott, M.D.; Sule, N.; Srivastava, D.K.; Mallik, S. Synthesis of barbiturate-based methionine aminopeptidase-1 inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 2373–2376. [Google Scholar] [CrossRef]
- Seeliger, F.; Berger, S.T.A.; Remennikov, G.Y.; Polborn, K.; Mayr, H. Electrophilicity of 5-Benzylidene-1,3-dimethylbarbituric and -thiobarbituric Acids. J. Org. Chem. 2007, 72, 9170–9180. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Tian, Y.; Tao, F.-r.; Yu, W.; You, K.-Y.; Zhou, L.-R.; Su, X.; Li, T.-d.; Cui, Y.-Z. Detection of nitroaromatics based on aggregation induced emission of barbituric acid derivatives. Spectrochim. Acta Part A 2019, 222, 117168. [Google Scholar] [CrossRef]
- Wróblewski, S.; Trzebiatowska, K.; Jędrzejewska, B.; Pietrzak, M.; Gawinecki, R.; Pączkowski, J. Development of fluorescence probes based on stilbazolium salts for monitoring free radical polymerization processes. J. Chem. Soc. Perkin Trans. 2 1999, 1909–1917. [Google Scholar] [CrossRef]
- Gawinecki, R.; Trzebiatowska, K. The effect of the amino group on the spectral properties of substituted styrylpyridinium salts. Dyes Pigm. 2000, 45, 103–107. [Google Scholar] [CrossRef]
- Gawinecki, R.; Kolehmainen, E.; Kauppinen, R. 1H and 13C NMR studies of para-substituted benzaldoximes for evaluation of the electron donor properties of substituted amino groups†. J. Chem. Soc. Perkin Trans. 2 1998, 25–30. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Skotnicka, A.; Laurent, A.D.; Pietrzak, M.; Jacquemin, D.; Ośmiałowski, B. Influence of the Nature of the Amino Group in Highly Fluorescent Difluoroborates Exhibiting Intramolecular Charge Transfer. J. Org. Chem. 2018, 83, 7779–7788. [Google Scholar] [CrossRef] [PubMed]
- Stortz, C. Conformational pathways of simple six-membered rings. J. Phys. Org. Chem. 2010, 23, 1173–1186. [Google Scholar] [CrossRef]
- Zhang, Y.; Jónsson, H.; Weber, P.M. Coherence in nonradiative transitions: Internal conversion in Rydberg-excited N-methyl and N-ethyl morpholine. PCCP 2017, 19, 26403–26411. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Gao, Y.; Stankus, B.; Xu, X.; Yong, H.; Weber, P.M. Ultrafast conformational dynamics of Rydberg-excited N-methyl piperidine. PCCP 2021, 23, 27417–27427. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Pietrzak, M.; Pączkowski, J. Solvent Effects on the Spectroscopic Properties of Styrylquinolinium Dyes Series. J. Fluoresc. 2010, 20, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Rotkiewicz, K.; Grellmann, K.H.; Grabowski, Z.R. Reinterpretation of the anomalous fluorescense of p-n,n-dimethylamino-benzonitrile. Chem. Phys. Lett. 1973, 19, 315–318. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef]
- Zhong, C. The driving forces for twisted or planar intramolecular charge transfer. PCCP 2015, 17, 9248–9257. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewska, B.; Grabarz, A.M.; Bartkowiak, W.; Ośmiałowski, B. Spectral and physicochemical properties of difluoroboranyls containing N,N-dimethylamino group studied by solvatochromic methods. Spectrochim. Acta Part A 2018, 199, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewska, B.; Krawczyk, P.; Józefowicz, M. Experimental and theoretical studies of the influence of solvent polarity on the spectral properties of two push-pull oxazol-5-(4H)-one compounds. Spectrochim. Acta Part A 2017, 171, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Kawski, A.; Bojarski, P. Comments on the determination of excited state dipole moment of molecules using the method of solvatochromism. Spectrochim. Acta Part A 2011, 82, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Beaujuge, P.M.; Amb, C.M.; Reynolds, J.R. Spectral Engineering in π-Conjugated Polymers with Intramolecular Donor−Acceptor Interactions. Acc. Chem. Res. 2010, 43, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, S.; Graham, K.R.; Shi, P.; Farley, R.T.; Steckler, T.T.; Brookins, R.N.; Taranekar, P.; Mei, J.; Padilha, L.A.; Ensley, T.R.; et al. Donor–Acceptor–Donor-based π-Conjugated Oligomers for Nonlinear Optics and Near-IR Emission. Chem. Mater. 2011, 23, 3805–3817. [Google Scholar] [CrossRef]
- Fromherz, P.; Heilemann, A. Twisted internal charge transfer in (aminophenyl)pyridinium. J. Phys. Chem. 1992, 96, 6864–6866. [Google Scholar] [CrossRef]
- Tahir, M.H.; Mubashir, T.; Shah, T.-U.-H.; Mahmood, A. Impact of electron-withdrawing and electron-donating substituents on the electrochemical and charge transport properties of indacenodithiophene-based small molecule acceptors for organic solar cells. J. Phys. Org. Chem. 2019, 32, e3909. [Google Scholar] [CrossRef]
- Haley, J.E.; Krein, D.M.; Monahan, J.L.; Burke, A.R.; McLean, D.G.; Slagle, J.E.; Fratini, A.; Cooper, T.M. Photophysical Properties of a Series of Electron-Donating and -Withdrawing Platinum Acetylide Two-Photon Chromophores. J. Phys. Chem. A 2011, 115, 265–273. [Google Scholar] [CrossRef]
- Kulinich, A.V.; Derevyanko, N.A.; Ishchenko, A.A. Electronic structure and solvatochromism of merocyanines based on N,N-diethylthiobarbituric acid. J. Photochem. Photobiol. A Chem. 2007, 188, 207–217. [Google Scholar] [CrossRef]
- El-Sayed, M.; Spange, S. Synthesis, properties, and solvatochromism of 1,3-dimethyl-5-{(thien-2-yl)-[4-(1-piperidyl) phenyl]methylidene}-(1H,3H)-pyrimidine-2,4,6-trione. J. Phys. Org. Chem. 2007, 20, 264–270. [Google Scholar] [CrossRef]
- Kulinich, A.V.; Ishchenko, A.A.; Groth, U.M. Electronic structure and solvatochromism of merocyanines: NMR spectroscopic point of view. Spectrochim. Acta Part A 2007, 68, 6–14. [Google Scholar] [CrossRef] [PubMed]

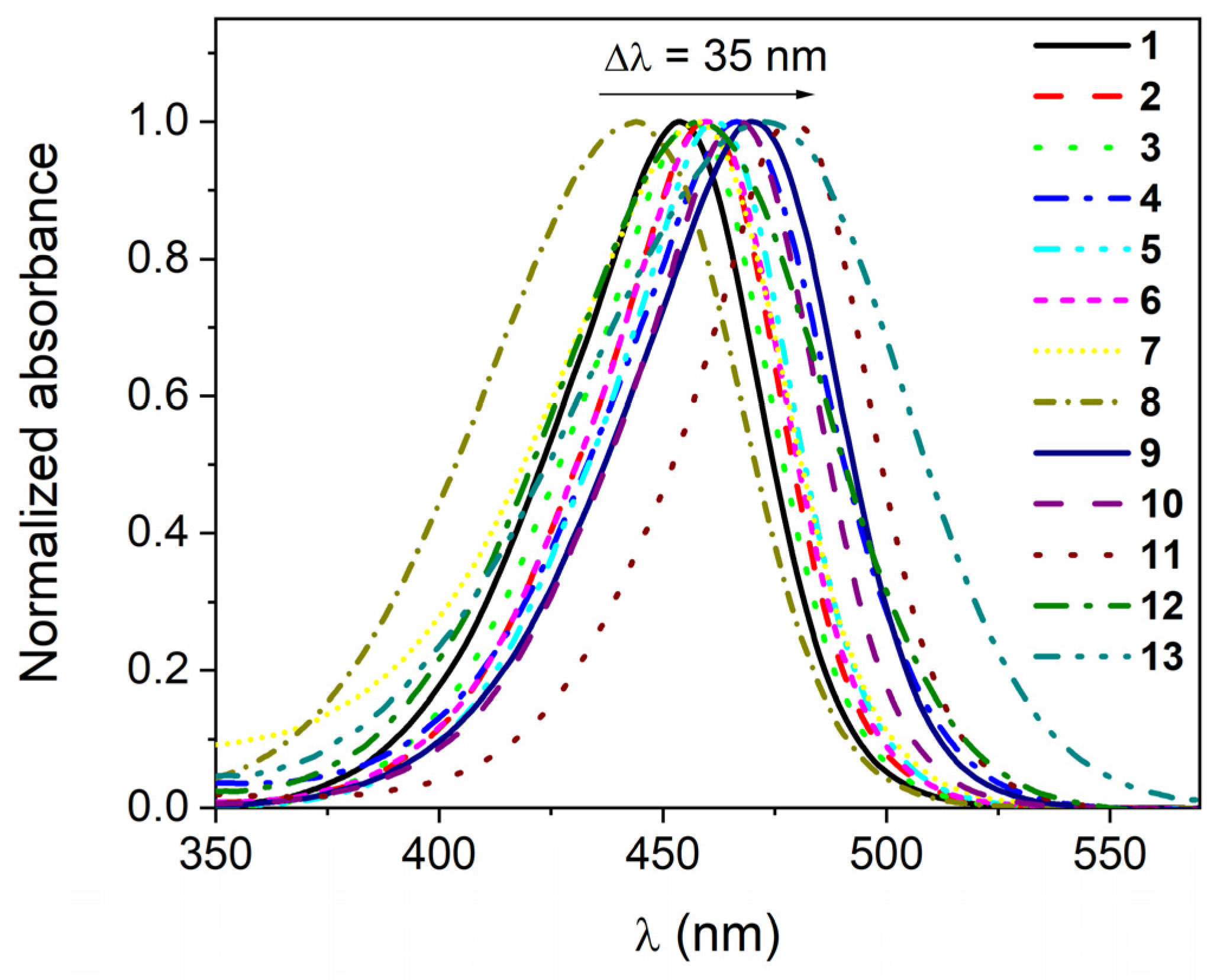



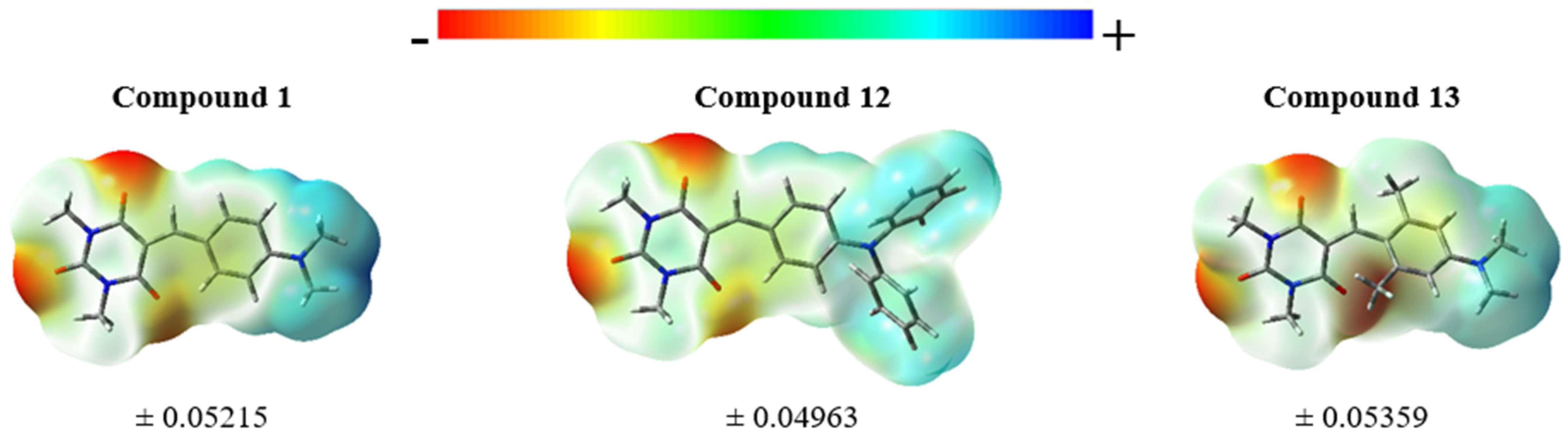
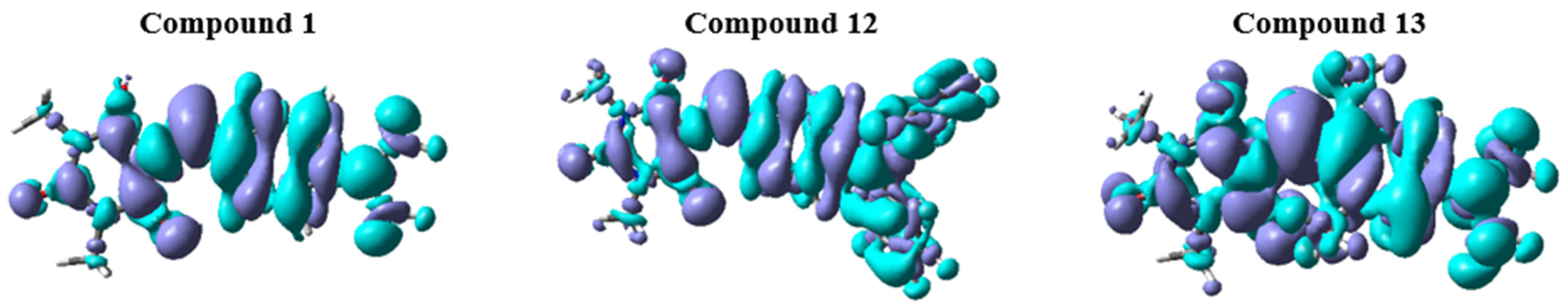
| No | εmax | FWHMab | ΔνSS | εmax | FWHMab | ΔνSS | εmax | FWHMab | ΔνSS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toluene | THF | DMF | |||||||||||||
| 1 | 452 | 7.03 | 2091 | 516 | 2744 | 453.5 | 7.15 | 2204 | 523 | 2930 | 463.5 | 7.17 | 2271 | 543 | 3159 |
| 2 | 457 | 9.03 | 1916 | 517 | 2539 | 459 | 8.95 | 2384 | 526 | 2775 | 469 | 7.93 | 2102 | 542 | 2872 |
| 3 | 454.5 | 7.89 | 2011 | 522 | 2845 | 455 | 7.58 | 2142 | 529 | 3074 | 465 | 7.03 | 2187 | 545 | 3157 |
| 4 | 464 | 5.99 | 2214 | 539 | 2999 | 466.5 | 5.87 | 2295 | 541 | 2952 | 477.5 | 5.69 | 2326 | 554 | 2892 |
| 5 | 459 | 8.10 | 1909 | 524 | 2703 | 461.5 | 8.43 | 2005 | 534 | 2942 | 471 | 7.44 | 2082 | 548 | 2983 |
| 6 | 459 | 7.83 | 1936 | 528 | 2847 | 460 | 7.68 | 2039 | 531 | 2907 | 468.5 | 7.36 | 2126 | 544 | 2962 |
| 7 | 456.5 | 5.87 | 2301 | 532 | 3109 | 458 | 6.42 | 2896 | 545 | 3485 | 468 | 5.66 | 2434 | 548 | 3119 |
| 8 | 441.5 | 5.15 | 2682 | 497 | 2529 | 443.5 | 4.82 | 2822 | 520 | 3317 | 453.5 | 4.96 | 2928 | 543 | 3635 |
| 9 | 468 | 6.93 | 2123 | 535 | 2676 | 469.5 | 6.42 | 2190 | 535 | 2608 | 482 | 6.82 | 2169 | 550 | 2565 |
| 10 | 464.5 | 7.81 | 1900 | 525 | 2481 | 466.5 | 7.72 | 2023 | 533 | 2675 | 477 | 7.65 | 2064 | 552 | 2848 |
| 11 | 476 | 9.33 | 1730 | 537 | 2386 | 479 | 8.88 | 1772 | 544 | 2494 | 489.5 | 8.60 | 1859 | 558 | 2508 |
| 12 | 463.5 | 3.67 | 2644 | 583 | 4422 | 458 | 2.59 | 2818 | 644 | 6306 | 460 | 3.05 | 2977 | 680 | 7033 |
| 13 | 476 | 2.27 | 2877 | 531 | 2176 | 473 | 1.94 | 3744 | 544 | 2759 | 481 | 1.73 | 3450 | 560 | 2933 |
| Compound | FQY (%) | ||
|---|---|---|---|
| Toluene | THF | DMF | |
| 1 | 0.031 | 0.035 | 0.040 |
| 2 | 0.029 | 0.045 | 0.046 |
| 3 | 0.034 | 0.052 | 0.041 |
| 4 | 0.024 | 0.025 | 0.024 |
| 5 | 0.043 | 0.060 | 0.063 |
| 6 | 0.039 | 0.046 | 0.044 |
| 7 | 0.043 | 0.049 | 0.046 |
| 8 | 0.033 | 0.035 | 0.045 |
| 9 | 0.049 | 0.035 | 0.039 |
| 10 | 0.046 | 0.036 | 0.048 |
| 11 | 0.067 | 0.054 | 0.055 |
| 12 | 0.257 | 0.534 | 0.109 |
| 13 | 0.048 | 0.001 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyszka, I.; Krawczyk, P.; Jędrzejewska, B. The Influence of the Alkylamino Group on the Solvatochromic Behavior of 5-(4-substituted-arylidene)-1,3-dimethylpyrimidine-2,4,6-triones: Synthesis, Spectroscopic and Computational Studies. Materials 2024, 17, 2447. https://doi.org/10.3390/ma17102447
Pyszka I, Krawczyk P, Jędrzejewska B. The Influence of the Alkylamino Group on the Solvatochromic Behavior of 5-(4-substituted-arylidene)-1,3-dimethylpyrimidine-2,4,6-triones: Synthesis, Spectroscopic and Computational Studies. Materials. 2024; 17(10):2447. https://doi.org/10.3390/ma17102447
Chicago/Turabian StylePyszka, Ilona, Przemysław Krawczyk, and Beata Jędrzejewska. 2024. "The Influence of the Alkylamino Group on the Solvatochromic Behavior of 5-(4-substituted-arylidene)-1,3-dimethylpyrimidine-2,4,6-triones: Synthesis, Spectroscopic and Computational Studies" Materials 17, no. 10: 2447. https://doi.org/10.3390/ma17102447
APA StylePyszka, I., Krawczyk, P., & Jędrzejewska, B. (2024). The Influence of the Alkylamino Group on the Solvatochromic Behavior of 5-(4-substituted-arylidene)-1,3-dimethylpyrimidine-2,4,6-triones: Synthesis, Spectroscopic and Computational Studies. Materials, 17(10), 2447. https://doi.org/10.3390/ma17102447