Corrosion Behavior and Mechanical Property of 5182 Aluminum/DP780 Steel Resistance Spot Welding Joints
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Electrochemical Polarization Test
2.3. Salt Spray Corrosion and Mechanical Test
2.4. Microstructure Examination
3. Results and Discussion
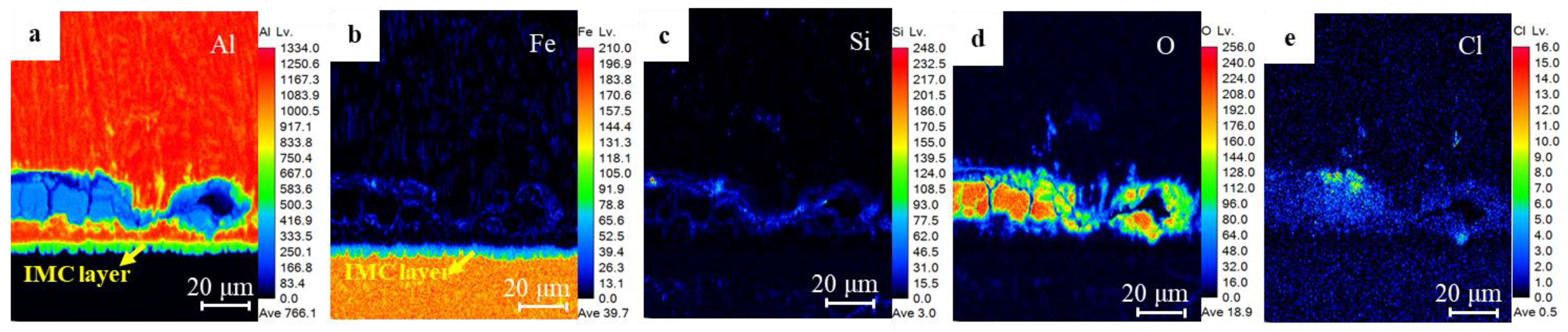
3.1. Microstructure Characteristics of RSW Joints
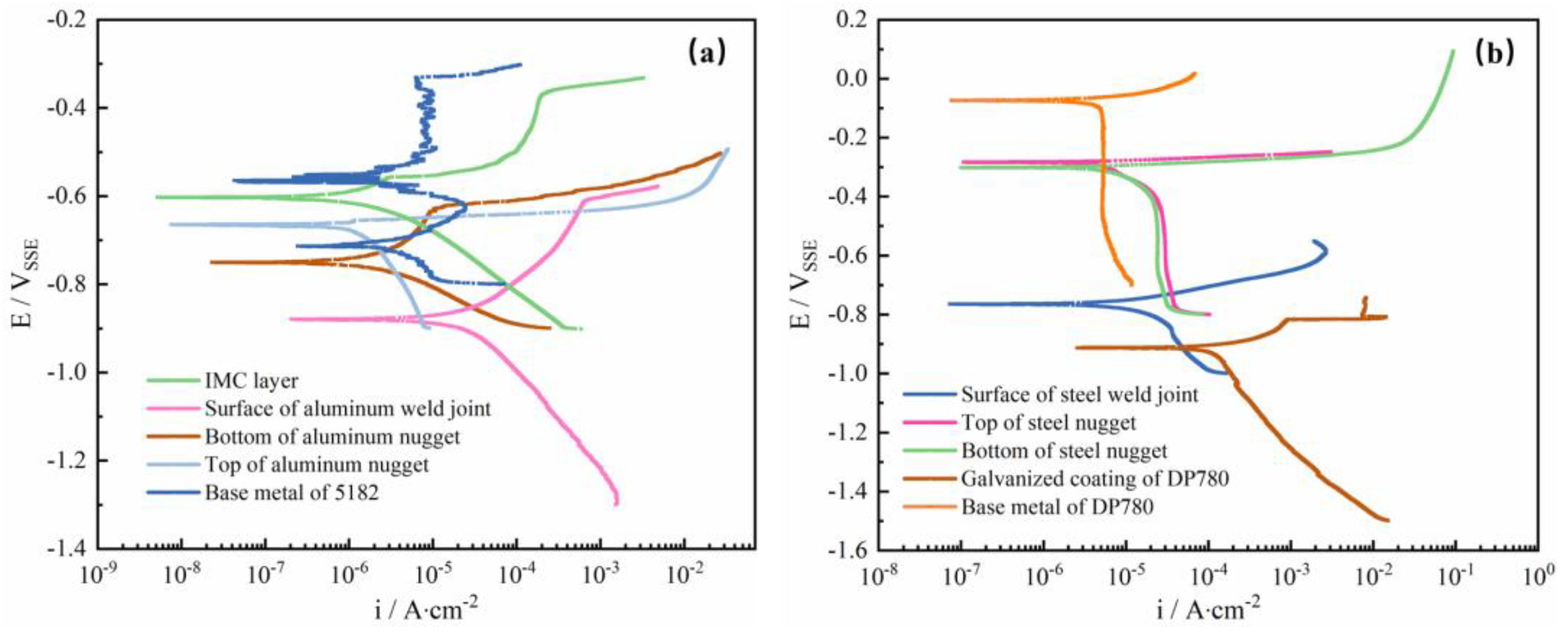
3.2. Potentiodynamic Polarization
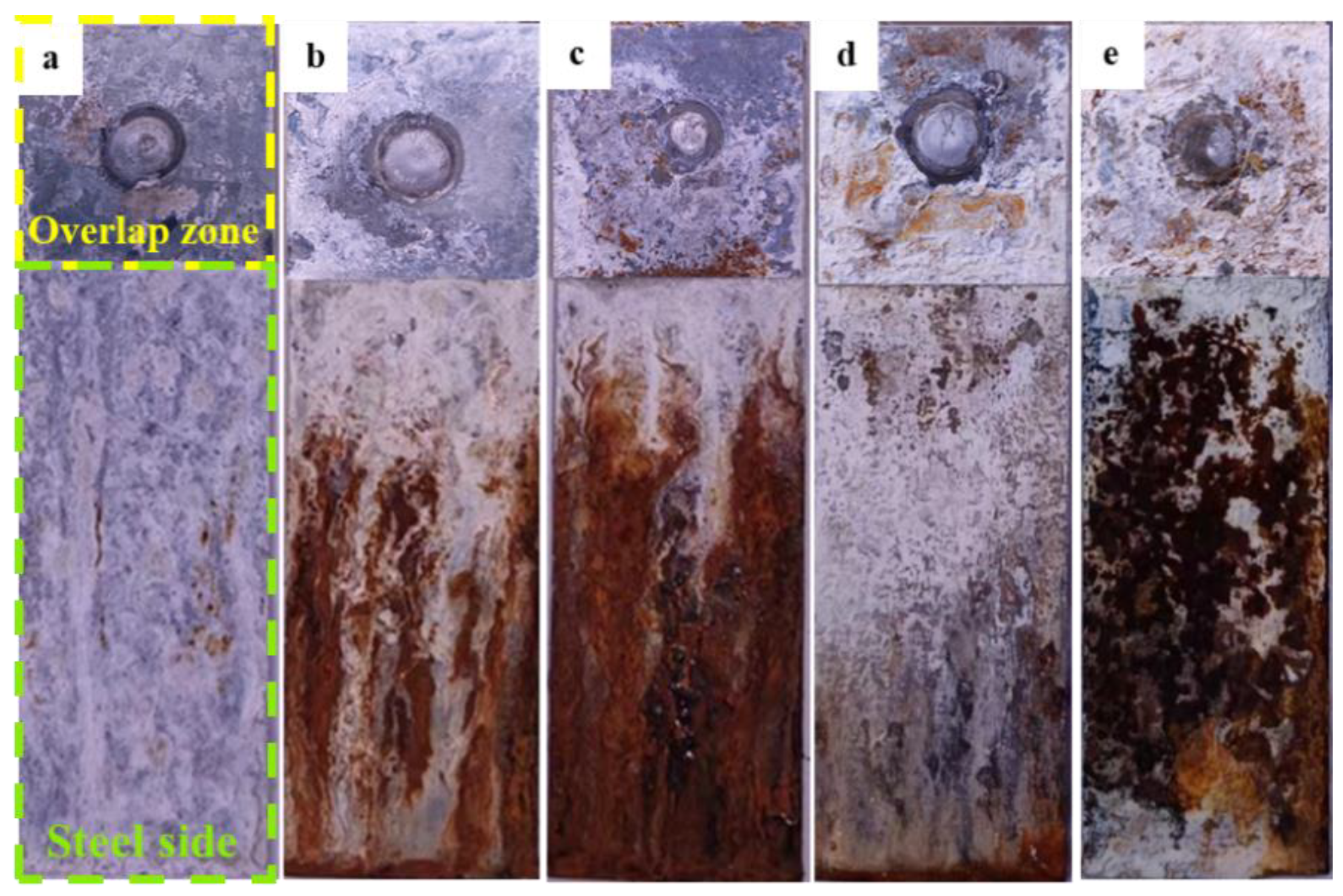
3.3. Salt Spray Corrosion Test
3.4. Fracture Characteristics and Mechanical Properties
4. Conclusions
- (1)
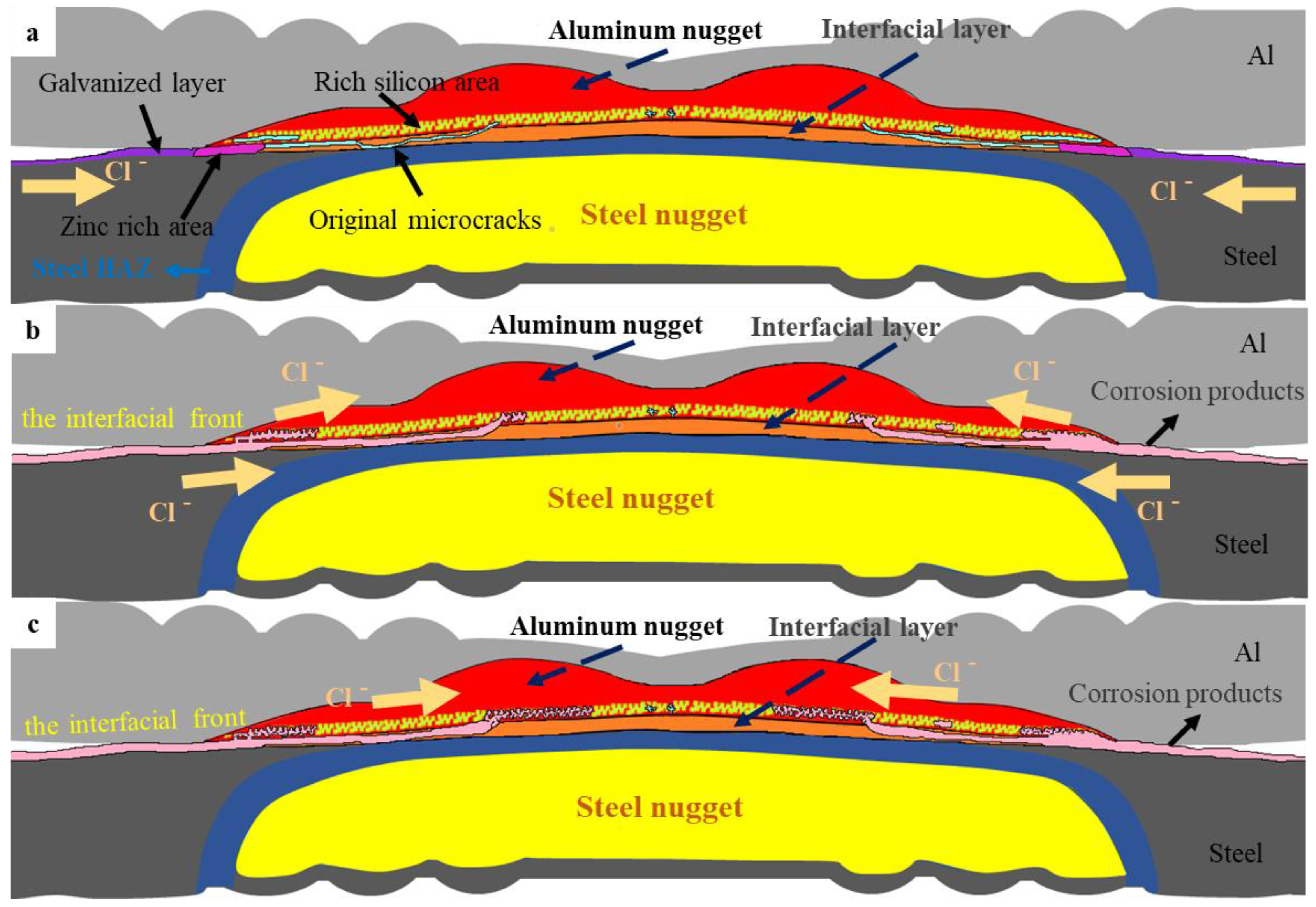
- According to microstructure observation, the joint between 5182 and DP780 is bonded by the formation of FeAl intermetallic compound layer. Si enrichment and a few oxidation defects were observed at the interface front on the aluminum nugget side.
- (2)
- The experimental result from the polarization test and salt spray test gives evidence to the fact that the interfacial compound layer was not susceptible to preferential corrosion due to its relative noble corrosion potential in the interfacial galvanic couple.
- (3)
- During the salt spray test, the corrosion of the joint initially occurred in the galvanized layer of the overlap region and then developed towards the nugget center of the joint along the original microcracks and the defects on the interface front of the aluminum nugget side. Such a result was consistent with the accelerated corrosion susceptibility of the aluminum nugget by anodic galvanic effect with a coupled interfacial IMC layer in the polarization test. According to the mechanical property test, the tensile shear strength decreased with an increase in the corroded area of the weld joint.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jochen Pflug, D.I. Recyclable hexagons for the automotive industry. Reinf. Plast. 2021, 65, 42–44. [Google Scholar] [CrossRef]
- Hoffmann, K.G.; Haag, K.; Müssig, J. Biomimetic approaches towards lightweight composite structures for car interior parts. Mater. Des. 2021, 212, 110281. [Google Scholar] [CrossRef]
- Li, M.; Tao, W.; Zhang, J.; Wang, Y.; Yang, S. Hybrid resistance-laser spot welding of aluminum to steel dissimilar materials: Microstructure and mechanical properties. Mater. Des. 2022, 221, 111022. [Google Scholar] [CrossRef]
- Yang, H.; Cai, D.; Li, J.; Kwok, C.Y.; Zhao, Y.; Li, Y.; Liu, H.; Lai, W. Fatigue behavior of Al–Al and Al–steel refill friction stir spot welding joints. Fatigue Fract. Eng. Mater. Struct. 2021, 3219–3223. [Google Scholar] [CrossRef]
- Sadeghian, A.; Iqbal, N. A review on dissimilar laser welding of steel-copper, steel-aluminum, aluminum-copper, and steel-nickel for electric vehicle battery manufacturing. Opt. Laser Technol. 2022, 146, 107595. [Google Scholar] [CrossRef]
- Khaleel, H.H.; Mahmood, I.A.; Khoshnaw, F. Fatigue and impact properties of single and double resistance spot welding for high-strength steel used in automotive applications. J. Braz. Soc. Mech. Sci. Eng. 2023, 45, 155. [Google Scholar] [CrossRef]
- Santoro, L.; Razza, V.; De Maddis, M. Frequency-based analysis of active laser thermography for spot weld quality assessment. Int. J. Adv. Manuf. Technol. 2024, 130, 3017–3029. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, G.; Ren, B.; Yu, W. Process optimization of aluminum/steel resistance spot welding based on dynamic resistance analysis. J. Mater. Sci. 2024, 58, 17908–17929. [Google Scholar] [CrossRef]
- Pan, B.; Sun, H.; Shang, S.-L.; Banu, M.; Wang, P.-C.; Carlson, B.E.; Liu, Z.-K.; Li, J. Understanding formation mechanisms of intermetallic compounds in dissimilar Al/steel joint processed by resistance spot welding. J. Manuf. Process. 2022, 83, 212–222. [Google Scholar] [CrossRef]
- Huang, R.; Tan, C.; Sun, Y.; Gong, X.; Wu, L.; Chen, B.; Zhao, H.; Song, X. Influence of processing window on laser welding-brazing of Al to press-hardened 22MnB5 steel. Opt. Laser Technol. 2021, 133, 106566. [Google Scholar] [CrossRef]
- Qian, C.; Ghassemi-Armaki, H.; Shi, L.; Kang, J.; Haselhuhn, A.S.; Carlson, B.E. Competing fracture modes in Al-steel resistance spot welded structures: Experimental evaluation and numerical prediction. Int. J. Impact Eng. 2024, 185, 104838. [Google Scholar] [CrossRef]
- Boettcher, S.W.; Oener, S.Z.; Lonergan, M.C.; Surendranath, Y.; Ardo, S.; Brozek, C.; Kempler, P.A. Potentially confusing: Potentials in electrochemistry. ACS Energy Lett. 2020, 6, 261–266. [Google Scholar] [CrossRef]
- Calabrese, L.; Proverbio, E.; Di Bella, G.; Galtieri, G.; Borsellino, C. Failure behavior of SPR joints after salt spray test. Eng. Struct. 2015, 82, 33–43. [Google Scholar] [CrossRef]
- Maddela, S.; Carlson, B.E. Corrosion characterization of resistance spot-welded aluminum and steel couple. J. Manuf. Sci. Eng. 2019, 141, 111010. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Zhang, G.; Huang, J. Corrosion Behavior of Aluminum-Steel Weld-Brazing Joint. J. Mater. Eng. Perform. 2016, 25, 1916–1923. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Wang, S.; Yang, J.; Dong, H.; Yan, D. Corrosion behavior of refilled friction stir spot welded joint between aluminum alloy and galvanized steel. Mater. Res. Express 2018, 5, 96524. [Google Scholar] [CrossRef]
- Lei, H.; Li, Y.; Carlson, B.; Guo, Y. Corrosion behaviors of CMT spot-joined AA6022-T4 to galvanized DC03. Weld. J. 2019, 98, 14–27. [Google Scholar]
- Ma, H.; Qin, G.; Geng, P.; Wang, S.; Zhang, D. Microstructural characterisation and corrosion behaviour of aluminium alloy/steel hybrid structure produced by friction welding. J. Manuf. Process. 2021, 61, 349–356. [Google Scholar] [CrossRef]
- Mahto, R.P.; Anishetty, S.; Sarkar, A.; Mypati, O.; Pal, S.K. Interfacial Microstructural and Corrosion Characterizations of Friction Stir Welded AA6061-T6 and AISI304 Materials. Met. Mater. Int. 2019, 25, 752–767. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Meng, X.; Chen, Y.; Cheng, C. Effect of intermetallic compound on the corrosion behavior of resistance spot welding joints between 5182 aluminum alloy and galvanized DP780 dual-phase steel. Mater. Res. Express 2023, 10, 26515. [Google Scholar] [CrossRef]
- Dang, Z.; Qin, G.; Lin, R. A comprehensive study of interfacial microstructure and corrosion behavior of AA2219 to 304SS inertia friction welded joints. Corros. Sci. 2023, 224, 111539. [Google Scholar] [CrossRef]
- Xue, J.; Li, Y.; Chen, H.; Zhu, Z. Effects of heat input on wettability, interface microstructure and properties of Al/steel butt joint in laser-metal inert-gas hybrid welding-brazing. J. Mater. Process. Technol. 2018, 255, 47–54. [Google Scholar] [CrossRef]
- Meng, X.; Li, S.; Shi, L.; Zhang, Y.; Chen, Y.; Zhang, S.; Wu, H. Effect of hold time on resistance spot weldability of aluminium to steel. Sci. Technol. Weld. Join. 2022, 27, 522–532. [Google Scholar] [CrossRef]
- Shi, L.; Kang, J.; Amirkhiz, B.S.; Sigler, D.R.; Haselhuhn, A.S.; Carlson, B.E. Influence of sheet thickness ratio on fracture mechanisms of Al-steel resistance spot welds produced using multi-ring domed electrode. Sci. Technol. Weld. Join. 2019, 25, 164–168. [Google Scholar] [CrossRef]
- Shi, L.; Kang, J.; Qian, C.; Liang, J.; Shalchi-Amirkhiz, B.; Haselhuhn, A.S.; Carlson, B.E. Role of Fe2Al5 in fracture of novel dissimilar aluminum-steel resistance spot welds using multi-ring domed electrodes. Mater. Sci. Eng. A 2022, 831, 142233. [Google Scholar] [CrossRef]
- Pan, B.; Sun, H.; Shang, S.-L.; Wen, W.; Banu, M.; Simmer, J.C.; Carlson, B.E.; Chen, N.; Liu, Z.-K.; Zheng, Z.; et al. Corrosion behavior in aluminum/galvanized steel resistance spot welds and self-piercing riveting joints in salt spray environment. J. Manuf. Process. 2021, 71, 608–620. [Google Scholar] [CrossRef]
- Hu, S.; Haselhuhn, A.S.; Ma, Y.; Li, Y.; Carlson, B.E.; Lin, Z. Sensitivity of dissimilar aluminum to steel resistance spot welds to weld gun deflection. J. Manuf. Process. 2021, 68, 534–545. [Google Scholar] [CrossRef]
- Zhang, H. Comparative Analysis of Salt Spray Test Standards ISO 9227:2006 and ISO 9227:1990. Mater. Prot. 2009, 42, 71–73. [Google Scholar]
- Gao, K.; Zhu, L. Numerical prediction for temperature and microstructure of A283GRC steel and 5052 aluminium alloy during induction-pressure welding. Int. J. Therm. Sci. 2022, 175, 107456. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Chen, S. Improving the performance of steel aluminum resistance spot welding joints based on steel coating design. Mater. Lett. 2023, 353, 135291. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, H.-P.; Chen, N.; Wang, M.; Carlson, B.E. Characterization of intermetallic compound at the interfaces of Al-steel resistance spot welds. J. Mater. Process. Technol. 2017, 242, 12–23. [Google Scholar] [CrossRef]
- Springer, H.; Kostka, A.; Payton, E.J.; Raabe, D.; Kaysser-Pyzalla, A.; Eggeler, G. On the formation and growth of intermetallic phases during interdiffusion between low-carbon steel and aluminum alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Z.; Bi, Y.; Zhang, Y. Nano-scale characterization of intermetallic compounds in dissimilar aluminum-steel resistance element welded joints. Mater. Charact. 2023, 204, 113227. [Google Scholar] [CrossRef]
- Rizi, E.C.; Ranjbarnodeh, E.; Mirsalehi, S.E. Predicting the quantity and type of intermetallic phases in aluminum–steel dissimilar joints produced by zinc interlayer. Weld World 2023, 67, 151–165. [Google Scholar] [CrossRef]
- Beygi, R.; Carbas, R.J.C.; Barbosa, A.Q.; Marques, E.A.S.; da Silva, L.F.M. A comprehensive analysis of a pseudo-brittle fracture at the interface of intermetallic of η and steel in aluminum/steel joints made by FSW: Microstructure and fracture behavior. Mater. Sci. Eng. A 2021, 824, 141812. [Google Scholar] [CrossRef]
- Doleker, K.M.; Erdogan, A.; Yener, T. Investigation of the Surface Degradation Properties of Aluminized Super Austenitic Stainless Steel. J. Manag. 2024, 76, 522–539. [Google Scholar] [CrossRef]
- Dang, H.; Yu, H. Effect of the post-weld heat treatment temperature on the mechanical properties and microstructure of magnetic pulse welded Al/Fe tubes. J. Mater. Res. Technol. 2021, 15, 4554–4563. [Google Scholar] [CrossRef]
- Zhang, N.; Hu, Q.; Ding, Z.; Lua, W.; Yangb, F.; Lia, J. 3D morphological evolution and growth mechanism of proeutectic FeAl3 phases formed at Al/Fe interface under different cooling rates. J. Mater. Sci. Technol. 2022, 116, 83–93. [Google Scholar] [CrossRef]
- Xu, P.; Hua, X.; Shen, C.; Mou, G.; Li, F. Formation of Fe5Si3 precipitate in the Fe2Al5 intermetallic layer of the Al/steel dissimilar arc welding joint: A transmission electron microscopy (TEM) study. Mater. Charact. 2021, 178, 111236. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, J.; Xie, J.; Gou, Y.; Zhang, H.; Chen, Y.; Yin, L.; Zhang, L.; Gao, L.; Wang, G. Intermetallic compounds growth and morphology evolution of Al6061/SS304 electromagnetic pulse welding joint interface during post-weld heat treatment. J. Mater. Res. Technol. 2024, 28, 4001–4011. [Google Scholar] [CrossRef]
- Tian, S.; Zhao, F.; Liu, X. Clarification of the solid-state diffusion behavior of a copper alloy/aluminum alloy composite interface assisted by position marking of the second phases. Mater. Charact. 2022, 192, 112173. [Google Scholar] [CrossRef]
- Beygi, R.; Carbas, R.; Queiros, A.; Marques, E.A.S.; da Silva, L.F.M. Comparative Study Between Stainless Steel and Carbon Steel During Dissimilar Friction Stir Welding with Aluminum: Kinetics of Al–Fe Intermetallic Growth. Met. Mater. Int. 2022, 28, 1948–1959. [Google Scholar] [CrossRef]
- Bobzin, K.; Öte, M.; Wiesner, S.; Gerdt, L.; Bührig-Polaczek, A. Effect of Alloying Elements on Growth Behavior of Intermetallic Compounds at the Cold-Sprayed Coating/Steel Interface During Immersion in Aluminum. Int. J. Met. 2018, 12, 712–721. [Google Scholar] [CrossRef]
- Wang, R.; Li, P.; Zhou, W.; Li, Y.; Gao, K.; Ouyang, Y. Study on oxidation mechanism of aluminum surface under applied electric field. Mater. Chem. Phys. 2024, 318, 129224. [Google Scholar] [CrossRef]
- Pradhan, D.; Mondal, A.; Chakraborty, A. Microstructural and Corrosion Resistance Evaluation of Hot-Dipped Al-Zn-Si Alloy-Coated Steel. J. Mater. Eng. Perform. 2022, 31, 2297–2308. [Google Scholar] [CrossRef]
- Wang, J.; Lv, M.; Du, M.; Li, Z.; Xu, T.; Li, G. Effects of cathodic polarization on X65 steel inhibition behavior and mechanism of mixed microorganisms induced corrosion in seawater. Corros. Sci. 2022, 208, 110670. [Google Scholar] [CrossRef]
- Zhao, R.; Xu, L.; Xin, Y.; Hou, J.; Fan, L.; Shao, Y.; Xuan, J.; Bai, S.; Xue, L.; Li, L.; et al. Effect of cathodic polarization on stress corrosion cracking susceptibility of 35CrMo steel for Class 12.9 fasteners in seawater. J. Mater. Res. Technol. 2023, 24, 9339–9355. [Google Scholar] [CrossRef]
- Zhang, X.; Mei, W.; Huang, L.; Tao, J.; Xiong, Y.; Wang, Z. Galvanic corrosion behavior of 5083 alloy/H62 brass couple under magnetic field. J. Mater. Res. Technol. 2023, 22, 192–205. [Google Scholar] [CrossRef]
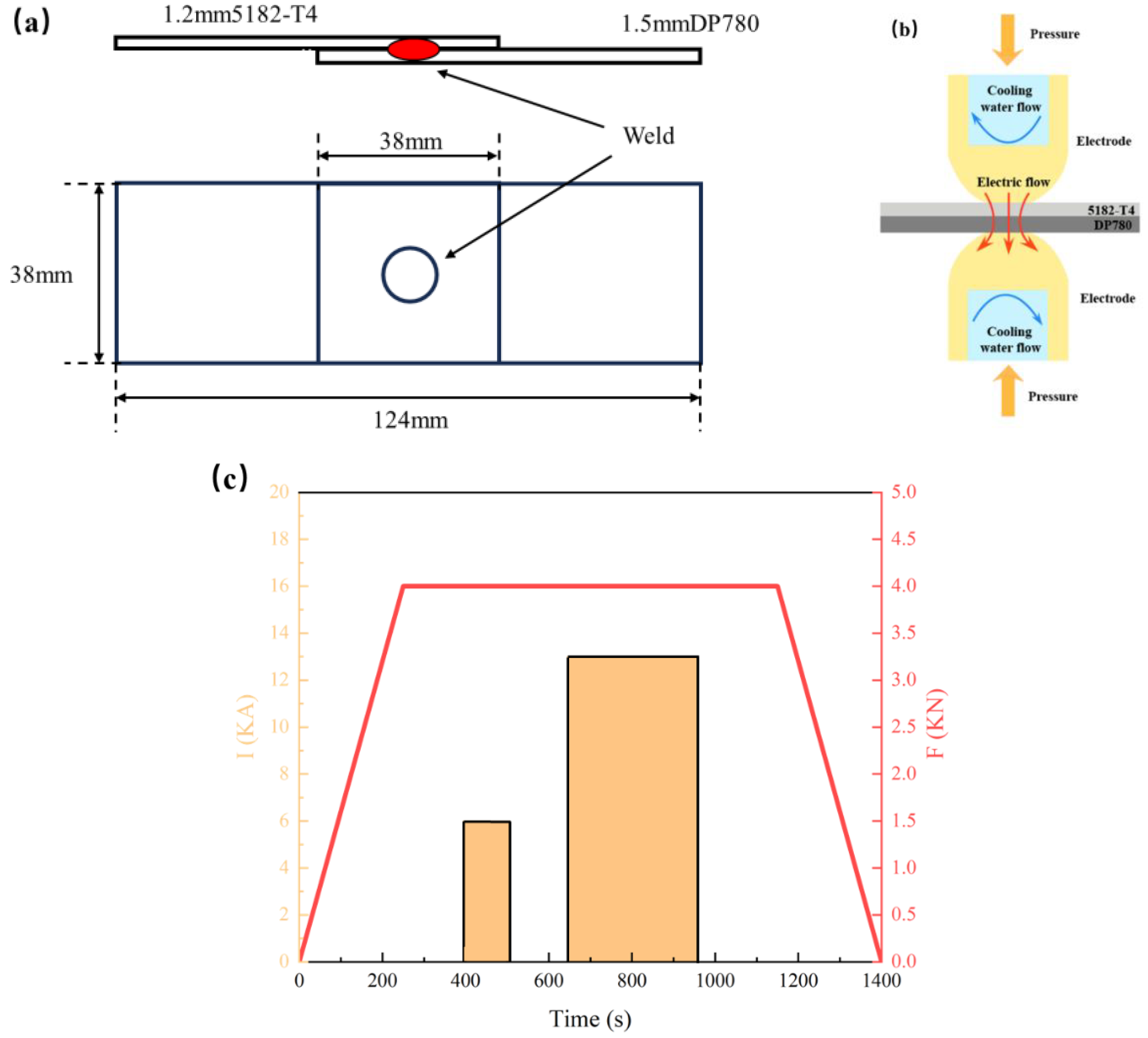

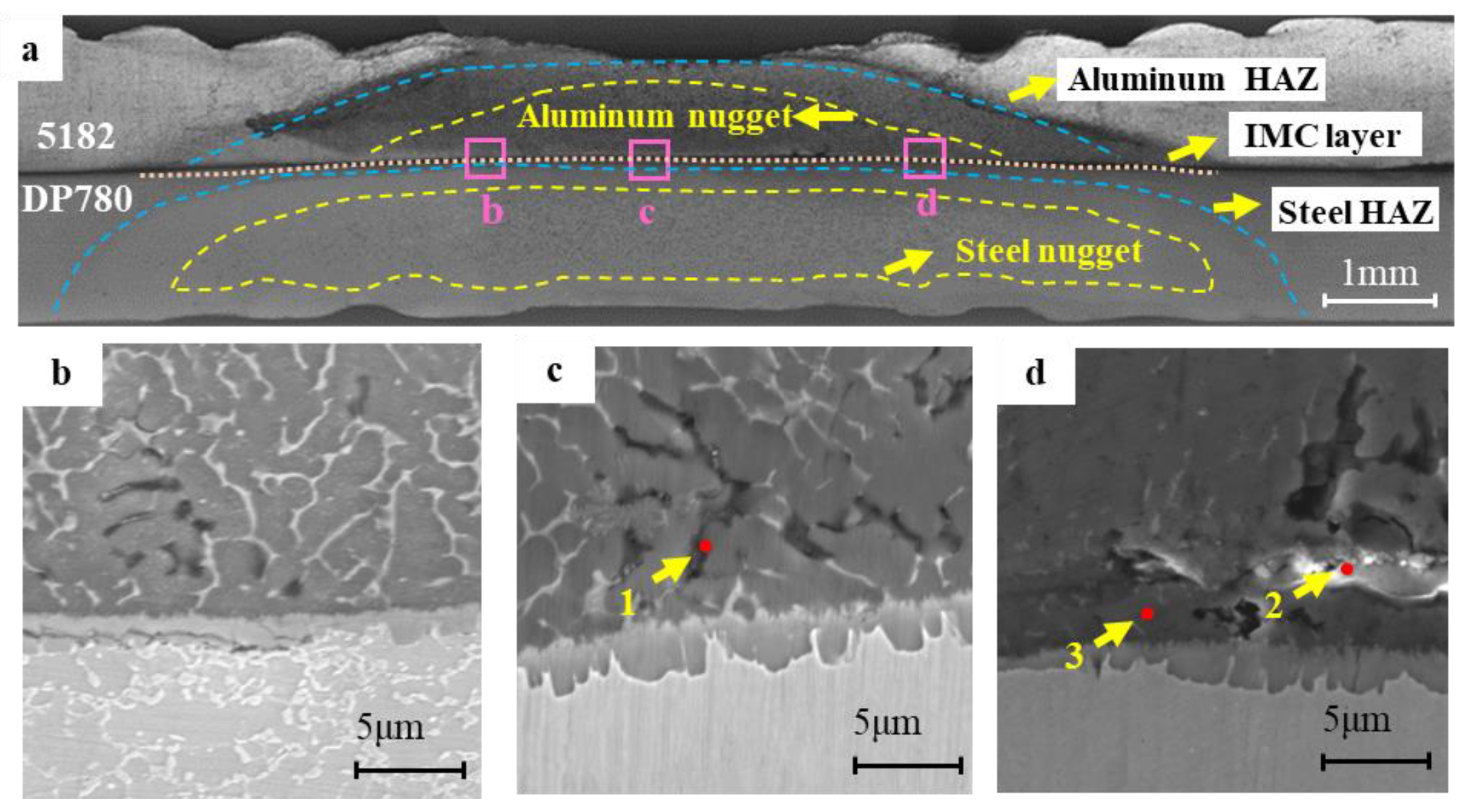
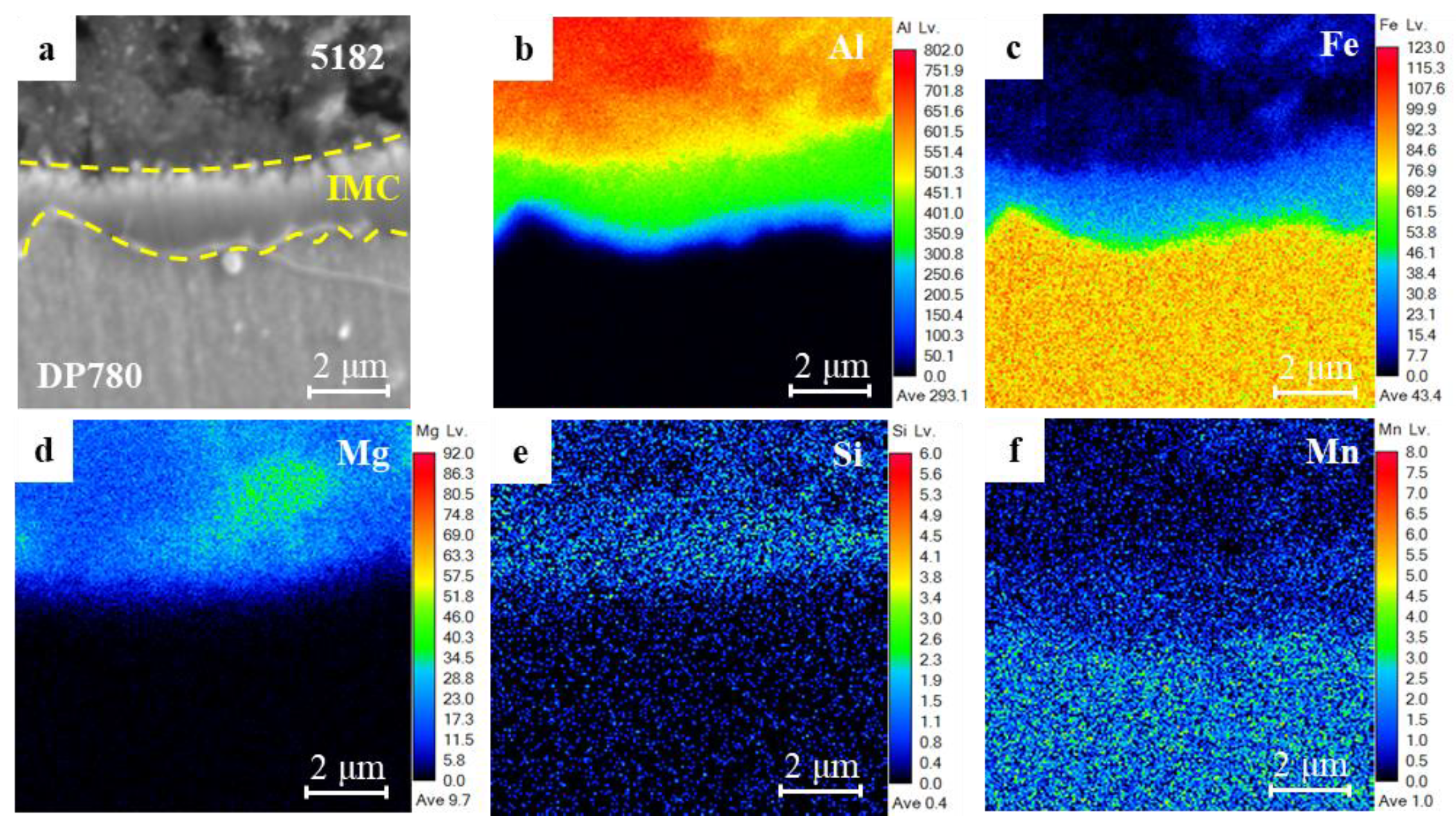




| Element | C | Al | Fe | Mg | Cu | Mn | Si | Cr | Zn | Mo | S | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5182-T4 | - | 94.81 | 0.17 | 4.53 | 0.04 | 0.22 | 0.19 | 0.03 | 0.01 | - | - | - |
| DP780 | 0.112 | 0.387 | 97.02 | - | - | 1.92 | 0.08 | 0.27 | - | 0.2 | 0.001 | 0.01 |
| Element | Al | Fe | O | Mn | Si | Mg | Cu | Cr | Zn |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 86 | 0.4 | 8.9 | 0.1 | 0.8 | 3.1 | 0.4 | --- | 0.3 |
| 2 | 18 | 3.6 | 64 | --- | 4.4 | 5.7 | --- | --- | 4.3 |
| 3 | 92 | 0.4 | 2.8 | --- | 0.04 | 4.7 | --- | --- | 0.06 |
| Region | Bottom of Al Nugget | Top of Al Nugget | Surface of Al Weld Joint | Base Metal of 5182 | IMC Layer | Galvanized Coating of DP 780 | Base Metal of DP780 | Surface of Steel Weld Joint | Bottom of Steel Nugget | Top of Steel Nugget |
|---|---|---|---|---|---|---|---|---|---|---|
| Ecorr/V | −0.746 | −0.672 | −0.881 | −0.573 | −0.603 | −0.913 | −0.074 | −0.767 | −0.303 | −0.282 |
| icorr/ (A/cm2) | 2.1 × 10−6 | 1.7 × 10−6 | 1.7 × 10−5 | 7.5 × 10−6 | 2.0 × 10−6 | 2.1 × 10−4 | 8.7 × 10−6 | 9.7 × 10−6 | 9.1 × 10−6 | 5.2 × 10−6 |
| Element | Al | Fe | O | Mg | Mn | Zn | Cl |
|---|---|---|---|---|---|---|---|
| 1 | 95 | 1.5 | 0.7 | 2.8 | - | - | - |
| 2 | 91 | 4.7 | 0.6 | 2.8 | 0.9 | - | - |
| 3 | 1.7 | 96 | - | - | 2.3 | - | - |
| 4 | 14 | 11 | 64 | 0.3 | - | 5.7 | 5 |
| 5 | 89 | 2 | 3 | 6 | - | - | - |
| 6 | 31 | 0.5 | 64 | 4.5 | - | - | - |
| 7 | 92 | - | - | 8 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Zhang, S.; Li, T.; Meng, X.; Cheng, C.; Pei, J.; Cao, T.; Zhao, J. Corrosion Behavior and Mechanical Property of 5182 Aluminum/DP780 Steel Resistance Spot Welding Joints. Materials 2024, 17, 2472. https://doi.org/10.3390/ma17112472
Shi X, Zhang S, Li T, Meng X, Cheng C, Pei J, Cao T, Zhao J. Corrosion Behavior and Mechanical Property of 5182 Aluminum/DP780 Steel Resistance Spot Welding Joints. Materials. 2024; 17(11):2472. https://doi.org/10.3390/ma17112472
Chicago/Turabian StyleShi, Xuan, Sai Zhang, Tao Li, Xianming Meng, Congqian Cheng, Jibin Pei, Tieshan Cao, and Jie Zhao. 2024. "Corrosion Behavior and Mechanical Property of 5182 Aluminum/DP780 Steel Resistance Spot Welding Joints" Materials 17, no. 11: 2472. https://doi.org/10.3390/ma17112472
APA StyleShi, X., Zhang, S., Li, T., Meng, X., Cheng, C., Pei, J., Cao, T., & Zhao, J. (2024). Corrosion Behavior and Mechanical Property of 5182 Aluminum/DP780 Steel Resistance Spot Welding Joints. Materials, 17(11), 2472. https://doi.org/10.3390/ma17112472





