Enhanced Photocatalytic Activity of V2C MXene-Coupled ZnO Porous Nanosheets with Increased Surface Area and Effective Charge Transfer
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
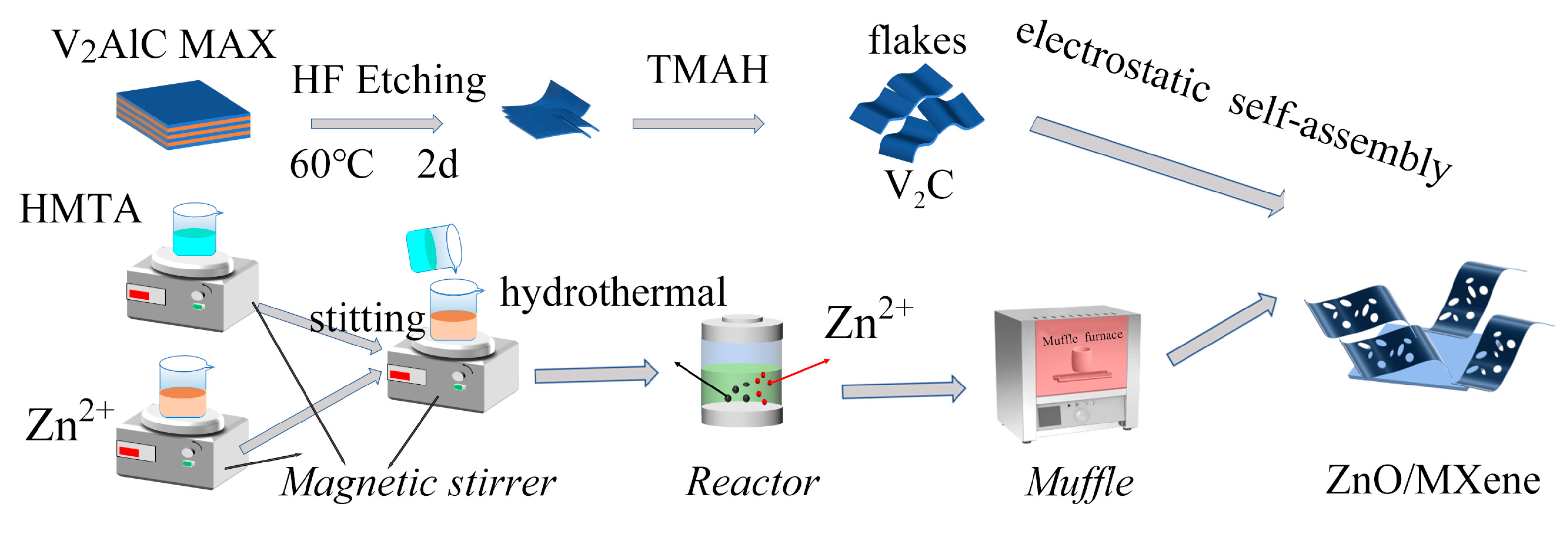
2.2. Preparation of ZnO/MXene Composite
2.3. Characterizations
2.4. Photocatalytic Activity of the ZnO/MXene Composite
3. Results and Discussion
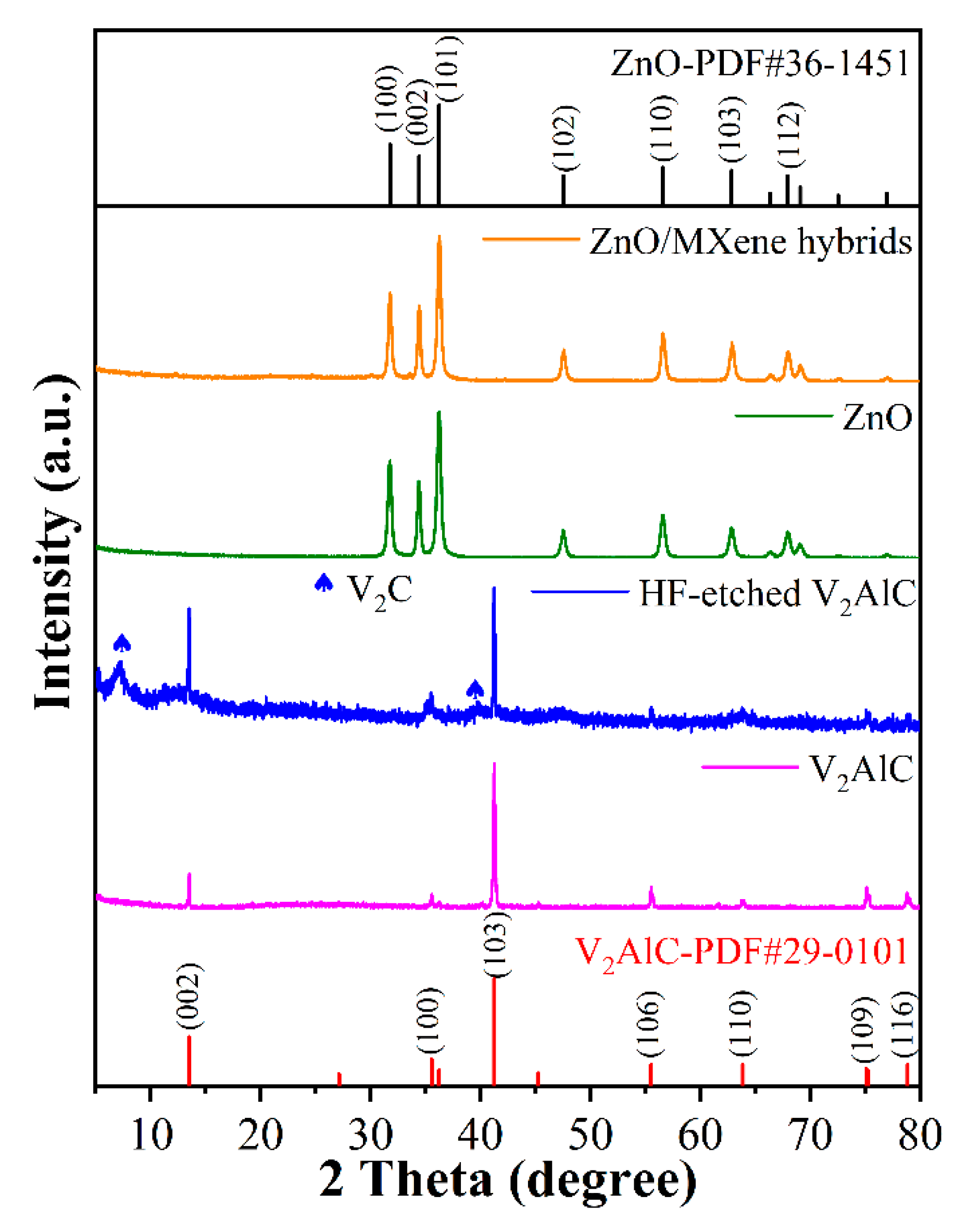
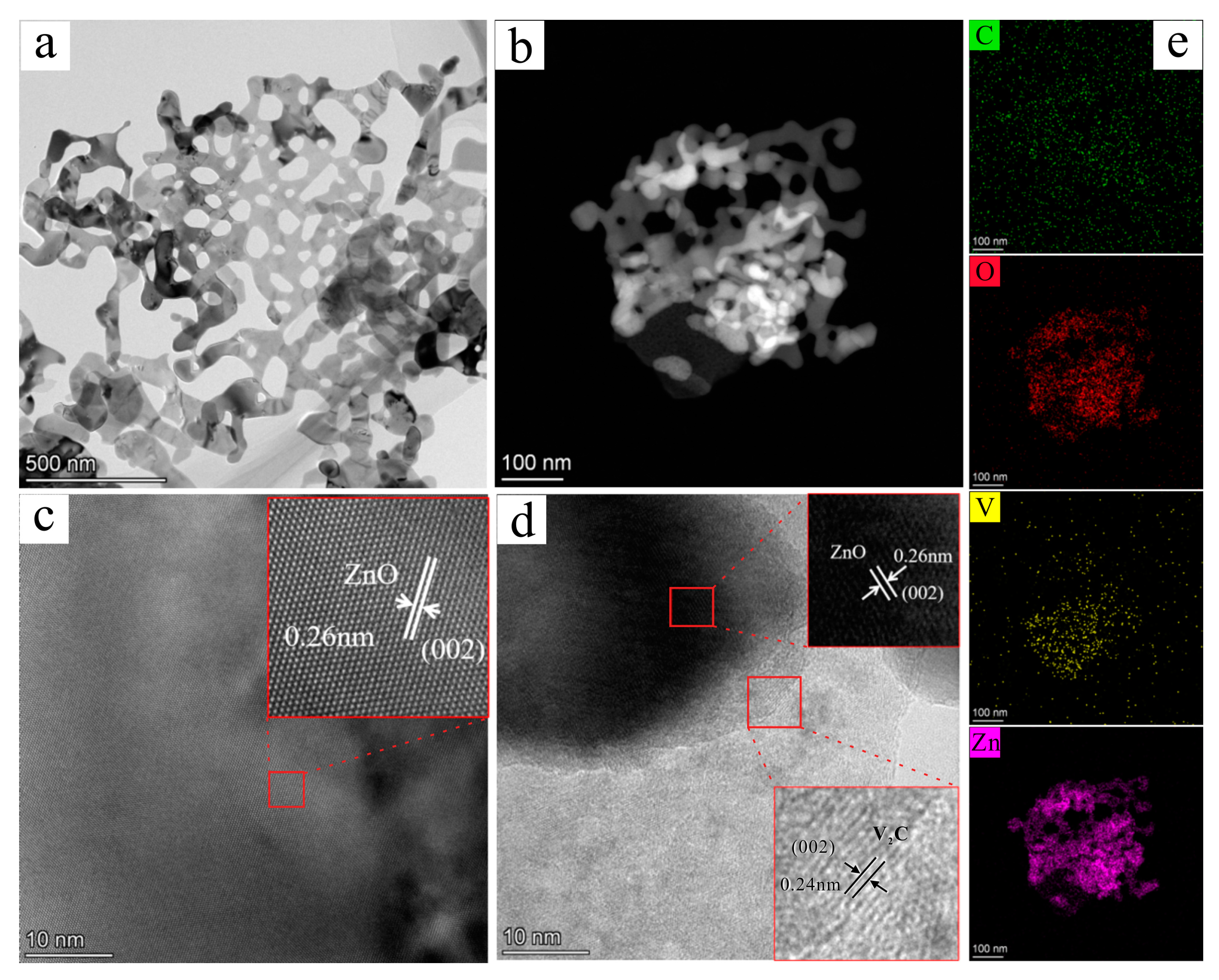
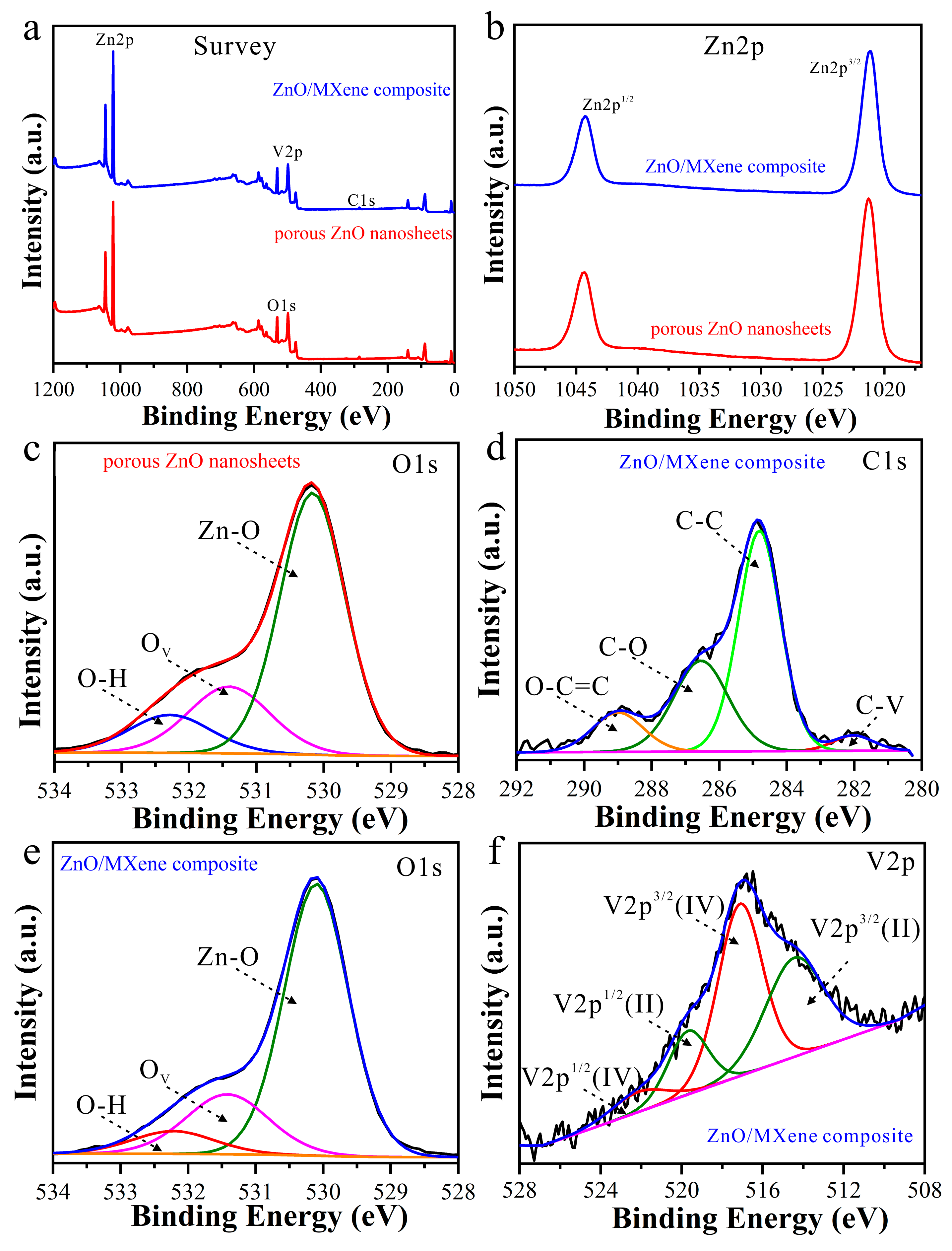
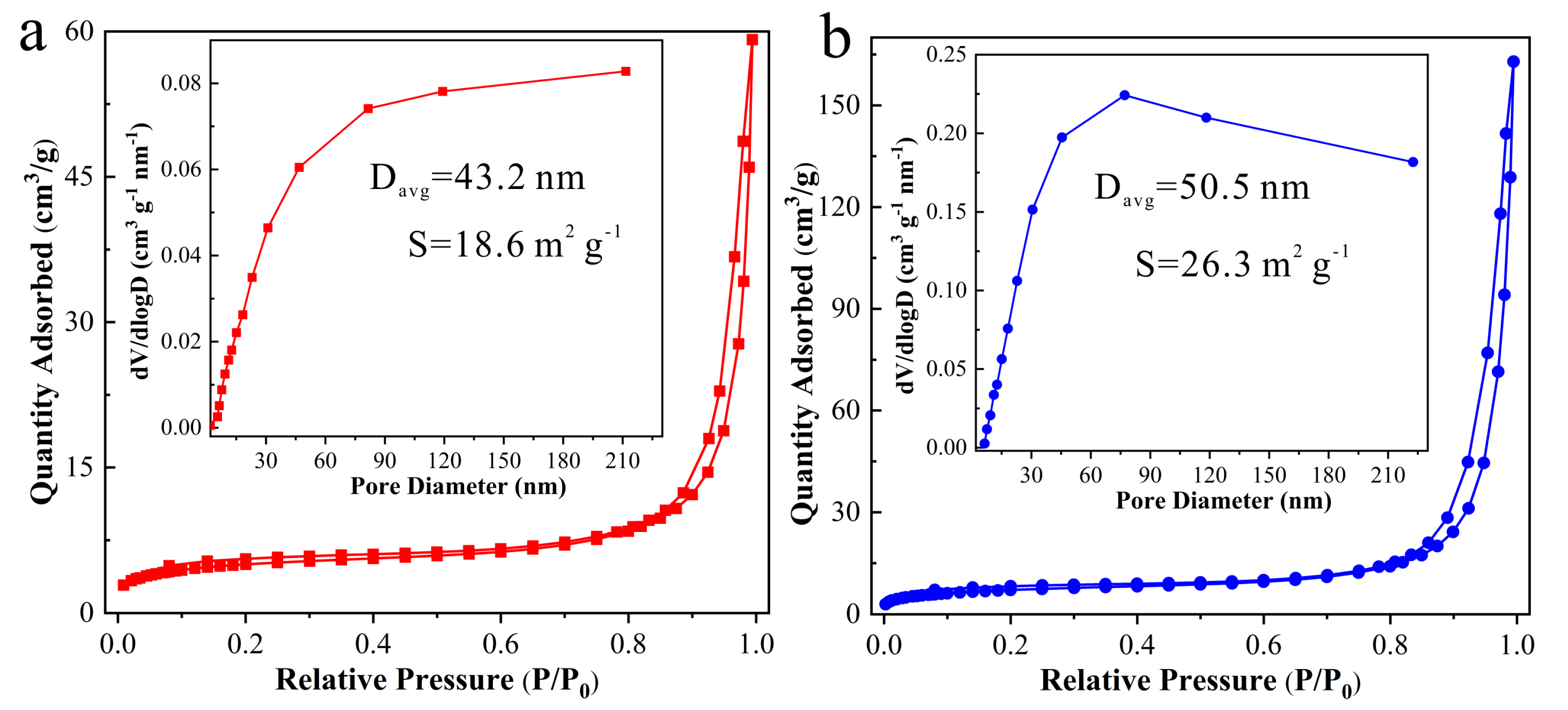
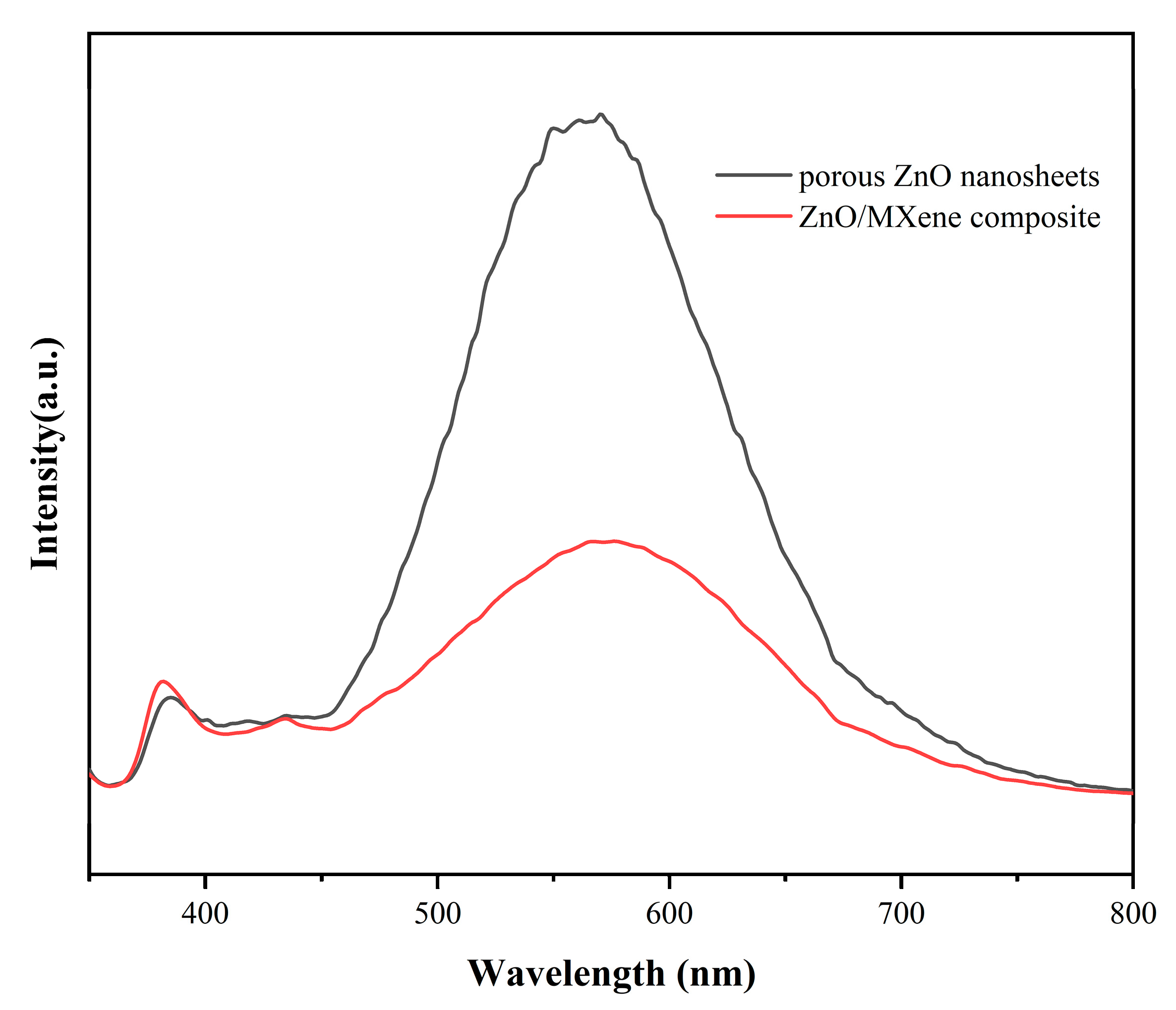
3.1. Structure and Morphology
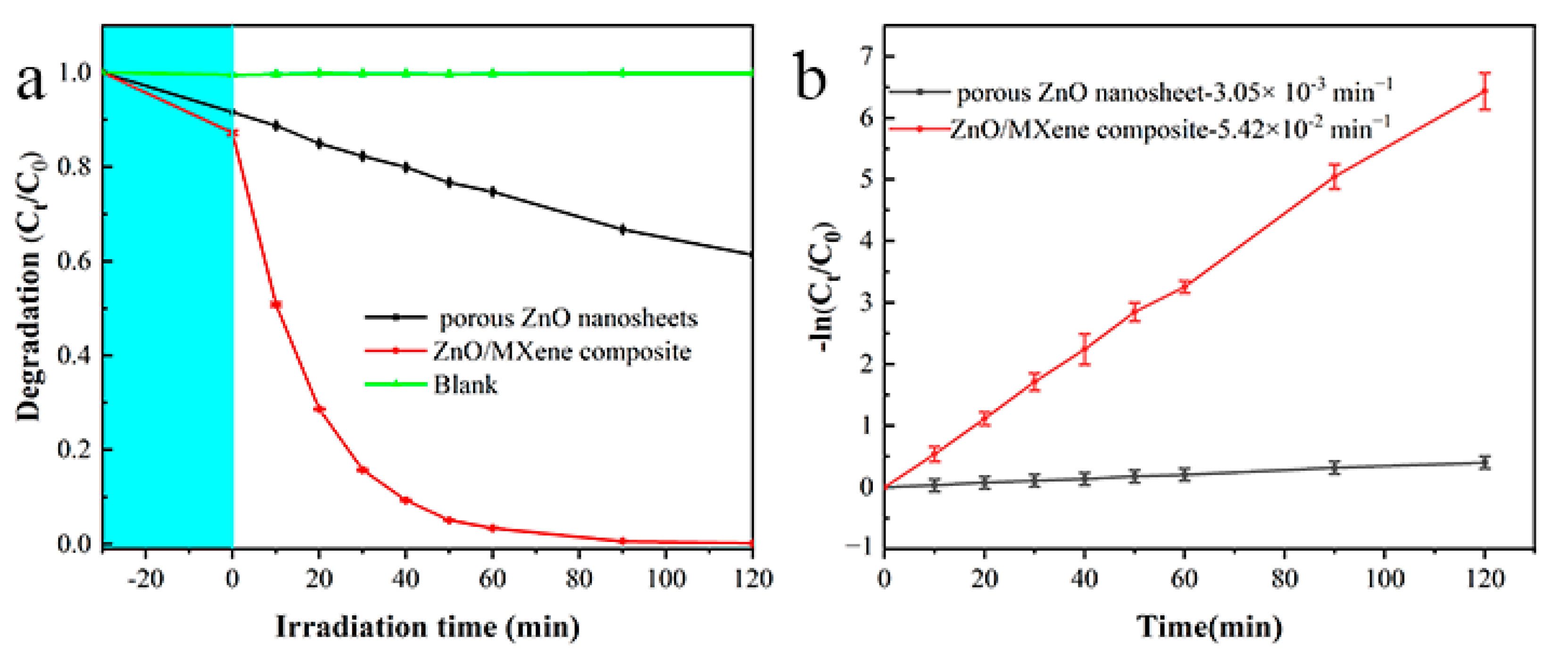
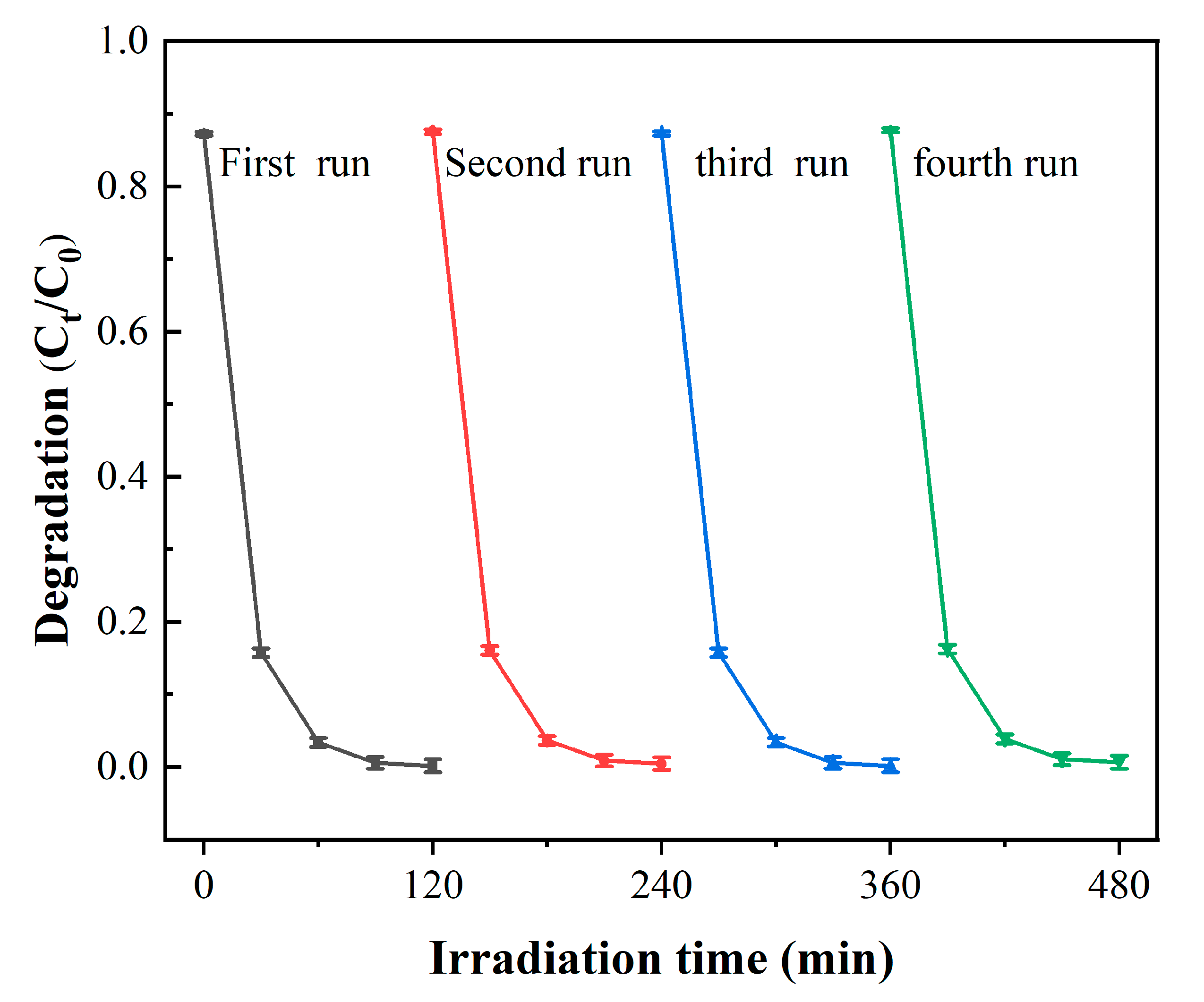
3.2. Photocatalytic Performance of ZnO/MXene Composite
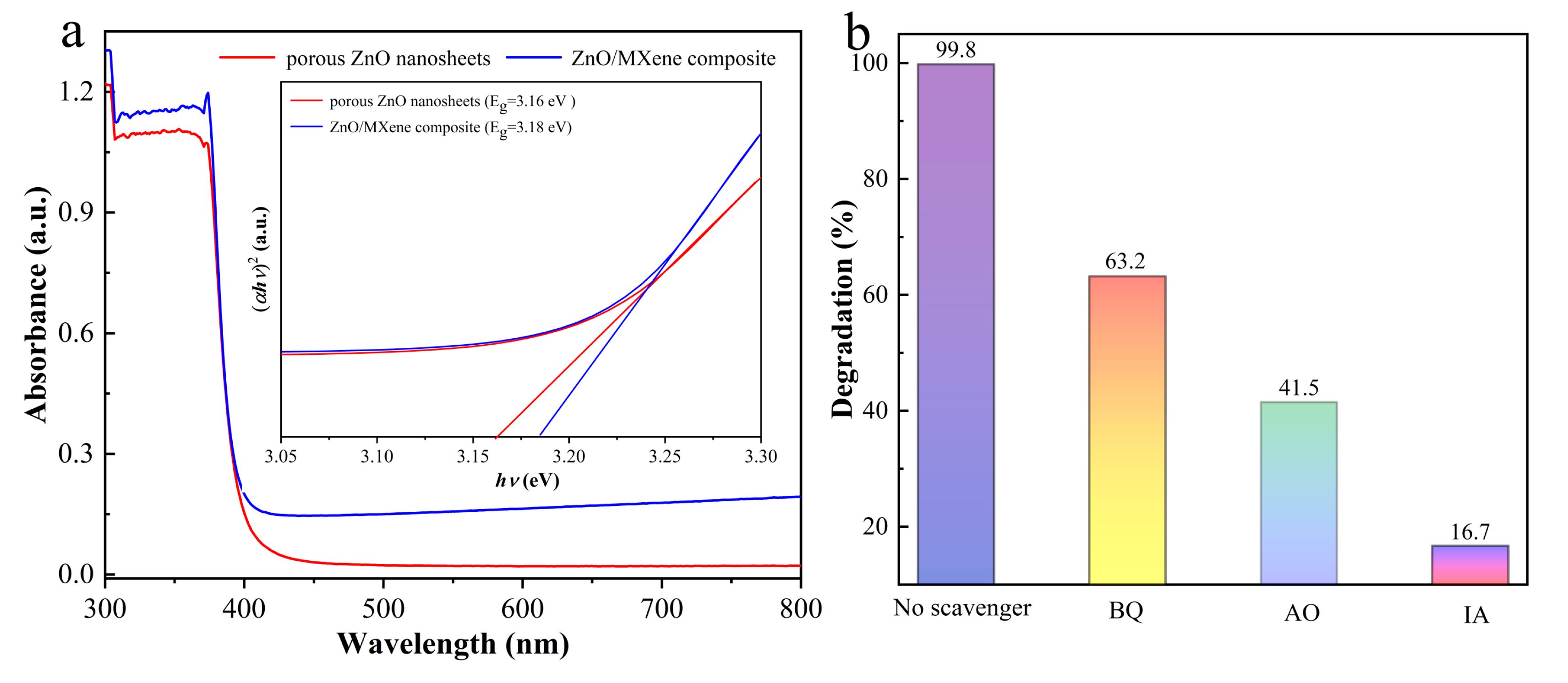
3.3. Possible Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehrabi, M.; Javanbakht, V. Photocatalytic degradation of cationic and anionic dyes by a novel nanophotocatalyst of TiO2/ZnTiO3/αFe2O3 by ultraviolet light irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 9908–9919. [Google Scholar] [CrossRef]
- Bayat, M.; Javanbakht, V.; Esmaili, J. Synthesis of zeolite/nickel ferrite/sodium alginate bionanocomposite via a co-precipitation technique for efficient removal of water-soluble methylene blue dye. Int. J. Biol. Macromol. 2018, 116, 607–619. [Google Scholar] [CrossRef]
- Lei, C.; Pi, M.; Jiang, C.; Cheng, B.; Yu, J. Synthesis of hierarchical porous zinc oxide (ZnO) microspheres with highly efficient adsorption of Congo red. J. Colloid Interface Sci. 2017, 490, 242–251. [Google Scholar] [CrossRef]
- Alguacil, F.J.; López, F.A. Organic dyes versus adsorption processing. Molecules 2021, 26, 5440. [Google Scholar] [CrossRef]
- Mohammad, S.; Suzylawati, I. Study of the adsorption/desorption of MB dye solution using bentonite adsorbent coating. J. Water Process Eng. 2020, 34, 101155. [Google Scholar]
- Ou, W.; Zhang, G.; Yuan, X.; Su, P. Experimental study on coupling photocatalytic oxidation process and membrane separation for the reuse of dye wastewater. J. Water Process Eng. 2015, 6, 120–128. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.J.; He, P.Y.; Li, C.J.; Li, H. Coupling of self-supporting geopolymer membrane with intercepted Cr(III) for dye wastewater treatment by hybrid photocatalysis and membrane separation. Appl. Surf. Sci. 2020, 515, 146024. [Google Scholar] [CrossRef]
- Rojviroon, T.; Rojviroon, O.; Sirivithayapakorn, S. Photocatalytic decolourisation of dyes using TiO2 thin film photocatalysts. Surf. Eng. 2016, 32, 562–569. [Google Scholar] [CrossRef]
- Ye, Z.; Kong, L.; Chen, F.; Chen, Z.; Lin, Y.; Liu, C. A comparative study of photocatalytic activity of ZnS photocatalyst for degradation of various dyes. Optik 2018, 164, 345–354. [Google Scholar] [CrossRef]
- Boutamine, Z.; Hamdaoui, O.; Merouani, S. Enhanced sonolytic mineralization of basic red 29 in water by integrated ultrasound/Fe2+/TiO2 treatment. Res. Chem. Intermed. 2017, 43, 1709–1722. [Google Scholar] [CrossRef]
- Abdelhay, A.; Jum’h, I.; Albsoul, A.; Abu Arideh, D.; Qatanani, B. Performance of electrochemical oxidation over BDD anode for the treatment of different industrial dye-containing wastewater effluents. Water Reuse 2021, 11, 110–121. [Google Scholar] [CrossRef]
- Moghaddas, S.M.T.H.; Elahi, B.; Javanbakht, V. Biosynthesis of pure zinc oxide nanoparticles using Quince seed mucilage for photocatalytic dye degradation. J. Alloys Compd. 2020, 821, 153519. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Zhang, W.; Sun, M.; Li, L.; Xu, N.; Wu, J.; Sun, J. High visible photoelectrochemical activity of Ag nanoparticle-sandwiched CdS/Ag/ZnO nanorods. ACS Appl. Mater. Interfaces 2017, 9, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yao, C.; Gan, J.; Jiang, K.; Hu, Z.; Lin, J.; Xu, N.; Sun, J.; Wu, J. ZnO colloids and ZnO nanoparticles synthesized by pulsed laser ablation of zinc powders in water. Mater. Sci. Semicond. Process. 2020, 109, 104918. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Wang, S.; Li, L. Dual templating fabrication of hierarchical porous three-dimensional ZnO/carbon nanocomposites for enhanced photocatalytic and photoelectrochemical activity. Appl. Catal. B Environ. 2018, 222, 209–218. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Xiang, L.; Komarneni, S. Synthesis, properties and applications of ZnO nanomaterials with oxygen vacancies: A review. Ceram. Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Periyat, P.; Naufal, B.; Lazar, M.A. Self-Doped ZnO Microrods High Temperature Stable Oxygen Deficient Platforms for Solar Photocatalysis. Ind. Eng. Chem. Res. 2016, 55, 6413–6421. [Google Scholar] [CrossRef]
- Tanji, K.; Navio, J.; Martín-Gómez, A.; Hidalgo, M.; Jaramillo-Páez, C.; Naja, J.; Hassoune, H.; Kherbeche, A. Role of Fe (III) in aqueous solution or deposited on ZnO surface in the photoassisted degradation of rhodamine B and caffeine. Chemosphere 2020, 241, 125009. [Google Scholar] [CrossRef]
- Tanji, K.; El Mrabet, I.; Fahoul, Y.; Soussi, A.; Belghiti, M.; Jellal, I.; Naciri, Y.; El Gaidoumi, A.; Kherbeche, A. Experimental and theoretical investigation of enhancing the photocatalytic activity of Mg doped ZnO for nitrophenol degradation. React. Kinet. Mech. Catal. 2023, 136, 1125–1142. [Google Scholar] [CrossRef]
- Nie, N.; Zhang, L.; Fu, J.; Cheng, B.; Yu, J. Self-assembled hierarchical direct Z-scheme g-C3N4/ZnO microspheres with enhanced photocatalytic CO2 reduction performance. Appl. Surf. Sci. 2018, 441, 12–22. [Google Scholar] [CrossRef]
- Hu, D.; Song, C.; Jin, X.; Huang, Q. Polymer solution-assisted assembly of hierarchically nanostructured ZnO onto 2D neat graphene sheets with excellent photocatalytic performance. J. Alloys Compd. 2020, 843, 156030. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Hou, F.; Li, H.; Yang, Y.; Zhang, X.; Yang, Y.; Wang, Y. Effects of Ag loading on structural and photocatalytic properties of flower-like ZnO microspheres. Appl. Surf. Sci. 2017, 391, 476–483. [Google Scholar] [CrossRef]
- Tsao, C.-W.; Fang, M.-J.; Hsu, Y.-J. Modulation of interfacial charge dynamics of semiconductor heterostructures for advanced photocatalytic applications. Coord. Chem. Rev. 2021, 438, 213876. [Google Scholar] [CrossRef]
- Wang, C.; Shen, J.; Chen, R.; Cao, F.; Jin, B. Self-assembled BiOCl/Ti3C2Tx composites with efficient photo-induced charge separation activity for photocatalytic degradation of p-nitrophenol. Appl. Surf. Sci. 2020, 519, 146175. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, J.; Zhang, P.; Zhu, Q.; Jiang, M.; Xu, B. 3D crumbled MXene for high-performance supercapacitors. Chin. Chem. Lett. 2020, 31, 2305–2308. [Google Scholar] [CrossRef]
- Zhou, H.; Jiresse, N.K.L.; Zhang, W.; Chen, Z.; Zhang, Y.; Zhang, J. MXene-derived TiO2/MXene-loaded Ag for the degradation of the methyl orange. J. Mater. Res. 2021, 36, 5002–5012. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Chen, W.; Tang, Y.; Li, L.; Wang, J. Enhanced photocatalytic degradation of ciprofloxacin by heterostructured BiOCl/Ti3C2Tx MXene nanocomposites. J. Alloys Compd. 2023, 950, 169797. [Google Scholar] [CrossRef]
- Nasri, M.S.I.; Samsudin, M.F.R.; Tahir, A.A.; Sufian, S. Effect of MXene loaded on g-C3N4 photocatalyst for the photocatalytic degradation of methylene blue. Energies 2022, 15, 955. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, B.; Zhu, J.; Li, K.; Tian, S. Enhanced photocatalytic activities of a hierarchical ZnO/V2C MXene hybrid with a close coupling heterojunction for the degradation of methyl orange, phenol and methylene blue dye. New J. Chem. 2022, 46, 14793–14804. [Google Scholar] [CrossRef]
- Wang, S.; Kuang, P.; Cheng, B.; Yu, J.; Jiang, C. ZnO hierarchical microsphere for enhanced photocatalytic activity. J. Alloys Compd. 2018, 741, 622–632. [Google Scholar] [CrossRef]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New two-dimensional niobium and vanadium carbides as promising materials for Li-ion batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Wu, Y.; Fu, Z.; Wang, W. Shape-controlled synthesis and photocatalytic properties of three-dimensional and porous zinc oxide. J. Alloys Compd. 2011, 509, 3576–3581. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Li, K.; Zhang, S.; Li, Y.; Fan, J.; Lv, K. MXenes as noble-metal-alternative co-catalysts in photocatalysis. Chin. J. Catal. 2021, 42, 3–14. [Google Scholar] [CrossRef]
- Lei, A.; Qu, B.; Zhou, W.; Wang, Y.; Zhang, Q.; Zou, B. Facile synthesis and enhanced photocatalytic activity of hierarchical porous ZnO microspheres. Mater. Lett. 2012, 66, 72–75. [Google Scholar] [CrossRef]
- Jin, X.; Liu, H. Preparation of flower-like Bi2WO6/ZnO heterojunction photocatalyst with improved photocatalytic performance. J. Mater. Sci. Mater. Electron. 2020, 31, 18745–18754. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, B.; Zhu, J.; Li, K.; Tian, S. Hierarchical ZnO/MXene (Nb2C and V2C) heterostructure with efficient electron transfer for enhanced photocatalytic activity. Appl. Surf. Sci. 2022, 590, 153095. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, L.; Wang, J.; Liu, F.; He, J.; Liu, A.; Lv, S.; You, R.; Yan, X.; Sun, P. Flexible resistive NO2 gas sensor of three-dimensional crumpled MXene Ti3C2Tx/ZnO spheres for room temperature application. Sens. Actuators B Chem. 2021, 326, 128828. [Google Scholar] [CrossRef]
- Feng, D.; Cheng, Y.; He, J.; Zheng, L.; Shao, D.; Wang, W.; Wang, W.; Lu, F.; Dong, H.; Liu, H. Enhanced photocatalytic activities of g-C3N4 with large specific surface area via a facile one-step synthesis process. Carbon 2017, 125, 454–463. [Google Scholar] [CrossRef]
- Kwon, D.; Kim, J. Silver-doped ZnO for photocatalytic degradation of methylene blue. Korean J. Chem. Eng. 2020, 37, 1226–1232. [Google Scholar] [CrossRef]
- Chand, P.; Singh, V.; Kumar, D. Rapid visible light-driven photocatalytic degradation using Ce-doped ZnO nanocatalysts. Vacuum 2020, 178, 109364. [Google Scholar]
- Karthik, K.; Raghu, A.; Reddy, K.R.; Ravishankar, R.; Sangeeta, M.; Shetti, N.P.; Reddy, C.V. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere 2022, 287, 132081. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Zou, Y. High photocatalytic activity of ZnO–graphene composite. J. Colloid Interface Sci. 2018, 529, 306–313. [Google Scholar] [CrossRef]
- Luo, Q.; Yang, J.; Wu, Y.; Cai, Q. Hybridisation of ZnO with Ti3C2 as a co-catalyst for enhanced photocatalytic activity. Micro Nano Lett. 2020, 15, 764–768. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, B.; Zhu, J.; Li, K. Synthesis of ZnO/Ti2C composites by electrostatic self-assembly for the photocatalytic degradation of methylene blue. J. Mater. Sci. 2022, 57, 3954–3970. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, T.; Zhang, K.; Meng, X.; Dai, W.; Wang, D.; Dong, H.; Zhang, X. Engineered exosome-mediated near-infrared-II region V2C quantum dot delivery for nucleus-target low-temperature photothermal therapy. Acs Nano 2019, 13, 1499–1510. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Nguyen Thi, V.N.; Tran, H.H.; Tran Thi, T.P.; Truong, T.T.; Vo, V. A facile synthesis of gC3N4/BaTiO3 photocatalyst with enhanced activity for degradation of methylene blue under visible light. Bull. Mater. Sci. 2021, 44, 28. [Google Scholar] [CrossRef]
- Elangovan, R.; Vijayan, V.; Bakthavatsalam, S.; Ramkumar, K.; Sathish, T.; Sudhakar, K. A Facile synthesis of MgFe2O4/ZnS heterojunction with effectively enhanced visible light photocatalytic activity for degradation of methylene blue and crystal violet dyes. J. Clust. Sci. 2023, 34, 991–999. [Google Scholar] [CrossRef]
- Aihemaiti, X.; Wang, X.; Wang, Z.; Bai, Y.; Qi, K.; Ma, Y.; Tao, K.; Simayi, M.; Kuerban, N. Effective prevention of charge trapping in red phosphorus with nanosized CdS modification for superior photocatalysis. J. Environ. Chem. Eng. 2021, 9, 106479. [Google Scholar] [CrossRef]


| Photocatalysts | Degradation Ratio (%) | Irradiation Time (min) | Performance Improvement | Light Sources | Ref |
|---|---|---|---|---|---|
| ZnO/V2C | 99.8 | 120 | 17.8 | 500 W Xenon lamp | This work |
| ZnO/Ag | 92.9 | 210 | 3 | Sunlight | [40] |
| ZnO/Ce | 94.68 | 60 | 9.1 | 300 W visible lamp | [41] |
| ZnO/Cu | 91 | 75 | 1.62 | 30 W 254 nm-lamp | [42] |
| ZnO/rGO | 99 | 120 | 12.03 | 300 W Xenon lamp | [43] |
| ZnO/Ti3C2 | 84 | 60 | 1.5 | 40 W 365 nm-lamp | [44] |
| ZnO/V2C | 98.93 | 120 | 14.27 | 500 W Xenon lamp | [30] |
| ZnO/Nb2C | 62.62 | 120 | 2.92 | 300 W Xenon lamp | [37] |
| ZnO/Ti2C | 99.16 | 120 | 14.76 | 300 W Xenon lamp | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Sun, L.; Li, K.; Tian, S. Enhanced Photocatalytic Activity of V2C MXene-Coupled ZnO Porous Nanosheets with Increased Surface Area and Effective Charge Transfer. Materials 2024, 17, 2529. https://doi.org/10.3390/ma17112529
Zhou W, Sun L, Li K, Tian S. Enhanced Photocatalytic Activity of V2C MXene-Coupled ZnO Porous Nanosheets with Increased Surface Area and Effective Charge Transfer. Materials. 2024; 17(11):2529. https://doi.org/10.3390/ma17112529
Chicago/Turabian StyleZhou, Weibing, Lilong Sun, Kang Li, and Shouqin Tian. 2024. "Enhanced Photocatalytic Activity of V2C MXene-Coupled ZnO Porous Nanosheets with Increased Surface Area and Effective Charge Transfer" Materials 17, no. 11: 2529. https://doi.org/10.3390/ma17112529
APA StyleZhou, W., Sun, L., Li, K., & Tian, S. (2024). Enhanced Photocatalytic Activity of V2C MXene-Coupled ZnO Porous Nanosheets with Increased Surface Area and Effective Charge Transfer. Materials, 17(11), 2529. https://doi.org/10.3390/ma17112529






