Abstract
A series of lead-free Rb2ZrCl6:xTe4+ (x = 0%, 0.1%, 0.5%, 1.0%, 2.0%, 3.0%, 5.0%, 10.0%) perovskite materials were synthesized through a hydrothermal method in this work. The substitution of Te4+ for Zr in Rb2ZrCl6 was investigated to examine the effect of Te4+ doping on the spectral properties of Rb2ZrCl6 and its potential applications. The incorporation of Te4+ induced yellow emission of triplet self-trapped emission (STE). Different luminescence wavelengths were regulated by Te4+ concentration and excitation wavelength, and under a low concentration of Te4+ doping (x ≤ 0.1%), different types of host STE emission and Te4+ triplet state emission could be achieved through various excitation energies. These luminescent properties made it suitable for applications in information encryption. When Te4+ was doped at high concentrations (x ≥ 1%), yellow triplet state emission of Te4+ predominated, resulting in intense yellow emission, which stemmed from strong exciton binding energy and intense electron-phonon coupling. In addition, a Rb2ZrCl6:2%Te4+@RTV scintillating film was fabricated and a spatial resolution of 3.7 lp/mm was achieved, demonstrating the potential applications of Rb2ZrCl6:xTe4+ in nondestructive detection and bioimaging.
1. Introduction
Perovskite had basic and practical significance in photoelectric research, such as solar power, information encryption, and scintillation applications [1]. There were many types of perovskite, which could be divided into Pb-based perovskites [2,3,4,5,6] and non-Pb perovskites [7,8,9,10] according to whether the compound contained lead (Pb). Lead halide perovskites (LHPs) garnered significant research interest in the optoelectronic field, including solar cells, LEDs, and anti-counterfeiting [11,12,13,14,15], due to their excellent light absorption properties, tunable bandgap width, and high photoluminescence quantum yield (PLQY). LHP nanocrystals were identified as high-performance scintillators due to their cost-effectiveness, tunable emission, and fast decay time [16,17]. However, unfortunately, the large-scale commercial application of this material was hindered by stability and toxicity issues [18]. Therefore, lead-free perovskite materials that displayed non-toxicity, stability, and exceptional luminescent properties became the focus of significant attention, emerging as a primary area of research. In recent years, researchers developed numerous lead-free perovskite materials by substituting lead with various low-toxicity and non-toxic elements, for example, tin-based [19,20], copper-based [21,22,23,24,25], manganese-based [26], and zirconium-based perovskites [27,28].
Due to its stability and distinctive luminescent properties, the all-inorganic Rb2ZrCl6 attracted widespread attention. Zhang synthesized Rb2ZrCl6 microcrystals via a hydrothermal method and utilized them as phosphors to prepare WLEDs [29]. The Rb2ZrCl6 microcrystals exhibited blue-white emission characteristics, with a high PLQY value of up to 60%. Notably, they demonstrated significant stability when exposed to heat, ultraviolet light, and environmental conditions involving water/oxygen. Zheng pioneered the environmentally friendly wet grinding method to synthesize a series of highly crystalline Rb2ZrCl6:xTe4+ microcrystals. Furthermore, the research indicated that the effective emission of these perovskite materials could be attributed to their direct bandgap characteristics and zero-dimensional structure [30]. Shi introduced the tunable emission characteristics of Rb2ZrCl6: xSb3+ phosphors that depend on the different excitation wavelengths [31]. In 2023, Liu synthesized Te4+ doped Rb2ZrCl6 microcrystals, and conducted a preliminary study on their optical properties to manufacture white light emitting diodes [32]. These studies on Rb2ZrCl6 proved that it has excellent luminescence properties.
However, the emission of Rb2ZrCl6 required excitation at high-energy wavelengths, posing a challenge for effective photon excitation when used in conjunction with commercial UV chips and electroluminescent devices [33]. Furthermore, the single emission of Rb2ZrCl6 could not meet the requirements of multiple anti-counterfeiting and information encryption applications. Therefore, the development of an effective wavelength tuning technique for Rb2ZrCl6 was of significant importance in expanding its applications. Doping technology emerged as an effective means of tuning the luminescent properties of perovskite halides. Intelligent light-emitting materials achieved an intelligent response through photochromism [34]. Photochromism entailed emitting different colors of light under various excitation wavelengths. Materials with photochromic properties were excellent candidates for anti-counterfeiting applications where light of different colors was emitted under different excitation wavelengths. It has been reported that Rb2ZrCl6:Te4+ exhibited characteristics of emitting blue and yellow light at 255 nm and 388 nm, respectively, making it a promising material for anti-counterfeiting applications [32]. In addition, Rb2ZrCl6 was a scintillator material with potential. However, the anti-counterfeiting of photochromism applications and scintillation properties of Rb2ZrCl6 powder samples had not been studied systematically.
In this study, the anti-counterfeiting application of Rb2ZrCl6:Te4+ was explored and the luminescence mechanism of Rb2ZrCl6:Te4+ was studied in detail, and its application in anti-counterfeiting and biological imaging was expanded and innovated. Rb2ZrCl6:xTe4+ microcrystals were synthesized through a hydrothermal method, and the crystal structure and spectral characteristics of Rb2ZrCl6:xTe4+ were analyzed. Remarkably, this modification resulted in the emergence of an additional yellow emission when subjected to low-energy excitation at 388 nm, facilitating the transition from the initial blue emission to a vibrant yellow emission. We designed an anti-counterfeiting information encryption and decryption experiment using Rb2ZrCl6:0.1%Te4+ powder to emit different colors under different excitations. Simultaneously, X-ray imaging tests were conducted on scintillating films of Rb2ZrCl6@RTV and Rb2ZrCl6:2%Te4+@RTV, and both demonstrated imaging capabilities. This discovery demonstrated the potential applications of Rb2ZrCl6:xTe4+ in information encryption, anti-counterfeiting, and bioimaging.
2. Experiment Section
2.1. Materials
Rubidium chloride (RbCl, 99.95%, CN), zirconium chloride (ZrCl4, 99.9%, CN), and antimony chloride (TeCl4, 99%, CN) were purchased from Aladdin Ltd. Hydrochloric acid (CP, 36.0~38.0%, CN) and methanol (GR, 99.7%, CN) was purchased from Sinopharm Chemical Reagent Co, Ltd. None of the materials required further purification.
2.2. Synthesis of Rb2ZrCl6:xTe4+ Microcrystals
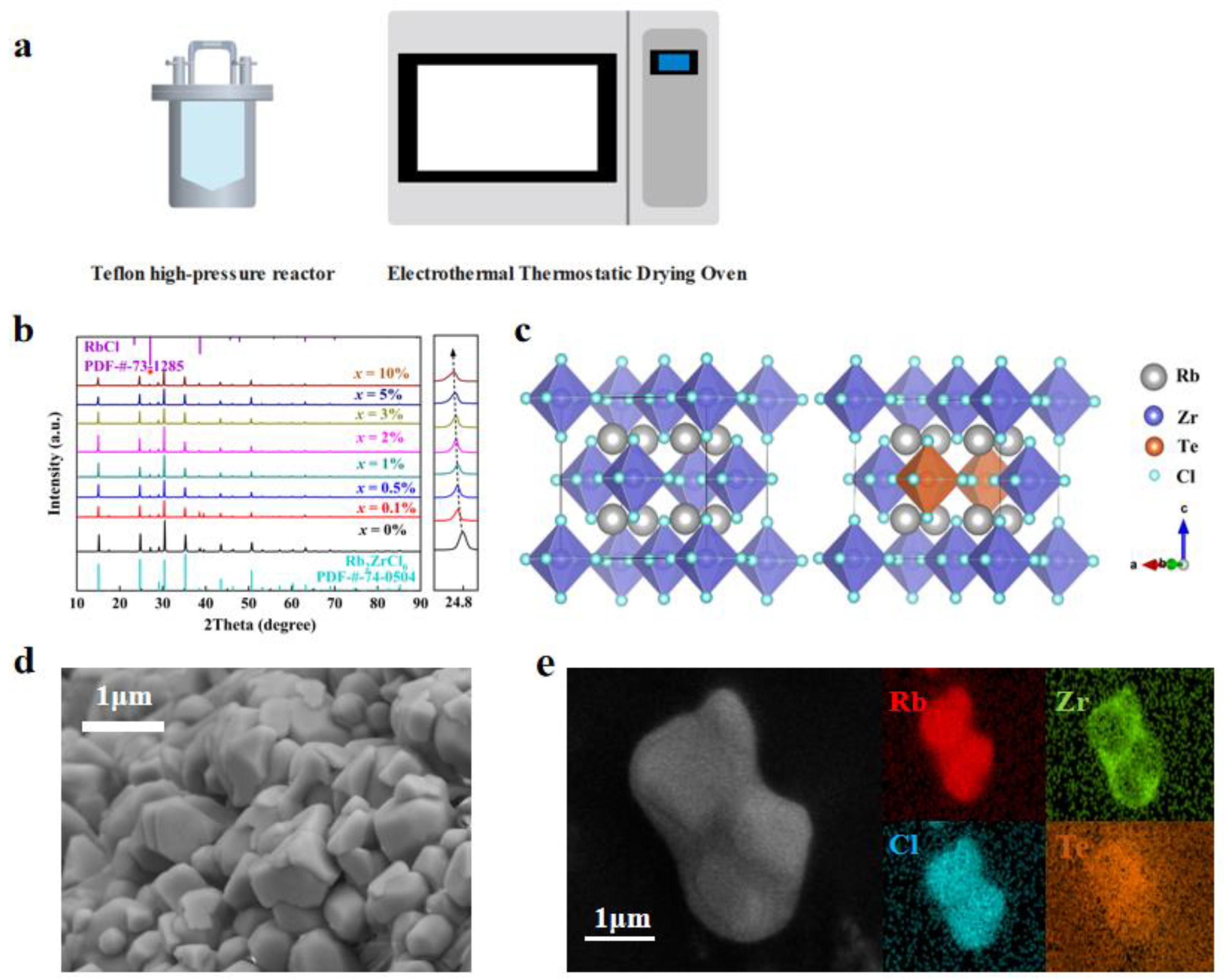
The synthesis of Rb2ZrCl6:xTe4+ involved a conventional hydrothermal method. Figure 1a schematically illustrates the Rb2ZrCl6:xTe4+ growth method. Specifically, 2 mmol of RbCl, 1 − x mmol of ZrCl4 and x mmol TeCl4 were dissolved in 5 mL of HCl and placed in a 25 mL Teflon high-pressure reactor. After being heated at 180 °C for 12 h, the solution was gradually cooled for over 8 h to reach room temperature. Rb2ZrCl6:xTe4+ microcrystals were observed to form once the temperature reduced to room temperature. Subsequently, Rb2ZrCl6:xTe4+ microcrystals were subjected to three methanol washes and then dried in an oven at 80 °C for a total for 6 h.
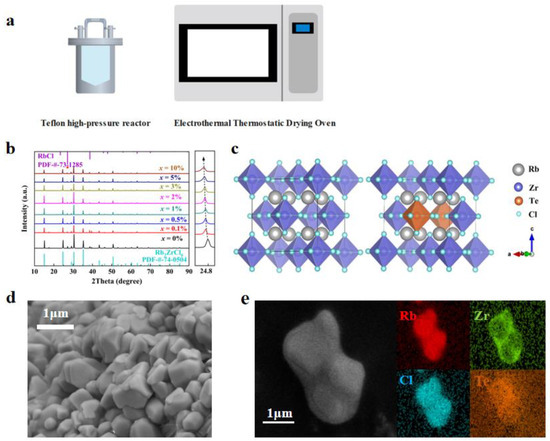
Figure 1.
(a) Schematic diagram of the synthesis method. (b) XRD patterns of Rb2ZrCl6:xTe4+ (x = 0%, 0.1%, 0.5%, 1.0%, 2.0%, 3.0%, 5.0%, 10.0%) [35]. (c) Crystal structure diagram of Rb2ZrCl6 and Rb2ZrCl6:2%Te4+. (d) SEM image, and (e) the elemental mappings of Rb2ZrCl6:2%Te4+ microcrystals.
2.3. Preparation of Rb2ZrCl6@RTV and Rb2ZrCl6:2%Te4+@RTV Scintillation Screens
The Rb2ZrCl6 powder was thoroughly mixed with RTV in a 2:1 ratio, and the Rb2ZrCl6@RTV scintillation film was fabricated using the screen-printing method. The production process of the Rb2ZrCl6:2%Te4+@RTV rigid scintillation screen follows the same method as that of the Rb2ZrCl6@RTV scintillation film.
2.4. Measurement and Characterization
The X-ray diffraction (XRD) diffractometer utilized in this study was the PANalytical instrument (Almelo, The Netherlands) employed for polycrystalline powder characterization. The testing conditions included a tube voltage set at 40 kV, with a Cu target material selected, emitting radiation at a wavelength of 1.540598 Å. Scanning was conducted within an angular range of 10° to 90° at a rate of 6°/min, with a step size of 0.02°. Scanning Electron Microscope (SEM) image measurements were performed using an SU3500 from Hitachi, Tokyo, Japan, and the samples were subjected to energy dispersive spectrometer (EDS) elemental mapping using a scanning electron microscope (Zeiss Gemini SEM 300, ZEISS, Jena, Germany). In this study, the experimental apparatus set to a voltage of 5 kV. PL, time-resolved photoluminescence (TRPL), and PLQY measurements were conducted using a PL spectrometer (Edinburgh, FLS 1000, Livingston, UK). Thermo-gravimetric (TG) data and Differential Scanning Calorimetry (DSC) data were collected with the aid of a Netzsch instrument (STA 449 F5 Jupiter, Burlington, MA, USA). The temperature range spanned from room temperature to 1273 K, with a heating rate of 10 K/min, under an Ar2 atmosphere for testing. The UV–VIS spectrum was obtained using a UV–VIS spectrophotometer (HITACHI, U-3900H). The imaging system used in this study was a custom-built platform consisting of an X-ray source, a scintillator screen, a CCD detector, as well as image processing and display equipment. The experimental setup involved a voltage of 100 kV, a current of 1000 μA, and a peak energy of 165 kVp.
3. Results and Discussion
Rb2ZrCl6:xTe4+ microcrystals were synthesized via a hydrothermal method, and the structural properties of Rb2ZrCl6:xTe4+ were characterized. Phase analysis of Rb2ZrCl6:xTe4+ was conducted, followed by an examination of the structural changes in the crystal before and after doping. Figure 1 presents the test results of structural performance characterization.
Figure 1b demonstrates the XRD patterns of Rb2ZrCl6:xTe4+ samples. Both the hydrothermally synthesized Rb2ZrCl6 and Rb2ZrCl6:xTe4+ samples exhibited a common hybrid peak at a diffraction angle of 27.072° [36]. When compared with the PDF card, this hybrid peak precisely matches the diffraction peak of RbCl (200) [31], which is not luminous. Due to the ionic radius of Te4+ being larger than Zr4+, the substitution of Te4+ for Zr4+ results in lattice expansion. Consequently, the diffraction peak position at (220) shifts notably towards smaller angles with increasing Te4+ content. In order to ascertain the successful incorporation of Zr into the lattice of Rb2ZrCl6, calculations of the lattice constants of the material were performed using the WinCSD software (Version 4). The computed results indicated that with the introduction of Zr, the lattice parameter of the material increased from 7.14081 Å at x = 0 to 7.17377 Å at x = 10%. As illustrated in Figure 1c, the Rb2ZrCl6 perovskite crystal structure is a typical cubic phase. Unlike the conventional 3D perovskite ABX3, Rb2ZrCl6 features a vacancy-ordered structure composed of [ZrCl6]2− octahedra and Rb+ ions. Te4+ replaces the Zr4+ sites. The Zr4+ in Rb2ZrCl6 substitutes for B2+ sites, leading to a 50% periodic vacancy in [ZrCl6]2−. This causes the octahedral clusters to be fully separated by the A-site cation Rb+, resulting in a distinct 0D perovskite structure. To gain a comprehensive understanding of the sample’s morphology and elemental distribution, perovskite microcrystalline powder was meticulously examined through SEM and EDS. As depicted in Figure 1d, the SEM image reveals that the perovskite exhibits a microcrystalline tetrahedral structure, and the average size of Rb2ZrCl6:2%Te4+ microcrystals was 2 μm. Moreover, in Figure 1e, the mapping of Cs, Zr, and Sb elements in a single crystal revealed that the Te4+ dopants were homogeneously introduced into the Rb2ZrCl6 host lattice.
Next, the optical properties of Rb2ZrCl6:xTe4+ were investigated. The ultraviolet fluorescence spectrum of Rb2ZrCl6:xTe4+ showed diverse luminescent characteristics under various excitation wavelengths—bright blue at 254 nm excitation and bright yellow at 365 nm excitation, which resulted in a luminescence wavelength range that could be adjusted within 455 nm to 575 nm, transitioning from blue to yellow, demonstrating excellent spectral tunability. Figure 2 displays the results of optical property testing.
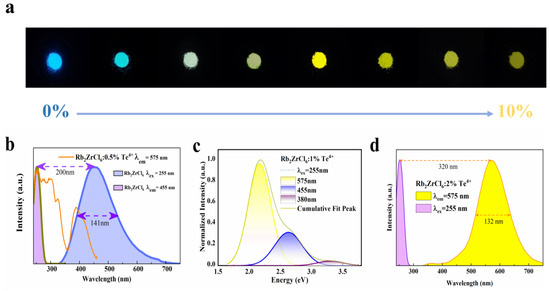
Figure 2.
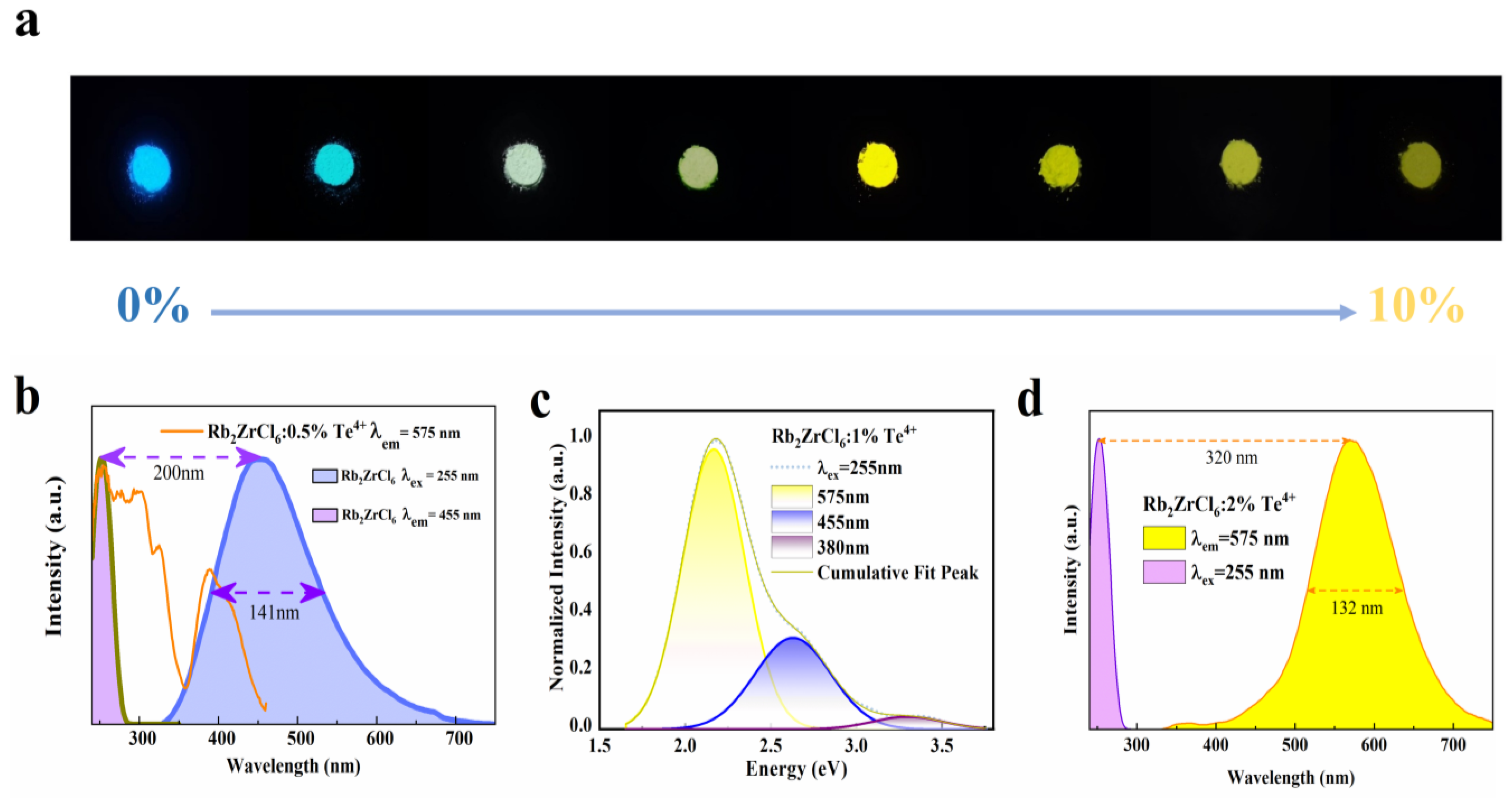
Optical characterizations of Rb2ZrCl6:xTe4+. (a) Photographs of the Rb2ZrCl6:xTe4+ powders under 254 nm UV light. (b) PL and PLE spectra of Rb2ZrCl6. (c) Schematic diagram of 255 nm excitation spectra Gaussian fitting process of Rb2ZrCl6:1%Te4+. (d) PL and PLE spectra of Rb2ZrCl6:2%Te4+.
As shown in Figure 2a, the precise control of the Te4+ doping level allowed for effective spectral tuning can result in the transformation from blue to yellow light emission.
As shown in Figure 2b, the emission spectrum of Rb2ZrCl6 at 255 nm partially overlaps with that excitation spectrum of Rb2ZrCl6:0.5%Te4+ at 575 nm, indicating that the blue emission of Rb2ZrCl6 can be absorbed by Te4+. The distinctive emission peak of Rb2ZrCl6 is situated at 455 nm, featuring a considerably wide full-width at half-maximum (FWHM) of 141 nm. Calculations reveal a substantial Stokes shift of 200 nm. The presence of such a substantial Stokes shift, along with the broad spectral characteristics, strongly implies that the PL mechanism in Rb2ZrCl6 may indeed originate from the emission of self-trapped excitons [37]. As shown in Figure S1, The UV–VIS absorption spectrum of Rb2ZrCl6:xTe4+ reveals a broad absorption range spanning from 250 to 475 nm. Compared to Rb2ZrCl6, the Te4+ doped Rb2ZrCl6 sample exhibits significantly enhanced absorption features in the wavelength range of 370–425 nm.
Figure 2c depicts the Gaussian peak-splitting curve for Rb2ZrCl6:1%Te4+. Under 255 nm excitation, three distinct peaks are readily apparent. Notably, the peak at 575 nm exhibits the highest intensity, corresponding to the 3P1→1S0 triplet energy transition of Te4+. The peaks at 380 nm and 455 nm originate from the 1P1→1S0 singlet transition of Te4+ and the STE emission of Rb2ZrCl6, respectively [32].
The characteristic emission peak of Rb2ZrCl6:2%Te4+ is centered at 575 nm, exhibiting a noteworthy full width at a half-peak of 132 nm, and a calculated Stokes shift of 187 nm (Figure 2d).
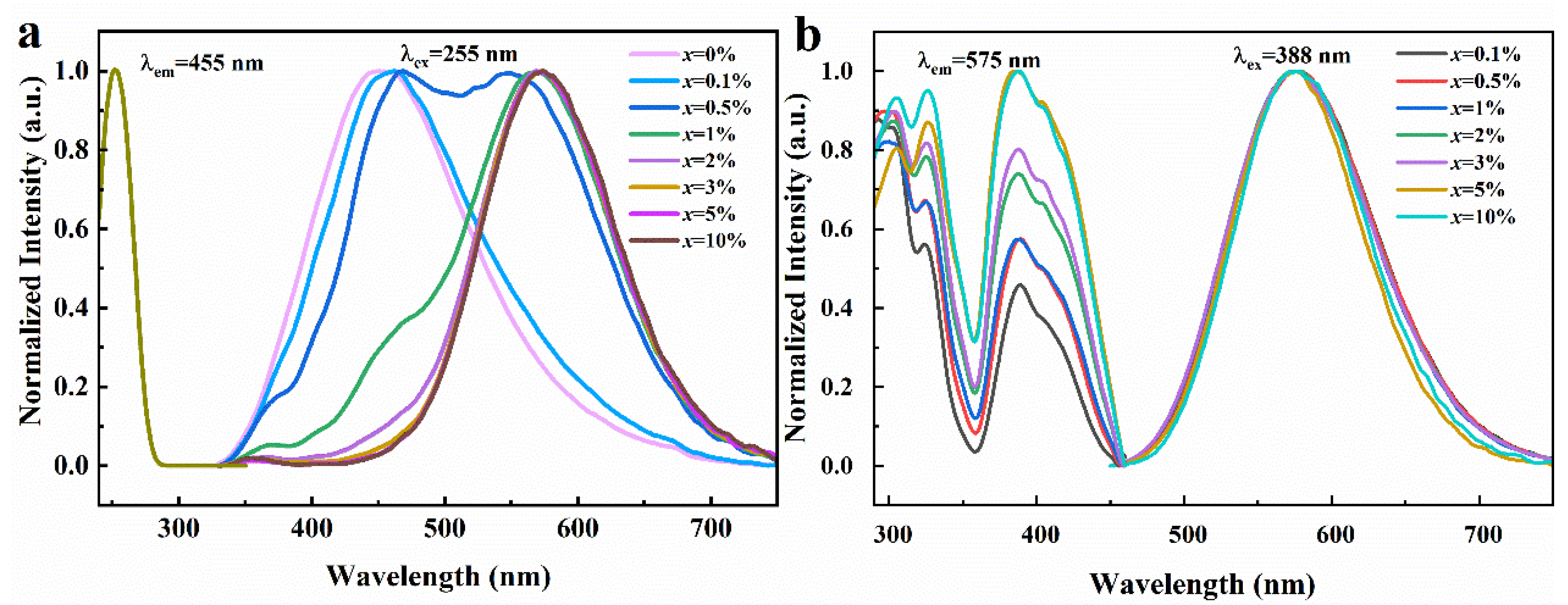
As depicted in Figure 3a, when excited under 255 nm UV light, the emission spectrum curves of Rb2ZrCl6 exhibit distinct variations based on different Te4+ doping concentrations. Rb2ZrCl6 itself exhibits an emission peak at 455 nm, while the introduction of Te4+ results in the emergence of a novel emission peak at 575 nm. As shown in Figure S2, as the concentration of Te4+ doping is increased, the intensity of the 455 nm peak gradually diminishes until it eventually fades away, while the 575 nm peak continues to rise until it reaches a maximum, after which it gradually decreases with higher doping ion concentrations. Therefore, the optical performance test in this paper is mainly based on Rb2ZrCl6:2%Te4+. It can be seen from Figure 3a that the two emission peaks change regularly with different doping concentrations, and the energy transfer between Rb2ZrCl6 and Te4+ is observed.
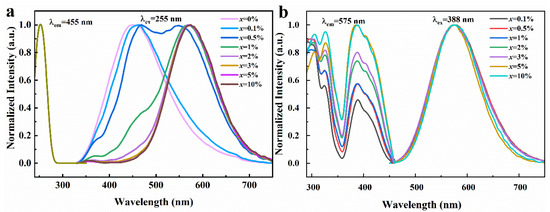
Figure 3.
(a) Normalized PLE and PL spectra of Rb2ZrCl6:xTe4+ (λem = 455 nm and λex = 255 nm). (b) Normalized PLE and PL spectra of Rb2ZrCl6:xTe4+ (λem = 575 nm and λex = 388 nm).
As depicted in Figure 3b, under UV excitation of 388 nm, Rb2ZrCl6 with different Te4+ doping concentrations presents a consistent emission peak at 575 nm. When the excitation and emission spectra of Rb2ZrCl6:xTe4+ are characterized, it is found that the excitation peak of 388 nm is detected at the emission wavelength of 575 nm, and this excitation peak is from the triplet emission of Te4+ [32].
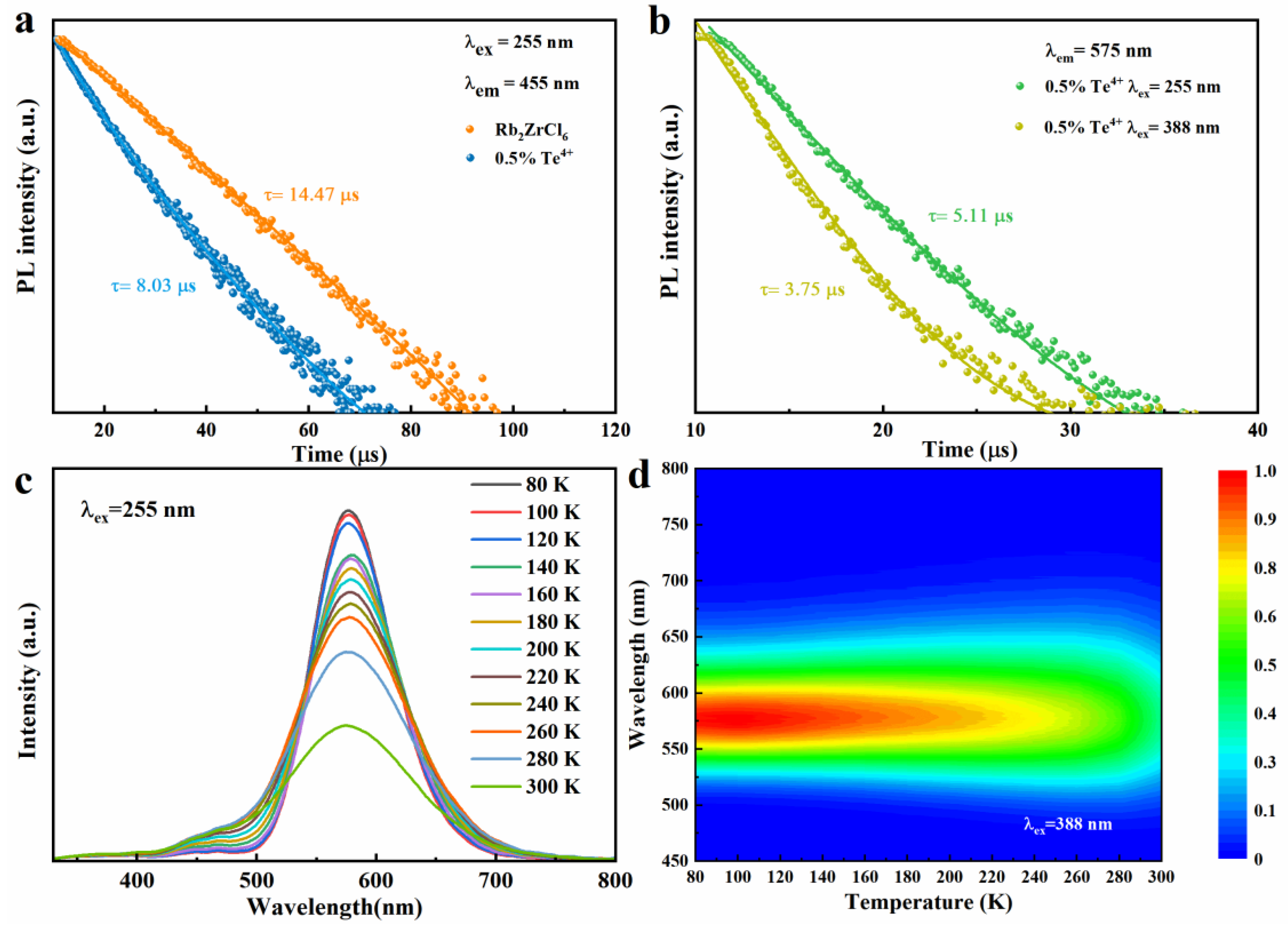
The decay times of Rb2ZrCl6 and Rb2ZrCl6:0.5%Te4+ PL at room temperature are studied, as shown in Figure 4a. The lifetime of Rb2ZrCl6 is about 14.47 μs (λex = 255 nm, λem = 455 nm), and the lifetime of Rb2ZrCl6:0.5%Te4+ is reduced to 8.03 μs (λex = 255 nm, λem = 455 nm) compared to Rb2ZrCl6. This is due to the partial transfer of the exciton of Rb2ZrCl6 to the Te4+ level, which would have led to the reduction of the luminous exciton of Rb2ZrCl6, resulting in faster decay. This also corresponds to the results of excitation and emission spectra as shown in Figure 3a. As shown in Figure 4b, the lifetimes of Rb2ZrCl6:0.5%Te4+ (λex = 255 nm, λem = 575 nm) and Rb2ZrCl6:0.5%Te4+ (λex = 388 nm, λem = 575 nm) are 5.11 μs and 3.75 μs, respectively. This is because under the 388 nm excitation, luminous excitons act directly on Te4+. Under 255 nm excitation, the excitons acting on Te4+ are partly derived from Rb2ZrCl6, so the lifetime is longer.
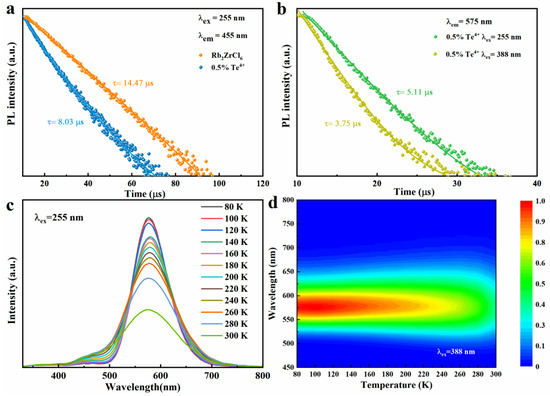
Figure 4.
(a) PL decay curve of Rb2ZrCl6 and Rb2ZrCl6:0.5%Te4+, respectively. (b) PL decay curve of Rb2ZrCl6:0.5%Te4+. (c) Temperature-dependent PL spectra of Rb2ZrCl6:1%Te4+ (λex = 255 nm). (d) Pseudo map of Rb2ZrCl6:1%Te4+ PL spectra at low temperature (T = 80–300 K, λex = 388 nm).
Under 255 nm ultraviolet excitation, Rb2ZrCl6:1%Te4+ exhibits peaks at 455 nm and 575 nm, testing the temperature-dependent (low-temperature) PL curve of Rb2ZrCl6:1%Te4+, as illustrated in Figure 4c. The PL spectra obtained under 255 nm excitation exhibit a trend where the PL intensity of the 1%Te4+ doped Rb2ZrCl6 increases as the temperature decreases. A similar change in trend was observed for samples excited at 388 nm (Figure S3). This behavior can be attributed to the reduction in electron–phonon coupling at lower temperatures, which is more conducive to the inter-system crossing (ISC) process, consequently leading to an enhancement in the strength of the triplet STE [38]. Figure 4d presents a false-color representation of the changes in PL intensity and wavelength in response to temperature variations for Rb2ZrCl6:1%Te4+ when excited with a 388nm light source. For a similar investigation under 255 nm excitation, please refer to Figure S4, which illustrates the pseudo-color diagram depicting PL intensity and wavelength changes as a function of temperature for Rb2ZrCl6:1%Te4+. As the temperature rises, there is a gradual reduction in the PL intensity attributed to heat-induced non-radiative recombination. Simultaneously, an increase in temperature is associated with a widening of the FWHM of the PL spectrum. This phenomenon indicates that at elevated temperatures, greater exciton–phonon coupling is at play, thereby promoting non-radiative recombination processes [39].
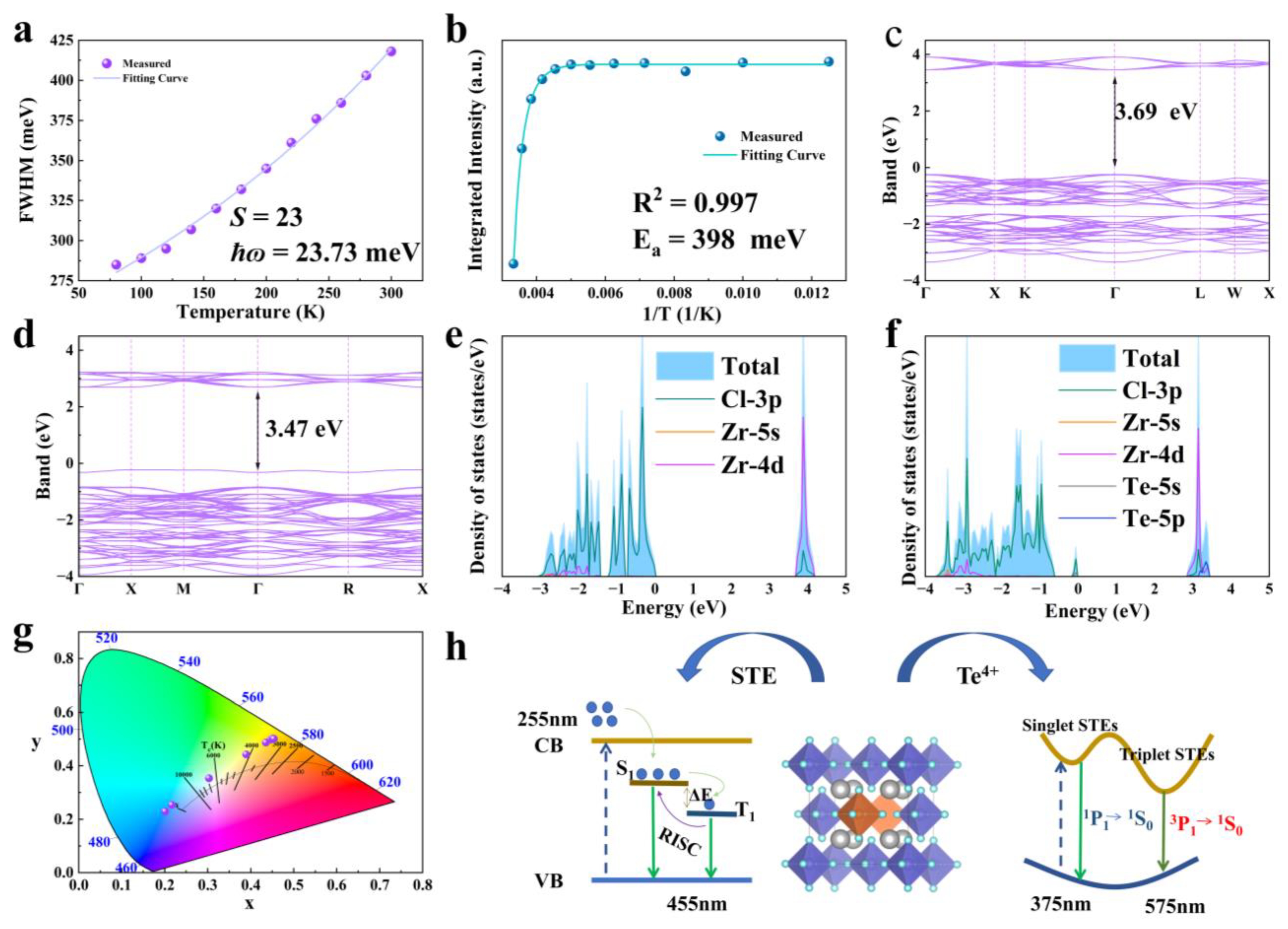
Next, in this study, the temperature-dependent relationship of FWHM and PL integral strength was analyzed. Band structure calculations and DOS were performed on Rb2ZrCl6:Te, Rb2ZrCl6 had a large band gap of 3.69 eV. After doping Te4+, a new impurity level was introduced, and the band gap was 3.47 eV. We analyzed the luminescence mechanism of Rb2ZrCl6:Te, in which yellow 1S0→3P1 emission originated from the triplet emission of Te4+. Figure 3 displays the results.
As depicted in Figure 5a, as the optimal doping concentration was determined to be 2% Te4+, the temperature-dependent curve of FWHM of the emission peak at 575 nm for Rb2ZrCl6:2%Te4+ was fitted to the phonon broadening model. The equation is provided below:
where S is the electron–phonon coupling, the ħɷ phonon is the phonon frequency, and kB is the Boltzmann constant. The fitting results are shown in Figure 5a. The Huang–Rhys factor (S) is found to be 23, the ħɷ phonon is calculated to be 23.73 meV, and this relatively high S value indicates the presence of a robust electron–phonon coupling in Rb2ZrCl6:xTe4+. Figure 5b shows the temperature-dependent curve of integral strength of the PL curve. It is shown that the luminescent intensity of Rb2ZrCl6:2%Te4+ microcrystals demonstrates a decrease as the temperature rises, indicating the occurrence of thermal quenching of luminescence (Figure 5b). The temperature dependency of PL intensity can be aptly described using the Arrhenius formula fitting [40]:
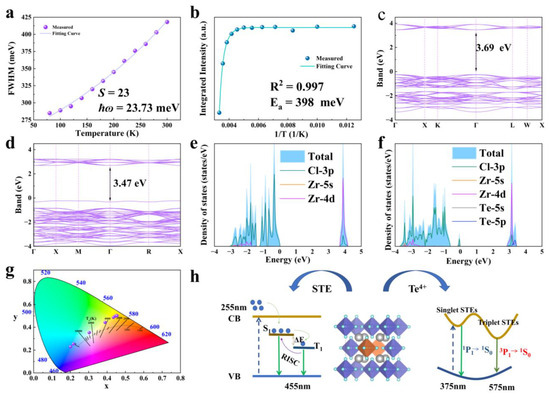
Figure 5.
(a) Temperature dependence curve of FWHM for the 575 nm luminescence peak of Rb2ZrCl6:2%Te4+. (b) Temperature-dependent curve of integral strength of PL curve. (c,d) Band structures of Rb2ZrCl6 and Rb2ZrCl6:2%Te4+, respectively. (e,f) DOS of Rb2ZrCl6 and Rb2ZrCl6:2%Te4+, respectively. (g) CIE color coordinates of the eight Rb2ZrCl6:xTe4+ samples under 255 nm excitation. (h) Schematic diagram of energy transfer process of Rb2ZrCl6:Te.
In the Arrhenius formula, I0 represents the PL intensity at 0 K, Ea stands for the activation energy, and kB denotes the Boltzmann constant. The Ea, determined through Arrhenius formula fitting, is calculated to be 398 meV. Notably, this Ea value significantly exceeds the thermal energy at room temperature, suggesting the formation of a stable STE with highly localized characteristics [41].
In Figure 5c, the Rb2ZrCl6 band results show the calculation results. The exchange-correlation functional employed the generalized gradient approximation (GGA), with a cutoff energy set at 550 eV. Brillouin zone sampling was achieved using a 4 × 4 × 4 Monkhorst-Pack κ-point grid. The convergence criteria for structural optimization were met when the energy per atom was below 10−8 eV and the forces were less than 10−3 eV/Å, which shows that Rb2ZrCl6 has a direct band gap of 3.69 eV, after doping 2%Te4+, it becomes 3.47 eV, as shown in Figure 5d. According to Figure 5e, the CBM is mainly composed of Zr 4d orbitals, supplemented by a small contraction of the Cl 3p orbital, while the VBM is mainly Cl 3p orbitals. As shown in Figure 5f, after doping with Te4+, an impurity level is introduced with the top 3.47 eV of the valence band, including the Te 5s and Cl 3p orbitals. The CBM is occupied by the Zr 4d, Cl 3p, and Te 5p orbitals.
By DOS analysis, Rb does not play a role in bandgap formation. This implies that electron bands predominantly arise from zirconium and halogen contributions within isolated [ZrCl6]2−octahedrons. The movement of electrons and holes is confined in three spatial dimensions, leading to the emergence of a 0D electronic structure. This unique structure fosters efficient emission of STEs [30]. Figure 5g depicts the CIE color coordinates of Rb2ZrCl6:xTe4+. Notably, these coordinates exhibit a systematic shift, transitioning from the cold white light region (0.20, 0.23) to the warm white light region (0.45, 0.50) as the Te4+ concentration increases. This shift in color coordinates demonstrates the tunable nature of the emission spectrum.
Based on the discussion above, the mechanism of STEs for Rb2ZrCl6:Te under UV excitation was obtained, as shown in Figure 5h. When stimulated by light, Rb2ZrCl6:Te4+ will undergo lattice distortion, resulting in STE emission. Owing to the narrow bandgap between the S1 and T1 states generated by STEs, the triplet exciton undergoes thermal activation to the vibrational level of the singlet state through RISC. Subsequently, it undergoes radiative transition from the S1 state to the ground state, emitting photons and resulting in a blue emission at 455 nm under 255 nm excitation. The introduction of Te4+ as a dopant creates new energy levels. Additionally, the formation of [TeCl6]2− octahedra induces Jahn–Teller distortion, further enhancing the generation of self-trapped excitons. Electrons are excited from the valence band of Rb2ZrCl6 to the conduction band, followed by a nonradiative relaxation process. This relaxation results in a dual outcome: one portion leads to bulk STE emission, specifically in the form of blue light emission, while the other fraction transitions to the Te4+ energy levels. In the case of Te4+, its ground state is categorized as the 1S0 energy level, and its excited state comprises three triplet energy levels, namely 3P0, 3P1, and 3P2, in addition to a singlet energy level denoted as 1P1. Based on transition rules, transitions from 1S0 to 1P1 and 3P1 are permitted. Transitions from 1S0 to 3P0 and 1S0 to 3P2 are entirely prohibited at the electric dipole transition level. Early studies indicate that the low-energy excitation region of Te4+ originates from the 1S0→3P1 transition [42], with the absorption peaks in the 370–425 nm range attributed to the 1S0→3P1 transition. Under 388 nm ultraviolet excitation, the 575 nm emission of Rb2ZrCl6:Te arises from the triplet transition of 1S0→3P1. Under 255 nm, some electrons in the singlet state 1P1 transition back to the ground state, resulting in PL at approximately 380 nm. Meanwhile, the remaining electrons undergo a process known as ISC, transitioning from the singlet state 1P1 to the triplet state 3P1. Subsequently, under 388 nm, the triplet state relaxes back to the ground state, emitting a yellow light at 575 nm. The PLQY for Rb2ZrCl6 at room temperature is notably high, reaching 62.64%. Figure S5 illustrates that the PLQY remains at 44.74% at room temperature even with a 2% Te4+ dopant.
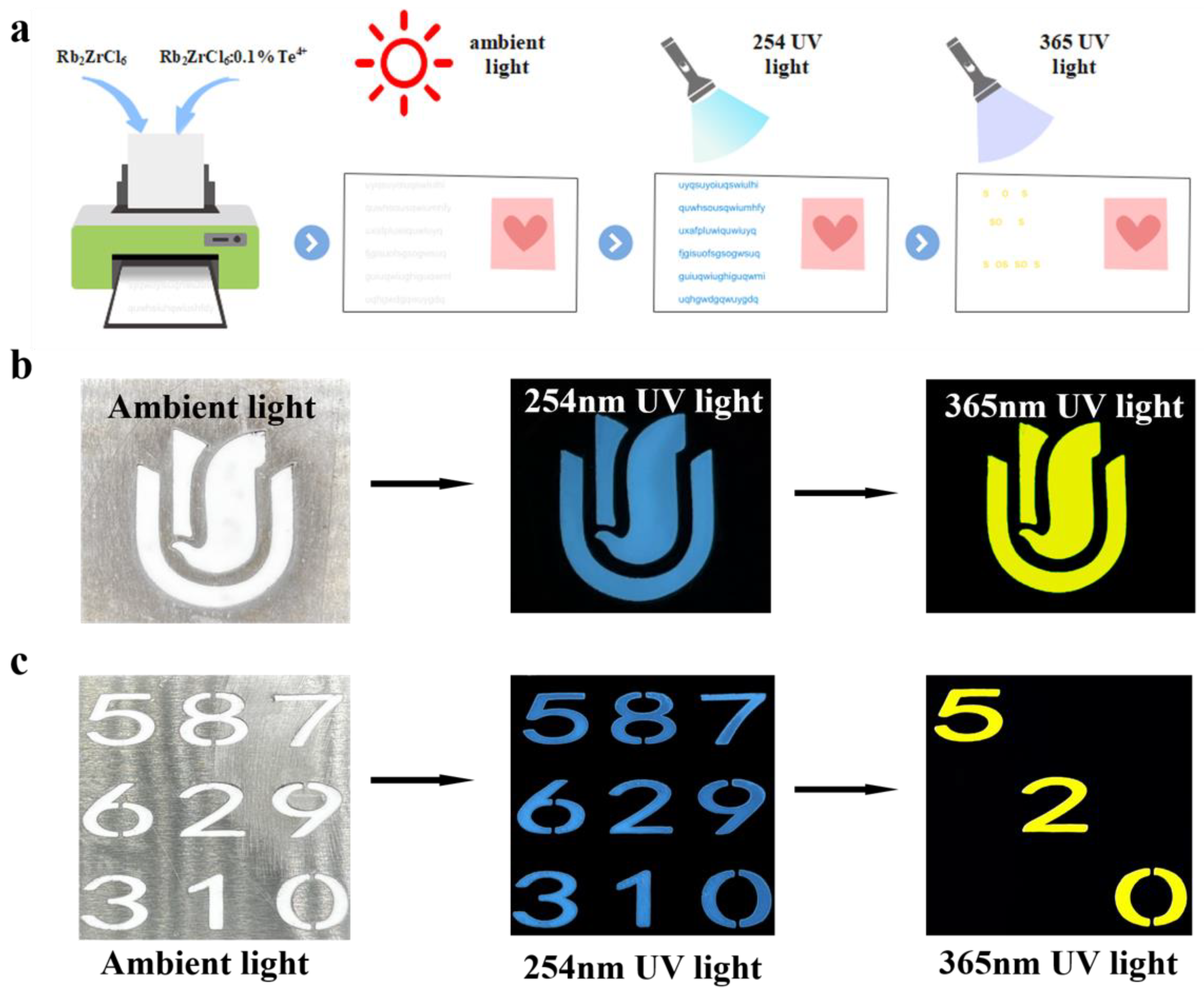
Next, Rb2ZrCl6:2%Te4+ was tested for stability, and exhibited good air stability over a period of four months. Moreover, the dual-state emission characteristic provides theoretical support for research and experimental design in information encryption applications. Figure 6 showed the wide application prospect of Rb2ZrCl6:2%Te4+ by designing anti-counterfeiting and information encryption experiments.
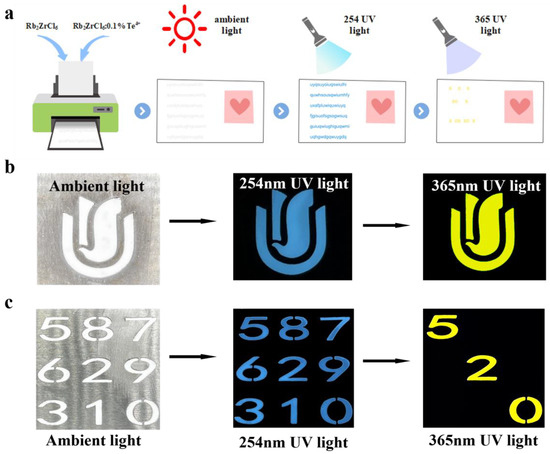
Figure 6.
(a) A Schematic representation of the concept of information encryption and decryption. (b) Anti-counterfeiting of Rb2ZrCl6:0.1%Te4+ pattern. (c) Information encryption and decryption test.
Using Rb2ZrCl6:0.1%Te4+ spectral adjustable properties, different colors were displayed through different wavelengths to achieve the function of anti-counterfeiting and information encryption. Figure 6a is a schematic diagram of the concept of information encryption and decryption. Figure 6b shows an example of anti-counterfeiting. The Rb2ZrCl6:0.1%Te4+ powder pattern appeared white under ambient light; at 254 nm UV light, it emitted blue light, and at 365 nm UV light, it emitted yellow light, which could be clearly distinguished by the naked eye. In order to further enhance the security level of optical information encryption, an optimization scheme was designed based on the dual excitation source of Rb2ZrCl6:0.1%Te4+ and the emission band luminescent material. As shown in Figure 6c, the “520” numbers were Rb2ZrCl6:0.1%Te4+ powder, and the rest were Rb2ZrCl6 powder. Since both Rb2ZrCl6:0.1%Te4+ and Rb2ZrCl6 powders appeared white under natural light, the nine digital patterns appeared white as a whole, representing the encryption process. When excited by 254 nm UV light, all nine numbers produced blue emission. Under excitation at 365 nm UV light, only the “520” filled with Rb2ZrCl6:0.1%Te4+ powder emitted yellow light, while other digits filled with Rb2ZrCl6 powder did not emit any light, this constituted the decryption process. The additional excitation and emission of luminescent information enhanced the overall security level, providing a more secure approach for anti-counterfeiting and confidential information encryption and decryption. Through this enhanced luminescent information excitation and emission process, the application prospects of Rb2ZrCl6:0.1%Te4+ in fields such as anti-counterfeiting and confidential information encryption and decryption were broadened.
We tested the stability of Rb2ZrCl6:xTe4+. The PL spectra of Rb2ZrCl6 powder, following a four-month exposure to atmospheric conditions (at 293 K and humidity levels of 20–30%), exhibited a 7% reduction in intensity when compared to freshly prepared powder. In contrast, the PL strength of Rb2ZrCl6:2%Te4+ decreased by only 6% after being stored for the same duration under identical environmental conditions (Figure S6). The exceptional stability of both Rb2ZrCl6 and Rb2ZrCl6:2%Te4+ could be attributed to their structural characteristics. Rb2ZrCl6 was a vacancy-ordered double perovskite, resulting from the combination of quaternary elements and vacancies; this unique structure contributes to its robust stability. In addition, Rb2ZrCl6 exhibited robust thermal stability both before and after the introduction of Te4+, as evidenced in Figure S7. Neither Rb2ZrCl6 nor Rb2ZrCl6:2%Te4+ underwent a phase transition, maintaining their structural integrity at temperatures up to 884 K, both before and after the doping process.
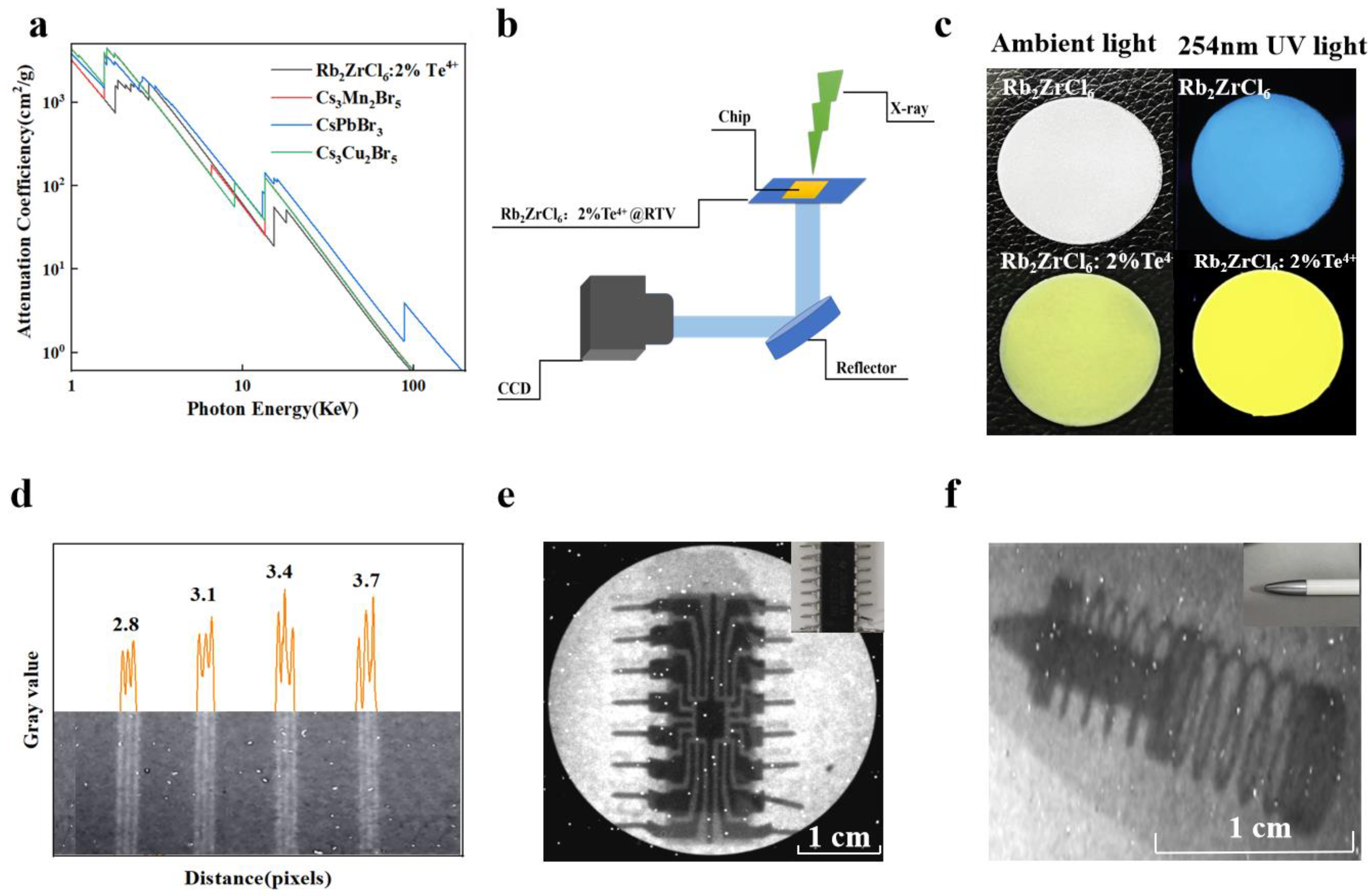
Additionally, a circular Rb2ZrCl6:2%Te4+@RTV thin film scintillation screen with a thickness of 70 μm and a diameter of 3 cm was also prepared, achieving a X-ray imaging spatial resolution of 3.7 lp/mm and a light output of 18,000 Photons/MeV for Rb2ZrCl6:2%Te4+, equivalent to 2.3 times that of BGO, demonstrating potential applications in X-ray imaging. Figure 7 showed the results of X-ray imaging testing.
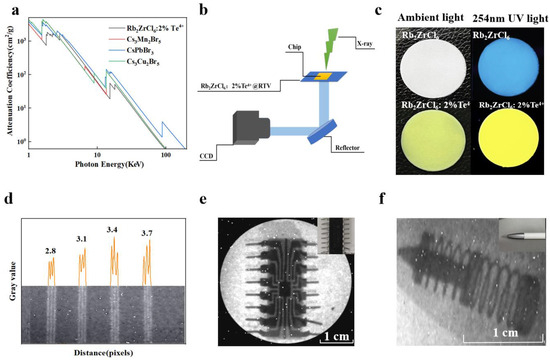
Figure 7.
(a) X-ray absorption coefficients of Rb2ZrCl6:2%Te4+, Cs3Mn2Br5, CsPbBr3, and Cs3Cu2I5. (b) X-ray imaging prototype equipment system diagram. (c) Photographs of Rb2ZrCl6 and Rb2ZrCl6:2%Te4+ @RTV film under ambient light (left) and 254 nm UV light (right). (d) The graph of the gray value change in standard X-ray resolution test pattern plate. (e) X-ray image of the chip by using Rb2ZrCl6:2%Te4+ @RTV film as the scintillator screen. (The upper right corner of the picture is the chip picture). (f) X-ray image of the ballpoint pen by using Rb2ZrCl6:2%Te4+@RTV film as the scintillator screen. (The upper right corner of the picture is the ballpoint pen).
Comparing Rb2ZrCl6, Rb2ZrCl6:2%Te4+, and commercial BGO in terms of XEL, it is observed that Rb2ZrCl6 achieves an optical yield of 89,000 photons/MeV, while Rb2ZrCl6:2%Te4+ reaches 18,000 photons/MeV, which is 11 times and 2.3 times of that of BGO, respectively, as shown in Figure S8. The future applications of Rb2ZrCl6:2%Te4+ and Rb2ZrCl6 in X-ray imaging were investigated. Considering that photodiodes generally peak in their responsiveness within the wavelength range of 500–600 nm [43]. Therefore, the emission light of Rb2ZrCl6 doped with Te4+ can better match the current mainstream CCD. Figure 7a compares the X-ray absorption capacity of Rb2ZrCl6:2%Te4+, Cs3Mn2Br5, CsPbBr3, and Cs3Cu2I5, Rb2ZrCl6:2%Te4+ showed similar X-ray absorption capacity to CsPbBr3. Figure 7b illustrates the X-ray imaging equipment utilized in this study. During the experimental procedures, the settings were configured with a voltage of 100 kV, a current of 1000 μA, and a peak energy of 165 kVp. The Rb2ZrCl6@RTV and Rb2ZrCl6:2%Te4+@RTV composite films with a diameter of 3 cm were prepared using a screen printing method. When observed under natural light, the Rb2ZrCl6 and Rb2ZrCl6:2%Te4+@RTV films appear white and light yellow respectively, and when excited by 254 nm UV light, they show blue and yellow respectively, as shown in Figure 7c. The thickness of Rb2ZrCl6 and Rb2ZrCl6:2%Te4+@RTV films is 70 μm, as shown in Figure S9. As shown in Figure 7d, Rb2ZrCl6:2%Te4+@RTV film as the scintillator screen is used to image the resolution plate, and a resolution of 3.7 lp/mm can be obtained. Its corresponding gray value is shown in Figure 7d. The resolution of Rb2ZrCl6@RTV film is 3.1 lp/mm, as shown in Figure S10. Figure 7e,f show the X-ray image of the chip and the ballpoint pen by using Rb2ZrCl6:2%Te4+@RTV film as the scintillator screen, showing clear internal structure. In the same way, the X-ray imaging test of Rb2ZrCl6@RTV film can also be used for the chip and the ballpoint pen imaging applications, as shown in Figure S11.
4. Conclusions
In summary, vacancy-ordered double perovskite Rb2ZrCl6 and Rb2ZrCl6:xTe4+ microcrystals were successfully prepared via hydrothermal method. The spectral properties of Rb2ZrCl6:xTe4+ were studied, and the application prospects of Rb2ZrCl6:xTe4+ in the fields of information encryption and X-ray detection are investigated. Rb2ZrCl6 has broadband STE emission, exhibiting a blue-white emission spectrum with fluorescence decay time and Stoker shift of 14.47 μs and 200 nm, respectively, without self-absorption. After doping, Rb2ZrCl6:xTe4+ presents different luminescence emissions at different excitation wavelengths. With the increase of Te4+ concentration, 3P1 to 1S0 emission of Rb2ZrCl6:xTe4+ gradually dominates, and the emission wavelength can be adjusted in the range of 455 nm to 575 nm, showing a transition from blue to yellow light, which means excellent spectral tunability. Rb2ZrCl6:xTe4+ microcrystals have broad spectrum emission, longer PL lifetime, and a significant Stokes shift. The strongest doping concentration, Rb2ZrCl6:2%Te4+, exhibits a strong yellow emission, and the decay time and Stokes shift of Rb2ZrCl6:2%Te4+ are 3.51 μs and 187 nm, respectively, attributed to its triplet self-trapping exciton emission. Therefore, Rb2ZrCl6:2%Te4+ can be applied to anti-counterfeiting and information encryption design, and can achieve specific color anti-counterfeiting and information encryption design by adjusting Te4+ concentration. For example, Rb2ZrCl6:0.1%Te4+ can produce different colors under different ultraviolet excitation energies, showing blue under 254 nm excitation and yellow under 365 nm excitation, which can realize the information encryption of blue and yellow color transformation. The application prospect in X-ray imaging was also investigated. The light yield of Rb2ZrCl6 and Rb2ZrCl6:2%Te4+ was 89,000 photon/MeV and 18,000 photon/MeV, respectively, which were 11 and 2.3 times that of BGO. Scintillation films with uniform thickness (70 μm) were prepared by combining the material with RTV, and spatial resolutions of 3.1 lp/mm and 3.7 lp/mm were achieved, respectively. Rb2ZrCl6:xTe4+ shows excellent application prospects in the field of information encryption and imaging.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma17112530/s1, Figure S1: Absorption spectra of Rb2ZrCl6:xTe4+; Figure S2: PL spectrum of Rb2ZrCl6: xTe4+ at 255 nm excitation; Figure S3: Temperature-dependent PL spectra of Rb2ZrCl6:1%Te4+ (λex = 388 nm); Figure S4: Pseudo color map of Rb2ZrCl6:1%Te4+ PL spectra at low temperature (T= 80 –300 K, λex = 255 nm); Figure S5: PLQY of sample Rb2ZrCl6 (λex = 255 nm) and sample Rb2ZrCl6:2%Te4+ (λex = 388 nm), respectively; Figure S6: Rb2ZrCl6 and Rb2ZrCl6:2%Te4+: Comparison of PL spectra of the fresh and 4 months after exposure to air. (temperature:293 K, humidity:20 ~ 30%). The insets show images of the corresponding fresh samples under 254 nm UV light; Figure S7: DSC and TGA curves of Rb2ZrCl6 and Rb2ZrCl6:2%Te4+, respectively; Figure S8: XEL curves for Rb2ZrCl6, Rb2ZrCl6:2%Te4+ and BGO; Figure S9: (a,b) respectively represent the thickness of the glass sheet, and the total thickness of the glass sheet and Rb2ZrCl6@RTV film. (c,d) respectively represent the thickness of the glass sheet, the total thickness of the glass sheet and Rb2ZrCl6:2%Te4+@RTV film; Figure S10: X-ray image of the resolution plate by using Rb2ZrCl6@RTV film as the scintillator screen; Figure S11: X-ray image of the chip (left) and the ballpoint pen (right) by using Rb2ZrCl6@RTV film as the scintillator screen.
Author Contributions
Conceptualization, G.P., Q.L. and H.F.; Methodology, M.L. and X.Y. (Xiaotong Yu); Validation, G.P., M.L., Y.Z., M.X., X.Y. (Xinxin Yang), Z.X., Q.L. and H.F.; Formal analysis, M.L.; Investigation, X.Y. (Xiaotong Yu), X.Y. (Xinxin Yang) and Z.X.; Resources, M.X.; Data curation, Y.Z.; Writing—original draft, G.P.; Writing—review & editing, G.P. and H.F.; Supervision, Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant numbers 12175130, 12375209 and 11905122).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Bohn, B.J.; Bladt, E.; Wang, K.; Müller-Buschbaum, P.; Bals, S.; Urban, A.S.; Polavarapu, L.; Feldmann, J. From Precursor Powders to CsPbX3 Perovskite Nanowires: One-Pot Synthesis, Growth Mechanism, and Oriented Self-Assembly. Angew. Chem. Int. Ed. 2017, 56, 13887–13892. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Liu, S.C.; Qiu, F.Z.; Zhang, Z.Y.; Xue, D.J.; Hu, J.S. High-Efficiency CsPbI2Br Perovskite Solar Cells with Dopant-Free Poly(3-hexylthiophene) Hole Transporting Layers. Adv. Energy Mater. 2020, 10, 2000501. [Google Scholar] [CrossRef]
- Peng, J.; Xia, C.Q.; Xu, Y.; Li, R.; Cui, L.; Clegg, J.K.; Herz, L.M.; Johnston, M.B.; Lin, Q. Crystallization of CsPbBr(3) single crystals in water for X-ray detection. Nat. Commun. 2021, 12, 1531. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Yan, Q.; Gao, T.; Ding, J.; Lv, Q.; Ning, C.; Li, Q.; Sun, J.-L. Perovskite CH3NH3PbI3(Cl) Single Crystals: Rapid Solution Growth, Unparalleled Crystalline Quality, and Low Trap Density toward 108 cm−3. J. Am. Chem. Soc. 2016, 138, 9409–9412. [Google Scholar] [CrossRef] [PubMed]
- Jun, T.; Sim, K.; Iimura, S.; Sasase, M.; Kamioka, H.; Kim, J.; Hosono, H. Lead-Free Highly Efficient Blue-Emitting Cs3Cu2I5 with 0D Electronic Structure. Adv. Mater. 2018, 30, e1804547. [Google Scholar] [CrossRef]
- Lin, R.; Guo, Q.; Zhu, Q.; Zhu, Y.; Zheng, W.; Huang, F. All-Inorganic CsCu2I3 Single Crystal with High-PLQY (approximately 15.7%) Intrinsic White-Light Emission via Strongly Localized 1D Excitonic Recombination. Adv. Mater. 2019, 31, e1905079. [Google Scholar] [CrossRef] [PubMed]
- Mccall, K.M.; Stoumpos, C.C.; Kontsevoi, O.Y.; Alexander, G.C.; Wessels, B.W.; Kanatzidis, M.G. From 0D Cs3Bi2I9 to 2D Cs3Bi2I6Cl3: Dimensional Expansion Induces a Direct Band Gap but Enhances Electron–Phonon Coupling. Chem. Mater. 2019, 31, 2644–2650. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, J.; Yang, B.; Niu, G.; Wu, H.; Zhao, X.; Yin, L.; Jin, T.; Liang, X.; Tang, J. Oriented-Structured CsCu2I3 Film by Close-Space Sublimation and Nanoscale Seed Screening for High-Resolution X-ray Imaging. Nano Lett. 2021, 21, 1392–1399. [Google Scholar] [CrossRef]
- Xu, L.; Chen, J.; Song, J.; Li, J.; Xue, J.; Dong, Y.; Cai, B.; Shan, Q.; Han, B.; Zeng, H. Double-Protected All-Inorganic Perovskite Nanocrystals by Crystalline Matrix and Silica for Triple-Modal Anti-Counterfeiting Codes. ACS Appl. Mater. Interfaces 2017, 9, 26556–26564. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.W.; Jung, H.S.; Shin, H.; Park, N.G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, H.; Wang, J.; Wang, R.; Li, Y.; Shan, Q.; Xu, X.; Dong, Y.; Wei, C.; Zeng, H. Perovskite White Light Emitting Diodes: Progress, Challenges, and Opportunities. ACS Nano 2021, 15, 17150–17174. [Google Scholar] [CrossRef]
- Li, L.; Ye, S.; Qu, J.; Zhou, F.; Song, J.; Shen, G. Recent Advances in Perovskite Photodetectors for Image Sensing. Small 2021, 17, e2005606. [Google Scholar] [CrossRef]
- Wei, K.; Faraj, Y.; Yao, G.; Xie, R.; Lai, B. Strategies for improving perovskite photocatalysts reactivity for organic pollutants degradation: A review on recent progress. Chem. Eng. J. 2021, 414, 128783. [Google Scholar] [CrossRef]
- Gandini, M.; Villa, I.; Beretta, M.; Gotti, C.; Imran, M.; Carulli, F.; Fantuzzi, E.; Sassi, M.; Zaffalon, M.; Brofferio, C.; et al. Efficient, fast and reabsorption-free perovskite nanocrystal-based sensitized plastic scintillators. Nat. Nanotechnol. 2020, 15, 462–468. [Google Scholar] [CrossRef]
- Heo, J.H.; Shin, D.H.; Park, J.K.; Kim, D.H.; Lee, S.J.; Im, S.H. High-Performance Next-Generation Perovskite Nanocrystal Scintillator for Nondestructive X-ray Imaging. Adv. Mater. 2018, 30, 1801743. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wu, H.; Luo, J.; Deng, Z.; Ge, C.; Chen, C.; Jiang, X.; Yin, W.-J.; Niu, G.; Zhu, L.; et al. Cs2AgBiBr6 single-crystal X-ray detectors with a low detection limit. Nat. Photonics 2017, 11, 726–732. [Google Scholar] [CrossRef]
- Dai, L.; Deng, Z.; Auras, F.; Goodwin, H.; Zhang, Z.; Walmsley, J.C.; Paul DBristowe Felix Deschler Greenham, N.C. Slow carrier relaxation in tin-based perovskite nanocrystals. Nat. Photonics 2021, 15, 696–702. [Google Scholar] [CrossRef]
- Dai, L.; Ye, J.; Greenham, N.C. Thermalization and relaxation mediated by phonon management in tin-lead perovskites. Light Sci. Appl. 2023, 12, 208. [Google Scholar] [CrossRef]
- Grandhi, G.K.; Viswanath NS, M.; Cho, H.B.; Han, J.H.; Kim, S.M.; Choi, S.; Bin Im, W. Mechanochemistry as a Green Route: Synthesis, Thermal Stability, and Postsynthetic Reversible Phase Transformation of Highly-Luminescent Cesium Copper Halides. J. Phys. Chem. Lett. 2020, 11, 7723–7729. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, J.; Pan, R.; Cheng, J.; Chen, R.; He, T. All-inorganic copper(i)-based ternary metal halides: Promising materials toward optoelectronics. Nanoscale 2020, 12, 15560–15576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mo, Q.; Cai, W.; Wang, H.; Zang, Z. Inorganic lead-free cesium copper chlorine nanocrystal for highly efficient and stable warm white light-emitting diodes. Photonics Res. 2021, 9, 187–192. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Pan, G.; Xu, M.; Wang, C.; Chen, Y.; Yang, X.; Xu, Z.; Zhao, J.; Li, Q.; et al. Temperature-Induced Reversible Fluorescence Discoloration of Cs3Cu2Br5 and Its Application in X-ray Imaging. Cryst. Growth Des. 2023, 23, 8024–8033. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, J.; Lin, Q.S.; Rao, G.; Zhao, J.; Yang, C.; He, W.; Wang, C.; Zhang, Z. Novel, Green, and Scalable Aqueous Synthesis of Yellow–Green Emitting Cs3Cu2Cl5 Scintillator and its Application in High-Resolution TFT Panel for X-ray Imaging Detector. Adv. Opt. Mater. 2022, 11, 2202059. [Google Scholar] [CrossRef]
- Kong, Q.; Meng, X.; Ji, S.; Wang, Q.; Yang, B.; Bai, T.; Wang, X.; Wang, Z.; Zhang, R.; Zheng, D.; et al. Highly Reversible Cesium Manganese Iodine for Sensitive Water Detection and X-ray Imaging. ACS Mater. Lett. 2022, 4, 1734–1741. [Google Scholar] [CrossRef]
- Shao, H.; Li, Y.; Yang, W.; He, X.; Wang, L.; Fu, J.; Fu, M.; Ling, H.; Gkoupidenis, P.; Yan, F.; et al. A Reconfigurable Optoelectronic Synaptic Transistor with Stable Zr-CsPbI3 Nanocrystals for Visuomorphic Computing. Adv. Mater. 2023, 35, e2208497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lai, J.A.; Huang, Q.; Wu, D.; Qi, F.; Zhang, N.; Pu, Y.; Tian, C.; Chen, W.; Liu, Y.; et al. Ultrahigh PLQY Lead-Free Organic–Inorganic Hybrid Zirconium-Based Perovskites in Anticounterfeiting Applications. Adv. Opt. Mater. 2023, 11, 2300399. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Z.; Liu, Z.; Jia, M.; Chen, X.; Wu, D.; Li, X.; Shi, Z. Highly stable vacancy-ordered double perovskite Rb2ZrCl6 with broadband emission for down-conversion white light-emitting diodes. J. Lumin. 2022, 251, 119150–119165. [Google Scholar] [CrossRef]
- Zheng, K.; Chen, B.; Xie, L.; Li, X.; Lu, B.; Wang, M.; Wu, Y.; Jiang, T.; Zhang, F.; Li, X.; et al. Vacancy-Ordered Double Perovskite Rb2ZrCl6−xBrx: Facile Synthesis and Insight into Efficient Intrinsic Self-Trapped Emission. Adv. Opt. Mater. 2021, 10, 2101661. [Google Scholar] [CrossRef]
- Shi, X.; Li, Z.; Cao, M.; Rao, Z.; Zhao, X.; Gong, X. Fast HCl-free Synthesis of Lead-free Rb2ZrCl6:xSb3+ Perovskites. Inorg. Chem. 2022, 61, 14095–14101. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, Q.; Xu, H.; Yu, H.; Du, B.; Han, Q.; Wu, W. Vacancy-ordered Te4+-doped Rb2ZrCl6 double perovskite microcrystals for solid-state lighting and non-contact optical thermometry. Appl. Phys. Lett. 2023, 123, 111903. [Google Scholar] [CrossRef]
- Murugan, S.; Lee, E.C. Recent Advances in the Synthesis and Application of Vacancy-Ordered Halide Double Perovskite Materials for Solar Cells: A Promising Alternative to Lead-Based Perovskites. Materials 2023, 16, 5275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zheng, X.; Sun, H.; Li, W.; Wang, X.; Hao, X.; An, S. Dual-Mode Luminescence Modulation upon Visible-Light-Driven Photochromism with High Contrast for Inorganic Luminescence Ferroelectrics. ACS Appl. Mater Interfaces 2016, 8, 4789–4794. [Google Scholar] [CrossRef] [PubMed]
- Engel, G. Die Kristallstrukturen einiger Hexachlorokomplexsalze. Z. Krist.-Cryst. Mater. 1935, 90, 341–373. [Google Scholar] [CrossRef]
- Cheng, P.; Zheng, D.; Feng, L.; Liu, Y.; Liu, J.; Li, J.; Yang, Y.; Wang, G.; Han, K. Doped all-inorganic cesium zirconium halide perovskites with high-efficiency and tunable emission. J. Energy Chem. 2022, 65, 600–604. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, Z.; Qin, C.; Cui, M.; Yang, D.; Wang, X.; Wang, L.; Ji, X.; Chen, X.; Sun, J.; et al. Stable Yellow Light-Emitting Devices Based on Ternary Copper Halides with Broadband Emissive Self-Trapped Excitons. ACS Nano 2020, 14, 4475–4486. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liu, Y.; Zhao, J.; Xia, Z. Sb3+ Doping-Induced Triplet Self-Trapped Excitons Emission in Lead-Free Cs2SnCl6 Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 7439–7444. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Zheng, M.; Zhang, W.; Yin, L.; Du, X.; Zhang, P.; Zhang, X.; Gao, J.; Zhang, D.; Gao, L.; et al. Efficient and Reabsorption-Free Radioluminescence in Cs3Cu2I5 Nanocrystals with Self-Trapped Excitons. Adv. Sci. 2020, 7, 2000195. [Google Scholar] [CrossRef] [PubMed]
- Ai, B.; Liu, C.; Deng, Z.; Wang, J.; Han, J.; Zhao, X. Low temperature photoluminescence properties of CsPbBr3 quantum dots embedded in glasses. Phys. Chem. 2017, 19, 17349–17355. [Google Scholar] [CrossRef]
- Chang, T.; Wei, Q.; Zeng, R.; Wang, J.; Han, J.; Zhao, X. Efficient Energy Transfer in Te4+Doped Cs2ZrCl6 Vacancy-Ordered Perovskites and Ultrahigh Moisture Stability via A-Site Rb-Alloying Strategy. J. Phys. Chem. Lett. 2021, 12, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Bai, K.; Wei, Q.; Chang, T.; Yan, J.; Ke, B.; Huang, J.; Wang, L.; Zhou, W.; Cao, S.; et al. Boosting triplet self-trapped exciton emission in Te(IV)-doped Cs2SnCl6 perovskite variants. Nano Res. 2020, 14, 1551–1558. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, J.; Yang, Z.; Liu, Y.; Liu, S. Halide Perovskite: A Promising Candidate for Next-Generation X-ray Detectors. Adv. Sci. 2022, 10, e2205536. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).