Preparation and Characterization of Nanofiber Coatings on Bone Implants for Localized Antimicrobial Activity Based on Sustained Ion Release and Shape-Preserving Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Cu2+ Ionic Incorporation in SNW Coatings
2.2. Structural Characterization of the Coatings
2.3. Bonding Strengths of the Coatings
2.4. Wettability of the Coatings
2.5. Cu2+ Released from the Coatings
2.6. In Vitro Antibacterial Test
3. Results
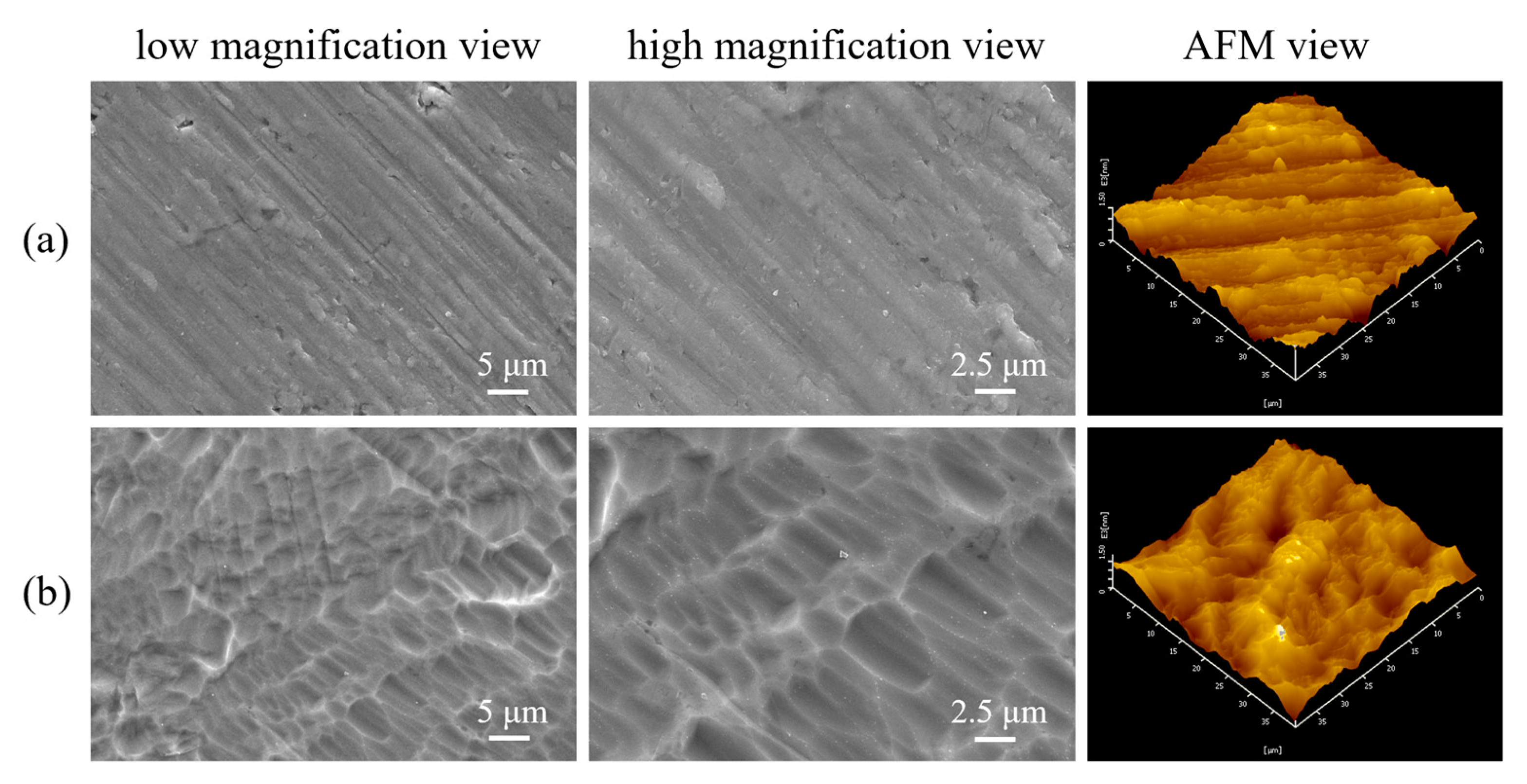
3.1. Topography of the P-Ti before and after Acid Etching
3.2. Structure and Topography of the HT Coatings before and after IE
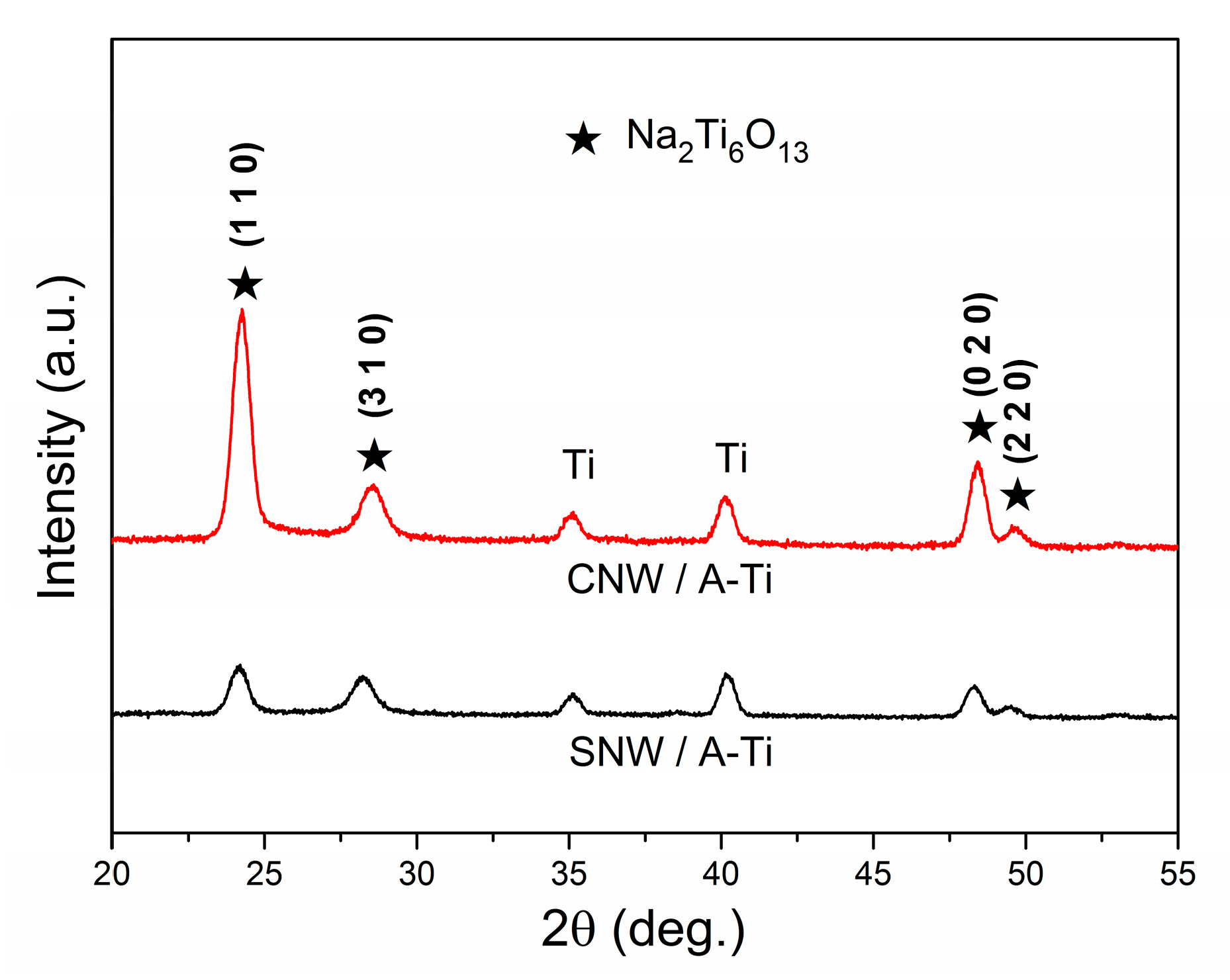
3.3. Phases and Surface Chemical Species of the Coatings
3.4. The Formation Mechanism of SNW/A-Ti and CNW/A-Ti
3.5. Hydrophilicity of Coatings
3.6. Bonding Integrity of the Coatings
3.7. Cu2+ Release of the Coatings
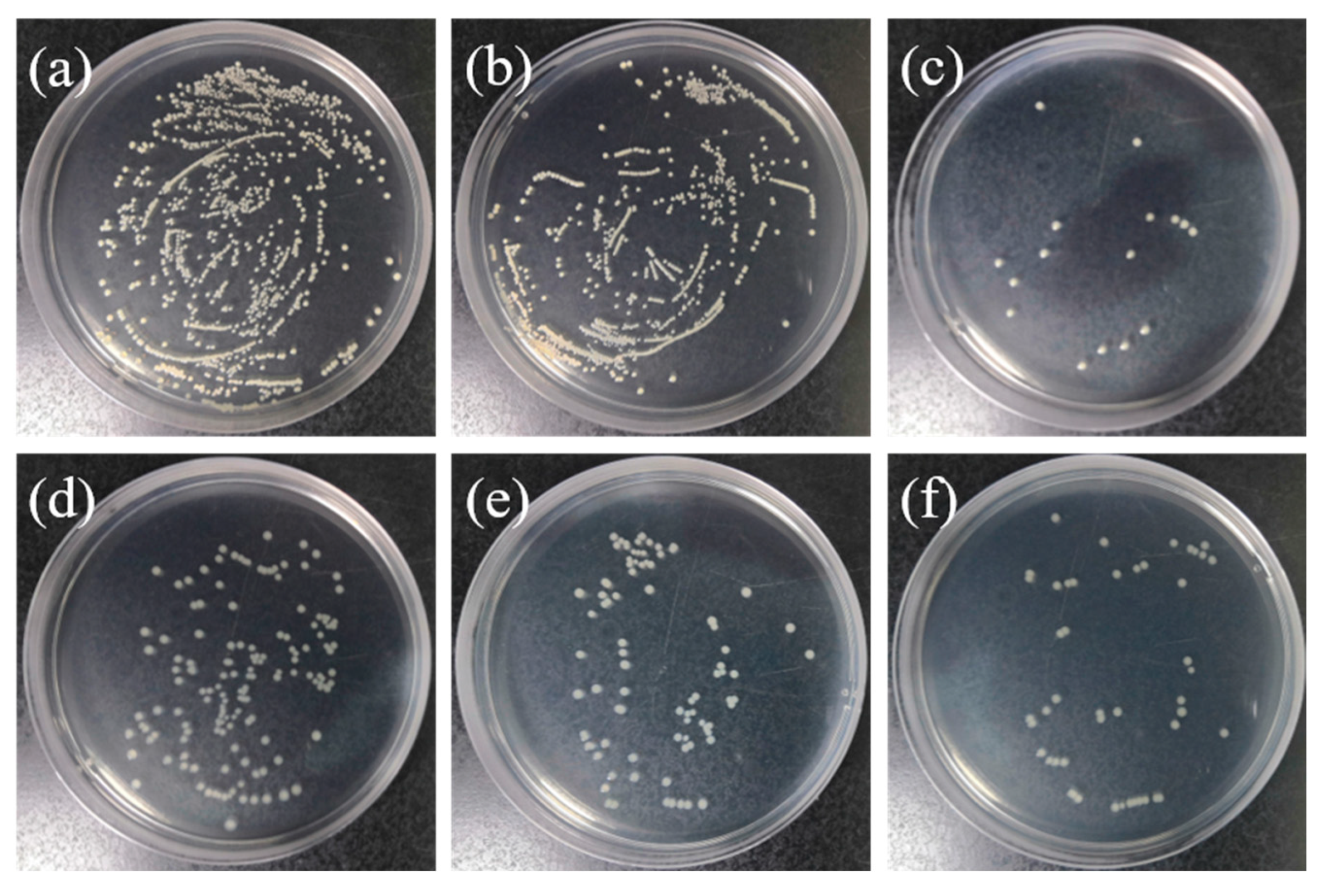
3.8. Bactericidal Effects of the Coatings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rammelt, S.; Illert, T.; Bierbaum, S.; Scharnweber, D.; Zwipp, H.; Schneiders, W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials 2006, 27, 5561–5571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Rani, V.V.; Vinoth-Kumar, L.; Anitha, V.C.; Manzoor, K.; Deepthy, M.; Shantikumar, V.N. Osteointegration of titanium implant is sensitive to specific nanostructure morphology. Acta Biomater. 2012, 8, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lim, J.Y.; Donahue, H.J.; Dhurjati, R.; Mastro, A.M.; Vogler, E.A. Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: Phenotypic and genotypic responses observed in vitro. Biomaterials 2007, 28, 4535–4550. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Oates, C.J.; Molenberg, A.; Dard, M.; Hamilton, D.W. The effect of enamel matrix proteins on the spreading, proliferation and differentiation of osteoblasts cultured on titanium surfaces. Biomaterials 2010, 31, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Ishizaki, K.; Iwasa, F.; Ueno, T.; Minamikawa, H.; Yamada, M.; Suzuki, T.; Ogawa, T. Effects of pico-to-nanometer-thin TiO2 coating on the biological properties of microroughened titanium. Biomaterials 2011, 32, 8374–8384. [Google Scholar] [CrossRef]
- Lim, J.Y.; Shaughnessy, M.C.; Zhou, Z.; Noh, H.; Vogler, E.A.; Donahue, H.J. Surface energy effects on osteoblast spatial growth and mineralization. Biomaterials 2008, 29, 1776–1784. [Google Scholar] [CrossRef]
- Setzer, B.; Bächle, M.; Metzger, M.C.; Kohal, R.J. The gene-expression and phenotypic response of hFOB 1.19 osteoblasts to surface-modified titanium and zirconia. Biomaterials 2009, 30, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Dolatshahi-Pirouz, A.; Jensen, T.; Kraft, D.C.; Foss, M.; Kingshott, P.; Hansen, J.L.; Larsen, A.N.; Chevallier, J.; Besenbacher, F. Fibronectin adsorption, cell adhesion, and proliferation on nanostructured tantalum surfaces. ACS Nano 2010, 4, 2874–2882. [Google Scholar] [CrossRef]
- Heydarkhan-Hagvall, S.; Gluck, J.M.; Delman, C.; Jung, M.; Ehsani, N.; Full, S.; Shemin, R.J. The effect of vitronectin on the differentiation of embryonic stem cells in a 3D culture system. Biomaterials 2012, 33, 2032–2040. [Google Scholar] [CrossRef]
- Kuo, S.W.; Lin, H.I.; Ho, J.H.; Shih, Y.R.; Chen, H.F.; Yen, T.J.; Lee, O.K. Regulation of the fate of human mesenchymal stem cells by mechanical and stereo-topographical cues provided by silicon nanowires. Biomaterials 2012, 33, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Jin, Q.; Jin, T.; Chang, S.; Zhang, Z.; Czajka-Jakubowska, A.; Giannobile, W.V.; Nör, J.E.; Clarkson, B.H. The stimulation of adipose-derived stem cell differentiation and mineralization by ordered rod-like fluorapatite coatings. Biomaterials 2012, 33, 5036–5046. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Ito, H.; Nagai, A.; Komotori, J.; Imai, H. Adhesion of osteoblast-like cells on nanostructured hydroxyapatite. Acta Biomater. 2010, 6, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Stroescu, H.; Anastasescu, M.; Calderon-Moreno, J.M.; Preda, S.; Stoica, M.; Fogarassy, Z.; Petrik, P.; Gheorghe, M.; Parvulescu, C.; et al. High-quality PMMA/ZnO NWs piezoelectric coating on rigid and flexible metallic substrates. Appl. Surf. Sci. 2020, 529, 147135. [Google Scholar] [CrossRef]
- Bavykin, D.; Walsh, F. Elongated Titanate Nanostructures and Their Applications. Eur. J. Inorg. Chem. 2009, 2009, 977–997. [Google Scholar] [CrossRef]
- Mark, K.; Park, J.; Bauer, S.; Schmuki, P. Nanoscale engineering of biomimetic surfaces: Cues from the extracellular matrix. Cell Tissue Res. 2010, 339, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Kim, Y.J.; Yeom, J.; Jeon, J.H.; Yi, G.C.; Je, J.H.; Hahn, S.K. The topographic effect of zinc oxide nanoflowers on osteoblast growth and osseointegration. Adv. Mater. 2010, 22, 4857–4861. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Castano, O.; Kim, H.W. Electrospun materials as potential platforms for bone tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, H.; Boughton, R.I.; Du, G.; Lin, J.; Wang, J.; Liu, D. One-dimensional single-crystalline Ti–O based nanostructures: Properties, synthesis, modifications and applications. J. Mater. Chem. 2010, 20, 5993–6008. [Google Scholar] [CrossRef]
- Woo, K.M.; Chen, V.J.; Ma, P.X. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J. Biomed. Mater. Res. Part A 2003, 67, 531–537. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, Q.; Pang, X.; Han, S.; Yan, Y. Corrosion behavior and biocompatibility of strontium and fluorine co-doped electrodeposited hydroxyapatite coatings. Appl. Surf. Sci. 2013, 282, 456–462. [Google Scholar] [CrossRef]
- Surmenev, R.; Surmeneva, M.; Ivanova, A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2013, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Goyenvalle, E.; Aguado, E.; Nguyen, J.-M.; Passuti, N.; Le Guéhennec, L.; Layrolle, P.; Daculsi, G. Osteointegration of femoral stem prostheses with a bilayered calcium phosphate coating. Biomaterials 2006, 27, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yu, M.; Wang, R.; Li, B.; Zhao, X.; Hao, Y.; Guo, Z.; Han, Y. Hydrothermally grown TiO2-nanorods on surface mechanical attrition treated Ti: Improved corrosion fatigue and osteogenesis. Acta Biomater. 2020, 116, 400–414. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Y.; Zhang, L. Formation and Bioactivity of SrTiO3 Nanotubes on Titanium by Modified Anodization and Hydrothermal Treatment. J. Mater. Sci. Technol. 2016, 32, 930–936. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, T.; Epstein, J.; Cooney, L.; Wang, H.; Li, Y.; Jiang, Y.-B.; Cogbill, A.; Varadan, V.; Tian, Z.R. Multifunctional Nanowire Bioscaffolds on Titanium. Chem. Mater. 2007, 19, 4454–4459. [Google Scholar] [CrossRef]
- Park, J.Y.; Davies, J.E. Red blood cell and platelet interactions with titanium implant surfaces. Clin. Oral Implants Res. 2000, 11, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, X.; Wu, H.; Tian, L.; Ma, Y.; Tang, B. Microstructure and antibacterial properties of Cu-doped TiO2 coating on titanium by micro-arc oxidation. Appl. Surf. Sci. 2014, 292, 944–947. [Google Scholar] [CrossRef]
- Finney, L.; Vogt, S.; Fukai, T.; Glesne, D. Copper and Angiogenesis: Unravelling a Relationship Key to Cancer Progression. Clin. Exp. Pharmacol. Physiol. 2009, 36, 88–94. [Google Scholar] [CrossRef]
- Wu, Q.; Li, J.; Zhang, W.; Qian, H.; She, W.; Pan, H.; Wen, J.; Zhang, X.; Liu, X.; Jiang, X. Antibacterial property, angiogenic and osteogenic activity of Cu-incorporated TiO2 coating. J. Mater. Chem. B 2014, 2, 6738–6748. [Google Scholar] [CrossRef] [PubMed]
- Heidenau, F.; Mittelmeier, W.; Detsch, R.; Haenle, M.; Stenzel, F.; Ziegler, G.; Gollwitzer, H. A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization. J. Mater. Sci. 2005, 16, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Mirastschijski, U.; Martin, A.; Jorgensen, L.N.; Sampson, B.; Ågren, M.S. Zinc, copper, and selenium tissue levels and their relation to subcutaneous abscess, minor surgery, and wound healing in humans. Biol. Trace Elem. Res. 2013, 153, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Venojarvi, M.; Trikha, P.; Ellison, E.C.; Hunt, T.K.; Roy, S. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1821–H1827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, J.; Huang, X.; Zhang, Y.; Han, Y. The dual function of Cu-doped TiO2 coatings on titanium for application in percutaneous implants. J. Mater. Chem. B 2016, 4, 3788–3800. [Google Scholar] [CrossRef] [PubMed]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Han, Y.; Qi, K. Formation Mechanism, Degradation Behavior, and Cytocompatibility of a Nanorod-Shaped HA and Pore-Sealed MgO Bilayer Coating on Magnesium. ACS Appl. Mater. Interfaces 2014, 6, 20. [Google Scholar] [CrossRef]
- Ye, J.; Li, B.; Li, M.; Zheng, Y.; Wu, S.; Han, Y. Formation of a ZnO nanorods-patterned coating with strong bactericidal capability and quantitative evaluation of the contribution of nanorods-derived puncture and ROS-derived killing. Bioact. Mater. 2022, 11, 181–191. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, J.; Ding, T.; Mao, M.; Zhu, S.; Zhou, J.; Zhang, L.; Han, Y. Building biointegration of Fe2O3–FeOOH coated titanium implant by regulating NIR irradiation in an infected model. Bioact. Mater. 2022, 8, 1–11. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- GB 4789.2—2010; Food Microbiological Examination: Aerobic Plate Count. Ministry of Health of the People’s Republic of China: Beijing, China, 2010.
- Han, G.; Liu, S.; Pan, Z.; Lin, Y.; Ding, S.; Li, L.; Luo, B.; Jiao, Y.; Zhou, C. Sulfonated chitosan and phosphorylated chitosan coated polylactide membrane by polydopamine-assisting for the growth and osteogenic differentiation of MC3T3-E1s. Carbohydr. Polym. 2020, 229, 115517. [Google Scholar] [CrossRef]
- Faia-Torres, A.B.; Guimond-Lischer, S.; Rottmar, M.; Charnley, M.; Goren, T.; Maniura-Weber, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; Neves, N.M. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials 2014, 35, 9023–9032. [Google Scholar] [CrossRef]
- Yeo, A.; Wong, W.J.; Khoo, H.H.; Teoh, S.H. Surface modification of PCL-TCP scaffolds improve interfacial mechanical interlock and enhance early bone formation: An in vitro and in vivo characterization. J. Biomed. Mater. Res. Part A 2010, 92, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Bose, S.; Bandyopadhyay, A. Surface modifications and cell-materials interactions with anodized Ti. Acta Biomater. 2007, 3, 573–585. [Google Scholar] [CrossRef]
- Wadge, M.D.; McGuire, J.; Hanby, B.V.T.; Felfel, R.M.; Ahmed, I.; Grant, D.M. Tailoring the degradation rate of magnesium through biomedical nano-porous titanate coatings. J. Magnes. Alloys 2021, 9, 336–350. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Qiao, Y.; Zhu, H.; Jiang, X.; Liu, X.; Ding, C. Chemically regulated bioactive ion delivery platform on a titanium surface for sustained controlled release. J. Mater. Chem. B 2014, 2, 283–294. [Google Scholar] [CrossRef]
- Xu, K.; Chen, W.; Hu, Y.; Shen, X.; Xu, G.; Ran, Q.; Yu, Y.; Mu, C.; Cai, K. Influence of strontium ions incorporated into nanosheet-pore topographical titanium substrates on osteogenic differentiation of mesenchymal stem cells in vitro and on osseointegration in vivo. J. Mater. Chem. B 2016, 4, 4549–4564. [Google Scholar] [CrossRef] [PubMed]
- Atuchin, V.; Kesler, V.; Pervukhina, N.; Zhang, Z. Ti 2p and O 1s Core Levels and Chemical Bonding in Titanium-Bearing Oxides. J. Electron Spectrosc. Relat. Phenom. 2006, 152, 18–24. [Google Scholar] [CrossRef]
- Meng, X.-D.; Wang, D.-Z.; Liu, J.-H.; Zhang, S.-Y. Preparation and characterization of sodium titanate nanowires from brookite nanocrystallites. Mater. Res. Bull. 2004, 39, 2163–2170. [Google Scholar] [CrossRef]
- Jose, M.; Szymańska, K.; Szymański, K.; Moszyński, D.; Mozia, S. Effect of copper salts on the characteristics and antibacterial activity of Cu-modified titanate nanotubes. J. Environ. Chem. Eng. 2020, 8, 104550. [Google Scholar] [CrossRef]
- Izawa, H.; Kikkawa, S.; Koizumi, M. Ion exchange and dehydration of layered [sodium and potassium] titanates, Na2Ti3O7 and K2Ti4O9. J. Phys. Chem 1982, 86, 5023–5026. [Google Scholar] [CrossRef]
- Tengvall, P.; Elwing, H.; Lundström, I. Titanium gel made from metallic titanium and hydrogen peroxide. J. Colloid Interface Sci. 1989, 130, 405–413. [Google Scholar] [CrossRef]
- Lee, K.; Yoo, D. Large-Area Sodium Titanate Nanorods Formed On Titanium Surface Via NaOH Alkali Treatment. Arch. Metall. Mater. 2015, 60, 1371–1374. [Google Scholar] [CrossRef]
- Kim, H.M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Effect of heat treatment on apatite-forming ability of Ti metal induced by alkali treatment. J. Mater. Sci. 1997, 8, 341–347. [Google Scholar] [CrossRef]
- Yada, M.; Inoue, Y.; Uota, M.; Torikai, T.; Watari, T.; Noda, I.; Hotokebuchi, T. Plate, Wire, Mesh, Microsphere, and Microtube Composed of Sodium Titanate Nanotubes on a Titanium Metal Template. Langmuir 2007, 23, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.; Victorio, E.; Jin, T. Synthesis of TiO2-Based Nanotube on Ti Substrate by Hydrothermal Treatment. J. Nanosci. Nanotechnol. 2007, 7, 668–672. [Google Scholar] [CrossRef]
- Umek, P.; Korosec, R.C.; Jancar, B.; Dominko, R.; Arcon, D. The influence of the reaction temperature on the morphology of sodium titanate 1D nanostructures and their thermal stability. J. Nanosci. Nanotechnol. 2007, 7, 3502–3508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, G.; Liu, H.; Liu, D.; Qin, S.; Wang, N.; Hu, C.; Tao, X.; Jiao, J.; Wang, J.; et al. Nanostructured Sheets of Ti-O Nanobelts for Gas Sensing and Antibacterial Applications. Adv. Funct. Mater. 2008, 18, 1131–1137. [Google Scholar] [CrossRef]
- De Souza, G.B.; Lepienski, C.M.; Foerster, C.E.; Kuromoto, N.K.; Soares, P.; Ponte, H.d.A. Nanomechanical and nanotribological properties of bioactive titanium surfaces prepared by alkali treatment. J. Mech. Behav. Biomed. Mater. 2011, 4, 756–765. [Google Scholar] [CrossRef]
- Szesz, E.M.; de Souza, G.B.; Santos, E.; Kuromoto, N.K. Nanomechanical Properties of Bioactive Ti Surfaces Obtained by NaOH-Based Anodic Oxidation and Alkali Treatment. Key Eng. Mater. 2012, 493–494, 524–529. [Google Scholar] [CrossRef]
- Ho, W.F.; Lai, C.H.; Hsu, H.C.; Wu, S.C. Surface modification of a Ti-7.5Mo alloy using NaOH treatment and Bioglass coating. J. Mater. Sci. 2010, 21, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, B.; Yu, M.; Guo, S.; Guo, Z.; Han, Y. Cubic multi-ions-doped Na2TiO3 nanorod-like coatings: Structure-stable, highly efficient platform for ions-exchanged release to immunomodulatory promotion on vascularized bone apposition. Bioact. Mater. 2022, 18, 72–90. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Wang, X.; Li, L.; Zhu, Y.; Li, M.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Tan, G.; et al. Concentration ranges of antibacterial cations for showing the highest antibacterial efficacy but the least cytotoxicity against mammalian cells: Implications for a new antibacterial mechanism. Chem. Res. Toxicol. 2015, 28, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, J.; Yan, T.; Han, Y. Fibroblast responses and antibacterial activity of Cu and Zn co-doped TiO2 for percutaneous implants. Appl. Surf. Sci. 2018, 434, 633–642. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Geng, Z.; Yin, Y.; Hang, R.; Huang, X.; Yao, X.; Tang, B. Preparation, antibacterial effects and corrosion resistant of porous Cu–TiO2 coatings. Appl. Surf. Sci. 2014, 308, 43–49. [Google Scholar] [CrossRef]





| Sample Name | Roughness (nm) | ||
|---|---|---|---|
| Ra | Rq | Rz | |
| P-Ti | 141.1 ± 7.4 | 181.1 ± 4.7 | 1074.0 ± 97.0 |
| A-Ti | 205.9 ± 12.8 | 256.3 ± 13.8 | 1180.9 ± 113.4 |
| SNW/A-Ti | 239.5 ± 9.9 | 312.6 ± 11.1 | 2325.7 ± 94.1 |
| CNW/A-Ti | 244.1 ± 12.0 | 321.0 ± 12.1 | 2401.0 ± 109.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Wang, H.; Cao, S.; Liu, Z.; Zhang, Y. Preparation and Characterization of Nanofiber Coatings on Bone Implants for Localized Antimicrobial Activity Based on Sustained Ion Release and Shape-Preserving Design. Materials 2024, 17, 2584. https://doi.org/10.3390/ma17112584
Cao Y, Wang H, Cao S, Liu Z, Zhang Y. Preparation and Characterization of Nanofiber Coatings on Bone Implants for Localized Antimicrobial Activity Based on Sustained Ion Release and Shape-Preserving Design. Materials. 2024; 17(11):2584. https://doi.org/10.3390/ma17112584
Chicago/Turabian StyleCao, Yubao, Hong Wang, Shuyun Cao, Zaihao Liu, and Yanni Zhang. 2024. "Preparation and Characterization of Nanofiber Coatings on Bone Implants for Localized Antimicrobial Activity Based on Sustained Ion Release and Shape-Preserving Design" Materials 17, no. 11: 2584. https://doi.org/10.3390/ma17112584
APA StyleCao, Y., Wang, H., Cao, S., Liu, Z., & Zhang, Y. (2024). Preparation and Characterization of Nanofiber Coatings on Bone Implants for Localized Antimicrobial Activity Based on Sustained Ion Release and Shape-Preserving Design. Materials, 17(11), 2584. https://doi.org/10.3390/ma17112584






