Deep Desulfurization of High-Sulfur Petroleum Coke via Alkali Catalytic Roasting Combined with Ultrasonic Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
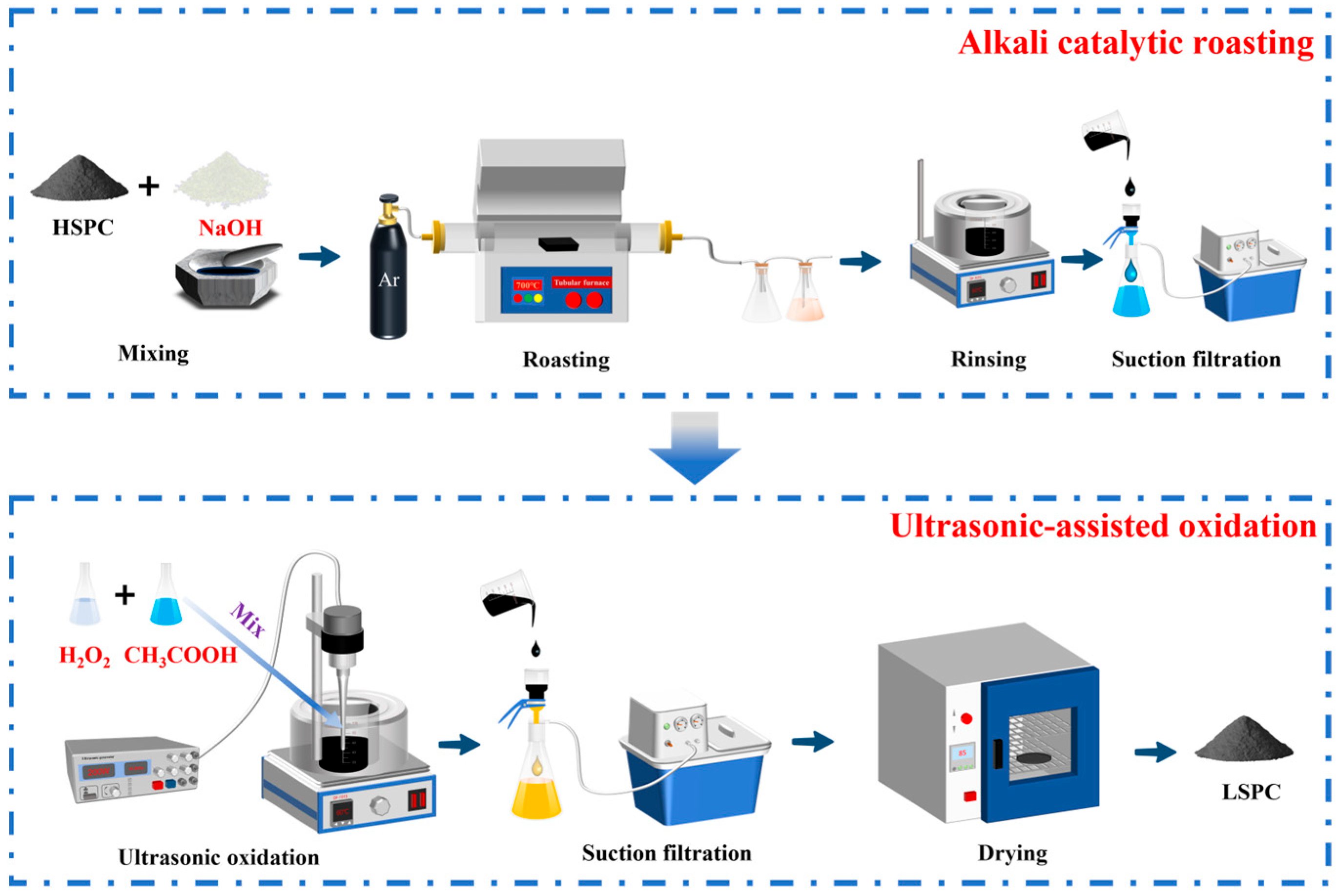
2.2. Experimental Methods
3. Results and Discussion
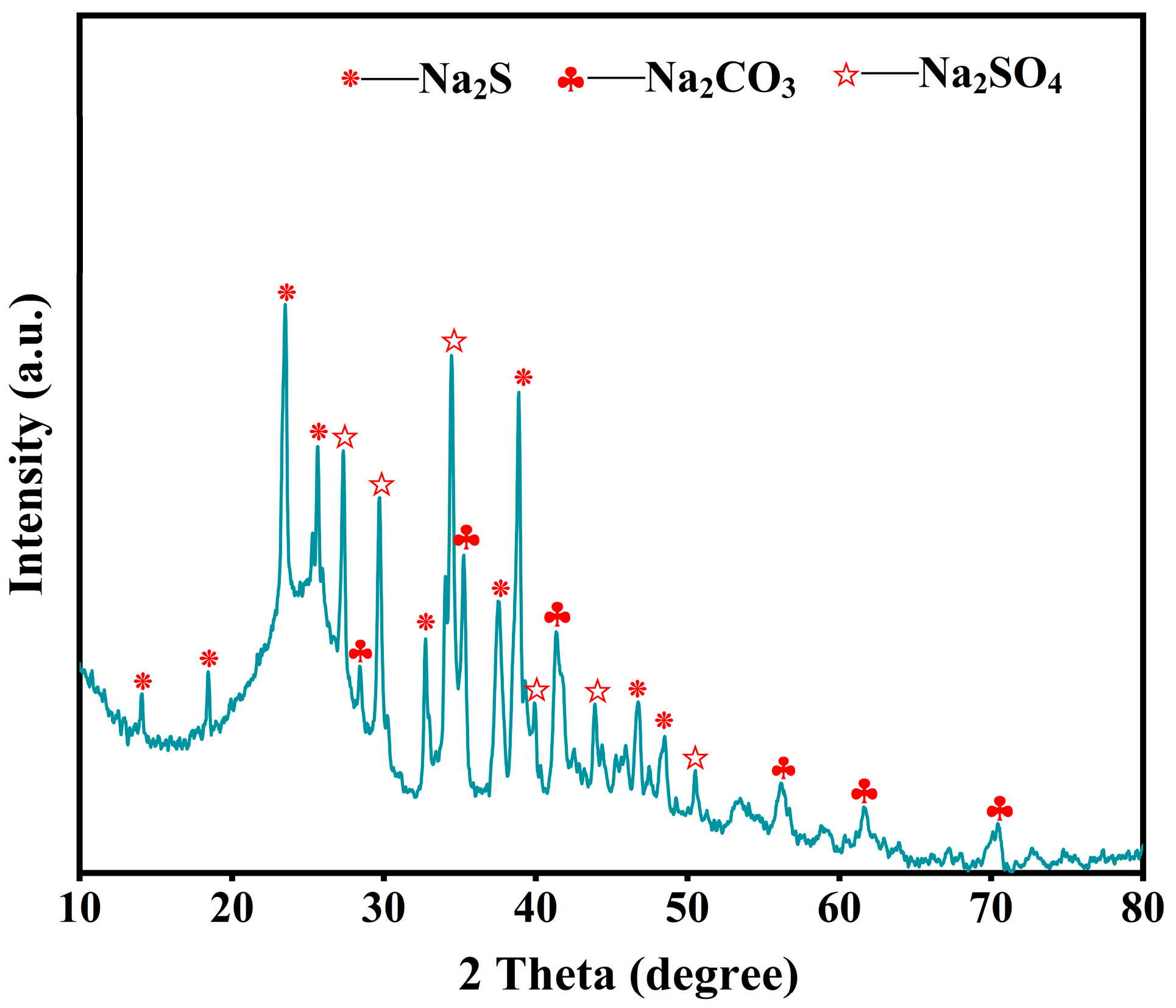
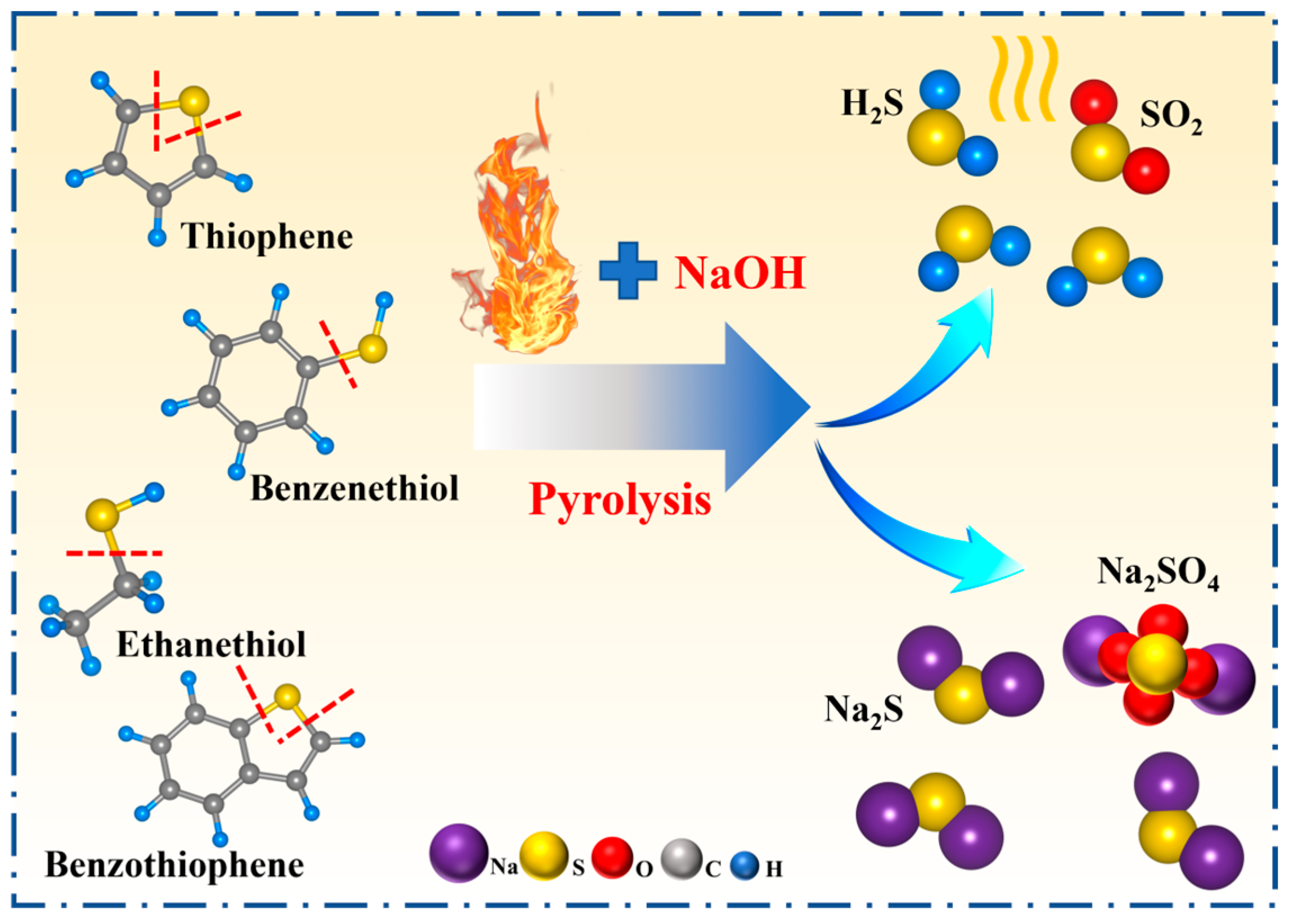
3.1. Effect of Alkali Catalytic Roasting on Desulfurization of Petroleum Coke
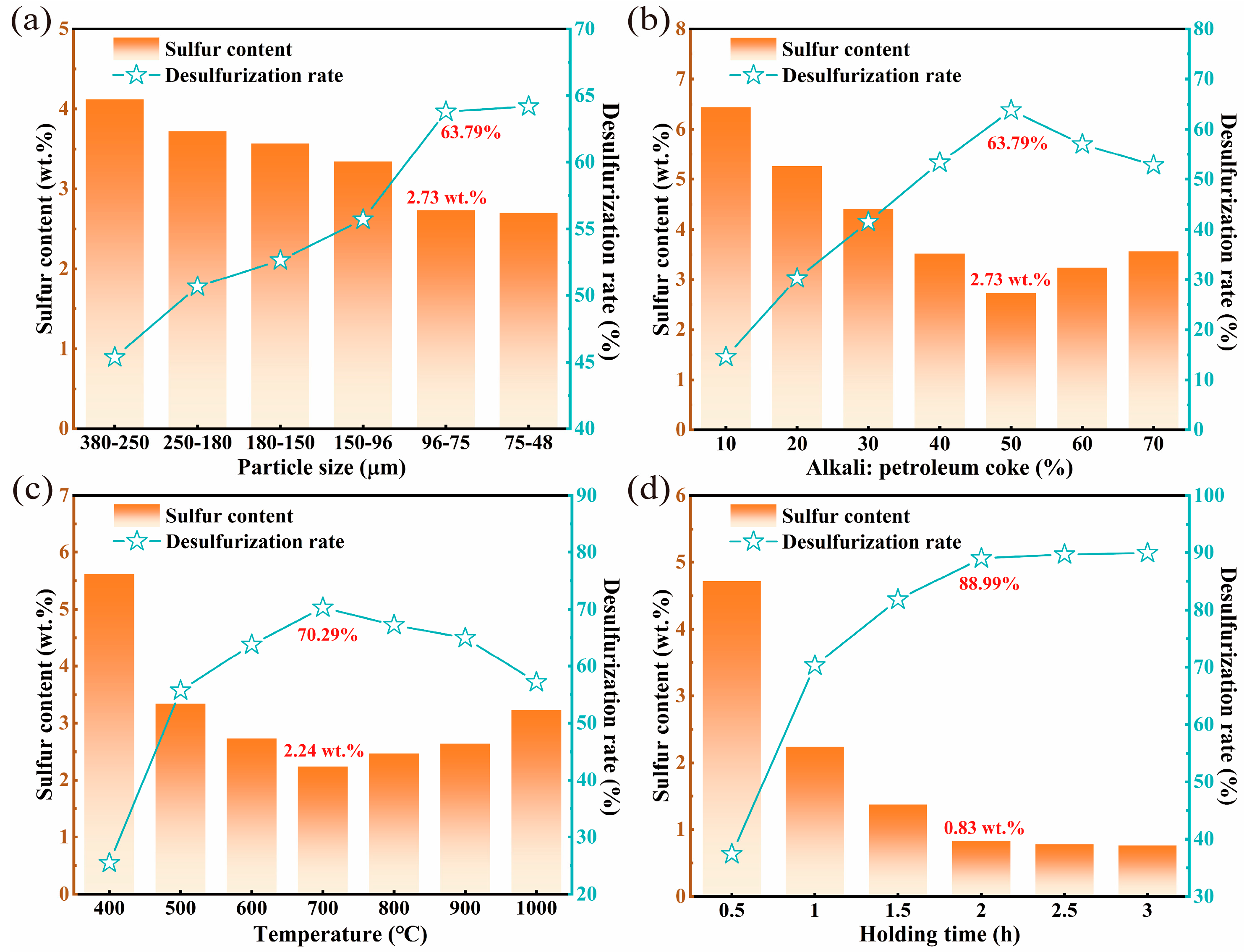
3.1.1. Particle Size of Petroleum Coke
3.1.2. Alkali-to-Petroleum Coke Ratio
3.1.3. Roasting Temperature
3.1.4. Holding Time
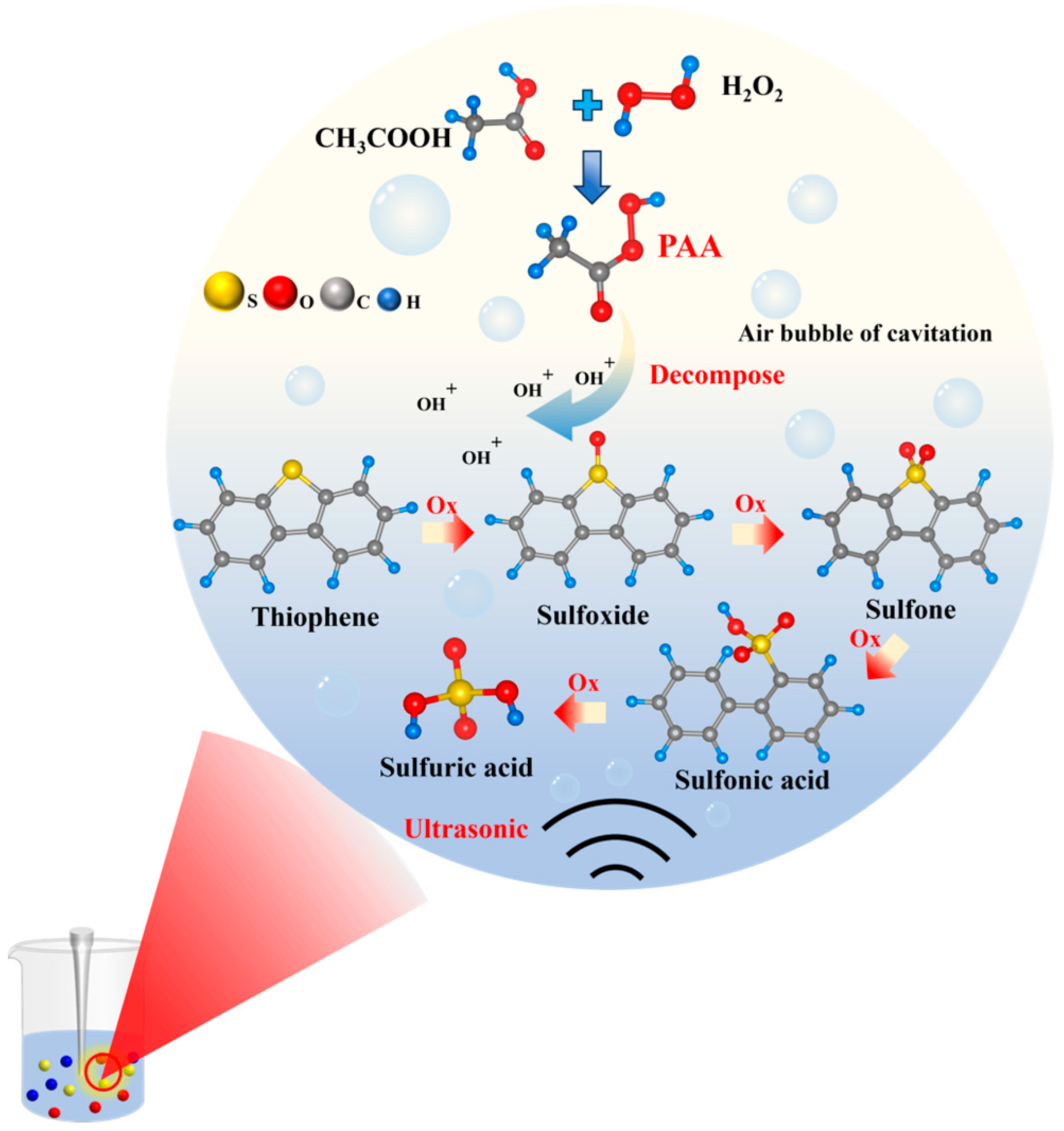
3.2. Study on Desulfurization of Petroleum Coke by Ultrasonic-Assisted Oxidation
3.2.1. Hydrogen Peroxide Concentration
3.2.2. Liquid-to-Solid Ratio
3.2.3. Acetic Acid Addition
3.2.4. Ultrasonic Power
3.2.5. Reaction Temperature
3.2.6. Comparison of the Desulfurization of Petroleum Coke via Conventional Leaching and Ultrasonic Leaching
3.3. Characterization of Petroleum Coke before and after Desulfurization
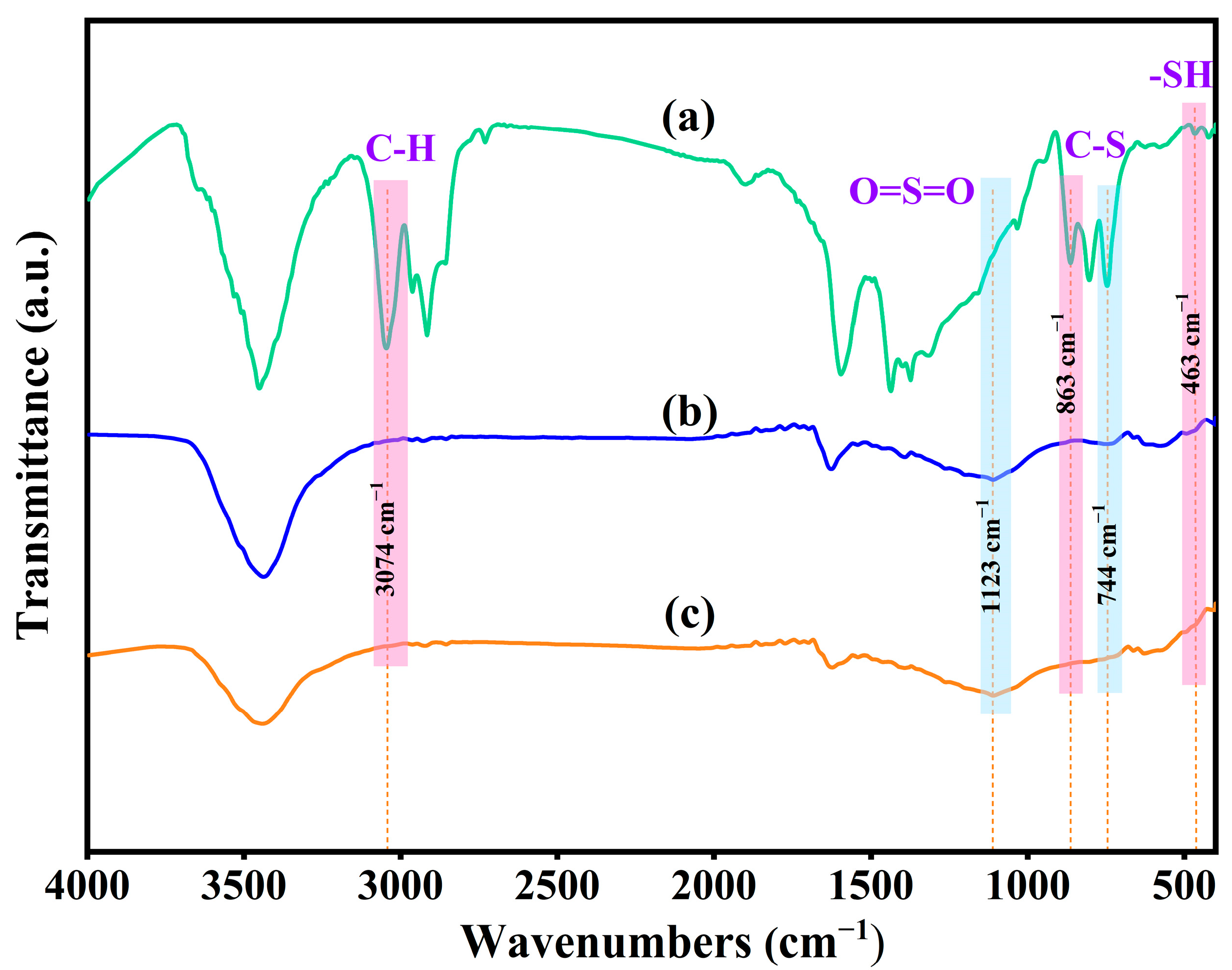
3.3.1. FTIR Spectroscopy Analysis
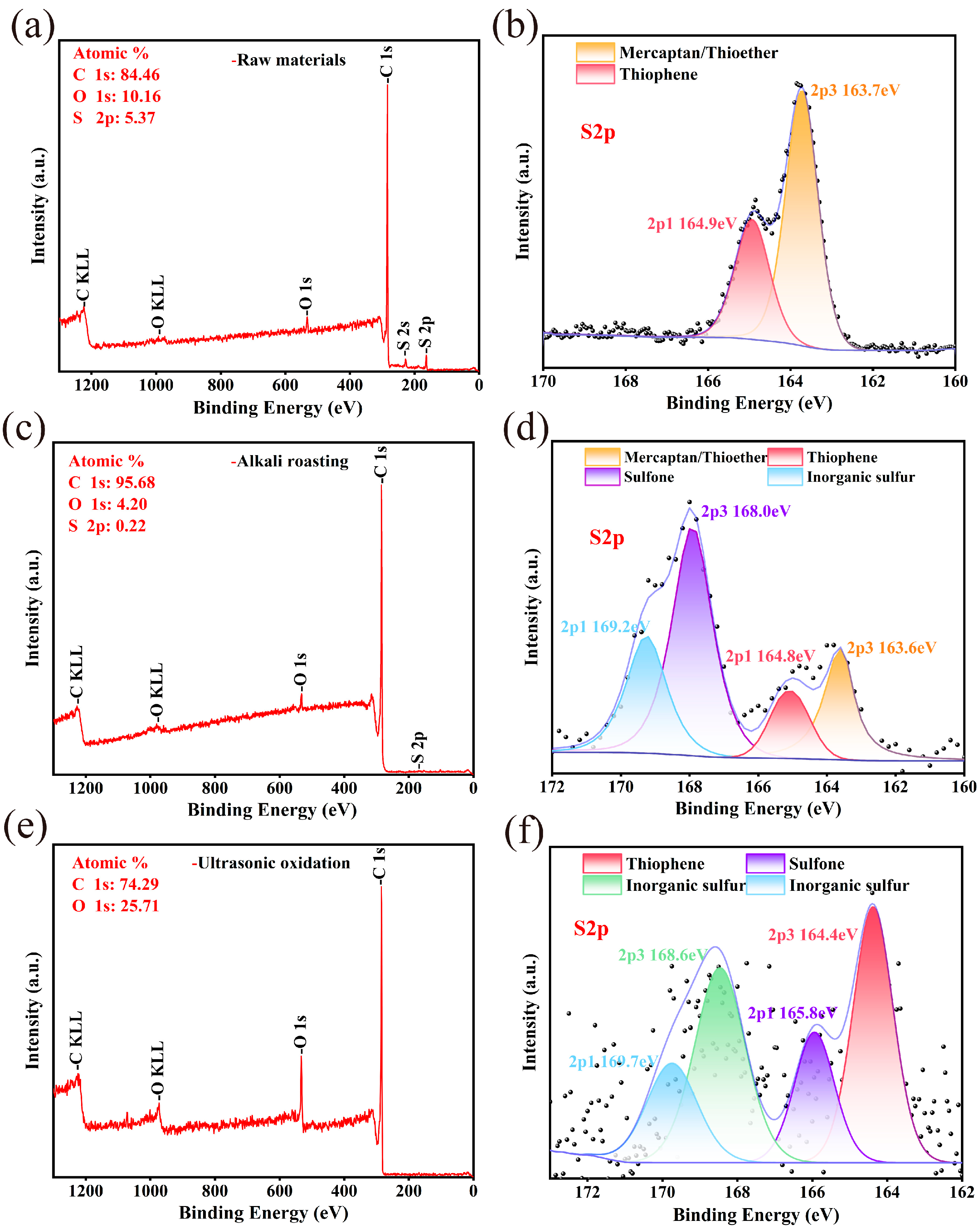
3.3.2. XPS Analysis
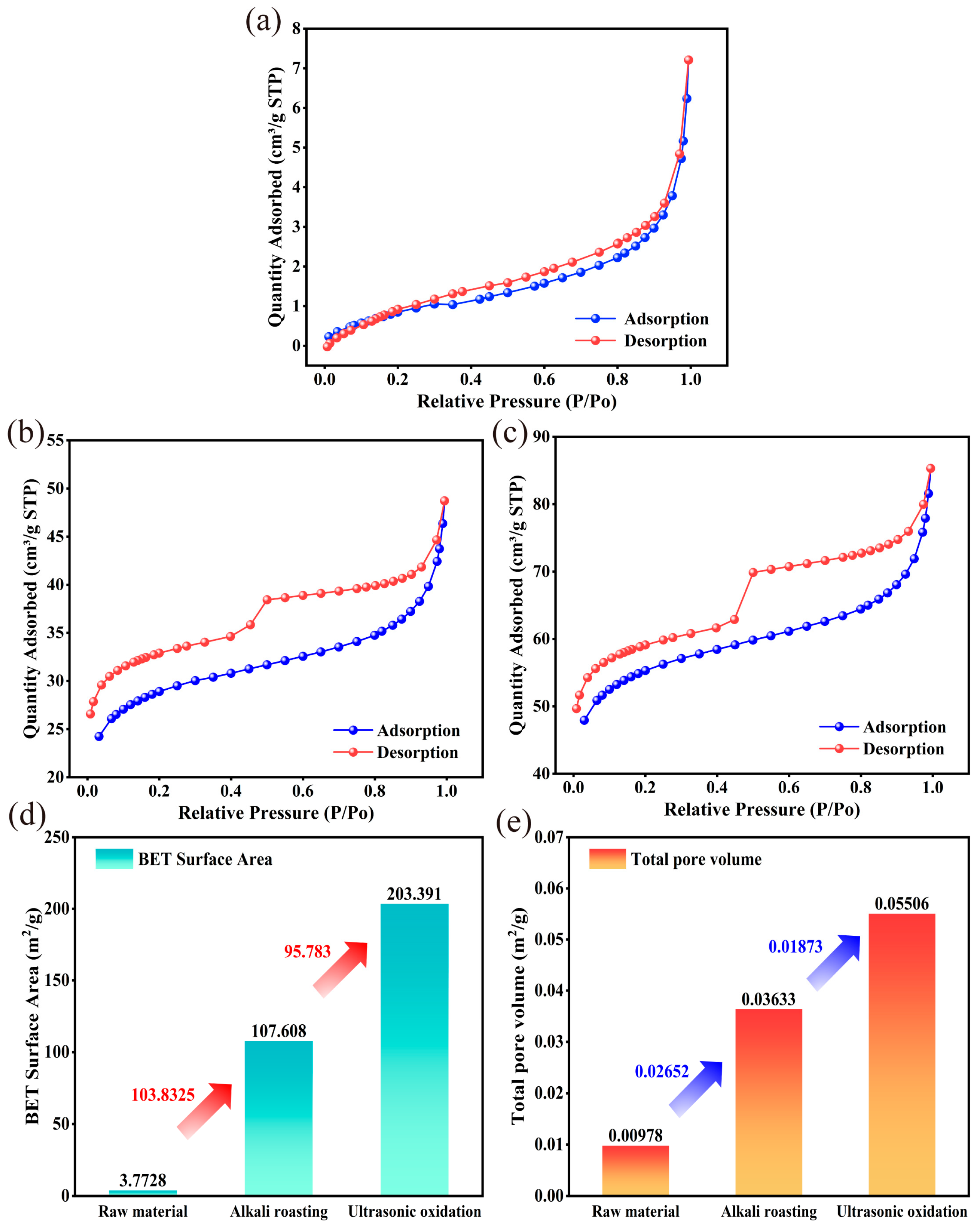
3.3.3. Textural Properties Analysis
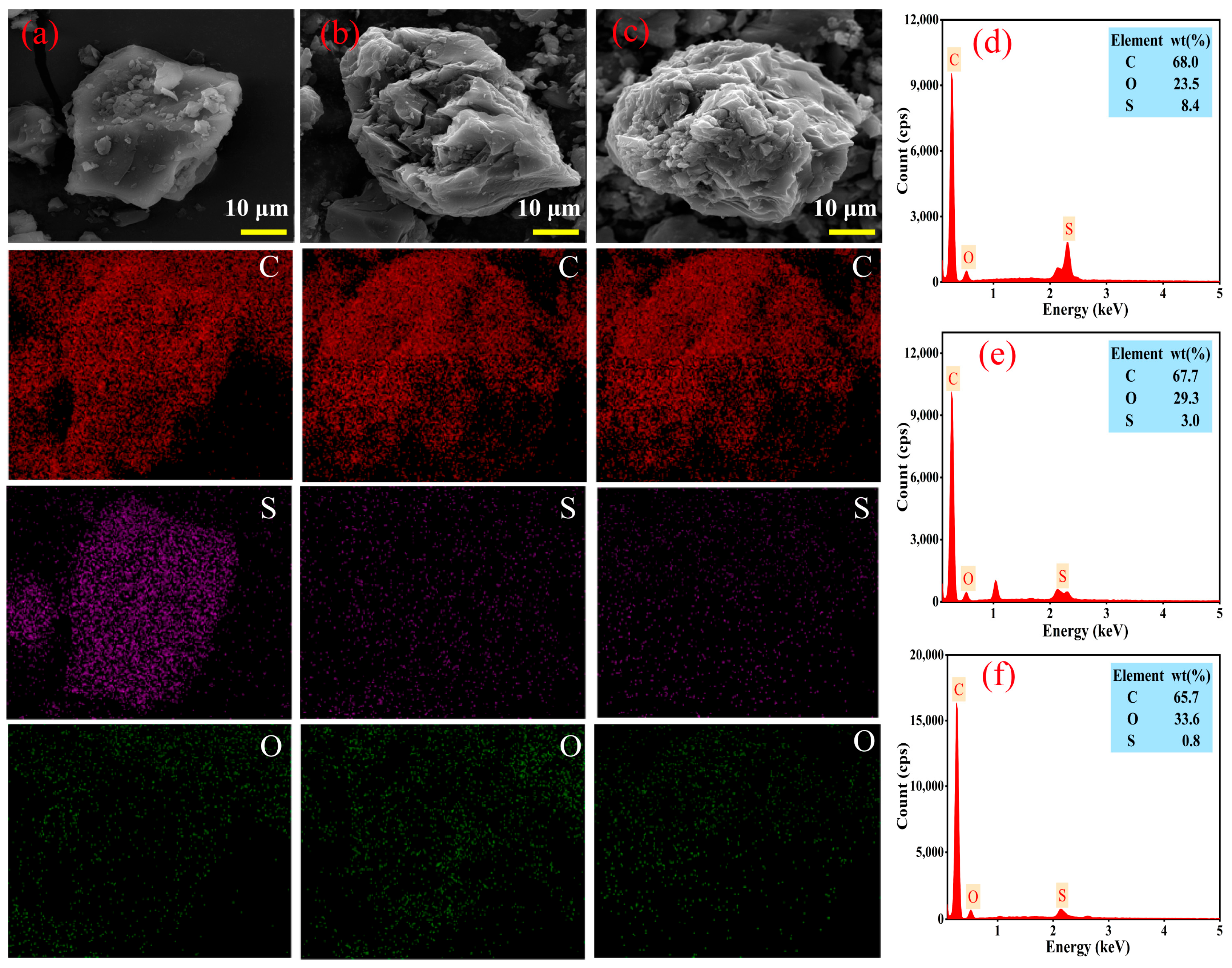
3.3.4. Micromorphology and SEM–EDS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milenkova, K.S.; Borrego, A.G.; Alvarez, D.; Menéndez, R.; Petersen, H.I.; Rosenberg, P. Coal Blending with Petroleum Coke in a Pulverized-Fuel Power Plant. Energy Fuels 2005, 19, 453–458. [Google Scholar] [CrossRef]
- Heintz, E.A. The characterization of petroleum coke. Carbon 1996, 34, 699–709. [Google Scholar] [CrossRef]
- Wang, M.; Wan, Y.; Guo, Q.; Bai, Y.; Yu, G.; Liu, Y.; Zhang, H.; Zhang, S.; Wei, J. Brief review on petroleum coke and biomass/coal co-gasification: Syngas production, reactivity characteristics, and synergy behavior. Fuel 2021, 304, 121517. [Google Scholar] [CrossRef]
- Priyadharshini, R.; Saravanathamizhan, R.; Manimozhi, V.; Manokaran, J.; Balasubramanian, N. Preparation of activated petroleum coke for supercapacitor application. Energy Storage 2020, 2, e151. [Google Scholar] [CrossRef]
- Shan, J.; Wang, J.-J.; Kiekens, P.; Zhao, Y.; Huang, J.-J. Effect of co-activation of petroleum coke and Artemisia Hedinii on potassium loss during activation and its promising application in anode material of potassium-ion batteries. Solid State Sci. 2019, 92, 96–105. [Google Scholar] [CrossRef]
- Nugroho, A.; Nursanto, E.B.; Pradanawati, S.A.; Oktaviano, H.S.; Nilasary, H.; Nursukatmo, H. Fe based catalysts for petroleum coke graphitization for Lithium Ion battery application. Mater. Lett. 2021, 303, 130557. [Google Scholar] [CrossRef]
- Xi, F.; Li, S.; Ma, W.; Chen, Z.; Wei, K.; Wu, J. A review of hydrometallurgy techniques for the removal of impurities from metallurgical-grade silicon. Hydrometallurgy 2021, 201, 105553. [Google Scholar] [CrossRef]
- Xu, K.; Li, Y.; Yang, F.; Yang, W.; Zhang, L.; Xu, C.; Kaneko, T.; Hatakeyama, R. Controllable synthesis of single- and double-walled carbon nanotubes from petroleum coke and their application to solar cells. Carbon 2014, 68, 511–519. [Google Scholar] [CrossRef]
- Chen, J.; Lu, X. Progress of petroleum coke combusting in circulating fluidized bed boilers—A review and future perspectives. Resour. Conserv. Recycl. 2007, 49, 203–216. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. The use of petroleum coke as fuel in chemical-looping combustion. Fuel 2007, 86, 1947–1958. [Google Scholar] [CrossRef]
- Ma, A.; Zhao, S.; Luo, H.; Sun, Z.; Xie, X.; Liao, Y.; Liang, X.; Li, H. Mercury removal from coal-fired flue gas of high-sulfur petroleum coke activated by pyrolysis and mechanochemical method. Chem. Eng. J. 2022, 429, 132154. [Google Scholar] [CrossRef]
- Twomey, C.; Birkinshaw, C.; Breen, S. The identity of the sulfur-containing phases present in cement clinker manufactured using a high sulfur petroleum coke fuel. J. Chem. Technol. Biotechnol. 2004, 79, 486–490. [Google Scholar] [CrossRef]
- Mandal, D.; Mahapatra, P.L.; Kumari, R.; Kumbhakar, P.; Biswas, A.; Lahiri, B.; Chandra, A.; Tiwary, C.S. Convert waste petroleum coke to multi-heteroatom self-doped graphene and its application as supercapacitors. Emergent Mater. 2021, 4, 531–544. [Google Scholar] [CrossRef]
- Manasrah, A.D.; Nassar, N.N.; Ortega, L.C. Conversion of petroleum coke into valuable products using oxy-cracking technique. Fuel 2018, 215, 865–878. [Google Scholar] [CrossRef]
- Zhong, Q.; Mao, Q.; Zhang, L.; Xiang, J.; Xiao, J.; Mathews, J.P. Structural features of Qingdao petroleum coke from HRTEM lattice fringes: Distributions of length, orientation, stacking, curvature, and a large-scale image-guided 3D atomistic representation. Carbon 2018, 129, 790–802. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, Y.; Wang, D.-Q.; Yang, H.-P.; Zhang, S.-H.; Chen, H.-P. Study on high temperature pyrolysis process and sulfur transformation property of high sulfur petroleum coke. J. Fuel Chem. Technol. 2020, 48, 683–688. [Google Scholar]
- Conn, R.E. Laboratory techniques for evaluating ash agglomeration potential in petroleum coke fired circulating fluidized bed combustors. Fuel Process. Technol. 1995, 44, 95–103. [Google Scholar] [CrossRef]
- Guo, Z.; Fu, Z.; Wang, S. Sulfur distribution in coke and sulfur removal during pyrolysis. Fuel Process. Technol. 2007, 88, 935–941. [Google Scholar] [CrossRef]
- Al-Haj Ibrahim, H.; Morsi, B. Desulfurization of Petroleum Coke: A Review. Ind. Eng. Chem. Res. 1992, 31, 1835–1840. [Google Scholar] [CrossRef]
- El-Kaddah, N.; Ezz, S.Y. Thermal desulphurization of ultra-high-sulphur petroleum coke. Fuel 1973, 52, 128–129. [Google Scholar] [CrossRef]
- Wang, B.; Zheng, P.; Fan, H.; Meng, Q.; Duan, A.; Chen, Z.; Xu, C. Insights into the effect of solvent on dibenzothiophene hydrodesulfurization. Fuel 2021, 287, 119459. [Google Scholar] [CrossRef]
- Farahani, S.; Sobati, M.A. A novel method for the management of sulfone-rich waste produced in the oxidative desulfurization (ODS) process. Chin. J. Chem. Eng. 2020, 28, 2447–2456. [Google Scholar] [CrossRef]
- He, X.; Li, R.; Qiu, J.; Xie, K.; Ling, P.; Yu, M.; Zhang, X.; Zheng, M. Synthesis of mesoporous carbons for supercapacitors from coal tar pitch by coupling microwave-assisted KOH activation with a MgO template. Carbon 2012, 50, 4911–4921. [Google Scholar] [CrossRef]
- Ityokumbul, M.T. Experimental evaluation of molten caustic leaching of an oil sand coke residue. Can. J. Chem. Eng. 1994, 72, 370–374. [Google Scholar] [CrossRef]
- George, Z.M.; Schneider, L.G.; Tollefson, E.L. Desulphurization of a fluid coke similar to the Athabasca oil sands coke. Fuel 1978, 57, 497–501. [Google Scholar] [CrossRef]
- Li, G.-Q.; Li, S.-S.; Qu, S.-W.; Liu, Q.-K.; Ma, T.; Zhu, L.; Liang, F.-L.; Liu, R.-L. Improved biodesulfurization of hydrodesulfurized diesel oil using Rhodococcuserythropolis and Gordonia sp. Biotechnol. Lett. 2008, 30, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.-J.; Ma, C.; Wang, J.-T.; Qiao, W.-M.; Ling, L.-C. Almost total desulfurization of high-sulfur petroleum coke by Na2CO3-promoted calcination combined with ultrasonic-assisted chemical oxidation. New Carbon Mater. 2018, 33, 587–594. [Google Scholar] [CrossRef]
- Ibrahim, H.A.-H. Thermal Treatment of Syrian Sponge Coke. J. King Saud Univ.—Eng. Sci. 2006, 18, 261–269. [Google Scholar] [CrossRef]
- Martins, M.A.; Oliveira, L.S.; Franca, A.S. Modeling and simulation of petroleum coke calcination in rotary kilns. Fuel 2001, 80, 1611–1622. [Google Scholar] [CrossRef]
- Xiao, J.; Deng, S.-Y.; Zhong, Q.-F.; Ye, S.-L. Effect of sulfur impurity on coke reactivity and its mechanism. Trans. Nonferrous Met. Soc. China 2014, 24, 3702–3709. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, J.; Zhong, Q.; Li, F.; Zhang, H.; Li, J. A Real-Time Mathematical Model for the Two-Dimensional Temperature Field of Petroleum Coke Calcination in Vertical Shaft Calciner. JOM 2016, 68, 2149–2159. [Google Scholar] [CrossRef]
- Yu, X.; Yu, D.; Yu, G.; Liu, F.; Han, J.; Wu, J.; Xu, M. Temperature-resolved evolution and speciation of sulfur during pyrolysis of a high-sulfur petroleum coke. Fuel 2021, 295, 120609. [Google Scholar] [CrossRef]
- Phillips, C.R.; Chao, K.S. Desulphurization of Athabasca petroleum coke by (a) chemical oxidation and (b) solvent extraction. Fuel 1977, 56, 70–72. [Google Scholar] [CrossRef]
- Agarwal, P.; Sharma, D. Studies on the Desulfurization of Petroleum Coke by Organorefining and Other Chemical and Biochemical Techniques Under Milder Ambient Pressure Conditions. Pet. Sci. Technol. 2011, 29, 1482–1493. [Google Scholar] [CrossRef]
- Nomura, N.; Takada, M.; Okada, H.; Shinohara, Y.; Nakajima-Kambe, T.; Nakahara, T.; Uchiyama, H. Identification and functional analysis of genes required for desulfurization of alkyl dibenzothiophenes of Mycobacterium sp. G3. J. Biosci. Bioeng. 2005, 100, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, T.; Izumi, Y. Microbial Desulfurization of Organic Sulfur Compounds in Petroleum. Biosci. Biotechnol. Biochem. 1999, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Huang, J.-J.; Li, J.-Z.; Li, G.; Zhao, J.-T.; Fang, Y.-t. Insight into transformation of sulfur species during KOH activation of high sulfur petroleum coke. Fuel 2018, 215, 258–265. [Google Scholar] [CrossRef]
- Wang, M.; Yang, A.; Wang, M.; Zhang, X.; Zhai, Y. Desulfurization of Petroleum Coke via Alkali Calcination. Adv. Mater. Res. 2014, 997, 526–529. [Google Scholar] [CrossRef]
- Askari, H.; Khorasheh, F.; Soltanali, S.; Tayyebi, S. Desulfurization of high sulfur petroleum coke by molten caustic leaching. Egypt. J. Pet. 2019, 28, 225–231. [Google Scholar] [CrossRef]
- Zhu, H.; Yao, Y.; Wang, R. Comparative Study of the Effects of Various Activation Methods on the Desulfurization Performance of Petroleum Coke. Aerosol Air Qual. Res. 2020, 20, 100479. [Google Scholar] [CrossRef]
- Cao, S.; Chen, Z.; Ma, W.; Zhang, H. Vacuum-assisted and alkali roasting for desulfurization of petroleum coke. J. Clean. Prod. 2022, 332, 130052. [Google Scholar] [CrossRef]
- GB/T 2001–2013; Coke—Determination of Proximate Analysis. Standardization Administration of China: Beijing, China, 2013.
- Pintowantoro, S.; Setiawan, M.A.; Abdul, F. Study of variation grain size in desulfurization process of calcined petroleum coke. AIP Conf. Proc. 2018, 1945, 020035. [Google Scholar]
- Li, M.; Yang, J.; Zhang, Q.; Chang, H.; Sun, H. XPS study on transformation of N- and S-functional groups during pyrolysis of high sulfur New Zealand coal. J. Fuel Chem. Technol. 2013, 41, 1287–1293. [Google Scholar]
- Zhang, C.; Yan, A.; Guo, H.; Lei, Y.; Hao, H.; Liu, F. DFT combined with XPS to investigate sulfur removal and sulfur-fixation mechanisms of copper oxide during coal pyrolysis and semi-coke combustion. Fuel 2022, 317, 123482. [Google Scholar] [CrossRef]
- Lichtman, D.; Craig, J.H.; Sailer, V.; Drinkwine, M. AES and XPS spectra of sulfur in sulfur compounds. Appl. Surf. Sci. 1981, 7, 325–331. [Google Scholar] [CrossRef]
- Kelemen, S.R.; George, G.N.; Gorbaty, M.L. Direct determination and quantification of organic sulfur forms by X-ray photoelectron spectroscopy (XPS) and sulfur k-edge absorption spectroscopy. Fuel Process. Technol. 1990, 24, 425–429. [Google Scholar] [CrossRef]
- Bejarano, C.; Jia, C.; Chung, K. Mechanistic Study of the Carbothermal Reduction of Sulfur Dioxide with Oil Sand Fluid Coke. Ind. Eng. Chem. Res. 2003, 42, 3731–3739. [Google Scholar] [CrossRef]
- Ma, L.-L.; Qin, Z.-H.; Zhang, L.; Liu, X.; Chen, H. Peak fitting methods and parameter settings in XPS analysis for organic sulfur in coal. J. Fuel Chem. Technol. 2014, 42, 277–283. [Google Scholar]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]

| Industrial Analysis (wt.%) | Elemental Analysis (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | Qcal/g | C | H | O | N | S |
| 0.36 | 0.38 | 9.9 | 89.36 | 8173.6 | 86.5 | 2.8 | 1.4 | 1.2 | 7.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, P.; Chen, Z.; Chen, X.; Ma, W. Deep Desulfurization of High-Sulfur Petroleum Coke via Alkali Catalytic Roasting Combined with Ultrasonic Oxidation. Materials 2024, 17, 2609. https://doi.org/10.3390/ma17112609
Luo P, Chen Z, Chen X, Ma W. Deep Desulfurization of High-Sulfur Petroleum Coke via Alkali Catalytic Roasting Combined with Ultrasonic Oxidation. Materials. 2024; 17(11):2609. https://doi.org/10.3390/ma17112609
Chicago/Turabian StyleLuo, Pen, Zhengjie Chen, Xiuhua Chen, and Wenhui Ma. 2024. "Deep Desulfurization of High-Sulfur Petroleum Coke via Alkali Catalytic Roasting Combined with Ultrasonic Oxidation" Materials 17, no. 11: 2609. https://doi.org/10.3390/ma17112609





