Fenton-like Degradation of Methylene Blue on Attapulgite Clay Composite by Loading of Iron–Oxide: Eco-Friendly Preparation and Its Catalytic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Fabrication of Attapulgite Iron–Oxide Composite
2.3. Characterization
2.4. Batch Degradation
3. Result and Discussion
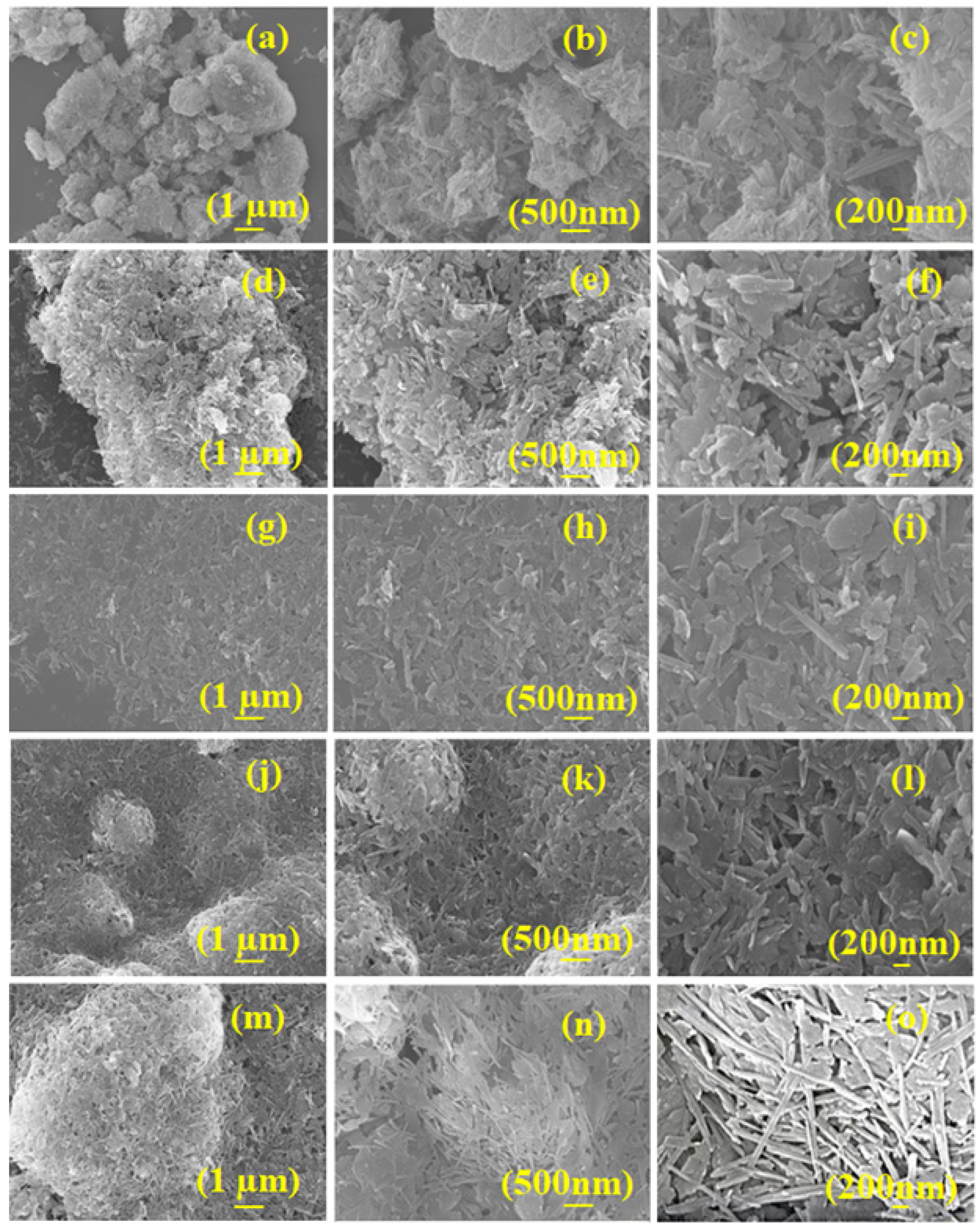
3.1. Structural Elucidation
3.2. Specific Surface Area Analysis for ATP Composites
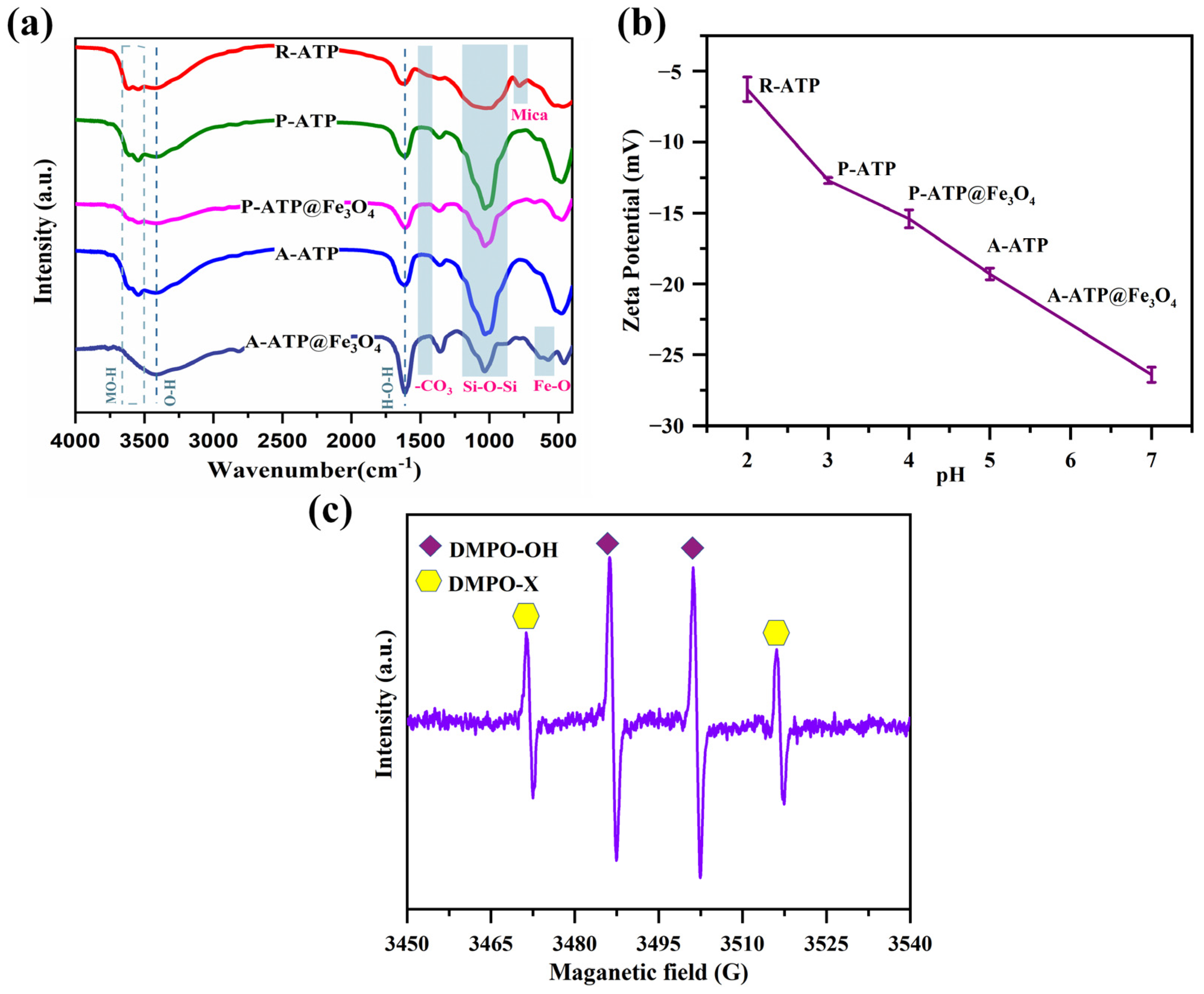
3.3. Surface Analysis
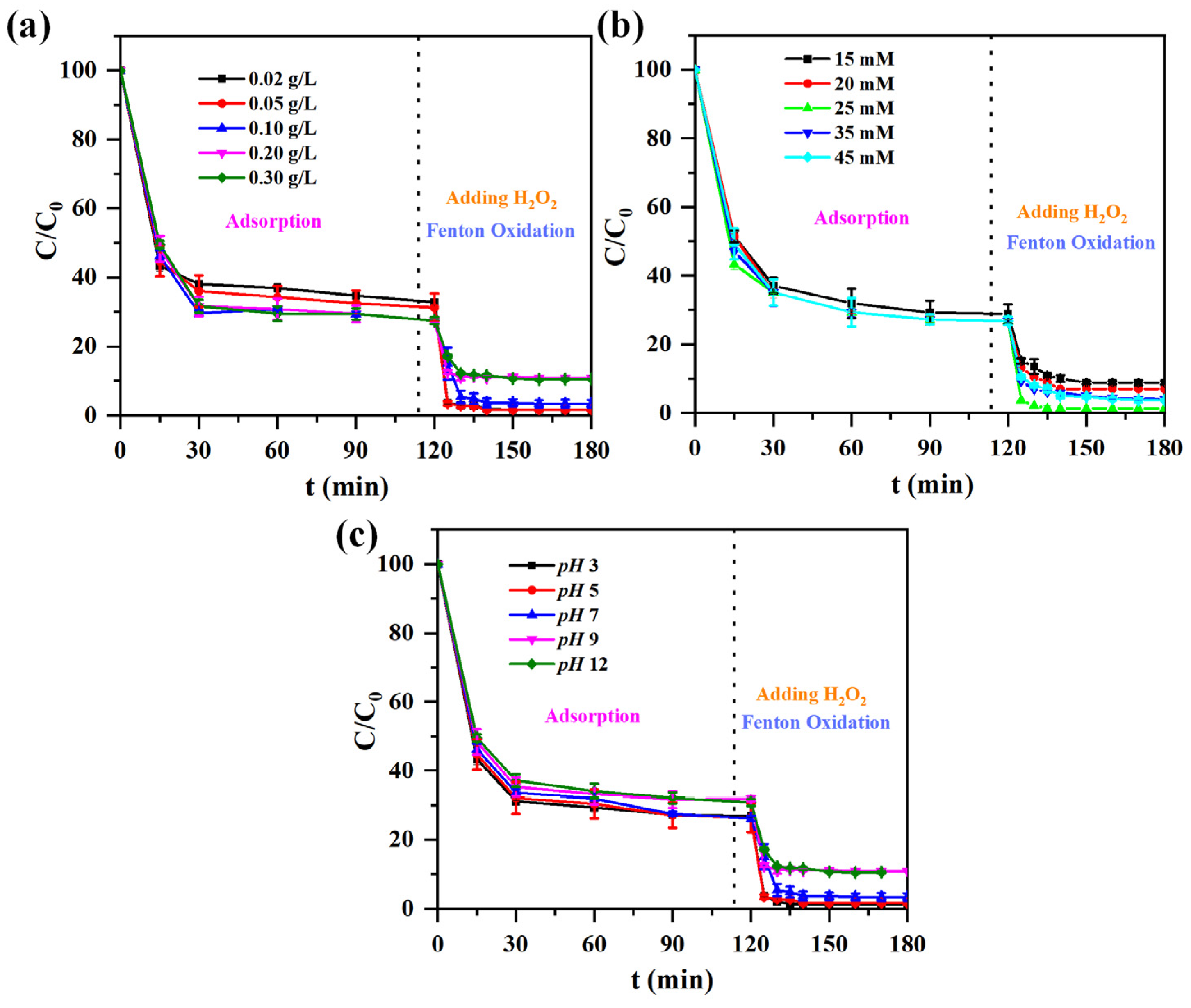
3.4. Adsorption and Degradation
3.5. Catalytic Activity
3.6. Degradation Mechanism
3.7. Reusability
3.8. Method Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mamajonova, N.; Oydin, M.; Usmonali, T.; Olimjon, A.; Madina, A.; Margʻuba, M. The role of green spaces in urban planning enhancing sustainability and quality of life. Hold. Reason. 2024, 2, 346–358. [Google Scholar]
- Mundetia, N.; Sharma, D.; Sharma, A. Groundwater sustainability assessment under climate change scenarios using integrated modelling approach and multi-criteria decision method. Ecol. Modell. 2024, 487, 110544. [Google Scholar] [CrossRef]
- Sabale, R.; Venkatesh, B.; Jose, M. Sustainable water resource management through conjunctive use of groundwater and surface water: A review. Innov. Infrastruct. Solut. 2023, 8, 17. [Google Scholar] [CrossRef]
- Sahoo, S.; Goswami, S. Theoretical framework for assessing the economic and environmental impact of water pollution: A detailed study on sustainable development of India. J. Environ. Sustain. 2024, 4, 23–34. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Oki, T. Water pricing reform for sustainable water resources management in China’s agricultural sector. Agric. Manag. Water Qual. 2023, 275, 108045. [Google Scholar] [CrossRef]
- Manny, L. Socio-technical challenges towards data-driven and integrated urban water management: A socio-technical network approach. Sustain. Cities Soc. 2023, 90, 104360. [Google Scholar] [CrossRef]
- Taiwo, R.; Ben Seghier, M.E.A.; Zayed, T. Toward sustainable water infrastructure: The state-of-the-art for modeling the failure probability of water pipes. Water Resour. Res. 2023, 59, e2022WR033256. [Google Scholar] [CrossRef]
- Wan Rosely, W.I.H.; Voulvoulis, N. Systems thinking for the sustainability transformation of urban water systems. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1127–1147. [Google Scholar] [CrossRef]
- Sharma, A.; Rasheed, S.; Mangla, D.; Choudhry, A.; Shukla, S.; Chaudhry, S.A. Cobalt ferrite incorporated ocimum sanctum nanocomposite matrix as an interface for adsorption of organic dyes: A sustainable alternative. ChemistrySelect 2023, 8, e202203709. [Google Scholar] [CrossRef]
- Sun, L.; Dong, H.; Lu, Y.; Zhang, L.; Yang, L.; Zhao, J.; Song, Y. A hydrate-based zero liquid discharge method for high-concentration organic wastewater: Resource recovery and water reclamation. NPJ Clean Water 2023, 6, 49. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Ma, S.; Tong, S. Ultrathin WS2 nanobowls-based hybrid aerogels for selective trapping of precious metals from electronic wastes and elimination of organic dyes. Chem. Eng. J. 2023, 451, 138539. [Google Scholar] [CrossRef]
- Pereira, L.; Alves, M. Dyes—Environmental impact and remediation. In Environmental Protection Strategies for Sustainable Development; Springer: Dordrecht, The Netherlands, 2012; pp. 111–162. [Google Scholar]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of textile dyes on health and ecosystem: A review of structure, causes, and potential solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef]
- Jack Clifton, I.; Leikin, J.B. Methylene blue. Am. J. Ther. 2003, 10, 289–291. [Google Scholar] [CrossRef]
- Kayabaşı, Y.; Erbaş, O. Methylene blue and its importance in medicine. Demiroglu Sci. Univ. Florence Nightingale J. Med. 2020, 6, 136–145. [Google Scholar] [CrossRef]
- Perendija, J.; Ljubić, V.; Popović, M.; Milošević, D.; Arsenijević, Z.; Đuriš, M.; Kovač, S.; Cvetković, S. Assessment of waste hop (Humulus lupulus) stems as a biosorbent for the removal of malachite green, methylene blue, and crystal violet from aqueous solution in batch and fixed-bed column systems: Biosorption process and mechanism. J. Mol. Liq. 2024, 394, 123770. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Nunes, M.I. Recent trends and developments in Fenton processes for industrial wastewater treatment—A critical review. Adv. Environ. Res. 2021, 197, 110957. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kumar, A. Research progress in synthesis strategies of magnesium oxide nanoparticles for water treatment applications. Environ. Sci. Water Res. Technol. 2024, 10, 577–587. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, T.; Dou, Z.; Yang, H. MnMg-MOF material photo-Fenton reaction degradation of methylene blue. Mater. Sci. Semicond. Process. 2024, 171, 108021. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R.; Ramesh, S.T. Degradation of dyes from aqueous solution by Fenton processes: A review. Environ. Sci. Pollut. Res. 2013, 20, 2099–2132. [Google Scholar] [CrossRef]
- Moradi, Z.; Ghorbani-Choghamarani, A.J.S.R. Fe3O4@SiO2@KIT-6@2-ATP@CuI as a catalyst for hydration of benzonitriles and reduction of nitroarenes. Sci. Rep. 2023, 13, 7645. [Google Scholar] [CrossRef]
- Alula, M.T.; Spende, H.; Aragaw, T.A.; Alene, A.N.; Aragaw, B.A.; Madiba, M.J.J. A highly stable silver nanoparticle loaded magnetic nanocomposite as a recyclable catalysts. J. Clust. Sci. 2023, 34, 2205–2214. [Google Scholar] [CrossRef]
- Ma, A.; Liao, X.; Qian, H.; Liu, J.; Liu, H.; Ren, S.J.M.S. An attapulgite composite with high degradation performance for Congo red without using oxidants and photoexcitation. Mater. Sci. Eeg. B 2023, 296, 116628. [Google Scholar] [CrossRef]
- Haden, W.L.; Schwint, I.A. Attapulgite: Its properties and applications. Ind. Eng. Chem. 1967, 59, 58–69. [Google Scholar] [CrossRef]
- Bradley, W.F. The structural scheme of attapulgite. Am. Mineral. 1940, 25, 405–410. [Google Scholar]
- Zhang, M.-H.; Dong, H.; Zhao, L.; Wang, D.-X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, F.; Zhang, W.; Li, R.; Zhong, H.; Zhao, Y.; Zhang, Y.; Wang, X. Photo degradation of methyl orange by attapulgite–SnO2–TiO2 nanocomposites. J. Hazard. Mater. 2009, 171, 294–300. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Zhang, Y.; Huang, H.; Ou, H.; Zhang, Y. In-situ adsorption-conversion recovery of heavy metal cadmium by natural clay mineral for multi-functional photocatalysis. Sep. Purif. Technol. 2023, 319, 124058. [Google Scholar] [CrossRef]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, J.; Chen, X.; Shi, X.; Wen, S. Degradation of tannic acid in heterogeneous Fenton system and persulfate system using iron (III) modified attapulgite: Influencing factors and degradation mechanism analysis. Desalination Water Treat. 2024, 317, 100160. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, J.; Chen, Z.; Mao, J.; Liu, J.; Zhang, Z.; Li, X.; Ren, S. Preparation, characterization, application and structure evolution of attapulgite: From nanorods to nanosheets. Appl. Surf. Sci. 2021, 565, 150398. [Google Scholar] [CrossRef]
- Biesinger, M.C.J.S.; Analysis, I. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Amin, M.O.; D’Cruz, B.; Madkour, M.; Al-Hetlani, E. Magnetic nanocomposite-based SELDI probe for extraction and detection of drugs, amino acids and fatty acids. Microchim. Acta 2019, 186, 503. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Handb. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Gupta, R.P.; Sen, S.K. Calculation of multiplet structure of core p-vacancy levels. II. Phys. Rev. B 1975, 12, 15. [Google Scholar] [CrossRef]
- Zaman, F.; Akhtar, N.; Guan, Y.; Huang, Y. Thermal degradation kinetic analysis and conversion of Aesculus indica to porous carbon. Ind. Crops Prod. 2020, 153, 112555. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Baxter, D.; Wang, X.; Mackinnon, I.D.R.; Xi, Y. Thermal behaviours of clay mixtures during brick firing: A combined study of in-situ XRD, TGA and thermal dilatometry. Constr. Build. Mater. 2021, 299, 124319. [Google Scholar] [CrossRef]
- De León, M.A.; Castiglioni, J.; Bussi, J.; Sergio, M. Catalytic activity of an iron-pillared montmorillonitic clay mineral in heterogeneous photo-Fenton process. Catal. Today 2008, 133–135, 600–605. [Google Scholar] [CrossRef]
- Shandilya, E.; Maiti, S. Self-regulatory micro-and macroscale patterning of ATP-mediated nanobioconjugate. ACS Nano 2023, 17, 5108–5120. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Portela, R.; Baeza, P.; Alcolea-Rodriguez, V.; Villarroel, M.; Ávila, P. Zeta potential as a tool for functional materials development. Catal. Today 2023, 423, 113862. [Google Scholar] [CrossRef]
- Teğin, İ.; Saka, C. Chemical and thermal activation of clay sample for improvement adsorption capacity of methylene blue. Int. J. Environ. Anal. Chem. 2023, 103, 4503–4514. [Google Scholar] [CrossRef]
- Xu, G.; Ma, C.; Wei, J.; Yang, X.; Wang, T.; Tan, C.; Yang, K.; Jiang, K.; Yu, G.; Zhang, C.J. Enhanced photocatalytic activity of 3D hierarchical RP/BP/BiOCOOH via oxygen vacancies and double heterojunctions. Chemosphere 2022, 300, 134485. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Qian, H.; Liu, H.; Ren, S. Degradation of malachite green by g-C3N4-modified magnetic attapulgite composites under visible-light conditions. Environ. Sci. Pollut. Res. 2023, 30, 96360–96375. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Wang, N.; Weng, Z.; Zou, X.; Huang, X. The synergistic activation of peroxymonosulfate for the degradation of Acid Scarlet GR by palygorskite/MnO2/Fe3O4 nanocomposites. Dalton Trans. 2023, 52, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Fan, K.; Niazi, N.K.; Gustave, W.; Li, H.; Wang, H.; Bolan, N.S.; Qin, J.; Qiu, R. The impact of rainwater-borne H2O2-induced Fenton process on root iron plaque formation and arsenic accumulation in rice. Sci. Total Environ. 2024, 908, 168300. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Fang, Z.; Tsang, P.E. Effect and mechanism of norfloxacin removal by Eucalyptus leaf extract enhanced the ZVI/H2O2 process. Sci. Total Environ. 2024, 914, 169820. [Google Scholar] [CrossRef] [PubMed]
- Ustun, S.O.; Buyukgungor, H. Comparative study of degradation of pharmaceutical and personal care products in wastewater by advanced oxidation processes: Fenton, UV/H2O2, UV/TiO2. Clean–Soil Air Water 2024, 52, 2300204. [Google Scholar] [CrossRef]
- Kaushik, R.; Sharma, K.; Siril, P.F.; Halder, A. Design and investigation of photoelectrochemical water treatment using self-standing Fe3O4/NiCo2O4 photoanode: In-situ H2O2 generation and fenton-like activation. Biochem. Eng. J. 2024, 479, 147575. [Google Scholar] [CrossRef]
- Freese, T.; Meijer, J.T.; Brands, M.B.; Alachouzos, G.; Stuart, M.C.; Tarozo, R.; Gerlach, D.; Smits, J.; Rudolf, P.; Reek, J.N.H.; et al. Iron oxide-promoted photochemical oxygen reduction to hydrogen peroxide (H2O2). EES Catal. 2024, 2, 262–275. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Z.; Liu, X.; Yang, G.; Jiang, C.; Pan, Z.; Liu, X.; Wang, Y.; Xing, B. Catalytic degradation of tetracycline hydrochloride using surface nitrogen-rich activated carbon based on polyaniline. Environ. Sci. Water Res. 2024, 10, 376–388. [Google Scholar] [CrossRef]
- Hamdi, S.; Gharbi-Khelifi, H.; Barreiro, A.; Mosbahi, M.; Cela-Dablanca, R.; Brahmi, J.; Sanjurjo, F.M.J.; Núñez-Delgado, A.; Issaoui, M.; Álvarez-Rodríguez, E. Tetracycline adsorption/desorption by raw and activated Tunisian clays. Adv. Environ. Res. 2024, 242, 117536. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-K.; Abbasi, Z.; Khushbakht, F.; Ullah, I.; Rehman, F.U.; Hafeez, M. Rapid pH-dependent photocatalytic degradation of methylene blue by CdS nanorods synthesized through hydrothermal process. Arab. J. Chem. 2024, 17, 105422. [Google Scholar] [CrossRef]
- Aboussabek, A.; Boukarma, L.; Qdhy, S.E.; Ousaa, A.; Zerbet, M.; Chiban, M. Experimental investigation, kinetics and statistical modeling of methylene blue removal onto Clay@Fe3O4: Batch, fixed bed column adsorption and photo-Fenton degradation studies. Case Stud. Chem. Environ. Eng. 2024, 9, 100580. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, K.; He, Y.; Li, Z.; Su, H.; Zhang, X.; Sun, Y.; Shi, W.; Ge, D. Fe3O4-based multifunctional nanospheres for amplified magnetic targeting photothermal therapy and Fenton reaction. ACS Biomater. Sci. Eng. 2018, 5, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Carbajo, J.; Silveira, J.; Pliego, G.; Zazo, J.; Casas, J. Increasing photo-Fenton process efficiency: The effect of high temperatures. Sep. Purif. Technol. 2021, 271, 118876. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Tian, Y.; Zhao, J.; Zhang, J.; Zuo, W. Precisely controlled fabrication of magnetic 3D γ-Fe2O3@ ZnO core-shell photocatalyst with enhanced activity: Ciprofloxacin degradation and mechanism insight. Biochem. Eng. J. 2017, 308, 377–385. [Google Scholar]
- Velo-Gala, I.; López-Peñalver, J.J.; Sánchez-Polo, M.; Rivera-Utrilla, J. Comparative study of oxidative degradation of sodium diatrizoate in aqueous solution by H2O2/Fe2+, H2O2/Fe3+, Fe (VI) and UV, H2O2/UV, K2S2O8/UV. Biochem. Eng. J. 2014, 241, 504–512. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, L.; Xiang, J.; Hu, S.; Su, S.; Zhang, A.J. The experimental and mechanism study of novel heterogeneous Fenton-like reactions using Fe3−xTixO4 catalysts for Hg0 absorption. Proc. Combust. Inst. 2015, 35, 2875–2882. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, J.; Chen, J.; Kong, L.; Lu, J.; Ding, F.; Xiong, Y.J.I.; Research, E.C. Fe2(MoO4)3 as an effective photo-Fenton-like catalyst for the degradation of anionic and cationic dyes in a wide pH range. Hold. Reason. 2013, 52, 13333–13341. [Google Scholar] [CrossRef]


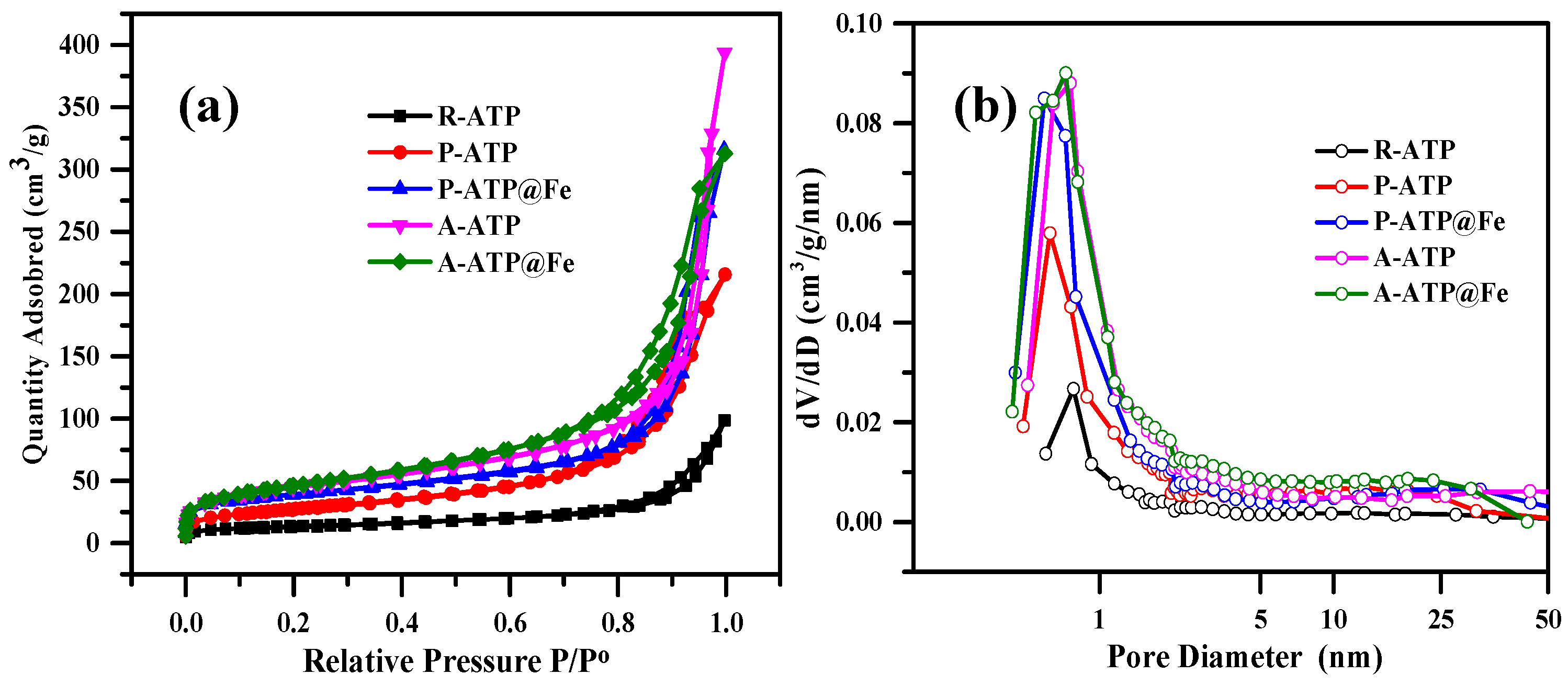





| No. | Sample | Surface Area (m2/g) | Total Pore Volume (mL/g) | Average Pore Size (nm) |
|---|---|---|---|---|
| 1 | R-ATP | 46.15 | 0.14 | 13.53 |
| 2 | P-ATP | 97.13 | 0.32 | 11.58 |
| 3 | P-ATP@Fe3O4 | 133.08 | 0.46 | 15.19 |
| 4 | A-ATP | 156.73 | 0.57 | 14.76 |
| 5 | A-ATP@Fe3O4 | 163.66 | 0.47 | 10.55 |
| % Atomic of Elements | ||||||
|---|---|---|---|---|---|---|
| Elements | % C | % O | % Al | % Si | % Mg | % Fe |
| R-ATP | 20.99 | 55.77 | 5.45 | 13.77 | 2.64 | 1.38 |
| P-ATP | 15.53 | 56.44 | 6.38 | 16.84 | 2.77 | 2.04 |
| P-ATP@Fe3O4 | 8.94 | 58.44 | 6.44 | 15.81 | 3.54 | 6.53 |
| A-ATP | 15.10 | 56.56 | 5.56 | 16.56 | 2.10 | 3.05 |
| A-ATP@Fe3O4 | 6.93 | 59.89 | 6.60 | 15.55 | 3.76 | 7.61 |
| Samples | qe,exp (mg/g) | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 | R2 | qe | k2 | R2 | ||
| R-ATP | 179.34 ± 1.45 | 155.96 | −0.0048 | 0.955 | 185.19 | 0.0006 | 0.981 |
| P-ATP | 320.35 ± 1.78 | 246.04 | −0.0048 | 0.967 | 322.58 | 0.0004 | 0.985 |
| P-ATP@Fe3O4 | 330.86 ± 1.56 | 272.77 | −0.0058 | 0.949 | 333.33 | 0.0004 | 0.986 |
| A-ATP | 342.39 ± 1.83 | 263.69 | −0.0053 | 0.961 | 344.83 | 0.0004 | 0.989 |
| A-ATP@Fe3O4 | 380.65 ± 1.89 | 291.21 | −0.0063 | 0.977 | 384.62 | 0.0004 | 0.993 |
| Catalyst | Reaction Conditions | Degradation Efficiency | Mechanism | Refs | |||
|---|---|---|---|---|---|---|---|
| Dyes | [Dyes] (mg L−1) | [Catalyst] (g L−1) | [H2O2] (mM) | ||||
| 3D γ-Fe2O3@ZnO | CIP | 15.0 | 1.25 | 35 | 18.30% (30 min) | Photocatalysis | [58] |
| Fe2+/H2O2 | MB | 50 | 1.5 | 35.3 | 99% (40 min) | Fenton-like | [59] |
| Fe3−xTixO4 | MB | 100 | 3.0 | 300 | 74.40% (100 min) | Adsorption Fenton-like | [60] |
| Fe2(MoO4)3 | MB | 100 | 1.0 | 18 | 91.3% (30 min) | Photo-Fenton-like | [61] |
| AATP@Fe3O4 | MB | 100 | 0.1 | 100 | 99.6% (20 min) | Adsorption Fenton-like | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karim, N.; Kyawoo, T.; Jiang, C.; Ahmed, S.; Tian, W.; Li, H.; Feng, Y. Fenton-like Degradation of Methylene Blue on Attapulgite Clay Composite by Loading of Iron–Oxide: Eco-Friendly Preparation and Its Catalytic Activity. Materials 2024, 17, 2615. https://doi.org/10.3390/ma17112615
Karim N, Kyawoo T, Jiang C, Ahmed S, Tian W, Li H, Feng Y. Fenton-like Degradation of Methylene Blue on Attapulgite Clay Composite by Loading of Iron–Oxide: Eco-Friendly Preparation and Its Catalytic Activity. Materials. 2024; 17(11):2615. https://doi.org/10.3390/ma17112615
Chicago/Turabian StyleKarim, Naveed, Tin Kyawoo, Chao Jiang, Saeed Ahmed, Weiliang Tian, Huiyu Li, and Yongjun Feng. 2024. "Fenton-like Degradation of Methylene Blue on Attapulgite Clay Composite by Loading of Iron–Oxide: Eco-Friendly Preparation and Its Catalytic Activity" Materials 17, no. 11: 2615. https://doi.org/10.3390/ma17112615
APA StyleKarim, N., Kyawoo, T., Jiang, C., Ahmed, S., Tian, W., Li, H., & Feng, Y. (2024). Fenton-like Degradation of Methylene Blue on Attapulgite Clay Composite by Loading of Iron–Oxide: Eco-Friendly Preparation and Its Catalytic Activity. Materials, 17(11), 2615. https://doi.org/10.3390/ma17112615









