Antipathogenic Applications of Copper Nanoparticles in Air Filtration Systems
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
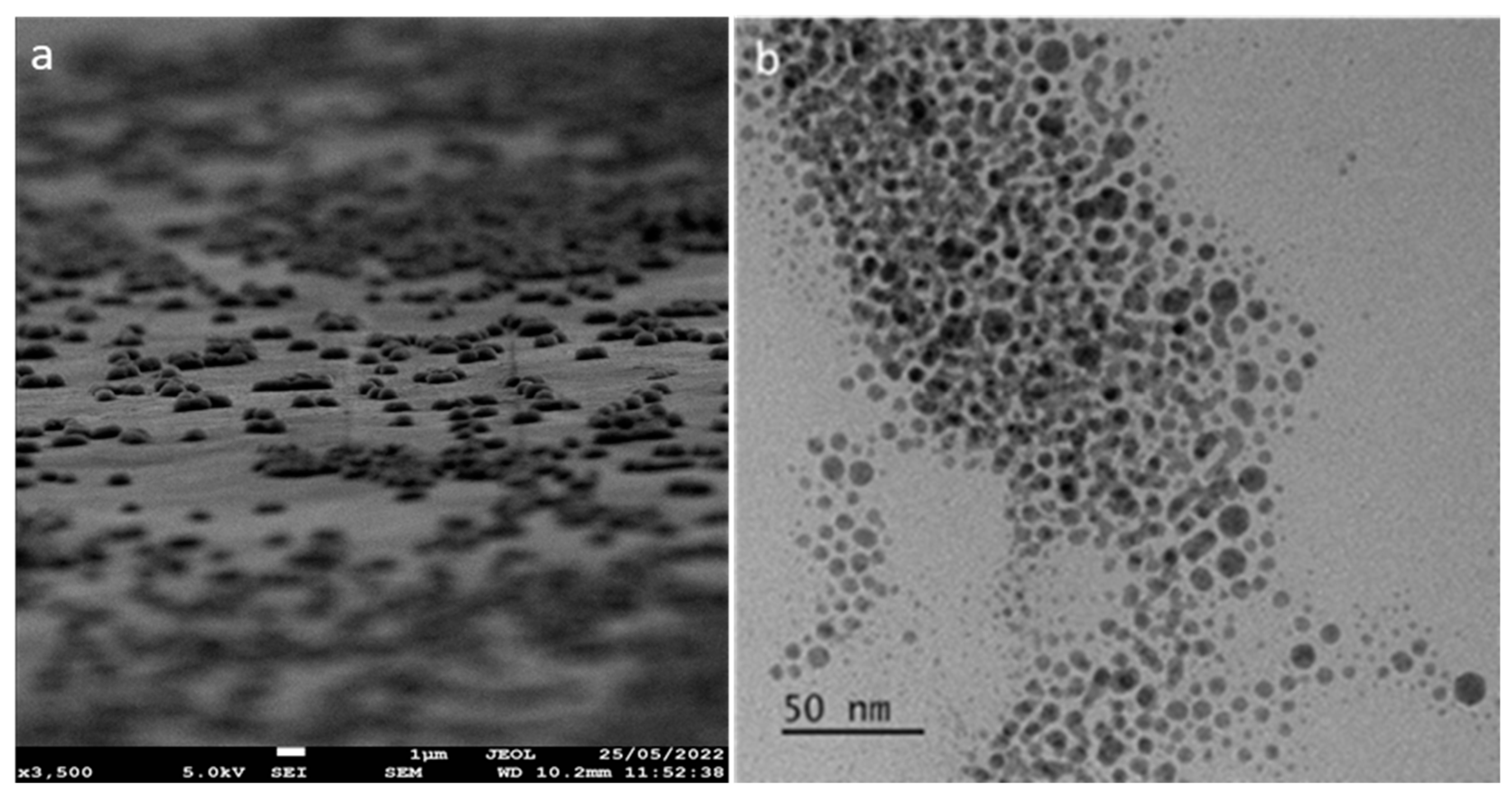
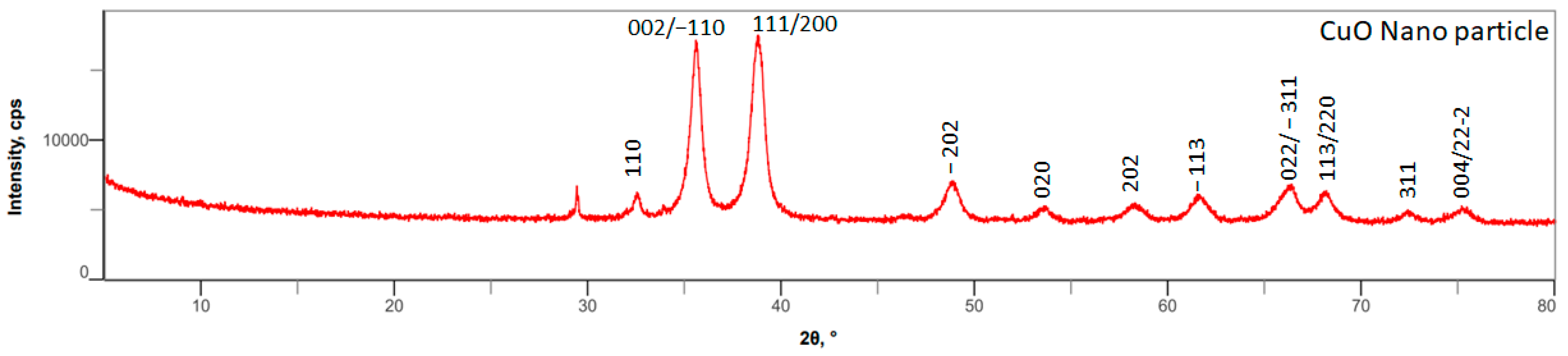
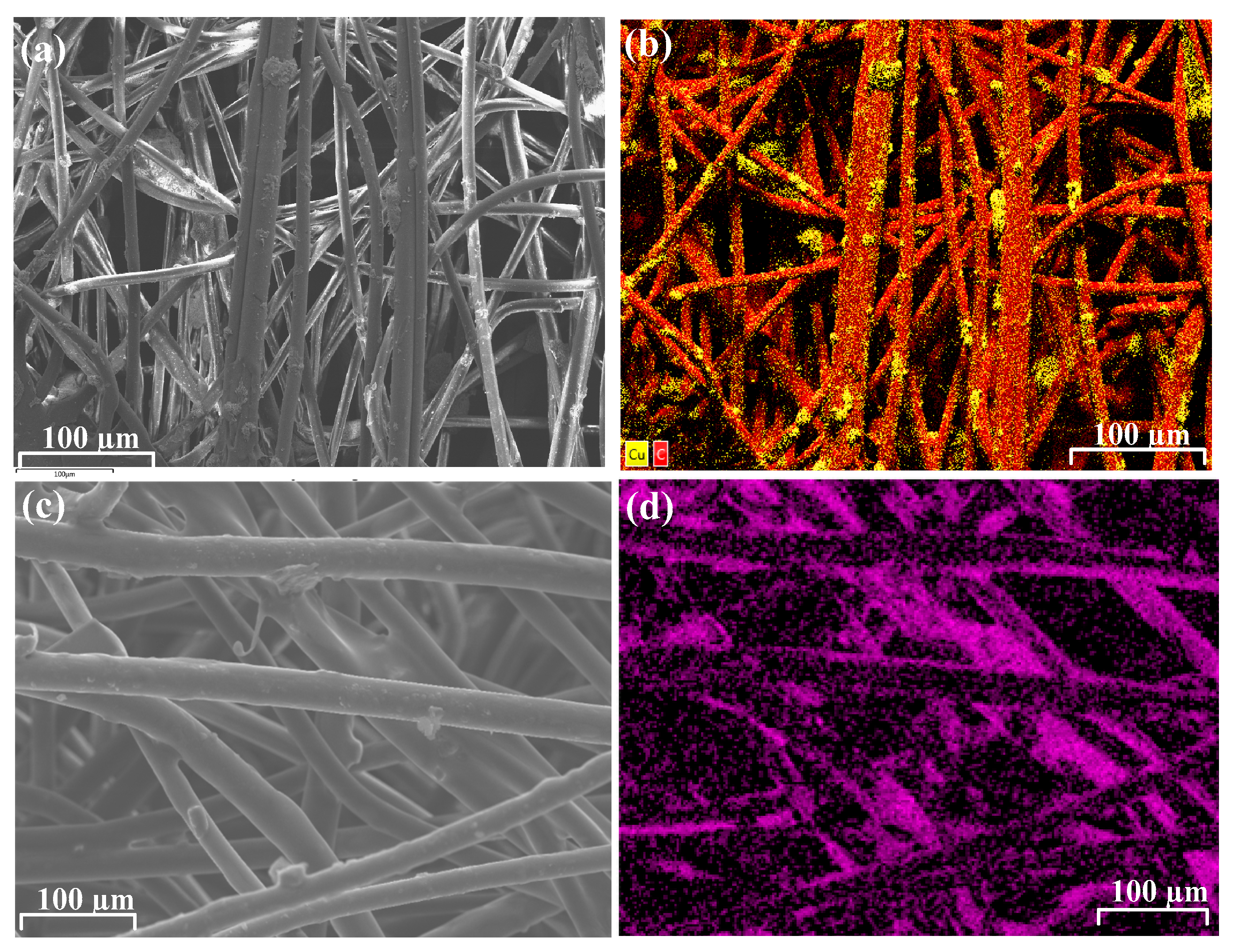
3.1. Synthesis and Characterization of Lysine–Copper Oxide-Coated Polymeric Air Filtration Media Fibers
3.2. Evaluation of Copper Leaching from Polymeric Fibers
3.3. Virucidal Activity Assessments of Polymeric Fibers
3.4. Antibacterial Activity Assessment of Polymeric Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yousefimashouf, M.; Yousefimashouf, R.; Alikhani, M.S.; Hashemi, H.; Karami, P.; Rahimi, Z.; Hosseini, S.M. Evaluation of the Bacterial Contamination of Face Masks Worn by Personnel in a Center of COVID 19 Hospitalized Patients: A Cross-Sectional Study. New Microbes New Infect. 2023, 52, 101090. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, C.; Guo, J.; Hou, J.; Guo, X.; Zhang, H.; Tan, J.; Li, M.; Li, X.; Zhu, H. Global Meta-Analysis of Airborne Bacterial Communities and Associations with Anthropogenic Activities. Environ. Sci. Technol. 2022, 56, 9891–9902. [Google Scholar] [CrossRef]
- Fennelly, K.P. Particle Sizes of Infectious Aerosols: Implications for Infection Control. Lancet Respir. Med. 2020, 8, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Xu, Y.; Yarlagadda, T.; Schuetz, M.; Spann, K.; Yarlagadda, P. Antiviral and Antibacterial Nanostructured Surfaces with Excellent Mechanical Properties for Hospital Applications. ACS Biomater. Sci. Eng. 2020, 6, 3608–3618. [Google Scholar] [CrossRef]
- Li, B.; Wang, D.; Lee, M.M.S.; Wei, W.; Tan, Q.; Zhao, Z.; Huang, X. Fabrics Attached with Highly Efficient Aggregation-Induced Emission Photosensitizer: Toward Self-Antiviral Personal Protective Equipment. ACS Nano 2021, 15, 13857–13870. [Google Scholar] [CrossRef]
- Keum, H.; Kim, D.; Whang, C.; Kang, A.; Lee, S.; Na, W.; Jon, S. Impeding the Medical Protective Clothing Contamination by a Spray Coating of Trifunctional Polymers. ACS Omega 2022, 7, 10526–10538. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Lloyd, K.; Oaten, L.C.; Andreeva, D.V.; Carr, C.; Farmery, A.D.; Kim, I.; Novoselov, K.S. Sustainable Personal Protective Clothing for Healthcare Applications: A Review. ACS Nano 2020, 14, 12313–12340. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Barbanera, M.; Carota, E.; Crognale, S.; Marconi, M.; Rossi, S.; Rubino, G.; Scungio, M.; Taborri, J.; Calabrò, G. Polymer Materials for Respiratory Protection: Processing, End Use, and Testing Methods. ACS Appl. Polym. Mater. 2021, 3, 531–548. [Google Scholar] [CrossRef]
- Shi, S.; Si, Y.; Li, Z.; Meng, S.; Zhang, S.; Wu, H.; Zhi, C.; Io, W.F.; Ming, Y.; Wang, D.; et al. An Intelligent Wearable Filtration System for Health Management. ACS Nano 2023, 17, 7035–7046. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.; Luijten, B.J.; Li, S.; De Moraes, A.C.M.; Silvaroli, A.J.; Wallace, S.G.; Hui, J.; Downing, J.R.; Shull, K.R.; Hersam, M.C. Sterilizable and Reusable UV-Resistant Graphene–Polyurethane Elastomer Composites. ACS Appl. Mater. Interfaces 2022, 14, 53241–53249. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.P.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. COVID-19 Pandemic Repercussions on the Use and Management of Plastics. Environ. Sci. Technol. 2020, 54, 7760–7765. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Mabury, S.A. Single-Use Face Masks as a Potential Source of Synthetic Antioxidants to the Environment. Environ. Sci. Technol. Lett. 2021, 8, 651–655. [Google Scholar] [CrossRef]
- Teo, J.C.M.; Kng, J.; Periaswamy, B.; Liu, S.; Lim, P.C.; Lee, C.E.; Tan, B.H.; Loh, X.J.; Ni, X.; Tiang, D.; et al. Exploring Reusability of Disposable Face Masks: Effects of Disinfection Methods on Filtration Efficiency, Breathability, and Fluid Resistance. Glob. Chall. 2021, 5, 2100030. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, N.; Haag, A.; Brang-Lamprecht, R.; Gebel, J.; Essert, S.M.; Kistemann, T.; Exner, M.; Mutters, N.T.; Engelhart, S. Air Filtration as a Tool for the Reduction of Viral Aerosols. Sci. Total Environ. 2021, 772, 144956. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhu, Z.; Wang, H.; Chen, J.; Zhang, M.; Han, M.; Shen, Y.; Shuai, D. Photosensitized Electrospun Nanofibrous Filters for Capturing and Killing Airborne Coronaviruses under Visible Light Irradiation. Environ. Sci. Technol. 2022, 56, 4295–4304. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Gao, F.; Qin, M.; Yu, Y.; Zhao, Y.; Shao, T.; Cai, C.; Zhang, W.; Xie, B.; Xiong, Y.; et al. How to Make Personal Protective Equipment Spontaneously and Continuously Antimicrobial (Incorporating Oxidase-like Catalysts). ACS Nano 2022, 16, 7755–7771. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, D.P.; Lee, D.H. Mechanistic Antimicrobial Approach of Extracellularly Synthesized Silver Nanoparticles against Gram Positive and Gram Negative Bacteria. J. Hazard. Mater. 2013, 260, 878–884. [Google Scholar] [CrossRef]
- Sadrhaghighi, A.; Sarvari, R.; Fakhri, E.; Poortahmasebi, V.; Sedighnia, N.; Torabi, M.; Mohammadzadeh, M.; Azhiri, A.H.; Eskandarinezhad, M.; Moharamzadeh, K.; et al. Copper-Nanoparticle-Coated Melt-Blown Facemask Filter with Antibacterial and SARS-CoV-2 Antiviral Ability. ACS Appl. Nano Mater. 2023, 6, 12849–12861. [Google Scholar] [CrossRef]
- Ammendolia, M.G.; De Berardis, B. Nanoparticle Impact on the Bacterial Adaptation: Focus on Nano-Titania. Nanomaterials 2022, 12, 3616. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, G.-H.; Ma, J.; Lee, S.; Kim, C.-S. Facile Nanostructured Zinc Oxide Coating Technique for Antibacterial and Antifouling Air Filters with Low Pressure Drop. J. Colloid Interface Sci. 2022, 612, 496–503. [Google Scholar] [CrossRef]
- Habib, Z.; Khan, S.J.; Ahmad, N.M.; Shahzad, H.M.A.; Jamal, Y.; Hashmi, I. Antibacterial Behaviour of Surface Modified Composite Polyamide Nanofiltration (NF) Membrane by Immobilizing Ag-Doped TiO2 Nanoparticles. Environ. Technol. 2019, 41, 3657–3669. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Baig, U.; Ansari, M.A. Fabrication of CuO Nanoparticles Immobilized Nanofiltration Composite Membrane for Dye/Salt Fractionation: Performance and Antibiofouling. J. Environ. Chem. Eng. 2022, 10, 106960. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Ku, J.W.K.; Zhang, H.; Salim, T.; Oo, G.; Zinn, A.A.; Boothroyd, C.; Tang, R.M.Y.; Gan, C.L.; Gan, Y.; et al. Copper-Nanoparticle-Coated Fabrics for Rapid and Sustained Antibacterial Activity Applications. ACS Appl. Nano Mater. 2022, 5, 12876–12886. [Google Scholar] [CrossRef]
- Goel, S.; Chen, F.; Cai, W. Synthesis and Biomedical Applications of Copper Sulfide Nanoparticles: From Sensors to Theranostics. Small 2013, 10, 631–645. [Google Scholar] [CrossRef]
- Laha, D.; Pramanik, A.; Chattopadhyay, S.; Dash, S.K.; Roy, S.; Pramanik, P.; Karmakar, P. Folic Acid Modified Copper Oxide Nanoparticles for Targeted Delivery in In Vitro and In Vivo Systems. RSC Adv. 2015, 5, 68169–68178. [Google Scholar] [CrossRef]
- Ammara, S.; Shamaila, S.; Zafar, N.; Bokhari, A.; Sabah, A. Nonenzymatic Glucose Sensor with High Performance Electrodeposited Nickel/Copper/Carbon Nanotubes Nanocomposite Electrode. J. Phys. Chem. Solids 2018, 120, 12–19. [Google Scholar] [CrossRef]
- Behzadinasab, S.; Chin, A.W.H.; Hosseini, M.H.; Poon, L.L.M.; Ducker, W.A. A Surface Coating That Rapidly Inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces 2020, 12, 34723–34727. [Google Scholar] [CrossRef]
- Jana, I.D.; Kumbhakar, P.; Banerjee, S.; Gowda, C.C.; Kedia, N.; Kuila, S.K.; Banerjee, S.; Das, N.C.; Das, A.K.; Manna, I.; et al. Copper Nanoparticle–Graphene Composite-Based Transparent Surface Coating with Antiviral Activity against Influenza Virus. ACS Appl. Nano Mater. 2020, 4, 352–362. [Google Scholar] [CrossRef]
- Hosseini, M.H.; Chin, A.W.H.; Behzadinasab, S.; Poon, L.L.M.; Ducker, W.A. Cupric Oxide Coating That Rapidly Reduces Infection by SARS-CoV-2 via Solids. ACS Appl. Mater. Interfaces 2021, 13, 5919–5928. [Google Scholar] [CrossRef]
- Liu, M.; Bauman, L.; Nogueira, C.L.; Aucoin, M.G.; Anderson, W.A.; Zhao, B. Antimicrobial Polymeric Composites for High-Touch Surfaces in Healthcare Applications. Curr. Opin. Biomed. Eng. 2022, 22, 100395. [Google Scholar] [CrossRef]
- Merkl, P.; Long, S.; McInerney, G.M.; Sotiriou, G.A. Antiviral Activity of Silver, Copper Oxide and Zinc Oxide Nanoparticle Coatings against SARS-CoV-2. Nanomaterials 2021, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, Y.; Sato, T.; Hayata, T.; Nagao, T.; Nakayama, M.; Nakayama, T.; Sugamata, R.; Suzuki, K. Novel Antiviral Characteristics of Nanosized Copper(I) Iodide Particles Showing Inactivation Activity against 2009 Pandemic H1N1 Influenza Virus. Appl. Environ. Microbiol. 2012, 78, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, Y.; Gao, D.; Ma, W.; Cheng-Zhu, J.; Jiang, X.; Lin, J.; Yang, F. Functionalization of Cellulosic Hydrogels with Cu2O@CuO Nanospheres: Toward Antifouling Applications. Carbohydr. Polym. 2022, 282, 119136. [Google Scholar] [CrossRef] [PubMed]
- Jardón-Maximino, N.; Cadenas-Pliego, G.; Ávila-Orta, C.A.; Comparán-Padilla, V.E.; Uribe, L.E.L.; Pérez-Álvarez, M.; Fernández, S.; De Jesús Sosa Santillán, G. Antimicrobial Property of Polypropylene Composites and Functionalized Copper Nanoparticles. Polymers 2021, 13, 1694. [Google Scholar] [CrossRef] [PubMed]
- Damm, C.; Münstedt, H.; Rösch, A. The Antimicrobial Efficacy of Polyamide 6/Silver-Nano- and Microcomposites. Mater. Chem. Phys. 2008, 108, 61–66. [Google Scholar] [CrossRef]
- Sierra-Ávila, R.; Pérez-Alvarez, M.; Valdez-Garza, J.; Avila-Orta, C.A.; Jiménez-Regalado, E.J.; Mata-Padilla, J.M.; Soto-Castruita, E.; Cadenas-Pliego, G. Synthesis and Thermomechanical Characterization of Nylon 6/Cu Nanocomposites Produced by an Ultrasound-Assisted Extrusion Method. Adv. Mat. Sci. Eng. 2018, 2018, 4792735. [Google Scholar] [CrossRef]
- Smith, N.; Raston, C.L.; Saunders, M.; Woodward, R. Synthesis of magnetic nanoparticles using spinning disc processing. In Technical Proceedings of the 2006 NSTI Nanotechnology Conference and Trade Show; Nano Science and Technology Institute: Washington, DC, USA, 2006; pp. 343–346. [Google Scholar]
- Cave, G.W.V.; Mundell, V.J. Coating Metal Oxide Particles. EP2825515A2, 15 March 2013. [Google Scholar]
- ISO 18184:2019; Textiles—Determination of Antiviral Activity of Textile Products. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/71292.html (accessed on 10 April 2024).
- Massart, R. Magnetic Fluids and Process For Obtaining Them. U.S. Patent 4329241, 11 May 1982. [Google Scholar]
- Cave, G.W.V. Reactor. U.S. Patent 10926236B2, 24 September 2021. [Google Scholar]
- ISO 17294-2:2023; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS). ISO: Geneva, Switzerland, 2023. Available online: https://www.iso.org/standard/82245.html (accessed on 10 April 2024).
- EN 14683:2019+AC:2019; Medical Face Masks Requirements and Test Methods. iTeh Standards: Etobicoke, ON, Canada, 2019.
- EN ISO 11737-1:2018; Sterilization of Health Care Products—Microbiological methods Part 1: Determination of a Population of Microorganisms on Products. iTeh Standards: Etobicoke, ON, Canada, 2018.
- ISO 22609:2004; Clothing for Protection against Infectious Agents—Medical Face Masks—Test Method for Resistance against Penetration by Synthetic Blood (Fixed Volume, Horizontally Projected). iTeh Standards: Etobicoke, ON, Canada, 2004.
- EN ISO 10993-10:2013; Biological Evaluation of Medical Devices—Part 10: Tests for Irritation and Skin Sensitization. iTeh Standards: Etobicoke, ON, Canada, 2013.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. iTeh Standards: Etobicoke, ON, Canada, 2009.
- ISO 20743:2021; Textiles—Determination of Antibacterial Activity of Textile Products. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/79819.html (accessed on 10 April 2024).
- ESCMID—European Society of Clinical Microbiology and Infectious Diseases 2008 Eucast: EUCAST. Available online: https://www.eucast.org/ (accessed on 10 April 2024).


| Virus Type | Filter Use | Test Condition | Virus Recovery Control (TCID50/Sample) | Antiviral Test (TCID50/Sample) | Contact Time | TCID50 (log10) | Mv | % Reduction |
|---|---|---|---|---|---|---|---|---|
| Influenza | HVAC | CNC-PP | N/A | (9.25 ± 7.91) × 104 | 7 h | 4.97 | 2.68 | 99.5 |
| Untreated control | (4.38 ± 2.06) × 107 | (1.71 ± 1.37) × 107 | 7 h | 7.64 | ||||
| SARS-CoV-2 | CNC-PP | N/A | (9.84 ± 4.22) × 103 | 2 h | 3.99 | 2.60 | 99.8 | |
| Untreated control | (3.94 ± 2.01) × 106 | (4.59 ± 1.88) × 106 | 2 h | 6.60 | ||||
| Influenza | Face mask | CNC-PE | N/A | (1.40 ± 0.715) × 104 | 7 h | 4.15 | 1.19 | 95.8 |
| Untreated control | (2.17 ± 0.64) × 105 | (3.30 ± 2.80) × 105 | 7 h | 5.34 | ||||
| SARS-CoV-2 | CNC-PE | N/A | (2.01 ± 1.22) × 104 | 2 h | 4.30 | 1.61 | 90 | |
| Untreated control | (8.15 ± 3.34) × 105 | (2.01 ± 1.22) × 105 | 2 h | 5.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekapothula, S.; Chrysanthou, E.; Hall, J.; Nekkalapudi, P.D.; McLean, S.; Cave, G.W.V. Antipathogenic Applications of Copper Nanoparticles in Air Filtration Systems. Materials 2024, 17, 2664. https://doi.org/10.3390/ma17112664
Mekapothula S, Chrysanthou E, Hall J, Nekkalapudi PD, McLean S, Cave GWV. Antipathogenic Applications of Copper Nanoparticles in Air Filtration Systems. Materials. 2024; 17(11):2664. https://doi.org/10.3390/ma17112664
Chicago/Turabian StyleMekapothula, Subbareddy, Elvina Chrysanthou, James Hall, Phani Durga Nekkalapudi, Samantha McLean, and Gareth W. V. Cave. 2024. "Antipathogenic Applications of Copper Nanoparticles in Air Filtration Systems" Materials 17, no. 11: 2664. https://doi.org/10.3390/ma17112664
APA StyleMekapothula, S., Chrysanthou, E., Hall, J., Nekkalapudi, P. D., McLean, S., & Cave, G. W. V. (2024). Antipathogenic Applications of Copper Nanoparticles in Air Filtration Systems. Materials, 17(11), 2664. https://doi.org/10.3390/ma17112664








