Use of Periodic Mesoporous Organosilica–Benzene Adsorbent for CO2 Capture to Reduce the Greenhouse Effect
Abstract
:1. Introduction
2. Materials and Methods
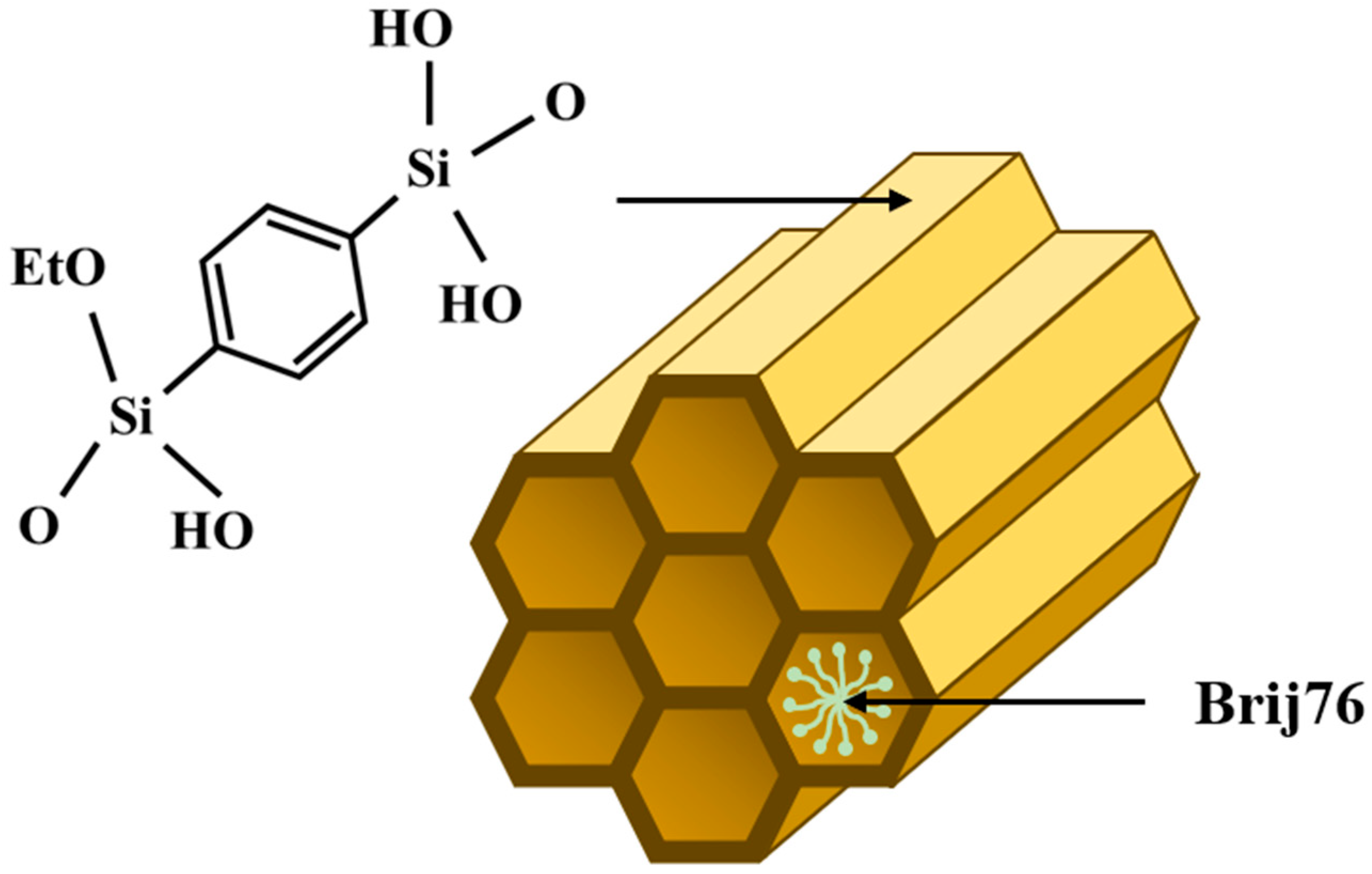
2.1. PMO–Benzene
2.2. Material Characterisation
2.3. Adsorption Isotherms
3. Results and Discussion
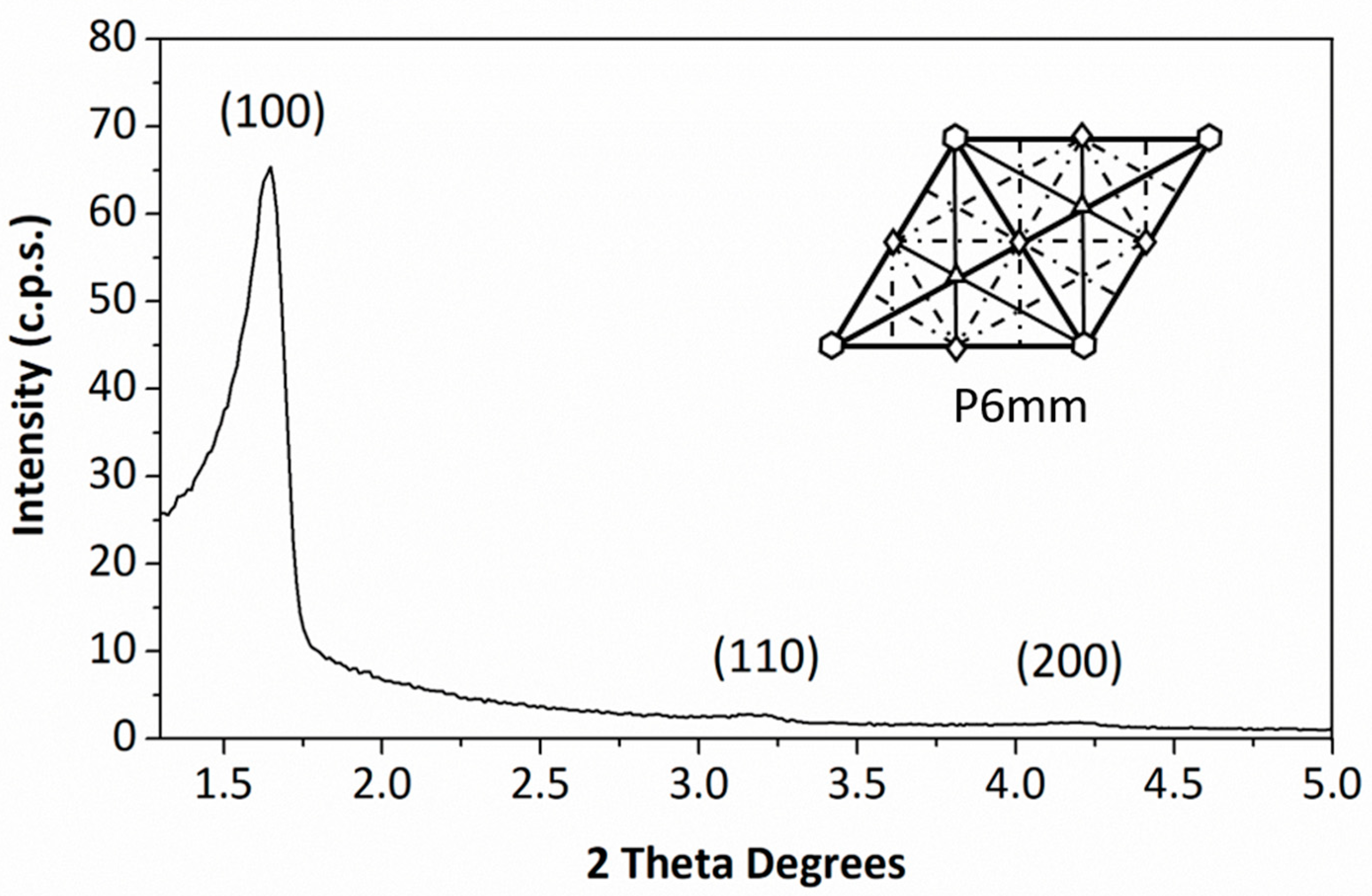
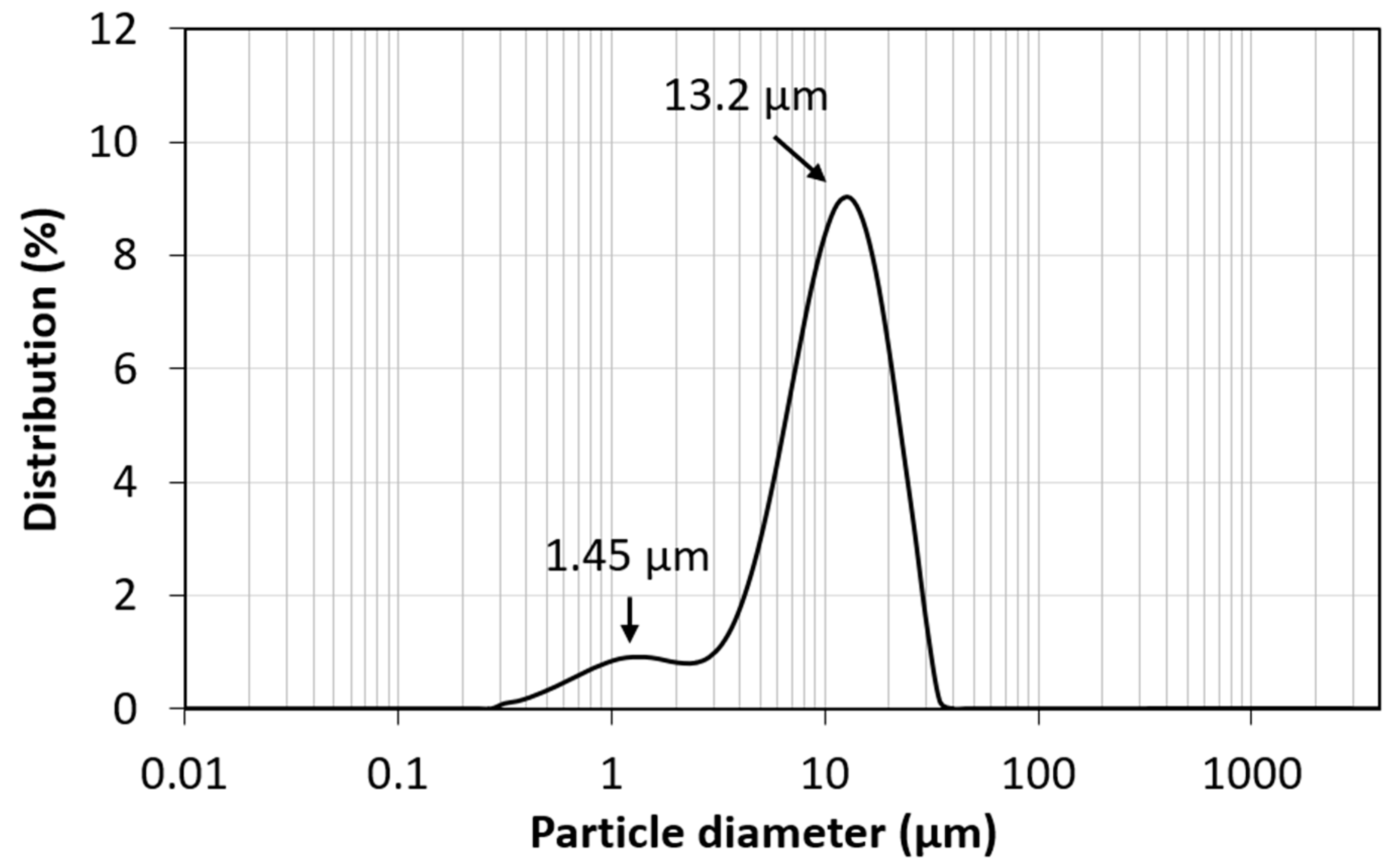
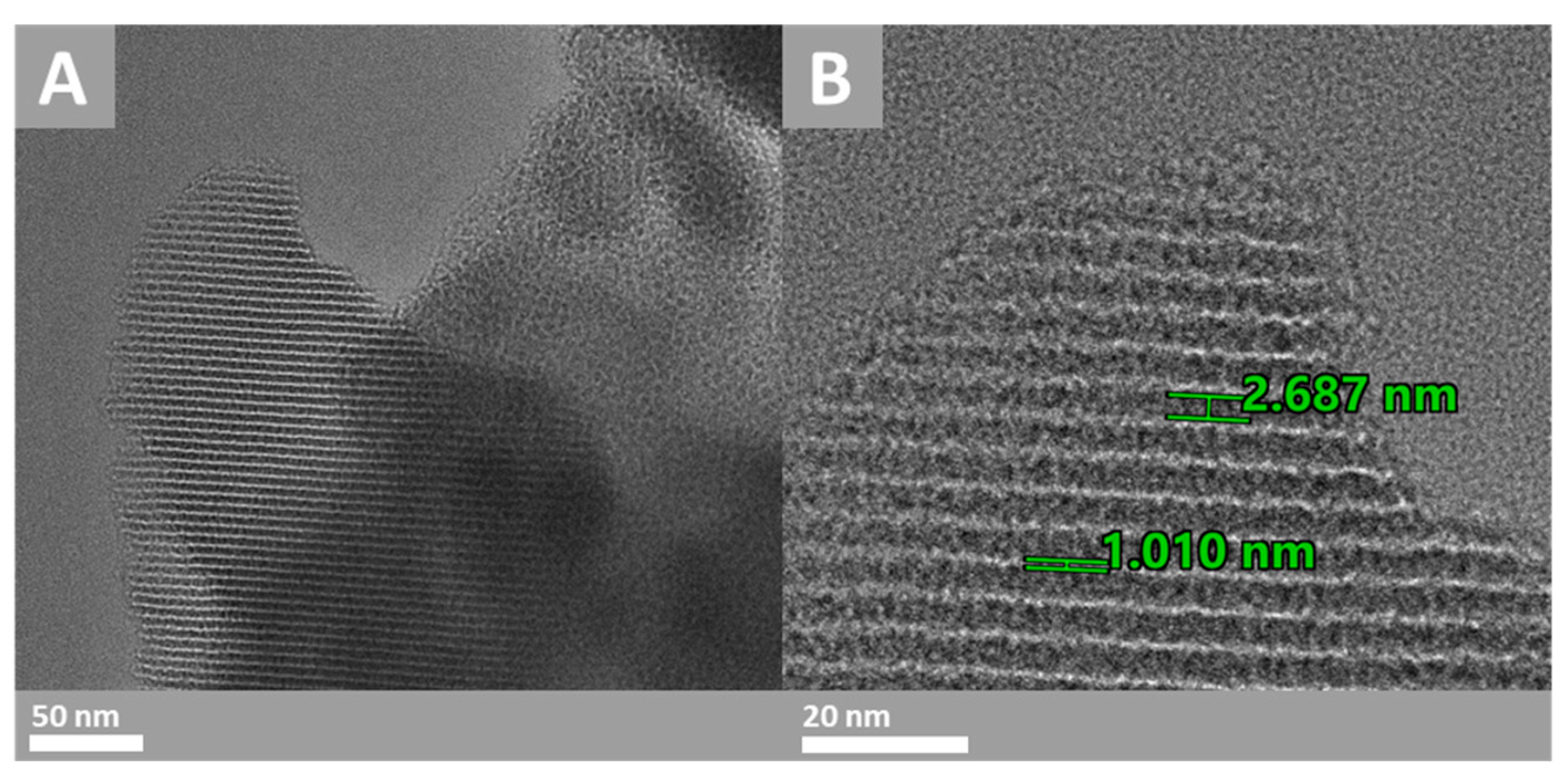
3.1. Characterization
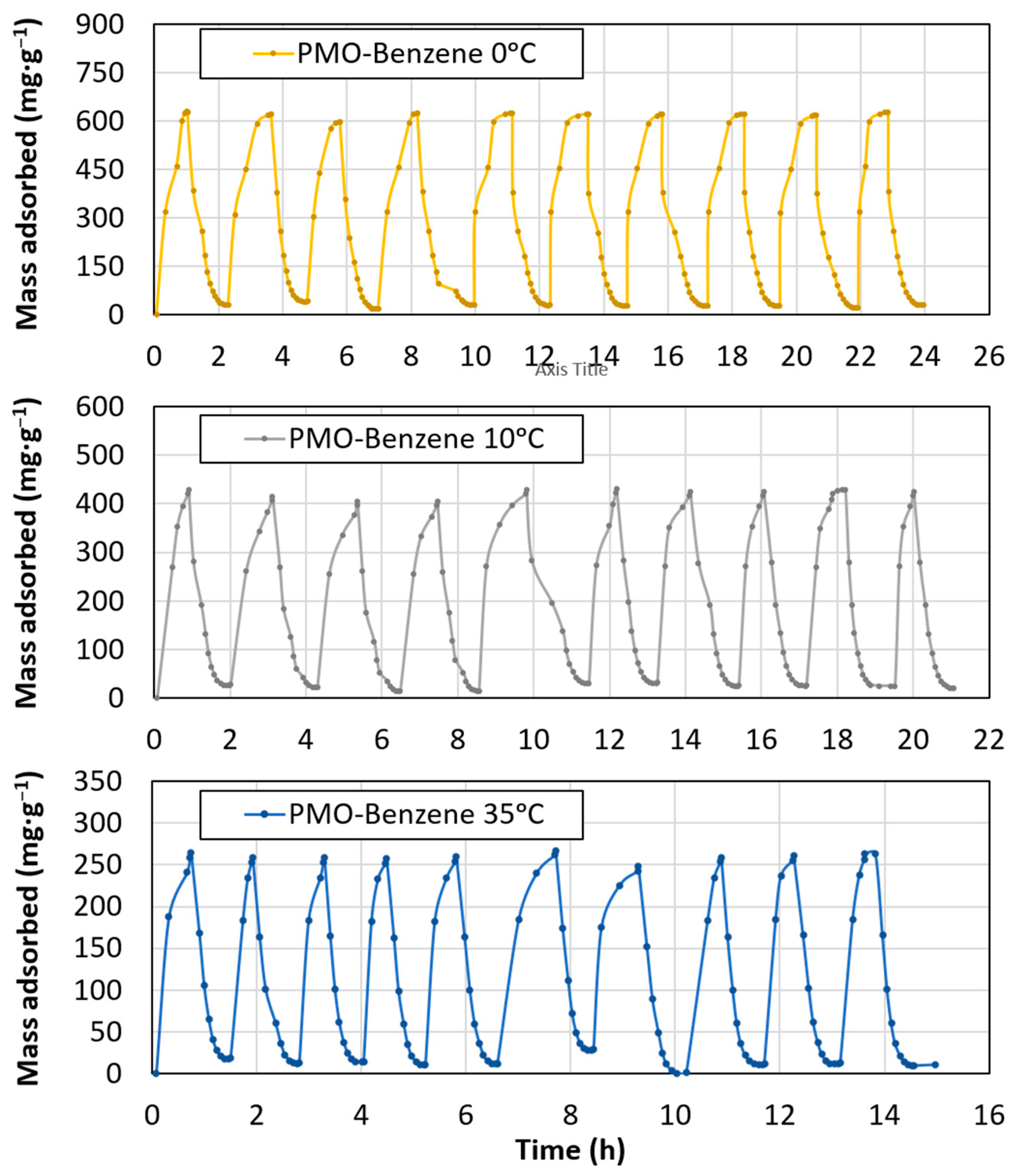
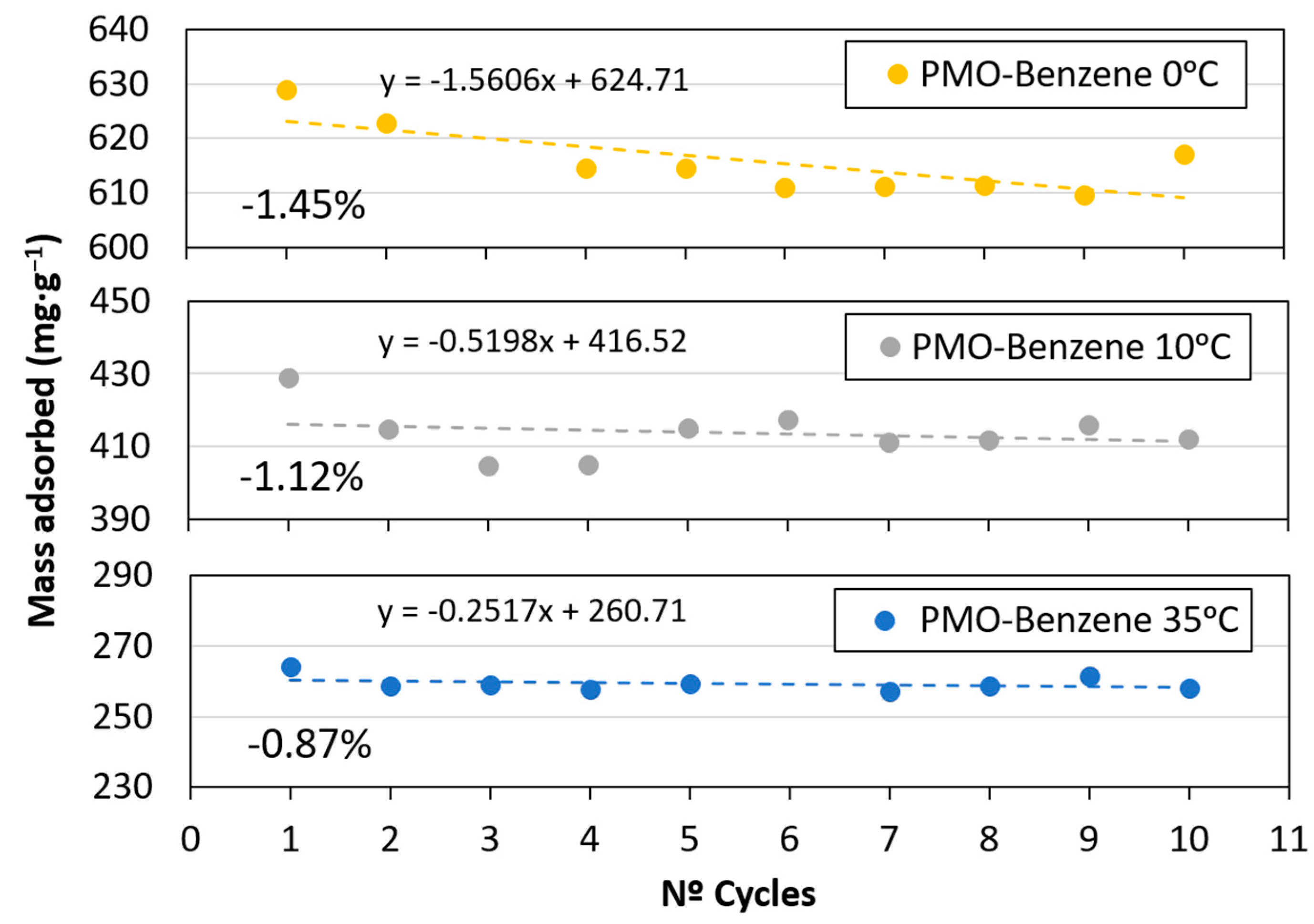
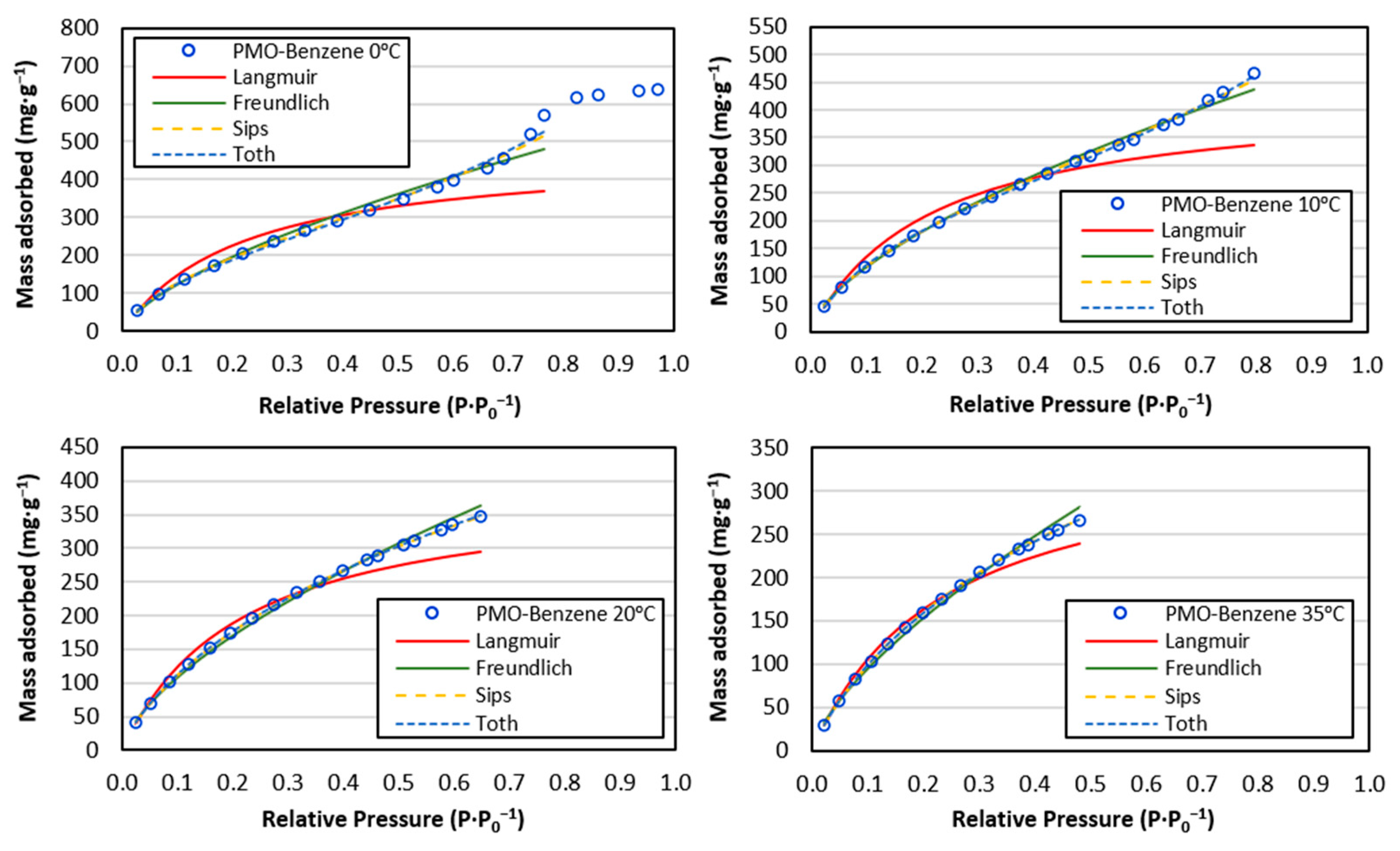
3.2. CO2 Adsorption
4. Conclusions
- The pore size was between 2.4 and 4.1 nm, while the total pore volume was 0.68 cm3·g−1 and SBET was 928 m2·g−1.
- All isotherms fitted the Freundlich model well. nf > 1 was very close to 1, indicating favorable and weak interactions.
- The Sips and Toth models improved the results obtained by other equations at temperatures between 0 °C and 20 °C, where multilayer formation occurred.
- The D-R and Temkin models showed a physical nature of adsorption (E < 8 kJ·mol−1).
- PMO–benzene featured the maximum adsorption (638.2 mg·g−1) at 0 °C and 34 atm.
- These results highlight that 0.43 g of PMO–benzene would be enough to reduce the CO2 level in 1 m3 of air to pre-industrial levels; 489.8 kg of PMO–benzene would be required to reduce the CO2 concentration of the volume of Wembley soccer stadium to pre-industrial levels.
- The maximum loss of adsorption capacity was 1.45% for the sample at 0 °C, after 10 adsorption–desorption cycles. Consequently, this material could be used in capture processes using changes in the pressure conditions.
- PMO–benzene could contribute to the development of CO2 capture and use (CCU) technology.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon Capture and Storage (CCS): The Way Forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- U.S. Department of Commerce, National Oceanic and Atmospheric Administration. Available online: https://www.noaa.gov/news-release/carbon-dioxide-now-more-than-50-higher-than-pre-industrial-levels#:~:text=PriortotheIndustrialRevolutionatmosphereforthousandsofyears (accessed on 24 October 2022).
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J.; et al. Global Carbon Budget 2021. Earth Syst. Sci. Data 2022, 14, 1917–2005. [Google Scholar] [CrossRef]
- Scripps Institution of Oceanography, UC San Diego. The Keeling Curve. Available online: https://keelingcurve.ucsd.edu (accessed on 24 October 2022).
- United Nations Paris Agreement. Available online: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 12 November 2020).
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D.C., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; IPCC: Geneva, Switzerland, 2018; ISBN 978-92-9169-151-7. [Google Scholar]
- Zhang, W.; Yang, Y.; Li, Y.; Li, F.; Luo, M. Recent Progress on Integrated CO2 Capture and Electrochemical Upgrading. Mater. Today Catal. 2023, 2, 100006. [Google Scholar] [CrossRef]
- Vega, L.F.; Bahamon, D.; Alkhatib, I.I.I. Perspectives on Advancing Sustainable CO2 Conversion Processes: Trinomial Technology, Environment, and Economy. ACS Sustain. Chem. Eng. 2024, 12, 5357–5382. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Alonso-Fariñas, B.; Vilches Arenas, L.F.; Navarrete, B. Carbon Capture and Utilization Technologies: A Literature Review and Recent Advances. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 41, 1403–1433. [Google Scholar] [CrossRef]
- Salih, H.A.; Alkhatib, I.I.I.; Zahra, M.A.; Vega, L.F. Diamine Based Hybrid-Slurry System for Carbon Capture. J. CO2 Util. 2023, 68, 102383. [Google Scholar] [CrossRef]
- Cox, M.; Mokaya, R. Ultra-High Surface Area Mesoporous Carbons for Colossal Pre Combustion CO2 Capture and Storage as Materials for Hydrogen Purification. Sustain. Energy Fuels 2017, 1, 1414–1424. [Google Scholar] [CrossRef]
- Zhu, B.; Huang, J.; Lu, J.; Zhao, D.; Lu, L.; Jin, S.; Zhou, Q. Worm-like Hierarchical Porous Carbon Derived from Bio-Renewable Lignin with High CO2 Capture Capacity. Int. J. Electrochem. Sci. 2017, 12, 11102–11107. [Google Scholar] [CrossRef]
- Chen, C.; Feng, N.; Guo, Q.; Li, Z.; Li, X.; Ding, J.; Wang, L.; Wan, H.; Guan, G. Template-Directed Fabrication of MIL-101(Cr)/Mesoporous Silica Composite: Layer-Packed Structure and Enhanced Performance for CO2 Capture. J. Colloid Interface Sci. 2018, 513, 891–902. [Google Scholar] [CrossRef]
- Kishor, R.; Ghoshal, A.K. APTES Grafted Ordered Mesoporous Silica KIT-6 for CO2 Adsorption. Chem. Eng. J. 2015, 262, 882–890. [Google Scholar] [CrossRef]
- Licciulli, A.; Notaro, M.; De Santis, S.; Terreni, C.; Kunjalukkal Padmanabhan, S. CO2 Capture on Amine Impregnated Mesoporous Alumina-Silica Mixed Oxide Spheres. Fuel Process. Technol. 2017, 166, 202–208. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Hou, X.J. Amine-Functionalized Metal Organic Framework as a Highly Selective Adsorbent for CO2 over CO. J. Phys. Chem. C 2012, 116, 19814–19821. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon Capture and Conversion Using Metal–Organic Frameworks and MOF-Based Materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; De Luna, P.; Daff, T.D.; Rother, J.; Liu, M.; Buchanan, W.; Hawari, A.I.; Woo, T.K.; Vaidhyanathan, R. A Single-Ligand Ultra-Microporous MOF for Precombustion CO2 Capture and Hydrogen Purification. Sci. Adv. 2015, 1, e1500421. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.; Jin, S.; Zhong, M.; Vu, A.T.; Lee, C.H. Sorption Capacity and Stability of Mesoporous Magnesium Oxide in Post-Combustion CO2 Capture. Mater. Chem. Phys. 2017, 198, 154–161. [Google Scholar] [CrossRef]
- Chowdhury, S.; Parshetti, G.K.; Balasubramanian, R. Post-Combustion CO2 Capture Using Mesoporous TiO2/Graphene Oxide Nanocomposites. Chem. Eng. J. 2015, 263, 374–384. [Google Scholar] [CrossRef]
- Liu, G.; Tatsuda, K.; Yoneyama, Y.; Tsubaki, N. Synthesis of Mesoporous Cerium Compound for CO2 Capture. E3S Web Conf. 2017, 22, 00106. [Google Scholar] [CrossRef]
- Quesada Carballo, L.; Perez Perez, M.d.R.; Cantador Fernández, D.; Caballero Amores, A.; Fernández Rodríguez, J.M. Optimum Particle Size of Treated Calcites for CO2 Capture in a Power Plant. Materials 2019, 12, 1284. [Google Scholar] [CrossRef]
- Cantador Fernandez, D.; Suescum Morales, D.; Jiménez, J.R.; Fernández-Rodriguez, J.M. CO2 Adsorption by Organohydrotalcites at Low Temperatures and High Pressure. Chem. Eng. J. 2022, 431, 134324. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Cantador-Fernández, D.; Jiménez, J.R.; Fernández, J.M. Mitigation of CO2 Emissions by Hydrotalcites of Mg3Al-CO3 at 0 °C and High Pressure. Appl. Clay Sci. 2021, 202, 105950. [Google Scholar] [CrossRef]
- Karimi, B.; Ganji, N.; Pourshiani, O.; Thiel, W.R. Periodic Mesoporous Organosilicas (PMOs): From Synthesis Strategies to Applications. Prog. Mater. Sci. 2022, 125, 100896. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, J.; Zhang, L.; Li, C. Functionalized Periodic Mesoporous Organosilicas for Catalysis. J. Mater. Chem. 2009, 19, 1945–1955. [Google Scholar] [CrossRef]
- Esquivel, D.; De Canck, E.; Jimenez-Sanchidrian, C.; Van Der Voort, P.; Romero-Salguero, F.J. Periodic Mesoporous Organosilicas as Catalysts for Organic Reactions. Curr. Org. Chem. 2014, 18, 1280–1295. [Google Scholar] [CrossRef]
- Huang, Y.; Yuan, P.; Wu, Z.; Yuan, X. Preparation of Surface-Silylated and Benzene-Bridged Ti-Containing Mesoporous Silica for Cyclohexene Epoxidation. J. Porous Mater. 2016, 23, 895–903. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Do Van, D.; Yonezawa, Y.; Saito, M.; Dohshi, S.; Kim, T.-H.; Matsuoka, M. Synthesis and Bifunctional Catalysis of Metal Nanoparticle-Loaded Periodic Mesoporous Organosilicas Modified with Amino Groups. RSC Adv. 2015, 5, 72653–72658. [Google Scholar] [CrossRef]
- López, M.; Esquivel, D.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.; Van Der Voort, P. A “one-step” Sulfonic Acid PMO as a Recyclable Acid Catalyst. J. Catal. 2015, 326, 139–148. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, X.; Chen, Y.; Zhou, L.; He, Y.; Ma, L.; Gao, J. Pickering Emulsion Stabilized by Lipase-Containing Periodic Mesoporous Organosilica Particles: A Robust Biocatalyst System for Biodiesel Production. Bioresour. Technol. 2014, 153, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Imamoglu, M.; Pérez-Quintanilla, D.; Sierra, I. Bifunctional Periodic Mesoporous Organosilicas with Sulfide Bridges as Effective Sorbents for Hg(II) Extraction from Environmental and Drinking Waters. Microporous Mesoporous Mater. 2016, 229, 90–97. [Google Scholar] [CrossRef]
- Esquivel, D.; Ouwehand, J.; Meledina, M.; Turner, S.; Van Tendeloo, G.; Romero-Salguero, F.J.; Clercq, J.D.; Voort, P. Van Der Thiol-Ethylene Bridged PMO: A High Capacity Regenerable Mercury Adsorbent via Intrapore Mercury Thiolate Crystal Formation. J. Hazard. Mater. 2017, 339, 368–377. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Bispo, C.; Bion, N.; Ferreira, P.; Batonneau-Gener, I. Periodic Mesoporous Organosilicas as Adsorbents for the Organic Pollutants Removal in Aqueous Phase. Microporous Mesoporous Mater. 2014, 200, 117–123. [Google Scholar] [CrossRef]
- Deka, J.R.; Liu, C.L.; Wang, T.H.; Chang, W.C.; Kao, H.M. Synthesis of Highly Phosphonic Acid Functionalized Benzene-Bridged Periodic Mesoporous Organosilicas for Use as Efficient Dye Adsorbents. J. Hazard. Mater. 2014, 278, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Otero, R.; Esquivel, D.; Ulibarri, M.A.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J.; Fernández, J.M. Adsorption of the Herbicide S-Metolachlor on Periodic Mesoporous Organosilicas. Chem. Eng. J. 2013, 228, 205–213. [Google Scholar] [CrossRef]
- Lourenço, M.A.O.; Silva, R.M.; Silva, R.F.; Pinna, N.; Pronier, S.; Pires, J.; Gomes, J.R.B.; Pinto, M.L.; Ferreira, P. Turning Periodic Mesoporous Organosilicas Selective to CO2/CH4 Separation: Deposition of Aluminium Oxide by Atomic Layer Deposition. J. Mater. Chem. A 2015, 3, 22860–22867. [Google Scholar] [CrossRef]
- Wu, L.; Yu, Z.; Ye, Y.; Yang, Y.; Zeng, H.; Huang, J.; Huang, Y.; Zhang, Z.; Xiang, S. Sulfonated Periodic-Mesoporous-Organosilicas Column for Selective Separation of C2H2/CH4 Mixtures. J. Solid State Chem. 2018, 264, 113–118. [Google Scholar] [CrossRef]
- Sanchez, C.; Jeremias, F.; Ernst, S.J.; Henninger, S.K. Synthesis, Functionalization and Evaluation of Ethylene-Bridged PMOs as Adsorbents for Sorption Dehumidification and Cooling Systems. Microporous Mesoporous Mater. 2017, 244, 151–157. [Google Scholar] [CrossRef]
- Lu, D.; Chen, H.; Yan, X.; Wang, L.; Zhang, J. Ratiometric Hg2+ Sensor Based on Periodic Mesoporous Organosilica Nanoparticles and Förster Resonance Energy Transfer. J. Photochem. Photobiol. A Chem. 2015, 299, 125–130. [Google Scholar] [CrossRef]
- Qiu, X.; Han, S.; Hu, Y.; Gao, M.; Wang, H. Periodic Mesoporous Organosilicas for Ultra-High Selective Copper(Ii) Detection and Sensing Mechanism. J. Mater. Chem. A 2014, 2, 1493–1501. [Google Scholar] [CrossRef]
- Grösch, L.; Lee, Y.J.; Hoffmann, F.; Fröba, M. Light-Harvesting Three-Chromophore Systems Based on Biphenyl-Bridged Periodic Mesoporous Organosilica. Chem. Eur. J. 2015, 21, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Taylor, R.N.K.; Kullmann, S.; Bao, H.; Hartmann, M. Mesoporous Organosilicas With Large Cage-Like Pores for High Efficiency Immobilization of Enzymes. Adv. Mater. 2011, 23, 2627–2632. [Google Scholar] [CrossRef]
- Aggad, D.; Jimenez, C.M.; Dib, S.; Croissant, J.G.; Lichon, L.; Laurencin, D.; Richeter, S.; Maynadier, M.; Alsaiari, S.K.; Boufatit, M.; et al. Gemcitabine Delivery and Photodynamic Therapy in Cancer Cells via Porphyrin-Ethylene-Based Periodic Mesoporous Organosilica Nanoparticles. ChemNanoMat 2018, 4, 46–51. [Google Scholar] [CrossRef]
- Inagaki, S.; Guan, S.; Fukushima, Y.; Ohsuna, T.; Terasaki, O. Novel Mesoporous Materials with a Uniform Distribution of Organic Groups and Inorganic Oxide in Their Frameworks. J. Am. Chem. Soc. 1999, 121, 9611–9614. [Google Scholar] [CrossRef]
- Melde, B.J.; Holland, B.T.; Blanford, C.F.; Stein, A. Mesoporous Sieves with Unified Hybrid Inorganic/Organic Frameworks. Chem. Mater. 1999, 11, 3302–3308. [Google Scholar] [CrossRef]
- Asefa, T.; MacLachlan, M.J.; Coombs, N.; Ozin, G.A. Periodic Mesoporous Organosilicas with Organic Groups inside the Channel Walls. Nature 1999, 402, 867–871. [Google Scholar] [CrossRef]
- Kuroki, M.; Asefa, T.; Whitnal, W.; Kruk, M.; Yoshina-Ishii, C.; Jaroniec, M.; Ozin, G.A. Synthesis and Properties of 1,3,5-Benzene Periodic Mesoporous Organosilica (PMO): Novel Aromatic PMO with Three Point Attachments and Unique Thermal Transformations. J. Am. Chem. Soc. 2002, 124, 13886–13895. [Google Scholar] [CrossRef] [PubMed]
- Esquivel Merino, M.D. Síntesis, Caracterización y Aplicaciones de Materiales Periódicos Mesoporosos Organosilícicos. Ph.D. Thesis, Universidad de Córdoba, Córdoba, Spain, 2011. Available online: https://helvia.uco.es/xmlui/bitstream/handle/10396/5222/9788469447673 (accessed on 24 October 2022).
- Sim, K.; Lee, N.; Kim, J.; Cho, E.B.; Gunathilake, C.; Jaroniec, M. CO2 Adsorption on Amine-Functionalized Periodic Mesoporous Benzenesilicas. ACS Appl. Mater. Interfaces 2015, 7, 6792–6802. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Cho, E.-B. Multifunctional Periodic Mesoporous Benzene-Silicas for Evaluation of CO2 Adsorption at Standard Temperature and Pressure. Microporous Mesoporous Mater. 2019, 293, 109816. [Google Scholar] [CrossRef]
- Kirren, P.; Barka, L.; Rahmani, S.; Bondon, N.; Donzel, N.; Trens, P.; Bessière, A.; Raehm, L.; Charnay, C.; Durand, J.O. Periodic Mesoporous Organosilica Nanoparticles for CO2 Adsorption at Standard Temperature and Pressure. Molecules 2022, 27, 4245. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, X.; Zhang, R.; Liu, Y.; Wang, W.; Ling, Y.; El-Toni, A.M.; Zhao, D. Periodic Mesoporous Organosilica Nanocubes with Ultrahigh Surface Areas for Efficient CO2 Adsorption. Sci. Rep. 2016, 6, 20769. [Google Scholar] [CrossRef]
- Liu, M.; Lu, X.; Shi, L.; Wang, F.; Sun, J. Periodic Mesoporous Organosilica with a Basic Urea-Derived Framework for Enhanced Carbon Dioxide Capture and Conversion Under Mild Conditions. ChemSusChem 2017, 10, 1110–1119. [Google Scholar] [CrossRef]
- Rekha, P.; Sharma, V.; Mohanty, P. Synthesis of Cyclophosphazene Bridged Mesoporous Organosilicas for CO2 Capture and Cr (VI) Removal. Microporous Mesoporous Mater. 2016, 219, 93–102. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2 Capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Lourenço, M.A.O.; Siquet, C.; Sardo, M.; Mafra, L.; Pires, J.; Jorge, M.; Pinto, M.L.; Ferreira, P.; Gomes, J.R.B. Interaction of CO2 and CH4 with Functionalized Periodic Mesoporous Phenylene-Silica: Periodic DFT Calculations and Gas Adsorption Measurements. J. Phys. Chem. C 2016, 120, 3863–3875. [Google Scholar] [CrossRef]
- Burleigh, M.C.; Markowitz, M.A.; Jayasundera, S.; Spector, M.S.; Thomas, C.W.; Gaber, B.P. Mechanical and Hydrothermal Stabilities of Aged Periodic Mesoporous Organosilicas. J. Phys. Chem. B 2003, 107, 12628–12634. [Google Scholar] [CrossRef]
- Burleigh, M.C.; Jayasundera, S.; Thomas, C.W.; Spector, M.S.; Markowitz, M.A.; Gaber, B.P. A Versatile Synthetic Approach to Periodic Mesoporous Organosilicas. Colloid Polym. Sci. 2004, 282, 728–733. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data (ICDD). The Powder Diffraction; ICDD: Newtown Square, PA, USA, 2003. [Google Scholar]
- Cantador-Fernandez, D.; Suescum-Morales, D.; Esquivel, D.; Jiménez, J.R.; Fernández-Rodriguez, J.M. CO2 Adsorption by Ethane Periodic Mesoporous Organosilica at Low Temperatures and High Pressure. J. Environ. Chem. Eng. 2023, 11, 110582. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Tóth, J. Uniform Interpretation of Gas/Solid Adsorption. Adv. Colloid Interface Sci. 1995, 55, 1–239. [Google Scholar] [CrossRef]
- Dubinin, M.M. Generalization of the Theory of Volume Filling of Micropores to Nonhomogeneous Microporous Structures. Carbon 1985, 23, 373–380. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Zaverina, E.D. Sorbtsiya I Struktura Aktivnykh Uglei. 6. Strukturnye Tipy Aktivnykh Uglei. J. Phys. Chem. 1949, 23, 1129–1140. [Google Scholar]
- Temkin, M.; Pyzhev, V. Kinetics of Ammonia Synthesis on Promoted Iron Catalyst. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]
- Temkin, M.; Levich, V. Adsorption Equilibrium on Heterogeneous Surfaces. J. Phys. Chem. 1946, 20, 961–974. [Google Scholar]
- Liang, Y.; Liu, X.; Allen, M.R. Measuring and Modeling Surface Sorption Dynamics of Organophosphate Flame Retardants on Impervious Surfaces. Chemosphere 2018, 193, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthes, V.; Krimissa, M. Sorption Isotherms: A Review on Physical Bases, Modeling and Measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar]
- Hu, Q.; Wang, Q.; Feng, C.; Zhang, Z.; Lei, Z.; Shimizu, K. Insights into Mathematical Characteristics of Adsorption Models and Physical Meaning of Corresponding Parameters. J. Mol. Liq. 2018, 254, 20–25. [Google Scholar] [CrossRef]
- Sparks, D.L. Environmental Soil Chemistry, 2nd ed.; Academic Press (Elsevier): Cambridge, MA, USA, 2003; ISBN 978-0-12-656446-4. [Google Scholar]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and Multilayer Adsorption Isotherm Models for Sorption from Aqueous Media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Lee, H.I.; Pak, C.; Yi, S.H.; Shon, J.K.; Kim, S.S.; So, B.G.; Chang, H.; Yie, J.E.; Kwon, Y.-U.; Kim, J.M. Systematic Phase Control of Periodic Mesoporous Organosilicas Using Gemini Surfactants. J. Mater. Chem. 2005, 15, 4711–4717. [Google Scholar] [CrossRef]
- Haul, R.S.J.; Gregg, K.S.W. Sing: Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982; Volume 86. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems. In Handbook of Heterogeneous Catalysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 1217–1230. ISBN 9783527610044. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. 786. Studies in Adsorption. Part XI. A System of Classification of Solution Adsorption Isotherms, and Its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Areas of Solids. J. Chem. Soc. 1960, 3973–3993. [Google Scholar] [CrossRef]
- Esfandiari, B.; Monajjemi, M. Physical Adsorption between Mono and Diatomic Gases inside of Carbon Nanotube with Respect to Potential Energy. J. Phys. Theor. Chem. 2013, 10, 31–42. [Google Scholar]
- Bhagiyalakshmi, M.; Lee, J.Y.; Jang, H.T. Synthesis of Mesoporous Magnesium Oxide: Its Application to CO2 Chemisorption. Int. J. Greenh. Gas Control 2010, 4, 51–56. [Google Scholar] [CrossRef]
- Wang, Y.; Du, T.; Song, Y.; Che, S.; Fang, X.; Zhou, L. Amine-Functionalized Mesoporous ZSM-5 Zeolite Adsorbents for Carbon Dioxide Capture. Solid State Sci. 2017, 73, 27–35. [Google Scholar] [CrossRef]
- Niu, M.; Yang, H.; Zhang, X.; Wang, Y.; Tang, A. Amine-Impregnated Mesoporous Silica Nanotube as an Emerging Nanocomposite for CO2 Capture. ACS Appl. Mater. Interfaces 2016, 8, 17312–17320. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jiang, J.; Tian, S.; Yan, F.; Chen, X. Polyethyleneimine–Nano Silica Composites: A Low-Cost and Promising Adsorbent for CO2 Capture. J. Mater. Chem. A 2015, 3, 2166–2175. [Google Scholar] [CrossRef]
- Lo, S.-H.; Chien, C.-H.; Lai, Y.-L.; Yang, C.-C.; Lee, J.J.; Raja, D.S.; Lin, C.-H. A Mesoporous Aluminium Metal–Organic Framework with 3 Nm Open Pores. J. Mater. Chem. A 2013, 1, 324–329. [Google Scholar] [CrossRef]
- Si, X.; Zhang, J.; Li, F.; Jiao, C.; Wang, S.; Liu, S.; Li, Z.; Zhou, H.; Sun, L.; Xu, F. Adjustable Structure Transition and Improved Gases (H2, CO2) Adsorption Property of Metal–Organic Framework MIL-53 by Encapsulation of BNHx. Dalt. Trans. 2012, 41, 3119–3122. [Google Scholar] [CrossRef]
- Bourrelly, S.; Llewellyn, P.L.; Serre, C.; Millange, F.; Loiseau, T.; Férey, G. Different Adsorption Behaviors of Methane and Carbon Dioxide in the Isotypic Nanoporous Metal Terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, S.; Xian, S.; Xi, H.; Li, Z. Adsorption Equilibrium and Kinetics of CO2 on Chromium Terephthalate MIL-101. Energy Fuels 2011, 25, 835–842. [Google Scholar] [CrossRef]
- Zhou, Z.; Mei, L.; Ma, C.; Xu, F.; Xiao, J.; Xia, Q.; Li, Z. A Novel Bimetallic MIL-101(Cr, Mg) with High CO2 Adsorption Capacity and CO2/N2 Selectivity. Chem. Eng. Sci. 2016, 147, 109–117. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Lin, Y.S. Adsorption and Diffusion of Carbon Dioxide on Metal−Organic Framework (MOF-5). Ind. Eng. Chem. Res. 2009, 48, 10015–10020. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh Porosity in Metal-Organic Frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [PubMed]
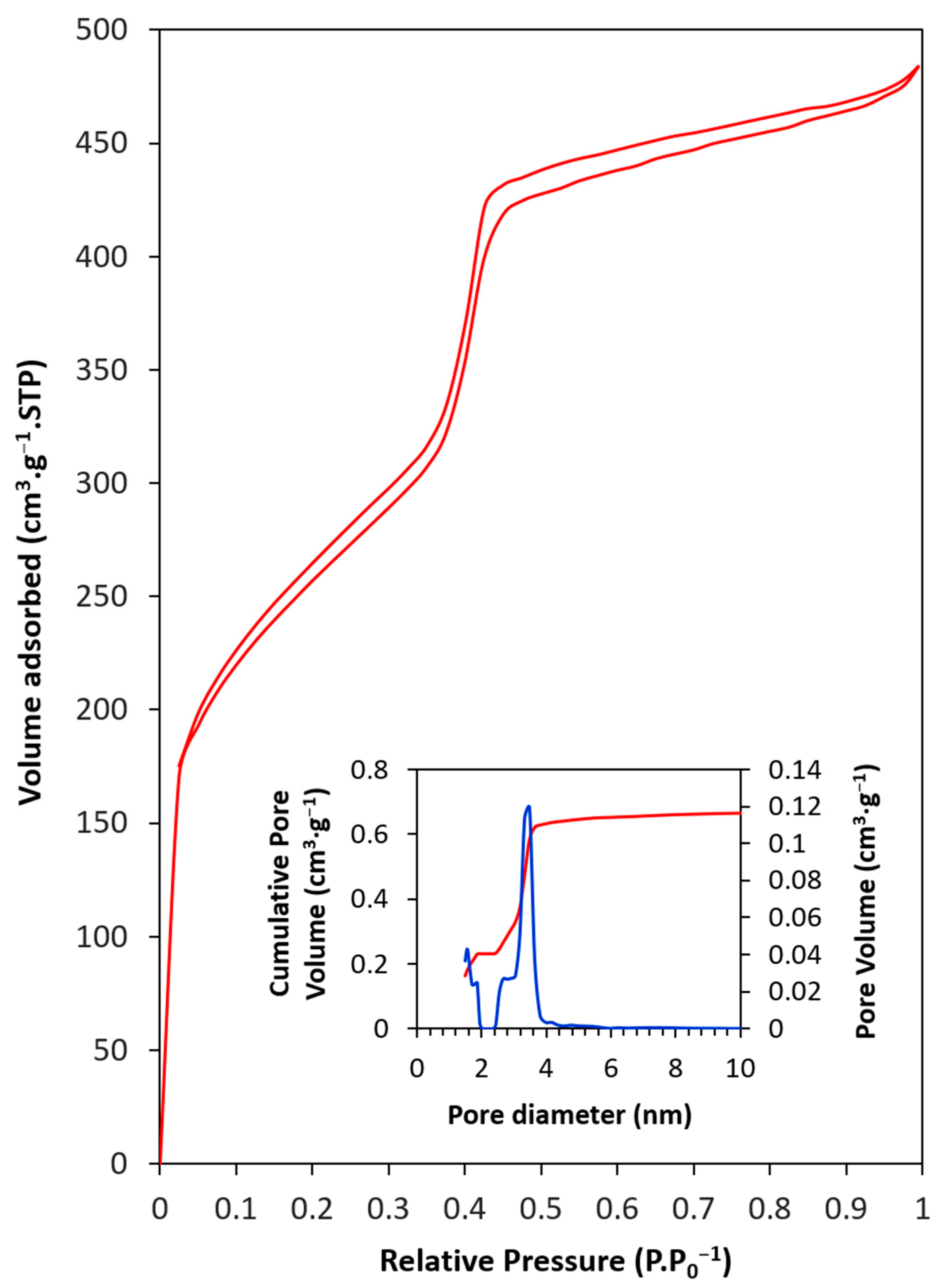

| SBET (m2·g−1) | Smc 1 (m2·g−1) | Vp 2 (cm3·g−1) | Dp 3 (nm) | |
|---|---|---|---|---|
| PMO–benzene | 928 | 139 | 0.68 | 3.47 |
| Langmuir | Freundlich | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qm | XL | qmc | KL | SL | R2 | Kf | n | nf | R2 | |
| (mg·g−1) | (mg·g−1) | (atm−1) | (m2·g−1) | (mg·g−1·atm(−1/n)) | (1/n) | |||||
| PMO–benzene 0 °C | 474.30 | 1.220 | 388.66 | 4.538 | 904.09 | 0.975 | 574.198 | 0.663 | 1.508 | 0.991 |
| PMO–benzene 10 °C | 429.93 | 1.218 | 353.01 | 4.590 | 821.18 | 0.982 | 505.880 | 0.643 | 1.556 | 0.998 |
| PMO–benzene 20 °C | 392.35 | 1.215 | 322.88 | 4.648 | 751.08 | 0.990 | 479.124 | 0.641 | 1.559 | 0.997 |
| PMO–benzene 35 °C | 357.14 | 1.238 | 288.57 | 4.208 | 671.27 | 0.997 | 468.582 | 0.692 | 1.445 | 0.996 |
| Sips | Toth | |||||||
|---|---|---|---|---|---|---|---|---|
| qS | KS | nS | R2 | qT | KT | nT | R2 | |
| (mg·g−1) | (atm−1) | (mg·g−1) | (atm−1) | |||||
| PMO–benzene 0 °C | 375.89 | 2.631 | 0.780 | 0.999 | 349.58 | 4.837 | 0.690 | 0.996 |
| PMO–benzene 10 °C | 384.79 | 1.981 | 0.780 | 1.000 | 357.72 | 4.303 | 0.760 | 0.999 |
| PMO–benzene 20 °C | 426.79 | 0.709 | 0.760 | 1.000 | 436.59 | 0.999 | 0.310 | 1.000 |
| PMO–benzene 35 °C | - | - | - | - | - | - | - | - |
| Dubinin–Raduskevich | Temkin | |||||||
|---|---|---|---|---|---|---|---|---|
| qD | β | E | R2 | KTk | B | bTk | R2 | |
| (mg·g−1) | (mol2·kJ−2) | (kJ·mol−1) | (atm−1) | (kJ·mol−1) | ||||
| PMO–benzene 0 °C | 440.54 | 0.035 | 3.780 | 0.897 | 27.711 | 143.096 | 0.016 | 0.847 |
| PMO–benzene 10 °C | 391.48 | 0.032 | 3.973 | 0.926 | 30.895 | 120.895 | 0.019 | 0.900 |
| PMO–benzene 20 °C | 344.76 | 0.028 | 4.208 | 0.961 | 37.432 | 100.181 | 0.024 | 0.949 |
| PMO–benzene 35 °C | 290.23 | 0.025 | 4.478 | 0.975 | 42.538 | 81.639 | 0.031 | 0.959 |
| Adsorbent | T Isotherm (°C) | Pressure (atm) | Capacity Adsorption (mg·g−1) | Ref. |
|---|---|---|---|---|
| PMO–benzene | 25 | 1 | 22 | [50] |
| PMO–benzene modified | 25 | 1 | 133.32 | |
| PMO–benzene (A-LB) | 25 | 1 | 77.44 | [51] |
| PMO–benzene (A-LBEO) | 25 | 1 | 69.52 | |
| PMO–Ethane Np py | 0 and 25 | 1 | 68.2 and 40.5 | [52] |
| PMO–Ethane Np Etbipy | 0 and 25 | 1 | 73.04 and 41.8 | |
| PMO–Ethane Np iPrbipy | 0 and 25 | 1 | 99.44 and 45.7 | |
| PMO-–Ethane | 0 | 1 | 62.48 | [53] |
| PMO-UDF | 0 | ≈1 | 52.8 | [54] |
| CPMOs | 0 | 1 | 96.36 | [55] |
| MCM-41-modified | 75 | 1 | 133 | [56] |
| NH2-Ph-PMO | 25 | ≈10 | 114.4 | [57] |
| TiO2/Graphene | 25 | 1 | 82.72 | [20] |
| MgO | 25 | 1 | 79.2 | [82] |
| ZSM-5 Mesoporous | 40 | 1 | 39.6 | [83] |
| MSiNTs-PEI50 | 85 | 0.6 | 121 | [84] |
| CeO2 Mesoporous | 25 | 10 | 391.6 | [21] |
| PEI50 | 75 | 1 | 138.16 | [85] |
| MOF-Al | 0 | 1 | 124.52 | [86] |
| MIL-53 (BNHx) | 0 | 1 | 198 | [87] |
| MIL-47 (V) | 31 | 20 | 506 | [88] |
| MIL-101 | 25 | 30 | 1007.6 | [89] |
| MIL-101 (Cr, Mg) | 25 | 1 | 145.2 | [90] |
| MOF-5 | 22.85 | 1 | 92.4 | [91] |
| MOF-74 | 25 | 42 | 457 | [92] |
| MOF-177 | 25 | 42 | 1493 | |
| MOF-200 | 25 | 50 | 2400 | [93] |
| HT-MgAl-CO3 | 0 | ≈35 | 142.02 | [24] |
| Organohydrotalcite TDD | 0 | 35 | 176.66 | [23] |
| PMO–benzene | 0 | ≈34 | 638.2 | This work |
| 10 | ≈34 | 465.2 | ||
| 20 | ≈34 | 346.7 | ||
| 35 | ≈34 | 266.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantador-Fernandez, D.; Esquivel, D.; Jiménez, J.R.; Fernández-Rodríguez, J.M. Use of Periodic Mesoporous Organosilica–Benzene Adsorbent for CO2 Capture to Reduce the Greenhouse Effect. Materials 2024, 17, 2669. https://doi.org/10.3390/ma17112669
Cantador-Fernandez D, Esquivel D, Jiménez JR, Fernández-Rodríguez JM. Use of Periodic Mesoporous Organosilica–Benzene Adsorbent for CO2 Capture to Reduce the Greenhouse Effect. Materials. 2024; 17(11):2669. https://doi.org/10.3390/ma17112669
Chicago/Turabian StyleCantador-Fernandez, David, Dolores Esquivel, José Ramón Jiménez, and José María Fernández-Rodríguez. 2024. "Use of Periodic Mesoporous Organosilica–Benzene Adsorbent for CO2 Capture to Reduce the Greenhouse Effect" Materials 17, no. 11: 2669. https://doi.org/10.3390/ma17112669
APA StyleCantador-Fernandez, D., Esquivel, D., Jiménez, J. R., & Fernández-Rodríguez, J. M. (2024). Use of Periodic Mesoporous Organosilica–Benzene Adsorbent for CO2 Capture to Reduce the Greenhouse Effect. Materials, 17(11), 2669. https://doi.org/10.3390/ma17112669







