Hexakis[p-(hydroxymethyl)phenoxy]cyclotriphosphazene as an Environmentally Friendly Modifier for Polyurethane Powder Coatings with Increased Thermal Stability and Corrosion Resistance
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Hexakis(4-(hydroxymethyl)phenoxy)cyclotriphosphazene (HHPCP)
2.3. Method of Preparation of Powder Coatings
2.4. Coatings Characterization
2.4.1. Polymerization Test
- The coating is quite soft and matt;
- The coating is matt and can be scratched with a fingernail;
- Gloss loss is less than 5 units;
- No noticeable changes. The coating cannot be scratched with a fingernail.
2.4.2. Glow Discharge Optical Emission Spectrometry (GD-EOS)
2.4.3. Performance Properties
2.4.4. Water Contact Angle
2.4.5. Thermogravimetric Analysis
2.4.6. Furnace Tests
2.4.7. Corrosion Resistance
3. Results and Discussion
3.1. Characteristics of the Powder Coatings Composition and Manufacturing Process
3.2. Powder Coatings Performance Properties
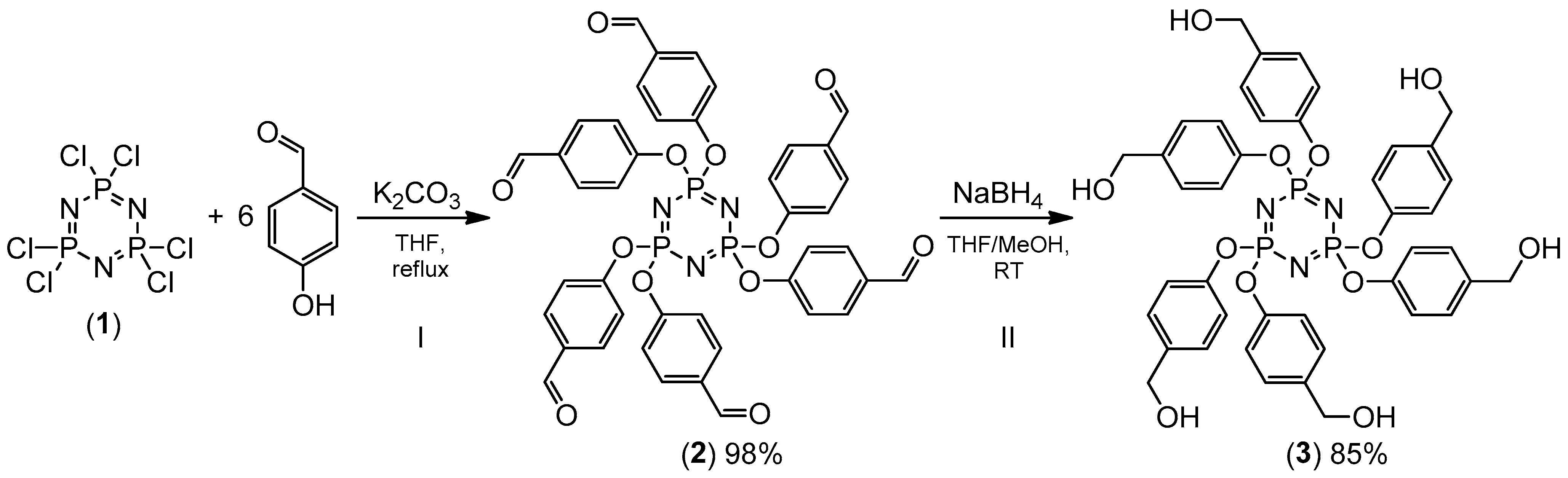
3.3. Phosphorus Distribution in the Cured Coatings
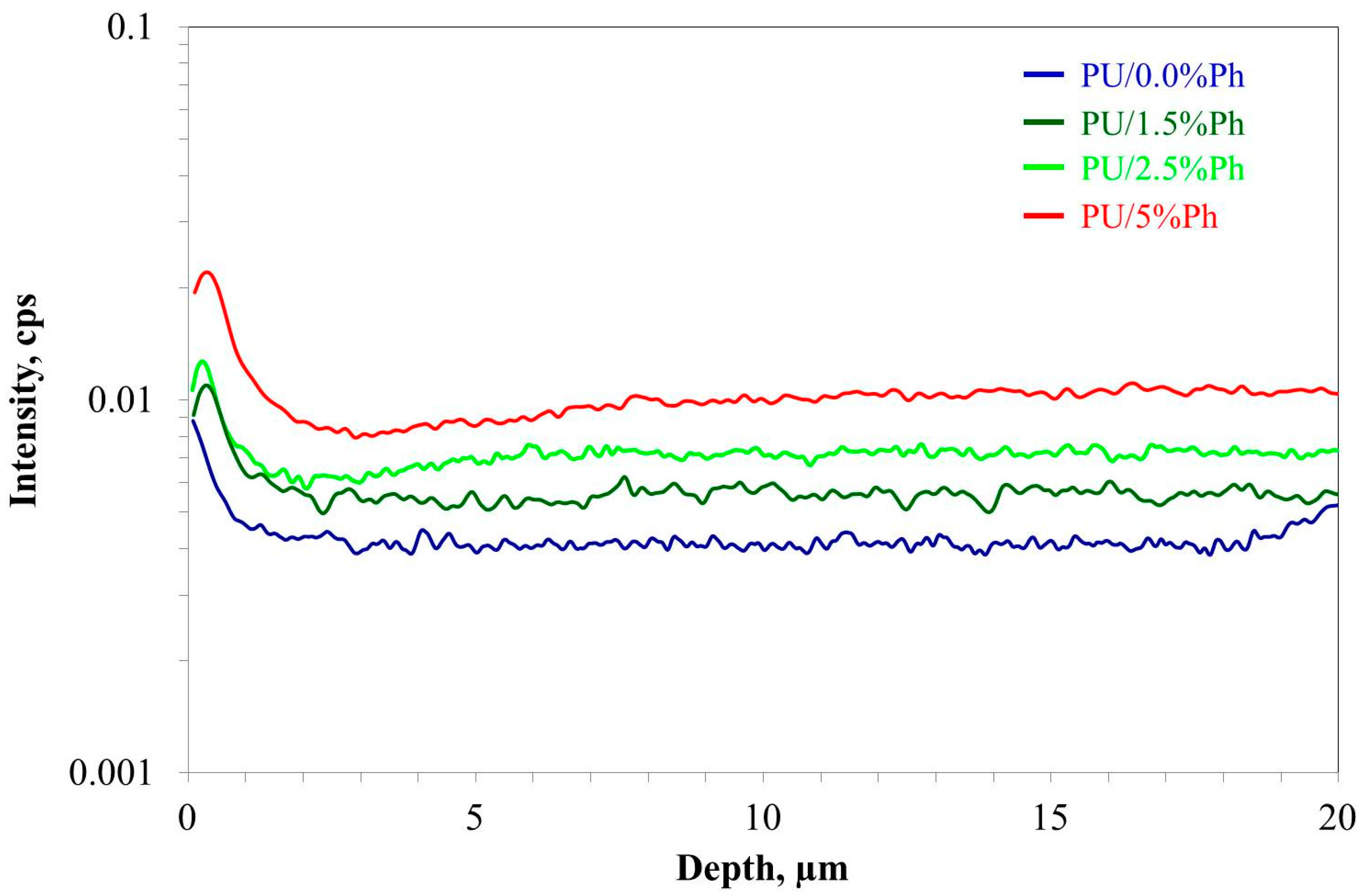
3.4. Thermal Stability
3.5. Thermal Protection
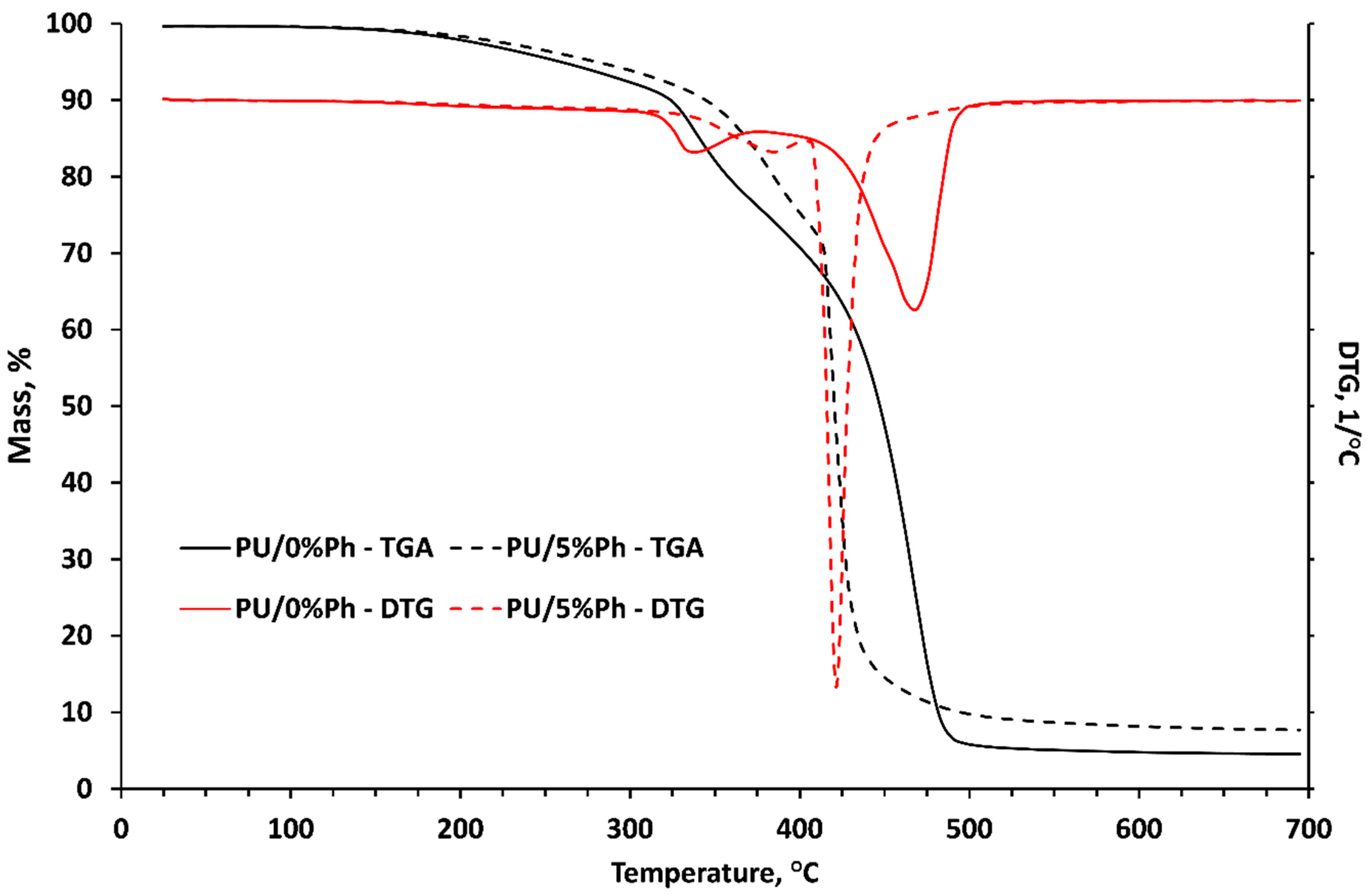
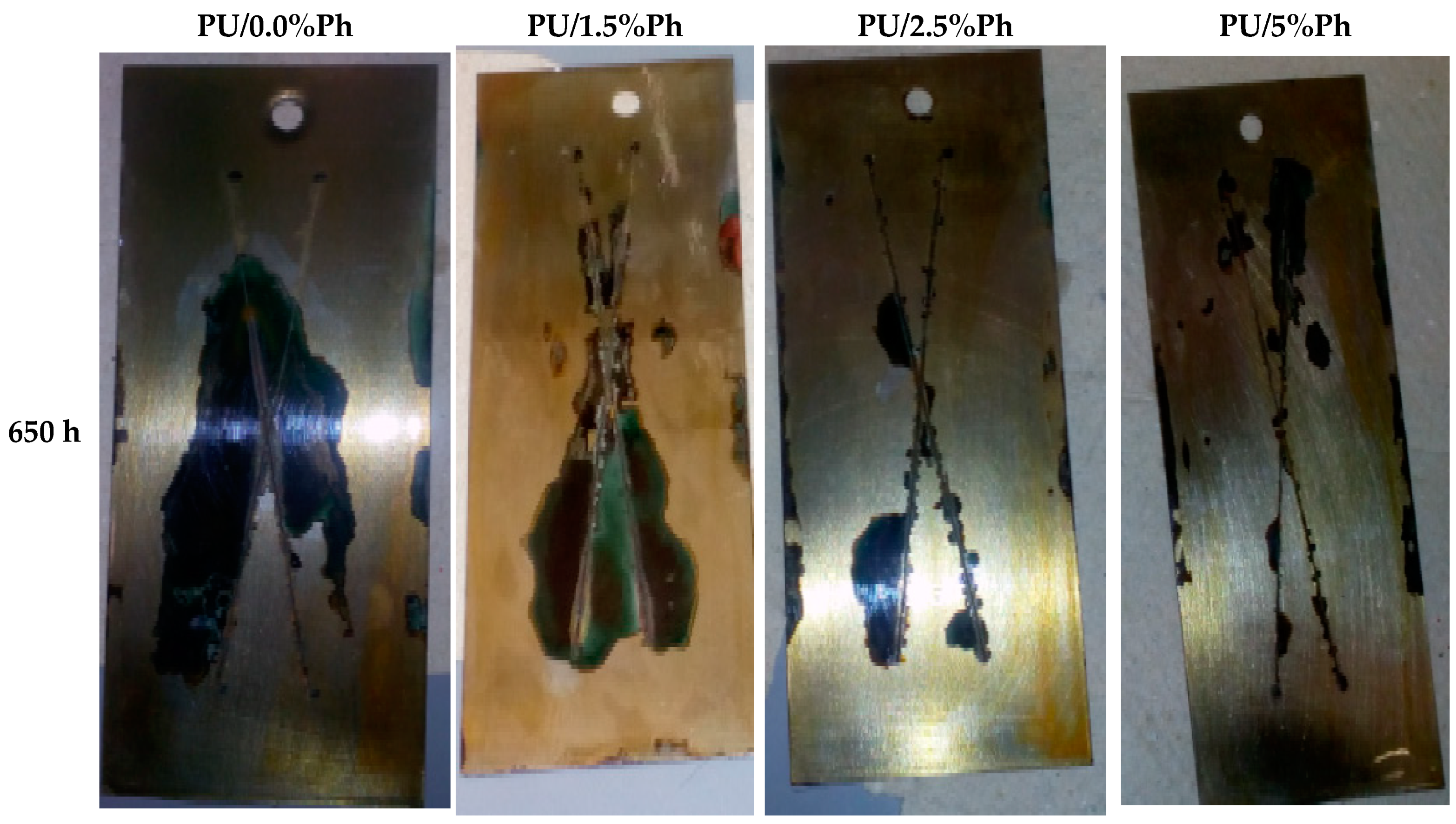
3.6. Corrosion Properties
- Ad—delamination area including scratch area, [mm2];
- Al—scratch area, [mm2], 30 mm2;
- l—scratch length, [mm], 100 mm.
- wc—average width of the corrosion zone, [mm];
- w—width of the original crack, [mm], 0.3 mm.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, J.; Patil, R. Enabling Green Manufacture of Polymer Products via Vegetable Oil Epoxides. Ind. Eng. Chem. Res. 2023, 62, 1725–1735. [Google Scholar] [CrossRef]
- Thomas, J.; Singh, V.; Jain, R. Synthesis and characterization of solvent free acrylic copolymer for polyurethane coatings. Prog. Org. Coat. 2020, 145, 105677. [Google Scholar] [CrossRef]
- Pilch-Pitera, B.; Czachor, D.; Kowalczyk, K.; Pavlova, E.; Wojturski, J.; Florczak, Ł.; Byczyński, Ł. Conductive polyurethane-based powder clear coatings modified with carbon nanotubes. Prog. Org. Coat. 2019, 137, 105367. [Google Scholar] [CrossRef]
- Pourhashem, S.; Ghasemy, E.; Rashidi, A.; Vaezi, M.R. A review on application of carbon nanostructures as nanofiller in corrosion-resistant organic coatings. J. Coat. Technol. Res. 2020, 17, 19–55. [Google Scholar] [CrossRef]
- Golgoon, A.; Aliofkhazrae, M.; Toorani, M. Nanocomposite Protective Coatings Fabricated by Electrostatic Spray Method. Protect. Met. Phys. Chem. Surf. 2018, 54, 192–221. [Google Scholar] [CrossRef]
- Stojanović, I.; Šimunović, V.; Alar, V.; Kapor, F. Experimental evaluation of polyester and epoxy-polyester powder coatings in aggressive media. Coatings 2018, 8, 98. [Google Scholar] [CrossRef]
- Binay, M.I.; Kirdeciler, S.K.; Akata, B. Development of antibacterial powder coatings using single and binary ion-exchanged zeolite A prepared from local kaolin. Appl. Clay Sci. 2019, 182, 105251. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Liu, C.; Yu, X. Durable superamphiphobic coatings from one-step electrostatic dusting. Soft Matter 2019, 15, 7374–7380. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, S.; Sun, G.; Zhong, Y.; You, B. Evaluation of scratch resistance of functionalized graphene oxide/polysiloxane nanocomposite coatings. Prog. Org. Coat. 2018, 117, 118–129. [Google Scholar] [CrossRef]
- Fernández-Álvarez, M.; Velasco, F.; Bautista, A.; Galiana, B. Functionalizing organic powder coatings with nanoparticles through ball milling for wear applications. Appl. Surf. Sci. 2020, 513, 145834. [Google Scholar] [CrossRef]
- Ahmad, F.; Zulkurnain, E.S.B.; Ullah, S.; Al-Sehemi, A.G.; Raza, M.R. Improved fire resistance of boron nitride/epoxy intumescent coating upon minor addition of nano-alumina. Mater. Chem. Phys. 2020, 256, 123634. [Google Scholar] [CrossRef]
- Tang, B.; Feng, W.; Guo, J.; Sun, J.; Zhang, S.; Gu, X.; Li, H.; Yang, W. Hydrophobic modification of pentaerythritol and its application in fire-retardant coatings for steel structures. Prog. Org. Coat. 2020, 138, 105391. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, X.; Sun, Z. Efficient flame-retardant and smoke-suppression properties of MgAlCO3-LDHs on the intumescent fire retardant coating for steel structures. Prog. Org. Coat. 2019, 135, 291–298. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, D.; Yan, L.; Xie, X. Synergistic effect of sepiolite and polyphosphate ester on the fire protection and smoke suppression properties of an amino transparent fire-retardant coating. Prog. Org. Coat. 2020, 141, 105572. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [PubMed]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Puri, R.G.; Khanna, A.S. Intumescent coatings: A review on recent progress. J. Coat. Technol. Res. 2017, 14, 1–20. [Google Scholar] [CrossRef]
- Horacek, H. Preparation and Fire Test of Intumescent Powder Coatings. OAlib 2014, 1, 1–15. [Google Scholar] [CrossRef]
- QUALICOAT. Specifications for a Quality Label for Liquid and Powder Organic Coatings on Aluminium for Architectural Applications; QUALICOAT: Zurich, Switzerland, 2022. [Google Scholar]
- Usri, S.N.K.; Jamain, Z.; Makmud, M.Z.H. A review on synthesis, structural, flame retardancy and dielectric properties of hexasubstituted cyclotriphosphazene. Polymers 2021, 13, 2916. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, G.; Yang, S.; Yang, L.; Cao, J.; Yang, K. The synthesis, curing kinetics, thermal properties and flame rertardancy of cyclotriphosphazene-containing multifunctional epoxy resin. Thermochim. Acta 2019, 680, 178348. [Google Scholar] [CrossRef]
- Xu, J.; He, Z.; Wu, W.; Ma, H.; Xie, J.; Qu, H.; Jiao, Y. Study of thermal properties of flame retardant epoxy resin treated with hexakis[p-(hydroxymethyl)phenoxy]cyclotriphosphazene. J. Therm. Anal. Calorim. 2013, 114, 1341–1350. [Google Scholar] [CrossRef]
- Chistyakov, E.M.; Filatov, S.N.; Yudaev, P.A.; Kireev, V.V. Synthesis, characterization and epoxidation of hexakis-4-(2-(4-((β-methallyl)oxy)phenyl)propan-2-yl)phenoxycyclotriphosphazene. Tetrahedron. Lett. 2019, 60, 444–448. [Google Scholar] [CrossRef]
- Rybyan, A.A.; Bilichenko, J.V.; Kireev, V.V.; Kolenchenko, A.A.; Chistyakov, E.M. Curing of DER-331 Epoxy Resin with Arylaminocyclotriphosphazenes Based on o-, m-, and p-methylanilines. Polymers 2022, 14, 5334. [Google Scholar] [CrossRef] [PubMed]
- Dagdag, O.; Kim, H. Progress in the Field of Cyclophosphazenes: Preparation, Properties, and Applications. Polymers 2023, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Malkappa, K.; Ray, S.S. Thermal Stability, Pyrolysis Behavior, and Fire-Retardant Performance of Melamine Cyanurate@Poly(cyclotriphosphazene-co-4,4′-sulfonyl diphenol) Hybrid Nanosheet-Containing Polyamide 6 Composites. ACS Omega 2019, 4, 9615–9628. [Google Scholar] [CrossRef]
- Wen, P.; Tai, Q.; Hu, Y.; Yuen, R.K.K. Cyclotriphosphazene-Based Intumescent Flame Retardant against the Combustible Polypropylene. Ind. Eng. Chem. Res. 2016, 55, 8018–8024. [Google Scholar] [CrossRef]
- Taip, N.A.M.; Jamain, Z.; Palle, I. Fire-Retardant Property of Hexasubstituted Cyclotriphosphazene Derivatives with Schiff Base Linking Unit Applied as an Additives in Polyurethane Coating for Wood Fabrication. Polymers 2022, 14, 3768. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Gao, X.-X.; Chen, H.-T.; Liu, X.-F.; Li, A.; Zhang, H.-J.; Wang, L.-N. Enhancement in mechanical and corrosion resistance properties of a biodegradable Zn-Fe alloy through second phase refinement. Mater. Sci. Eng. C 2020, 116, 111197. [Google Scholar] [CrossRef]
- Tomachuk, C.R.; Elsner, C.I.; Di Sarli, A.R.; Ferraz, O.B. Corrosion resistance of Cr(III) conversion treatments applied on electrogalvanised steel and subjected to chloride containing media. Mater. Chem. Phys. 2010, 119, 19–29. [Google Scholar] [CrossRef]
- Chen, K.H.; Fang, H.C.; Zhang, Z.; Chen, X.; Liu, G. Effect of of Yb, Cr and Zr additions on recrystallization and corrosion resistance of Al–Zn–Mg–Cu alloys. Mater. Sci. Eng. A 2008, 497, 426–431. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Sonawane, N.; Siju, C.R. Epoxy powder coatings containing polyaniline for enhanced corrosion protection. Prog. Org. Coat. 2009, 64, 383–386. [Google Scholar] [CrossRef]
- Chang, K.-C.; Hsu, M.-H.; Lu, H.-I.; Lai, M.-C.; Liu, P.-J.; Hsu, C.-H.; Ji, W.-F.; Chuang, T.-L.; Wei, Y.; Yeh, J.-M.; et al. Room-temperature cured hydrophobic epoxy/graphene composites as corrosion inhibitor for cold-rolled steel. Carbon N. Y. 2014, 66, 144–153. [Google Scholar] [CrossRef]
- Cai, S.; Wang, G.; Zhang, S.; Ni, Y.; Huang, J.; Li, Z.; Jin, R. Flexible Ceramic Wear-Resistant Heat-Proof Dual-Anticorrosive. Coating. Patent CN 1279130 C, 11 October 2016. [Google Scholar]
- Chen, J.; Liu, X.; Zhao, Z.; Huo, B.; Li, Z.; Sun, H. A Kind of Montmorillonite Intercalation Polymeric Modification Epoxy Resin Based Powder Coating for Pipeline Corrosion Protection and Preparation Method. Thereof. Patent CN 106189682 C, 7 December 2016. [Google Scholar]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Byczyński, Ł.; Dutkiewicz, M.; Januszewski, R.; Rojewski, S. Polyurethane high-solids coatings modified with silicon-containing functionalized cyclotriphosphazene. Prog. Org. Coat. 2022, 172, 107139. [Google Scholar] [CrossRef]
- ISO 12085; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Motif Parameters. Technical Committees ISO/TC 213; ISO: Geneva, Switzerland, 1996.
- ISO 2813; Paints and Varnishes—Determination of Gloss Value at 20°, 60° and 85°. Technical Committee: ISO/TC 35/SC 9; ISO: Geneva, Switzerland, 2014.
- ISO 2808; Paints and Varnishes—Determination of Film Thickness. Technical Committee: ISO/TC 35/SC 9; ISO: Geneva, Switzerland, 2019.
- ISO 2409; Paints and Varnishes—Cross-Cut Test. Technical Committee: ISO/TC 35/SC 9; ISO: Geneva, Switzerland, 2020.
- ISO 1522; Paints and Varnishes—Pendulum Damping Test. Technical Committee: ISO/TC 35/SC 9; ISO: Geneva, Switzerland, 2022.
- ISO 1520; Paints and Varnishes—Cupping Test. Technical Committee: ISO/TC 35/SC 9; ISO: Geneva, Switzerland, 2006.
- ISO 1518-1; Paints and Varnishes—Determination of Scratch Resistance. Technical Committee: ISO/TC 35/SC 9; ISO: Geneva, Switzerland, 2023.
- EN 828; Adhesives—Wettability—Determination by Measurement of Contact Angle and Surface Free Energy of Solid Surface. European Committee for Standardization: Brussels, Belgium, 2014.
- ISO 834-2; Fire-Resistance Tests—Elements of Building Construction—Part 2: Requirements and Recommendations for Measuring Furnace Exposure on Test Samples. Technical Committee: ISO/TC 92/SC 2; ISO: Geneva, Switzerland, 2019.
- ISO 17872; Paints and Varnishes—Guidelines for the Introduction of Scribe Marks through Coatings on Metallic Panels for Corrosion Testing. Technical Committee: ISO/TC 35/SC 9; ISO: Geneva, Switzerland, 2019.
- ISO 2812-1; Paints and Varnishes—Determination of Resistance to Liquids—Part 1: Immersion in Liquids Other than Water. Technical Committee: ISO/TC 35/SC 9; ISO: Geneva, Switzerland, 2017.
- ISO 4628-8; Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance—Part 8: Assessment of Degree of Delamination and Corrosion around a Scribe or Other Artificial Defect. Technical Committee: ISO/TC 35/SC 9; ISO: Geneva, Switzerland, 2012.
- Czachor-Jadacka, D.; Pilch-Pitera, B.; Byczyński, Ł.; Kisiel, M.; Zioło, A. Hydrophobic polyurethane powder clear coatings with lower curing temperature: Study on the synthesis of new blocked polyisocyanates. Prog. Org. Coat. 2021, 159, 106402. [Google Scholar] [CrossRef]
- Byczyński, Ł.; Dutkiewicz, M.; Januszewski, R. Thermal behaviour and flame retardancy of polyurethane high-solid coatings modified with hexakis(2,3-epoxypropyl)cyclotriphosphazene. Prog. Org. Coat. 2017, 108, 51–58. [Google Scholar] [CrossRef]
- ISO 4628 1-10; Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance. Technical Committee: ISO/TC 35/SC 9; ISO: Geneva, Switzerland, 2016.
- Tansuğ, G.; Tüken, T.; Özyılmaz, A.T.; Erbil, M.; Yazıcı, B. Mild steel protection with epoxy top coated polypyrrole and polyaniline in 3.5% NaCl. Curr. Appl. Phys. 2007, 7, 440–445. [Google Scholar] [CrossRef]



| Materials | Vestagon B 1530 [%] | Sirales PE 6110 [%] | Phosphazene Modifier [%] | Benzoin [%] | Resiflow PV 88 [%] |
|---|---|---|---|---|---|
| Coating Symbol | |||||
| PU/0.0%Ph | 17.75 | 81.50 | 0.00 | 0.25 | 0.50 |
| PU/1.5%Ph | 19.23 | 78.72 | 1.50 | 0.25 | 0.50 |
| PU/2.5%Ph | 20.55 | 76.20 | 2.50 | 0.25 | 0.50 |
| PU/5%Ph | 20.25 | 74.00 | 5.00 | 0.25 | 0.50 |
| Symbol of Coating | PU/0.0%Ph | PU/1.5%Ph | PU/2.5%Ph | PU/5%Ph | |
|---|---|---|---|---|---|
| Roughness ISO 12085 | Ra Rz | 1.20 ± 0.02 4.30 ± 0.20 | 1.09 ± 0.02 3.21 ± 0.18 | 1.12 ± 0.03 3.33 ± 0.17 | 0.97 ± 0.05 2.40 ± 0.14 |
| Gloss for the angle of 60° ISO 2813 | GU | 101.6 ± 1.3 | 106.2 ± 1.5 | 105.9 ± 1.4 | 107.1 ± 1.5 |
| Adhesion to the steel surface ISO 2409 | 0—best 5—worst | 0 | 0 | 0 | 0 |
| Hardness ISO 1522 | - | 0.808 ± 0.002 | 0.806 ± 0.003 | 0.806 ± 0.003 | 0.807 ± 0.003 |
| Cupping ISO 1520 | mm | 10.66 ± 0.10 | 10.63 ± 0.07 | 10.63 ± 0.06 | 10.62 ± 0.09 |
| Scratch resistance ISO 1518 | g | 350 | 350 | 350 | 350 |
| Water contact angle EN 828 | deg | 84.9 ± 2.3 | 84.7 ± 2.5 | 84.0 ± 2.1 | 83.0 ± 2.1 |
| Sample | Time | Coating Thickness, µm |
| Uncoated steel sample | 5 min 40 s ± 1 s | - |
| PU/0.0%Ph | 5 min 25 s ± 1 s | 73.6 ± 1.2 |
| PU/1.5%Ph | 6 min ± 1 s | 71.9 ± 1.0 |
| PU/2.5%Ph | 6 min 12 s ± 1 s | 73.2 ± 1.1 |
| PU/5%Ph | 6 min 13 s ± 1 s | 72.1 ± 1.0 |
| Symbol of Coating | Degree of Delamination around the Scratches, mm | Degree of Corrosion, around the Scratches, mm | ||
|---|---|---|---|---|
| 650 h | 720 h | 650 h | 720 h | |
| PU/0.0%Ph | 6.80 ± 0.05 | 8.20 ± 0.04 | 2.63 ± 0.02 | 3.75 ± 0.02 |
| PU/1.5%Ph | no damages | 6.23 ± 0.05 | 1.08 ± 0.02 | 1.80 ± 0.02 |
| PU/2.5%Ph | no damages | 6.85 ± 0.03 | 0.52 ± 0.01 | 1.73 ± 0.01 |
| PU/5%Ph | no damages | 3.05 ± 0.02 | 0.10 ± 0.01 | 0.43 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilch-Pitera, B.; Czachor-Jadacka, D.; Byczyński, Ł.; Dutkiewicz, M.; Januszewski, R.; Kowalczyk, K.; Nowak, W.J.; Pojnar, K. Hexakis[p-(hydroxymethyl)phenoxy]cyclotriphosphazene as an Environmentally Friendly Modifier for Polyurethane Powder Coatings with Increased Thermal Stability and Corrosion Resistance. Materials 2024, 17, 2672. https://doi.org/10.3390/ma17112672
Pilch-Pitera B, Czachor-Jadacka D, Byczyński Ł, Dutkiewicz M, Januszewski R, Kowalczyk K, Nowak WJ, Pojnar K. Hexakis[p-(hydroxymethyl)phenoxy]cyclotriphosphazene as an Environmentally Friendly Modifier for Polyurethane Powder Coatings with Increased Thermal Stability and Corrosion Resistance. Materials. 2024; 17(11):2672. https://doi.org/10.3390/ma17112672
Chicago/Turabian StylePilch-Pitera, Barbara, Dominika Czachor-Jadacka, Łukasz Byczyński, Michał Dutkiewicz, Rafał Januszewski, Krzysztof Kowalczyk, Wojciech J. Nowak, and Katarzyna Pojnar. 2024. "Hexakis[p-(hydroxymethyl)phenoxy]cyclotriphosphazene as an Environmentally Friendly Modifier for Polyurethane Powder Coatings with Increased Thermal Stability and Corrosion Resistance" Materials 17, no. 11: 2672. https://doi.org/10.3390/ma17112672
APA StylePilch-Pitera, B., Czachor-Jadacka, D., Byczyński, Ł., Dutkiewicz, M., Januszewski, R., Kowalczyk, K., Nowak, W. J., & Pojnar, K. (2024). Hexakis[p-(hydroxymethyl)phenoxy]cyclotriphosphazene as an Environmentally Friendly Modifier for Polyurethane Powder Coatings with Increased Thermal Stability and Corrosion Resistance. Materials, 17(11), 2672. https://doi.org/10.3390/ma17112672








