Enhancing Wastewater Depollution: Sustainable Biosorption Using Chemically Modified Chitosan Derivatives for Efficient Removal of Heavy Metals and Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Shrimp Shells Preparation
2.1.2. Chitosan Extraction Steps
2.2. Structural Characterizations
2.3. Adsorption Experiments of Metal Ions
2.4. Adsorption Experiments of Dyes
2.5. Physical and Chemical Characterization of the Commercial, Extracted, and Hydrogel Chitosan
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whelan, M.J.; Linstead, C.; Worrall, F.; Ormerod, S.J.; Durance, I.; Johnson, A.C.; Johnson, D.; Owen, M.; Wiik, E.; Howden, N.J.K.; et al. Is water quality in British rivers “better than at any time since the end of the Industrial Revolution”? Sci. Total Environ. 2022, 843, 157014. [Google Scholar] [CrossRef]
- Pradhan, B.; Chand, S.; Chand, S.; Rout, P.R.; Naik, S.K. Emerging groundwater contaminants: A comprehensive review on their health hazards and remediation technologies. Groundw. Sustain. Dev. 2023, 20, 100868. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M.; Popa, M.; Calderon Moreno, J.M. Chitosan Hydrogels for Water Purification Applications. Gels 2023, 9, 664. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Alves, D.C.; Healy, B.; Pinto, L.A.D.A.; Cadaval, T.R.S.; Breslin, C.B. Recent Developments in Chitosan-Based Adsorbents for the Removal of Pollutants from Aqueous Environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef] [PubMed]
- Nechita, P. Applications of Chitosan in Wastewater Treatment. In Biological Activities and Application of Marine Polysaccharides; Shalaby, E.A., Ed.; InTech: Houston, TX, USA, 2017. [Google Scholar]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Elemike, E.E.; Onwudiwe, D.C.; Onyango, M.S. Metal oxide-cellulose nanocomposites for the removal of toxic metals and dyes from wastewater. Int. J. Biol. Macromol. 2020, 164, 2477–2496. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2017, 4, 411–427. [Google Scholar] [PubMed]
- Vicente, F.A.; Ventura, S.P.M.; Passos, H.; Dias, A.C.R.V.; Torres-Acosta, M.A.; Novak, U.; Likozar, B. Crustacean waste biorefinery as a sustainable cost-effective business model. Chem. Eng. J. 2022, 442, 135937. [Google Scholar] [CrossRef]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent advances in heavy metal removal by chitosan based adsorbents. Carbohydr. Polym. 2021, 251, 117000. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Borsagli, F.G.L.M.; Borsagli, A. Chemically Modified Chitosan Bio-Sorbents for the Competitive Complexation of Heavy Metals Ions: A Potential Model for the Treatment of Wastewaters and Industrial Spills. J. Polym. Environ. 2019, 27, 1542–1556. [Google Scholar] [CrossRef]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Grégorio, C. (Eds.) Eaux Industrielles Contaminées: Réglementation, Paramètres Chimiques et Biologiques & Procédés D’épuration Innovants; Presses Universitaires de Franche-Comté: Besançon, France, 2017. [Google Scholar]
- Popuri, S.R.; Vijaya, Y.; Boddu, V.M.; Abburi, K. Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads. Bioresour. Technol. 2009, 100, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Chinnathambi, A.; Alahmadi, T.A. Zinc nanoparticles green-synthesized by Alhagi maurorum leaf aqueous extract: Chemical characterization and cytotoxicity, antioxidant, and anti-osteosarcoma effects. Arab. J. Chem. 2021, 14, 103083. [Google Scholar] [CrossRef]
- Baldino, L.; Concilio, S.; Cardea, S.; De Marco, I.; Reverchon, E. Complete glutaraldehyde elimination during chitosan hydrogel drying by SC-CO2 processing. J. Supercrit. Fluids 2015, 103, 70–76. [Google Scholar] [CrossRef]
- Pellá, M.C.G.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Mohammadzadeh Pakdel, P.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, V.; Park, D.; Lee, Y.-C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Zhang, T.-Y.; Dao, G.-H.; Xu, X.-Q.; Wang, X.-X.; Hu, H.-Y. Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl. Microbiol. Biotechnol. 2017, 101, 2659–2675. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, W.; Zou, B.; Zhou, L.; Ye, Z.; Zhao, Q. Preparation of EDTA-modified magnetic attapulgite chitosan gel bead adsorbent for the removal of Cu(II), Pb(II), and Ni(II). Int. J. Biol. Macromol. 2021, 182, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [PubMed]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination of degree of deacetylation of chitosan—Comparison of methods determination. Prog. Chem. Appl. Chitin Its Deriv. 2012, 17, 5–20. [Google Scholar]
- Chang, S.-H.; Lin, H.-T.V.; Wu, G.-J.; Tsai, G.J. pH Effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohydr. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.N.; Agnely, F.; Yagoubi, N.; Grossiord, J.L.; Couarraze, G. Water state characterization, swelling behavior, thermal and mechanical properties of chitosan based networks. Eur. J. Pharm. Sci. 2002, 15, 425–432. [Google Scholar] [CrossRef]
- Reynel-Avila, H.E.; Bonilla-Petriciolet, A.; de la Rosa, G. Analysis and modeling of multicomponent sorption of heavy metals on chicken feathers using Taguchi’s experimental designs and artificial neural networks. Desalination Water Treat. 2015, 55, 1885–1899. [Google Scholar] [CrossRef]
- Eddya, M.; Tbib, B.; EL-Hami, K. A comparison of chitosan properties after extraction from shrimp shells by diluted and concentrated acids. Heliyon 2020, 6, e03486. [Google Scholar] [CrossRef] [PubMed]
- Mahaninia, M.H.; Wilson, L.D. Modular Cross-Linked Chitosan Beads with Calcium Doping for Enhanced Adsorptive Uptake of Organophosphate Anions. Ind. Eng. Chem. Res. 2016, 55, 11706–11715. [Google Scholar] [CrossRef]
- Muley, A.B.; Chaudhari, S.A.; Mulchandani, K.H.; Singhal, R.S. Extraction and characterization of chitosan from prawn shell waste and its conjugation with cutinase for enhanced thermo-stability. Int. J. Biol. Macromol. 2018, 111, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Nandhakumar, S.; Dhanaraju, M. Formulation and Characterization of Glutaraldehyde Cross-Linked Chitosan Biodegradable Microspheres Loaded with Famotidine. Trop. J. Pharm. Res. 2011, 10. [Google Scholar] [CrossRef]
- Ahmed, A.; Hassan, A.; Nour, M. Utilization of chitosan extracted from shrimp shell waste in wastewater treatment as low cost biosorbent. Egypt. J. Chem. 2020, 64, 981–988. [Google Scholar] [CrossRef]
- Phongying, S.; Aiba, S.; Chirachanchai, S. Direct chitosan nanoscaffold formation via chitin whiskers. Polymer 2007, 48, 393–400. [Google Scholar] [CrossRef]
- Sumaila, A.; Ndamitso, M.M.; Iyaka, Y.A.; Abdulkareem, S.A.; Tijani, J.O.; Idris, M.O. Extraction and Characterization of Chitosan from Crab Shells: Kinetic and Thermodynamic Studies of Arsenic and Copper Adsorption from Electroplating Wastewater. Iraqi J. Sci. 2020, 61, 2156–2171. [Google Scholar] [CrossRef]
- Li, P.-C.; Liao, G.; Kumar, S.R.; Shih, C.-M.; Yang, C.-C.; Wang, D.-M.; Lue, S.J. Fabrication and Characterization of Chitosan Nanoparticle-Incorporated Quaternized Poly(vinyl alcohol) Composite Membranes as Solid Electrolytes for Direct Methanol Alkaline Fuel Cells. Electrochim. Acta 2016, 187, 616–628. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Nikpour, M.R.; Rabiee, S.M.; Jahanshahi, M. Synthesis and characterization of hydroxyapatite/chitosan nanocomposite materials for medical engineering applications. Compos. Part B Eng. 2012, 43, 1881–1886. [Google Scholar] [CrossRef]
- Matijevic, E. (Ed.) Medical Applications of Colloids; Springer: New York, NY, USA, 2008. [Google Scholar]
- Rouxhet, P.G.; Genet, M.J. XPS analysis of bio-organic systems. Surf. Interface Anal. 2011, 43, 1453–1470. [Google Scholar] [CrossRef]
- Hoffmann, B.; Seitz, D.; Mencke, A.; Kokott, A.; Ziegler, G. Glutaraldehyde and oxidised dextran as crosslinker reagents for chitosan-based scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2009, 20, 1495–1503. [Google Scholar] [CrossRef]
- Vondran, J.L.; Sun, W.; Schauer, C.L. Crosslinked, electrospun chitosan–poly(ethylene oxide) nanofiber mats. J. Appl. Polym. Sci. 2008, 109, 968–975. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Bernabé, P.; Becherán, L.; Cabrera-Barjas, G.; Nesic, A.; Alburquenque, C.; Tapia, C.V.; Taboada, E.; Alderete, J.; De Los Ríos, P. Chilean crab (Aegla cholchol) as a new source of chitin and chitosan with antifungal properties against Candida spp. Int. J. Biol. Macromol. 2020, 149, 962–975. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural differences between chitin and chitosan extracted from three different marine sources. Int. J. Biol. Macromol. 2014, 65, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef]
- Sila, A.; Mlaik, N.; Sayari, N.; Balti, R.; Bougatef, A. Chitin and Chitosan Extracted from Shrimp Waste Using Fish Proteases Aided Process: Efficiency of Chitosan in the Treatment of Unhairing Effluents. J. Polym. Environ. 2014, 22, 78–87. [Google Scholar] [CrossRef]
- Younes, I.; Ghorbel-Bellaaj, O.; Nasri, R.; Chaabouni, M.; Rinaudo, M.; Nasri, M. Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochem. 2012, 47, 2032–2039. [Google Scholar] [CrossRef]
- Doustdar, F.; Olad, A.; Ghorbani, M. Effect of glutaraldehyde and calcium chloride as different crosslinking agents on the characteristics of chitosan/cellulose nanocrystals scaffold. Int. J. Biol. Macromol. 2022, 208, 912–924. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Deshmukh, P.G.; Jakhade, A.P.; Kulkarni, S.D.; Chikate, R.C. 1,5 Diphenyl carbazide immobilized cross-linked chitosan films: An integrated approach towards enhanced removal of Cr(VI). J. Mol. Liq. 2017, 247, 254–261. [Google Scholar] [CrossRef]
- Borja-Urzola, A.D.C.; García-Gómez, R.S.; Flores, R.; Durán-Domínguez-de-Bazúa, M.D.C. Chitosan from shrimp residues with a saturated solution of calcium chloride in methanol and water. Carbohydr. Res. 2020, 497, 108116. [Google Scholar] [CrossRef] [PubMed]
- Komkiene, J.; Baltrenaite, E. Biochar as adsorbent for removal of heavy metal ions [Cadmium(II), Copper(II), Lead(II), Zinc(II)] from aqueous phase. Int. J. Environ. Sci. Technol. 2016, 13, 471–482. [Google Scholar] [CrossRef]
- Pyrzynska, K. Removal of cadmium from wastewaters with low-cost adsorbents. J. Environ. Chem. Eng. 2019, 7, 102795. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Suganya, E.; Selvaraju, N.; Varghese, L.A. Significance of exploiting non-living biomaterials for the biosorption of wastewater pollutants. World J. Microbiol. Biotechnol. 2014, 30, 1669–1689. [Google Scholar] [CrossRef]
- El malti, W.; Hijazi, A.; Khalil, Z.A.; Yaghi, Z.; Medlej, M.K.; Reda, M. Comparative study of the elimination of copper, cadmium, and methylene blue from water by adsorption on the citrus Sinensis peel and its activated carbon. RSC Adv. 2022, 12, 10186–10197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.; Yang, L.; Chu, Y. Research on Progress in Combined Remediation Technologies of Heavy Metal Polluted Sediment. Int. J. Environ. Res. Public Health 2019, 16, 5098. [Google Scholar] [CrossRef] [PubMed]
- Zapién Serrano, L.; Ortiz Lara, N.; Ríos Vera, R.; Cholico-González, D. Removal of Fe(III), Cd(II), and Zn(II) as Hydroxides by Precipitation–Flotation System. Sustainability 2021, 13, 11913. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Li, J.; Yang, Y.; Liu, X. Surface modified leaves with high efficiency for the removal of aqueous Cr (VI). Appl. Surf. Sci. 2019, 484, 189–196. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Shen, F.; Wu, C.; Gu, S. Equilibrium, kinetics and thermodynamics of cadmium ions (Cd2+) removal from aqueous solution using earthworm manure-derived carbon materials. J. Mol. Liq. 2017, 241, 612–621. [Google Scholar] [CrossRef]
- Dada, A.O.; Adekola, F.A.; Odebunmi, E.O. Liquid Phase Scavenging of Cd(II) and Cu(II) Ions onto Novel Nanoscale Zerovalent Manganese (nZVMn): Equilibrium, kinetic and thermodynamic studies. Environ. Nanotechnol. Monit. Manag. 2017, 8, 63–72. [Google Scholar] [CrossRef]
- Azizian, S.; Eris, S.; Wilson, L.D. Re-Evaluation of the Century-Old Langmuir Isotherm for Modeling Adsorption Phenomena in Solution. Chem. Phys. 2018, 513, 99–104. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Elanchezhiyan, S.S.D.; Preethi, J.; Talikdar, K.; Meenakshi, S.; Park, C.M. Two-Dimensional (2D) Ti3C2Tx MXene Nanosheets with Superior Adsorption Behavior for Phosphate and Nitrate Ions from the Aqueous Environment. Ceram. Int. 2021, 47, 732–739. [Google Scholar] [CrossRef]
- Castaldi, P.; Silvetti, M.; Garau, G.; Demurtas, D.; Deiana, S. Copper(II) and lead(II) removal from aqueous solution by water treatment residues. J. Hazard. Mater. 2015, 283, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of Heavy Metal Ions by Various Low-Cost Adsorbents: A Review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Pavithra, S.; Thandapani, G.; Sugashini, S.; Sudha, P.N.; Alkhamis, H.H.; Alrefaei, A.F.; Almutairi, M.H. Batch Adsorption Studies on Surface Tailored Chitosan/Orange Peel Hydrogel Composite for the Removal of Cr(VI) and Cu(II) Ions from Synthetic Wastewater. Chemosphere 2021, 271, 129415. [Google Scholar] [CrossRef] [PubMed]
- Pietrelli, L.; Francolini, I.; Piozzi, A.; Sighicelli, M.; Silvestro, I.; Vocciante, M. Chromium(III) Removal from Wastewater by Chitosan Flakes. Appl. Sci. 2020, 10, 1925. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Kamari, A.; Koay, Y.J. Equilibrium and kinetics studies of adsorption of copper (II) on chitosan and chitosan/PVA beads. Int. J. Biol. Macromol. 2004, 34, 155–161. [Google Scholar] [CrossRef]
- Radha, E.; Gomathi, T.; Sudha, P.N.; Latha, S.; Ghfar, A.A.; Hossain, N. Adsorption studies on removal of Pb(II) and Cd(II) ions using chitosan derived copoymeric blend. Biomass Convers. Biorefin. 2021, 7, 1–6. [Google Scholar] [CrossRef]
- Ling, S.L.Y.; Yee, C.Y.; Eng, H.S. Removal of A Cationic Dye using Deacetylated Chitin (Chitosan). J. Appl. Sci. 2011, 11, 1445–1448. [Google Scholar] [CrossRef]
- Mu, R.; Liu, B.; Chen, X.; Wang, N.; Yang, J. Adsorption of Cu (II) and Co (II) from aqueous solution using lignosulfonate/chitosan adsorbent. Int. J. Biol. Macromol. 2020, 163, 120–127. [Google Scholar] [CrossRef]
- Kuczajowska-Zadrożna, M.; Filipkowska, U.; Jóźwiak, T. Adsorption of Cu (II) and Cd (II) from aqueous solutions by chitosan immobilized in alginate beads. J. Environ. Chem. Eng. 2020, 8, 103878. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Saleh, A.S.; Elsharma, E.M.; Metwally, E.; Siyam, T. Chitosan-g-maleic acid for effective removal of copper and nickel ions from their solutions. Int. J. Biol. Macromol. 2019, 121, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.-W.; Kan, C.-C.; Rogel, B.D.; Dalida, M.L.P. Adsorption of copper (II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohydr. Polym. 2010, 80, 891–899. [Google Scholar] [CrossRef]
- Doshi, B.; Ayati, A.; Tanhaei, B.; Repo, E.; Sillanpää, M. Partially carboxymethylated and partially cross-linked surface of chitosan versus the adsorptive removal of dyes and divalent metal ions. Carbohydr. Polym. 2018, 197, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.-L.; Ren, J.-Q.; Li, H.-J.; Wu, D.-G.; Wu, Y.-C. Mesoporous crosslinked chitosan-activated clinoptilolite biocomposite for the removal of anionic and cationic dyes. Colloids Surf. B Biointerfaces 2022, 216, 112579. [Google Scholar] [CrossRef]
- Pal, A.; Pan, S.; Saha, S. Synergistically improved adsorption of anionic surfactant and crystal violet on chitosan hydrogel beads. Chem. Eng. J. 2013, 217, 426–434. [Google Scholar] [CrossRef]
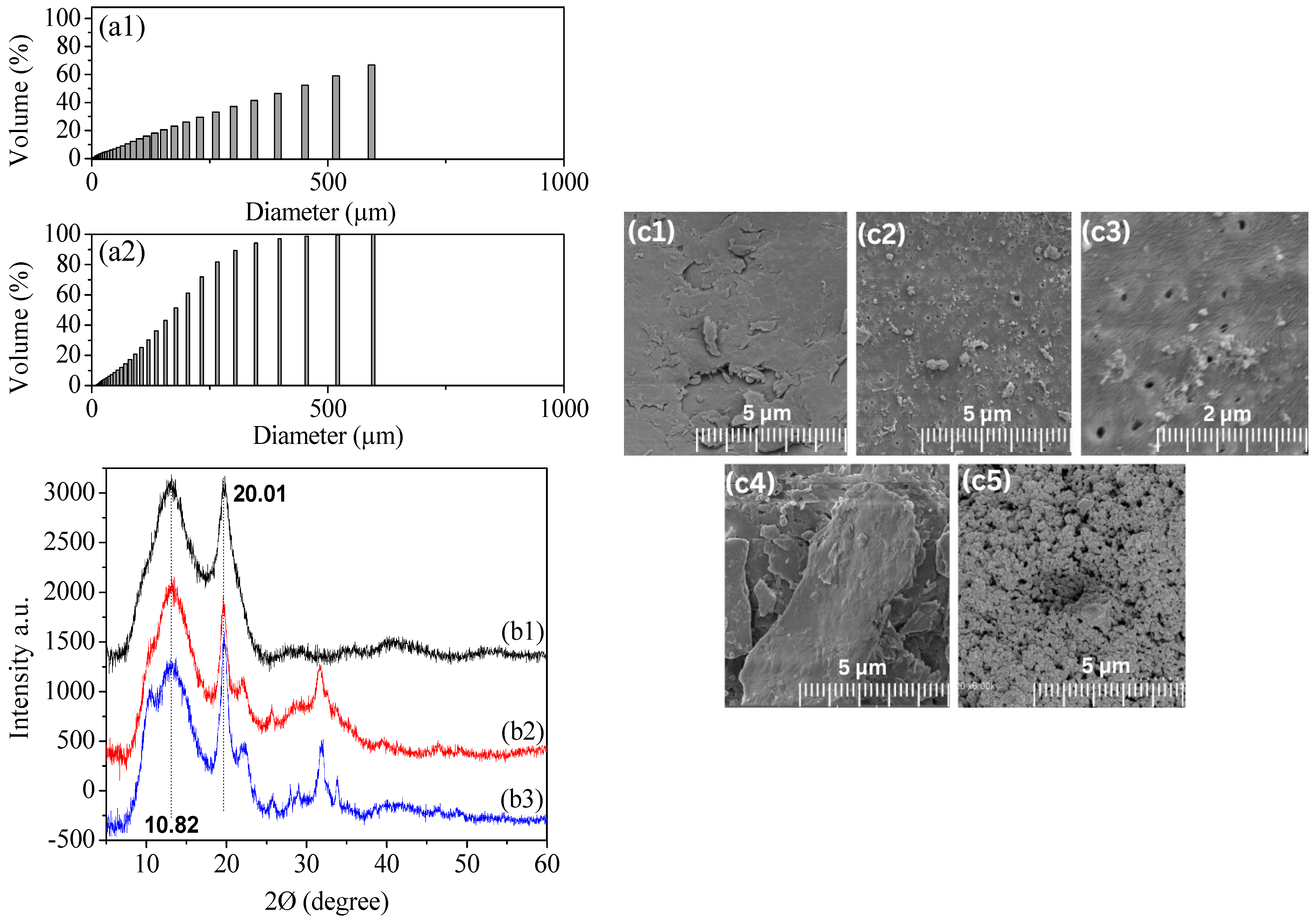

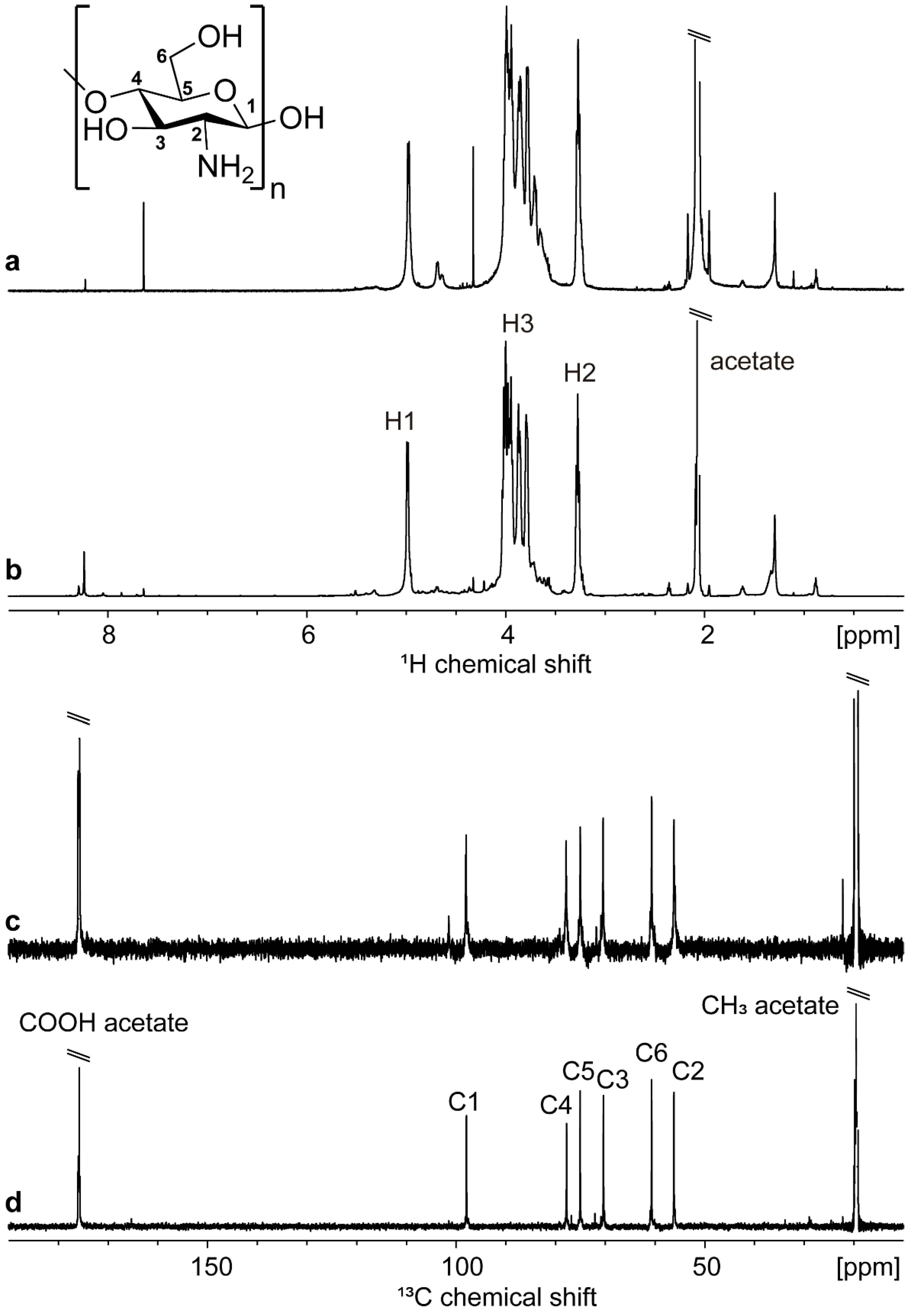
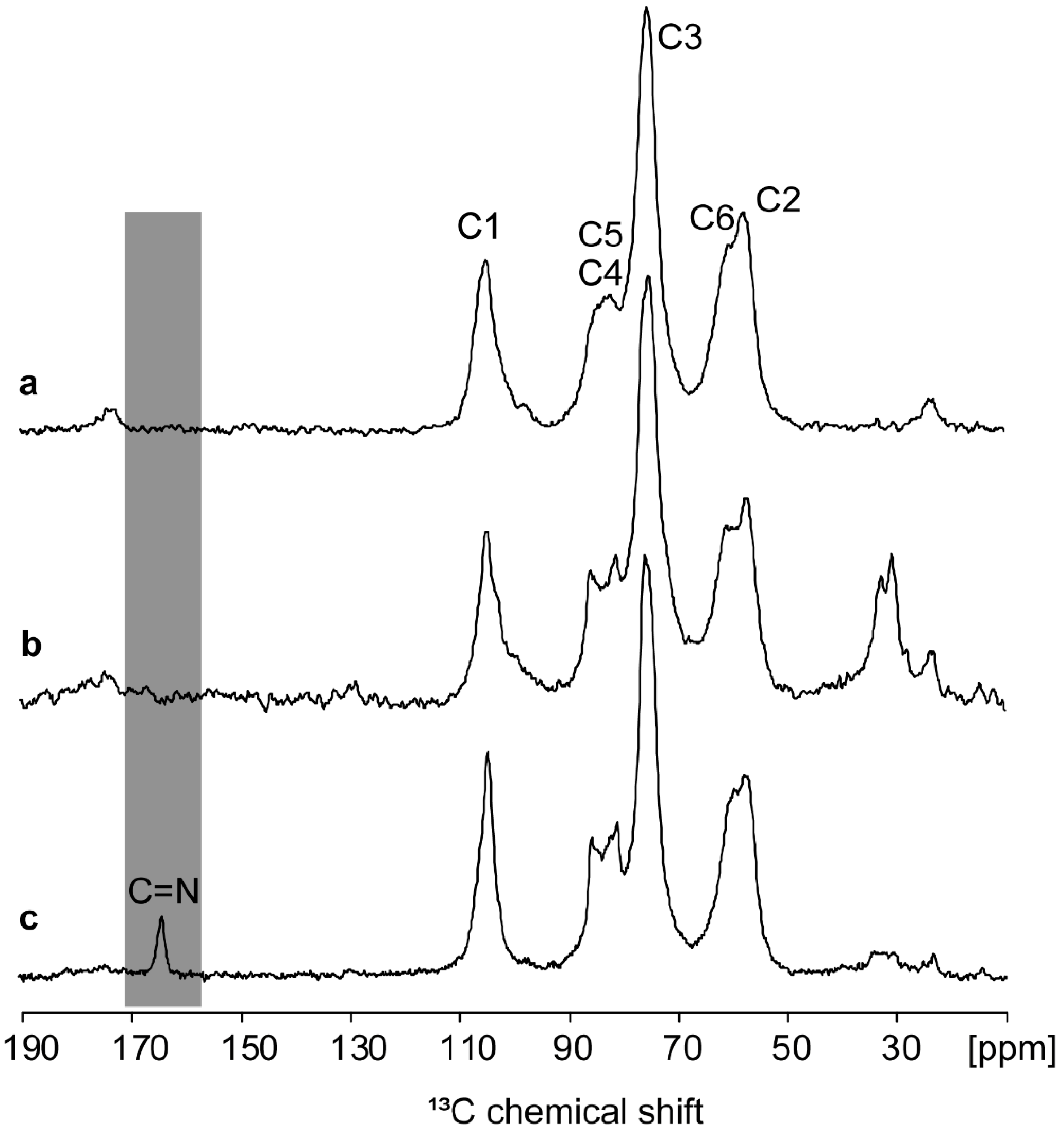
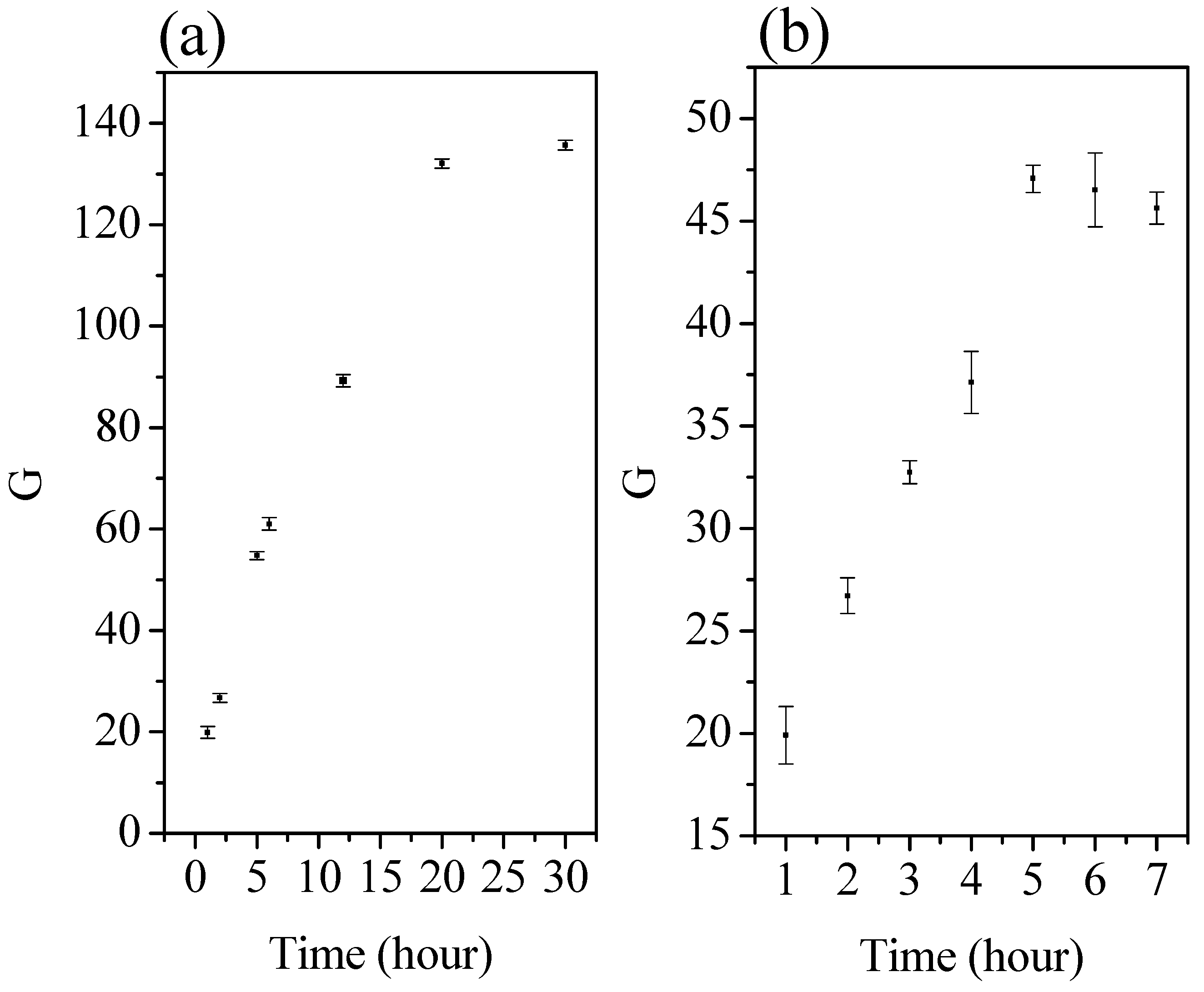

| Parameter | Commercial Chitosan | Extracted Chitosan | |
|---|---|---|---|
| BET surface area (m2/g) | 0.27 | 7.43 | |
| Total pore volume (cm3/g) | 0.01 | 0.021 | |
| Average pore diameter (nm) | 7.02 | 9.46 | |
| Elemental analysis (XPS in %) | O 1s | 35.31 | 29.08 |
| N 1s | 3.50 | 6.65 | |
| C 1s | 61.19 | 64.27 | |
| Factors | Chitosan Commercial | Chitosan Extracted | Chitosan Hydrogel | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pb2+ | Cu2+ | MB | CV | Pb2+ | Cu2+ | MB | CV | Pb2+ | Cu2+ | MB | CV | ||
| 1 | Time of contact (min) | 60 | 60 | 120 | 120 | 60 | 60 | 120 | 120 | 60 | 60 | 120 | 120 |
| Maximum Ads % | 93 | 94 | 95 | 76 | 98 | 99 | 99 | 98 | 75 | 80 | 83 | 95 | |
| 2 | pH | 7 | 7 | 4.5 | 4.5 | 7 | 7 | 4.5 | 4.5 | 7 | 7 | 4.5 | 4.5 |
| Maximum Ads % | 60 | 75 | 99 | 95 | 98 | 80 | 95 | 96 | 92 | 99 | 85 | 99 | |
| 3 | Temperature | 40 | 25 | 40 | 25 | 40 | 25 | 40 | 25 | 40 | 25 | 40 | 25 |
| Maximum Ads % | 82 | 94 | 99 | 98 | 95 | 96 | 99 | 90 | 95 | 98 | 83 | 97 | |
| 4 | Concentration | 8 | 4 | 10 | 2 | 8 | 4 | 10 | 2 | 8 | 4 | 10 | 2 |
| Maximum Ads % | 80 | 92 | 99 | 98 | 80 | 96 | 99 | 98 | 85 | 96 | 92 | 98 | |
| Isotherm Models | Freundlich | Dubinin–Radushkevich | ||||||
|---|---|---|---|---|---|---|---|---|
| Adsorbates | Parameters | Parameters | ||||||
| Kf | n | R2 | β | qm (mg/g) | E (KJ/mol) | R2 | ||
| Cu2+ | Commercial | 4.501 | 0.572 | 0.9724 | 0.1549 | 4.737 | 1.97 | 0.941 |
| Extracted | 8.323 | 0.265 | 0.9514 | 0.4024 | 13.994 | 1.115 | 0.9152 | |
| Pb2+ | Commercial | 6.482 | 0.338 | 0.9842 | 0.3021 | 8.696 | 1.286 | 0.9853 |
| Extracted | 10.402 | 0.450 | 0.9312 | 0.1935 | 9.845 | 1.607 | 0.9644 | |
| MB | Commercial | 6.785 | 0.604 | 0.9785 | 0.286 | 5.847 | 1.521 | 0.9985 |
| Extracted | 12.101 | 0.351 | 0.9842 | 0.3581 | 15.845 | 1.254 | 0.9854 | |
| CV | Commercial | 8.569 | 0.851 | 0.9957 | 0.451 | 6.487 | 1.99 | 0.9965 |
| Extracted | 14.526 | 0.541 | 0.9888 | 0.2547 | 7.257 | 1.758 | 0.9954 | |
| Chitosan from Other Studies | Present Study | |||||
|---|---|---|---|---|---|---|
| Name | Adsorbate | Adsorption Capacity (%) | References | Name | Adsorbate | Adsorption Capacity (%) |
| Chitosan | Cu2+ | 9.29 | [71] | Chitosan powder | Cu2+ | 98.98 |
| Pb2+ | 89 | [72] | Chitosan cross-linked gel GA | Cu2+ | 85 | |
| CV | 8.91 | [73] | ||||
| Chitosan/poly(vinyl alcohol) | Cu2+ | 13.52 | [71] | Chitosan powder | Pb2+ | 80 |
| Chitosan cross-linked gel GA | Pb2+ | 95 | ||||
| Sodium lignosulfonate/chitosan | Cu2+ | 89.9 | [74] | Chitosan powder | MB | 98 |
| Chitosan cross-linked gel GA | MB | 85 | ||||
| Chitosan immobilized in alginate beads | Cu2+ | 77 | [75] | Chitosan powder | CV | 86 |
| Chitosan cross-linked gel GA | CV | |||||
| Chitosan/Acrylic acid | Cu2+ | 88 | [75] | |||
| Chitosan-grafted maleic acid | Cu2+ | 88 | [76] | |||
| Chitosan/cellulose | Cu2+ | 7.46 | [77] | |||
| Pb2+ | 7.41 | |||||
| Chitosan/sand | Cu2+ | 13.47 | [78] | |||
| Pb2+ | 3.47 | |||||
| Glutaraldehyde-cross-linked chitosan | MB | 95.22 | [79] | |||
| CV | 87 | |||||
| Cross-linked chitosan/Activated clinoptilolite | MB | 40.47 | [80] | |||
| Chitosan hydrogel beads | CV | 1.54 | [81] | |||
| Chitosan–graphite oxide modified polyurethane | CV | 18.29 | [41] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayach, J.; Duma, L.; Badran, A.; Hijazi, A.; Martinez, A.; Bechelany, M.; Baydoun, E.; Hamad, H. Enhancing Wastewater Depollution: Sustainable Biosorption Using Chemically Modified Chitosan Derivatives for Efficient Removal of Heavy Metals and Dyes. Materials 2024, 17, 2724. https://doi.org/10.3390/ma17112724
Ayach J, Duma L, Badran A, Hijazi A, Martinez A, Bechelany M, Baydoun E, Hamad H. Enhancing Wastewater Depollution: Sustainable Biosorption Using Chemically Modified Chitosan Derivatives for Efficient Removal of Heavy Metals and Dyes. Materials. 2024; 17(11):2724. https://doi.org/10.3390/ma17112724
Chicago/Turabian StyleAyach, Jana, Luminita Duma, Adnan Badran, Akram Hijazi, Agathe Martinez, Mikhael Bechelany, Elias Baydoun, and Hussein Hamad. 2024. "Enhancing Wastewater Depollution: Sustainable Biosorption Using Chemically Modified Chitosan Derivatives for Efficient Removal of Heavy Metals and Dyes" Materials 17, no. 11: 2724. https://doi.org/10.3390/ma17112724






