Possibilities of Managing Waste Iron Sorbent FFH after CO2 Capture as an Element of a Circular Economy
Abstract
:1. Introduction
2. Materials and Methods
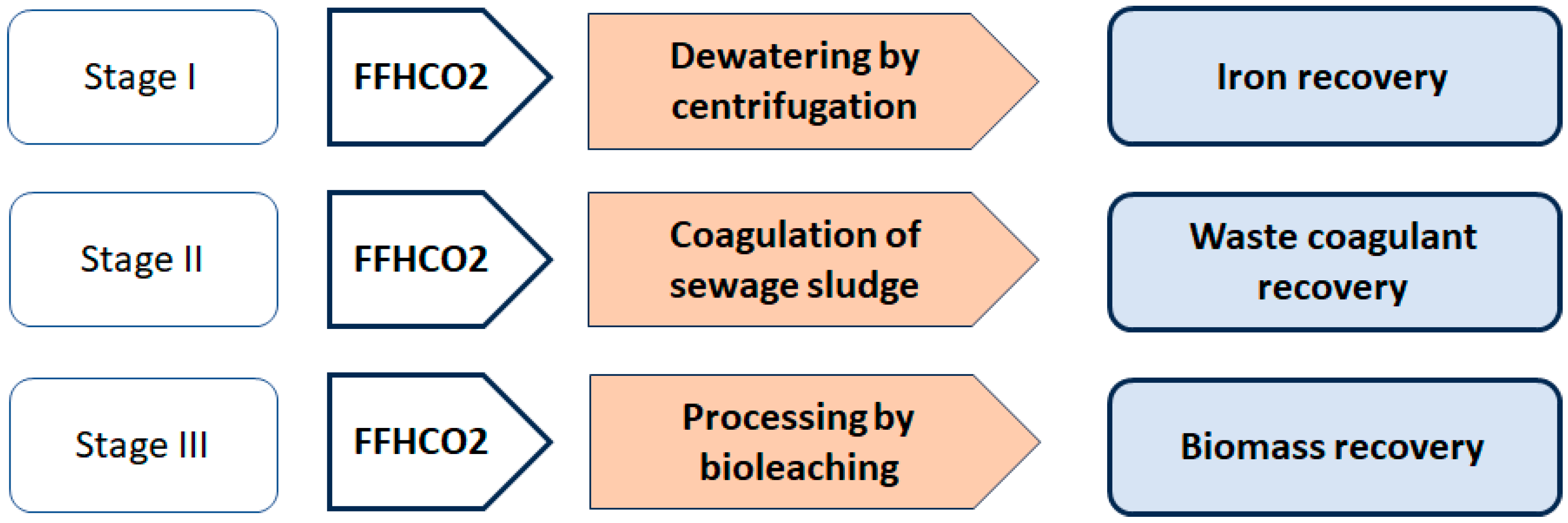
2.1. Research Stages
2.2. The FFHCO2 Substrate
2.3. Materials and Reagents Used in Stage I Research
2.4. Materials and Reagents Used in Stage II Research
2.5. Materials and Reagents Used in Stage III Research
2.5.1. Cultivation of Bioleaching Bacteria in Stage III
2.5.2. Inoculation (Stage III)
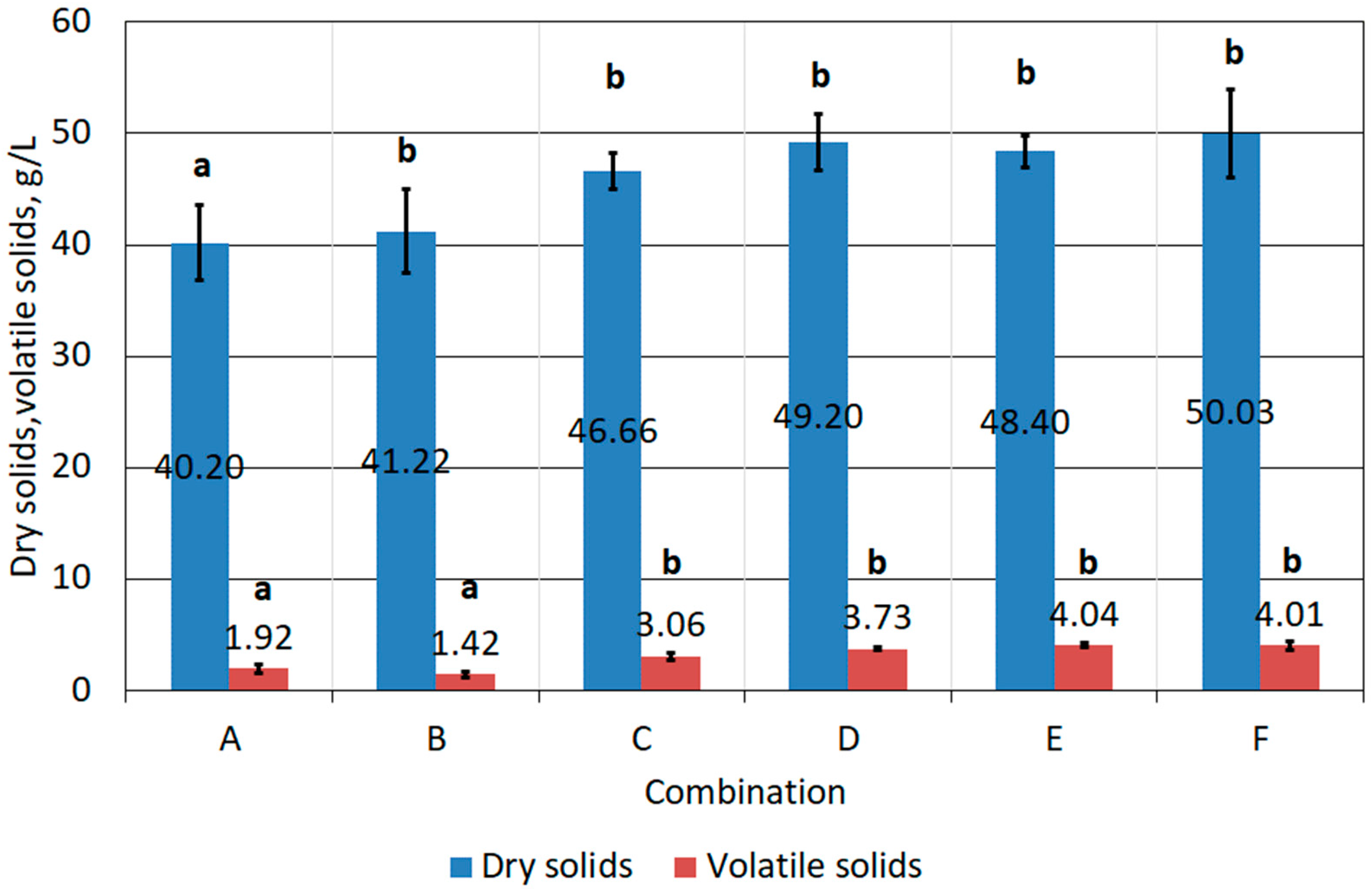
2.5.3. Research Combinations (Stage III)
2.6. Methodology
- dry solids (DS) [29].
- volatile solids (VS), [30].
- capillary suction time (CST), [31].
- specific resistance of filtration (SRT), [32].
- pH was determined using the potentiometric method (Elmetron CPC-505, Zabrze, Poland).
- Conductance (C) was determined using direct conductivity (Hydromet CD-210 probe, Gliwice, Poland).
- Reduction–oxidation potential (redox) was determined by an electrochemical method (Hydromet ERPt—11X probe, Gliwice, Poland).
- Alkalinity was determined by titration using a pH meter in accordance with the standard [33].
- The content of sulfur and iron compounds was determined using cuvette tests for a HACH DR 6000 spectrophotometer (Hach Company, Loveland, CO, USA); LCK 153—sulfates, LCK, LCK 321—iron, and LCK 654—sulfites.
- The turbidity (T) of the filtrate after separation of the solid phase in the vacuum filtration process was determined using a Hach 2100N turbidity meter (Hach Company, Loveland, CO, USA).
- The efficiency of phase separation by a centrifugation (Centrifugation efficiency, CE) method was determined using the following formula:
2.7. Statistical Analysis
3. Results and Discussion
3.1. Results of Stage I Tests
3.2. Results of Stage II Tests
3.3. Results of Stage III Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sattari, A.; Ramazani, A.; Aghahosseini, H.; Aroua, M.K. The application of polymer containing materials in CO2 capturing via absorption and adsorption methods. J. CO2 Util. 2021, 48, 101526. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Karayil, A.; Elseragy, A.; Aliyu, A.M. An Assessment of CO2 Capture Technologies towards Global Carbon Net Neutrality. Energies 2024, 17, 1460. [Google Scholar] [CrossRef]
- Budilovskis, D.; Šalkauskas, M. Nanopraticle Ferriferous Hydrosol as an Advanced Reagent for Waste Water Treatment. In Proceedings of the Linnaeus ECO-TECH’10, Kalmar, Sweden, 22–24 November 2010. [Google Scholar] [CrossRef]
- Mendoza, E.Y.M.; Santos, A.S.; López, E.V.; Drozd, V.; Durygin, A.; Chen, J.; Saxena, S.K. Iron oxides as efficient sorbents for CO2 capture. J. Mater. Res. Technol. 2019, 8, 2944–2956. [Google Scholar] [CrossRef]
- Alfe, M.; Ammendola, P.; Gargiulo, V.; Raganati, F.; Chirone, R. Magnetite loaded carbon fine particles as low-cost CO2 adsorbent in a sound assisted fluidized bed. Proc. Combust. Inst. 2015, 35, 2801–2809. [Google Scholar] [CrossRef]
- Wienchol, P.; Szlęk, A.; Ditaranto, M. Waste-to-energy technology integrated with carbon capture—Challenges and opportunities. Energy 2020, 198, 117352. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, Y.; Adewuyi, Y.G. State-of-the-art review on capture of CO2 using adsorbents prepared from waste materials. Process. Saf. Environ. Prot. 2020, 139, 1–25. [Google Scholar] [CrossRef]
- Mukherjee, C.; Denney, J.; Mbonimpa, E.G.; Slagley, J.; Bhowmik, R. A review on municipal solid waste-to-energy trends in the USA. Renew. Sustain. Energy Rev. 2020, 119, 109512. [Google Scholar] [CrossRef]
- Alsarhan, L.M.; Alayyar, A.S.; Alqahtani, N.B.; Khdary, N.H. Circular Carbon Economy (CCE): A Way to Invest CO2 and Protect the Environment, a Review. Sustainability 2021, 13, 11625. [Google Scholar] [CrossRef]
- Glavic, P.; Pintaric, Z.N.; Bogataj, M. Process Design and Sustainable Development—A European Perspective. Processes 2021, 9, 148. [Google Scholar] [CrossRef]
- Thomas, A.; Mishra, U. A green energy circular system with carbon capturing and waste minimization in a smart grid power management. Energy Rep. 2022, 8, 14102–14123. [Google Scholar] [CrossRef]
- Yu, Z.; Khan, S.A.R.; Ponce, P.; Zia-Ul-Haq, H.M.; Ponce, K. Exploring essential factors to improve waste-to-resource recovery: A roadmap towards sustainability. J. Clean. Prod. 2022, 350, 131305. [Google Scholar] [CrossRef]
- Magnanelli, E.; Mosby, J.; Becidan, M. Scenarios for carbon capture integration in a waste-to-energy plant. Energy 2021, 227, 120407. [Google Scholar] [CrossRef]
- Zabochnicka, M.; Krzywonos, M.; Romanowska-Duda, Z.; Szufa, S.; Darkalt, A.; Mubashar, M. Algal biomass utilization toward circular economy. Life 2022, 12, 1480. [Google Scholar] [CrossRef]
- Zabochnicka, M. An innovative look into ammonium nitrogen removal by algae and zeolites as an element of a circular bioeconomy. Appl. Sci. 2023, 13, 10220. [Google Scholar] [CrossRef]
- Bello, A.S.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. Sustainable and long-term management of municipal solid waste: A review. Bioresour. Technol. Rep. 2022, 18, 101067. [Google Scholar] [CrossRef]
- Miliute-Plepiene, J.; Sundqvist, J.-O. Assessing the Potential Climate Impacts and Benefits of Waste Prevention and Management: A Case Study of Sweden. Sustainability 2024, 16, 3799. [Google Scholar] [CrossRef]
- Cannon, C.E.B. Critical Environmental Injustice: A Case Study Approach to Understanding Disproportionate Exposure to Toxic Emissions. Toxics 2024, 12, 295. [Google Scholar] [CrossRef]
- Zabochnicka-Świątek, M. Utilization of Chlorella vulgaris and sediments after N-NH4 removal containing clinoptilolite for sorption of heavy metals from wastewater. Rocz. Ochr. Srodowiska 2013, 15, 324–347. [Google Scholar]
- Zabochnicka, M. Industrial wastewater as a growth medium for microalgal biomass for a sustainable circular bioeconomy. Appl. Sci. 2022, 12, 10299. [Google Scholar] [CrossRef]
- Donat, F.; Florin, N.H.; Anthony, E.J.; Fennell, P.S. Influence of High-Temperature Steam on the Reactivity of CaO Sorbent for CO2 Capture. Environ. Sci. Technol. 2012, 46, 1262–1269. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent Advances in Solid Sorbents for CO2 Capture and New Development Trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Cavazos, P.A.S.; Hunter-Sellars, E.; Iacomi, P.; McIntyre, S.R.; Danaci, D.; Williams, D.R. Evaluating solid sorbents for CO2 capture: Linking material properties and process efficiency via adsorption performance. Front. Energy Res. 2023, 11, 1167043. [Google Scholar] [CrossRef]
- Shewchuk, S.; Mukherjee, A.; Dalai, A. Selective carbon-based adsorbents for carbon dioxide capture from mixed gas streams and catalytic hydrogenation of CO2 into renewable energy source: A review. Chem. Eng. Sci. 2021, 243, 116735. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Li, B.; Sun, C.; Deng, F.; Song, C.; Wang, S. Isolation of Thiobacillus spp. and its application in the removal of heavy metals from activated sludge. Afr. J. Biotechnol. 2012, 11, 16336–16341. [Google Scholar]
- Gu, T.; Rastegar, S.O.; Mousavi, S.M.; Li, M.; Zhou, M. Advances in bioleaching for recovery of metals and bioremediation of fuel ash and sewage sludge. Bioresour. Technol. 2018, 261, 428–440. [Google Scholar] [CrossRef]
- Shi, S.; Pan, J.; Dong, B.; Zhou, W.; Zhou, C. Bioleaching of Rare Earth Elements: Perspectives from Mineral Characteristics and Microbial Species. Minerals 2023, 13, 1186. [Google Scholar] [CrossRef]
- PN-EN 12880:2004; Characterization of Sewage Sludge–Determination of Dry Solids Residue and Water Content. Polish Committee for Standardization: Warsaw, Poland, 2004. (In Polish)
- PN-EN 12879:2004; Characterization of Sewage Sludge—Determination of Losses on Ignition of Dry Sludge Mass. Polish Committee for Standardization: Warsaw, Poland, 2004. (In Polish)
- PN-EN 14701-1:2007; Characterization of Sewage Sludge—Filtration Properties—Part 1: Capillary Suction Time (CST). Polish Committee for Standardization: Warsaw, Poland, 2007. (In Polish)
- PN-EN 14701-2:2013-07; Characterization of Sewage Sludge—Filtration Properties—Part 2: Determination of Specific Filtration Resistance. Polish Committee for Standardization: Warsaw, Poland, 2013.
- PN-EN ISO 9963-1:2001; Water Quality—Determination of Alkalinity—Part 1: Determination of Total Alkalinity and Alkalinity against Phenolphthalein. Polish Committee for Standardization: Warsaw, Poland, 2004. (In Polish)
- Zhang, X.; Ye, P.; Wu, Y. Enhanced technology for sewage sludge advanced dewatering from an engineering practice perspective: A review. J. Environ. Manag. 2022, 321, 115938. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zhang, T.; Zhang, W.; Wang, D. Enhanced technology based for sewage sludge deep dewatering: A critical review. Water Res. 2021, 189, 116650. [Google Scholar] [CrossRef] [PubMed]
- Munien, C.; Kweinor Tetteh, E.; Govender, T.; Jairajh, S.; Mguni, L.L.; Rathilal, S. Turbidity and COD Removal from Municipal Wastewater Using a TiO2 Photocatalyst—A Comparative Study of UV and Visible Light. Appl. Sci. 2023, 13, 4766. [Google Scholar] [CrossRef]
- Warwick, C.; Guerreiro, A.; Gomez-Caballero, A.; Wood, E.; Kitson, J.; Robinson, J.; Soares, A. Conductance based sensing and analysis of soluble phosphates in wastewater. Biosens. Bioelectron. 2014, 52, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Shekhar, S. Iron contamination in the waters of Upper Yamuna basin. Groundw. Sustain. Dev. 2018, 7, 421–429. [Google Scholar] [CrossRef]
- Kulczycka, J.; Lewandowska, A.; Joachimiak-Lechman, K.; Kurczewski, P. The Circularity of Materials from the Perspective of a Product Life Cycle: A Life Cycle Assessment Case Study of Secondary Fence Boards—Part 1 (Baseline Scenario). Resources 2024, 13, 50. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Q.; Zhang, W.; Yang, P.; Du, Y.; Wang, D. Highly effective enhancement of waste activated sludge dewaterability by altering proteins properties using methanol solution coupled with inorganic coagulants. Water Res. 2018, 138, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Chen, H.; Chen, Z. Performance of sludge drying reed beds for the leachate purification: Effects of sludge loading frequencies and plant species. Environ. Res. 2021, 194, 110452. [Google Scholar] [CrossRef]
- Shi, C.; Sun, W.; Sun, Y.; Chen, L.; Xu, Y.; Tang, M. Synthesis, Characterization, and Sludge Dewaterability Evaluation of the Chitosan-Based Flocculant CCPAD. Polymers 2019, 11, 95. [Google Scholar] [CrossRef]
- Deng, H.; Wei, H.; Chen, L.; Li, S.; Liu, H.; Lu, H. Preparation, Properties, and Application of Biochar for Improving Sewage Sludge Dewatering Performance: A Review. Water 2023, 15, 1796. [Google Scholar] [CrossRef]
- Krebs, W.; Brombacher, C.; Bosshard, P.P.; Bachofen, R.; Brandl, H. Microbial recovery of metals from solids. FEMS Microbiol. Rev. 1997, 20, 605–617. [Google Scholar] [CrossRef]
- Gu, W.; Bai, J.; Lu, L.; Zhuang, X.; Zhao, J.; Yuan, W.; Zhang, C.; Wang, J. Improved bioleaching efficiency of metals from waste printed circuit boards by mechanical activation. Waste Manag. 2019, 98, 21–28. [Google Scholar] [CrossRef]
- Hong, Y.; Valix, M. Bioleaching of electronic waste using acidophilic sulfur oxidising bacteria. J. Clean. Prod. 2014, 65, 465–472. [Google Scholar] [CrossRef]
- Ishigaki, T.; Nakanishi, A.; Tateda, M.; Ike, M.; Fujita, M. Bioleaching of metal from municipal waste incineration fly ash using a mixed culture of sulfur-oxidizing and iron-oxidizing bacteria. Chemosphere 2005, 60, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Fan, J.; Liu, X.; Hu, Y.; Wei, X.; Hu, Y.; Wang, W.; Ren, Z. Recovery of lithium from salt-lake brines using solvent extraction with TBP as extractant and FeCl3 as co-extraction agent. Hydrometallurgy 2020, 191, 105244. [Google Scholar] [CrossRef]
- Ji, X.; Yang, M.; Wan, A.; Yu, S.; Yao, Z. Bioleaching of Typical Electronic Waste—Printed Circuit Boards (WPCBs): A Short Review. Int. J. Environ. Res. Public Health 2022, 19, 7508. [Google Scholar] [CrossRef]
- Pawlowicz, R. The electrical conductivity of seawater at high temperatures and salinities. Desalination 2012, 300, 32–39. [Google Scholar] [CrossRef]
- Kamizela, T.; Grobelak, A.; Worwag, M. Use of Acidithiobacillus thiooxidans and Acidithiobacillus ferrooxidans in the Recovery of Heavy Metals from Landfill Leachates. Energies 2021, 14, 3336. [Google Scholar] [CrossRef]
- Kamizela, T.; Worwag, M. Processing of Water Treatment Sludge by Bioleaching. Energies 2020, 13, 6539. [Google Scholar] [CrossRef]
- Kamizela, T.; Kowalczyk, M. Water purification sludge as a substrate in metal bioleaching. Desalination Water Treat. 2023, 301, 125–134. [Google Scholar] [CrossRef]
- Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C.; Gómez-Arroyave, L. Bioleaching Techniques for Sustainable Recovery of Metals from Solid Matrices. Sustainability 2023, 15, 10222. [Google Scholar] [CrossRef]
- Bień, B.; Bień, J.D. Analysis of Reject Water Formed in the Mechanical Dewatering Process of Digested Sludge Conditioned by Physical and Chemical Methods. Energies 2022, 15, 1678. [Google Scholar] [CrossRef]
- Pathak, A.; Dastidar, M.; Sreekrishnan, T. Bioleaching of heavy metals from sewage sludge: A review. J. Environ. Manag. 2009, 90, 2343–2353. [Google Scholar] [CrossRef]
- Phyo, A.K.; Jia, Y.; Tan, Q.; Sun, H.; Liu, Y.; Dong, B.; Ruan, R. Competitive Growth of Sulfate-Reducing Bacteria with Bioleaching Acidophiles for Bioremediation of Heap Bioleaching Residue. Int. J. Environ. Res. Public Health 2020, 17, 2715. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Patil, R.S.; Patil, M.; John, J. Addressing the Sustainability Conundrums and Challenges within the Polymer Value Chain. Sustainability 2023, 15, 15758. [Google Scholar] [CrossRef]


| Dry solids, DS, g/L | 45.30 ± 0.30 |
| Volatile solids, VS, g/L | 4.95 ± 0.22 |
| pH | 8.80 ± 0.1 |
| Alkalinity, mg CaCO3/L | 28,000.0 ± 707.0 |
| Magnetic field lines marked by FFHCO2 particles. (Stir bar, diameter × length: 0.7 × 40 mm.) |  |
| Dry solids, DS g/L | 22.6 ± 0.6 |
| Volatile solids, VS g/L | 17.3 ± 0.7 |
| pH | 6.40 ± 0.21 |
| Capillary suction time (CST), s | 634.0 ± 32.0 |
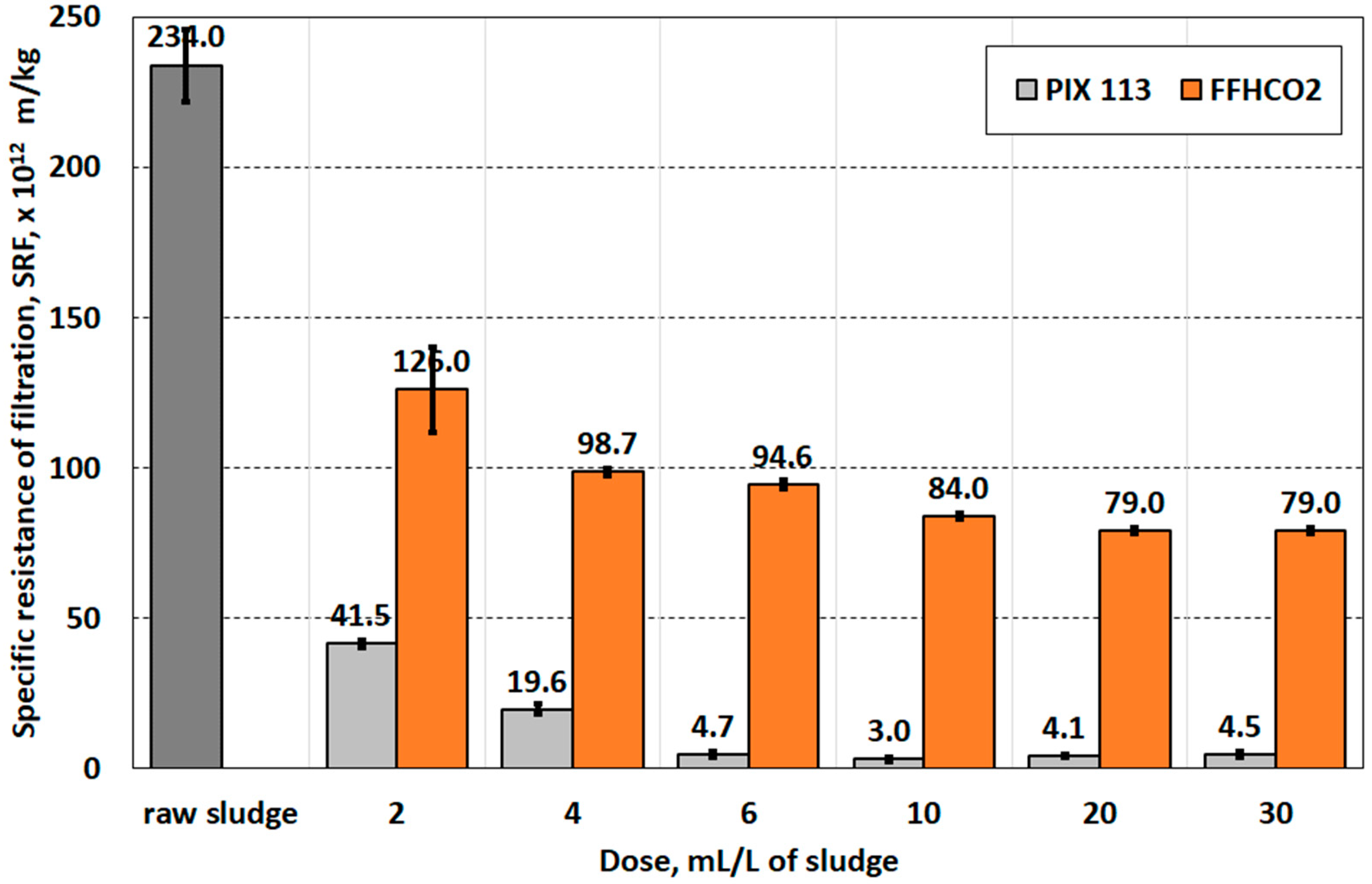
| Specific resistance of filtration, (SRT), E12 m/kg | 234 ± 1.2 |
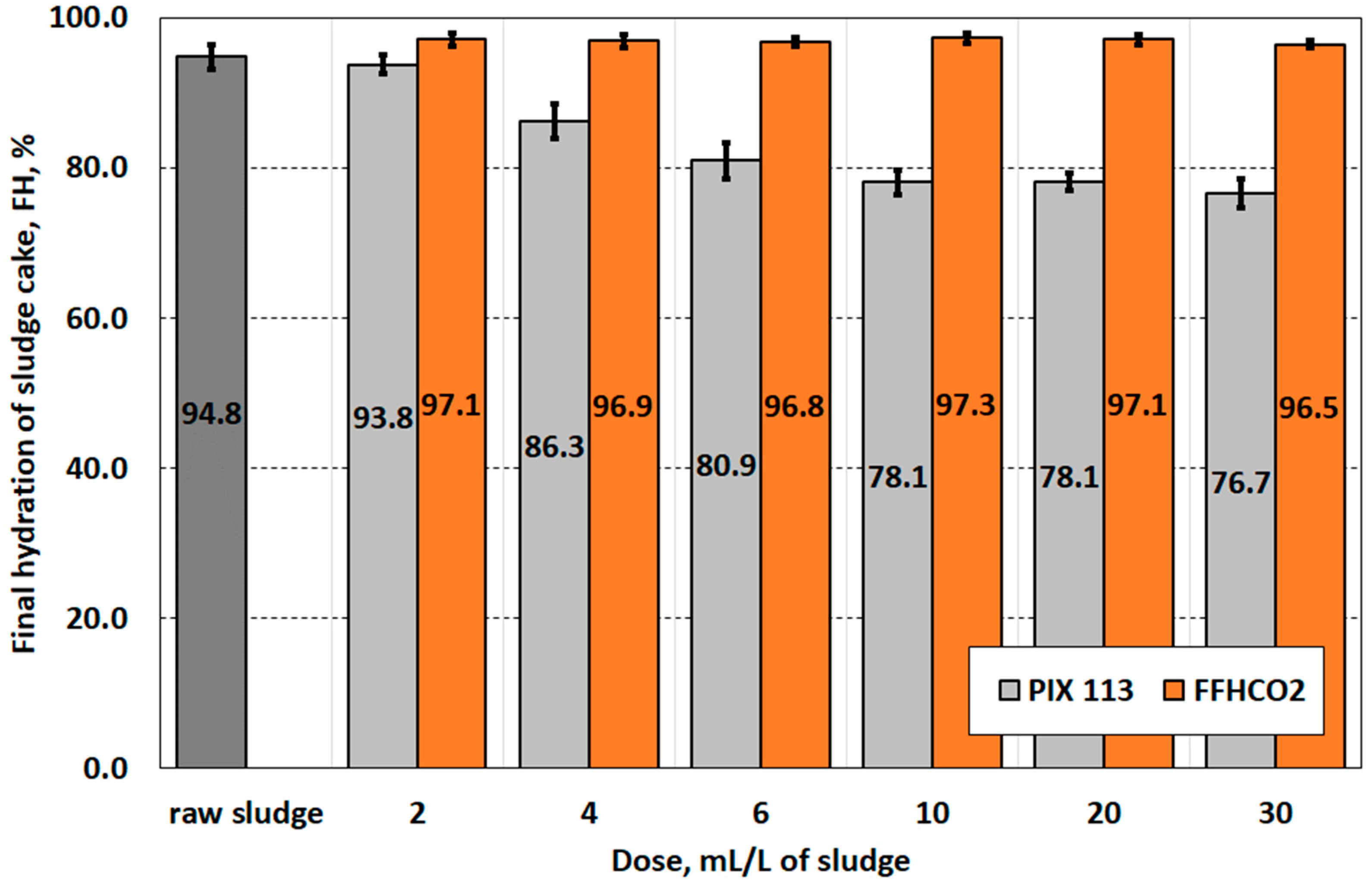
| Final hydration of sludge cake (FH), % | 94.8 ± 1.6 |
| Dry Solids, g/L | Volatile Solids, g/L | Volatile Solids Content, % DS | pH |
|---|---|---|---|
| 10.92 ± 0.34 | 8.03 ± 0.94 | 73.5 | 1.81 ± 0.12 |
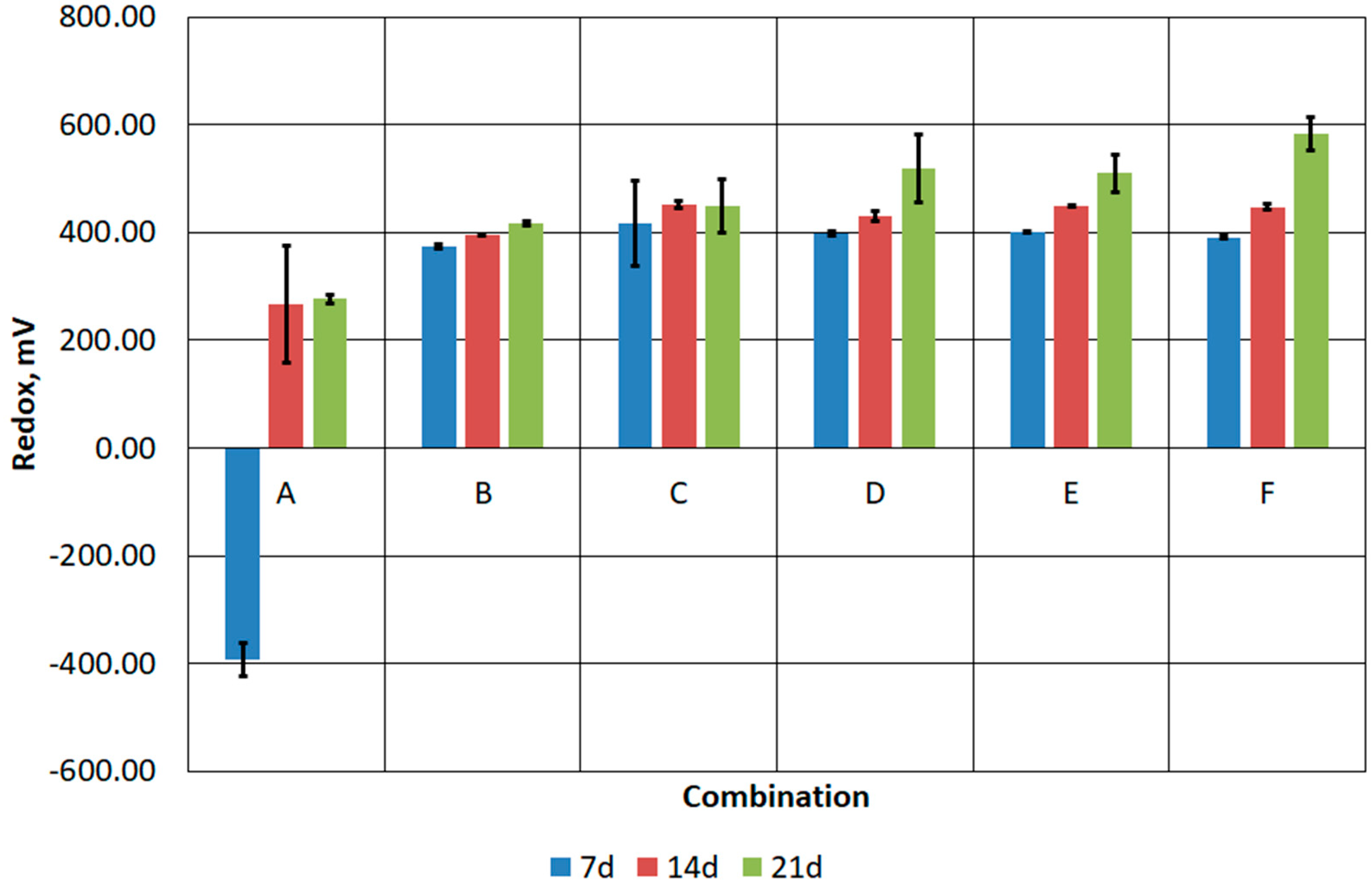
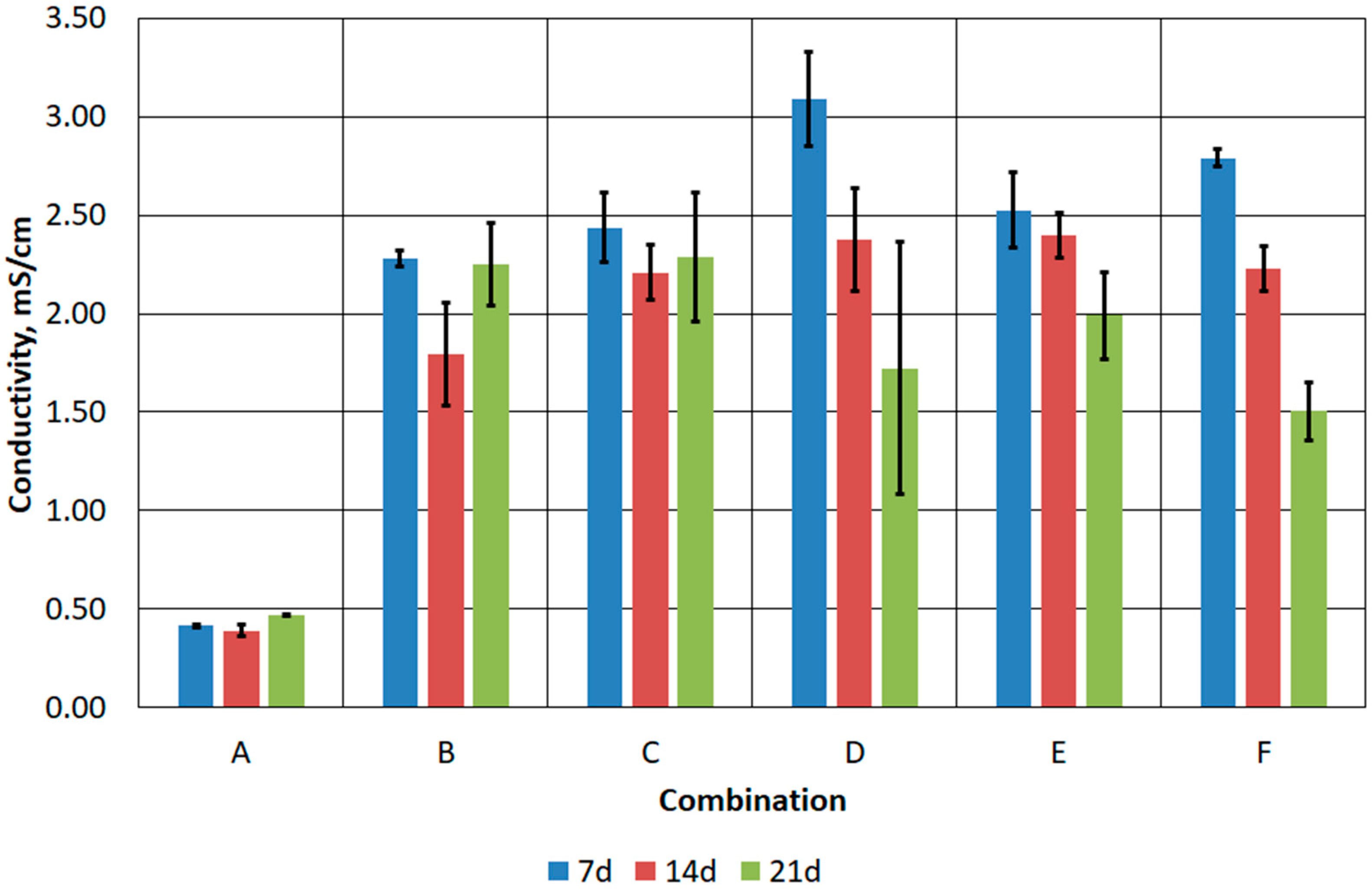
| Combination | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| FFH, % of sample volume | 100 | 100 | 50 | 50 | 50 | 50 |
| Inoculum, % of sample volume | 0 | 0 | 50 | 50 | 50 | 50 |
| pH correction to | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | |
| Addition of FeSO4·7H2O, g/L | 44.0 | 44.0 | ||||
| Addition of S0, g/L | 10.0 | 10.0 |
| Centrifugation Efficiency, % | Statistical Group | ||||||
|---|---|---|---|---|---|---|---|
| Centrifugation Time, min. | 1 min. | 98.3 | 98.3 | 98.5 | 98.7 | 98.8 | a |
| 2 min. | 98.5 | 98.6 | 98.8 | 98.9 | 99.0 | a | |
| Parameter | Centrifugation Time | 1000 | 3000 | 5000 | 7000 | 9000 | Statistical Group |
|---|---|---|---|---|---|---|---|
| pH | 1 min. | 8.0 ± 0.1 | 8.2 ± 0.1 | 8.2 ± 0.1 | 8.3 ± 0.2 | 8.3 ± 0.12 | a |
| 2 min. | 8.0 ± 0.2 | 8.1 ± 0.1 | 8.2 ± 0.1 | 8.2 ± 0.1 | 8.3 ± 0.2 | a | |
| Turbidity, NTU | 1 min. | 157 ± 10 | 153 ± 15 | 138 ± 14 | 100 ± 11 | 89 ± 8 | a |
| 2 min. | 137 ± 18 | 121 ± 19 | 96 ± 12 | 78 ± 9 | 68 ± 12 | b | |
| Conductance, uS/cm | 1 min. | 374 ± 32 | 380 ± 17 | 358 ± 23 | 350 ± 26 | 353 ± 13 | a |
| 2 min. | 368 ± 26 | 364 ± 29 | 344 ± 21 | 348 ± 11 | 346 ± 17 | a | |
| Total iron content, mg Fe/L | 1 min. | 3.0 ± 0.34 | 2.8 ± 0.14 | 2.3 ± 0.25 | 1.8 ± 0.18 | 1.5 ± 0.11 | a |
| 2 min. | 2.6 ± 0.24 | 1.9 ± 0.15 | 1.8 ± 0.11 | 1.5 ± 0.26 | 1.4 ± 0.09 | b |
| Parameter | Conditioner | Dose of Conditioner, mL/L of Sludge | Statistical Group | |||||
|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 10 | 20 | 30 | |||
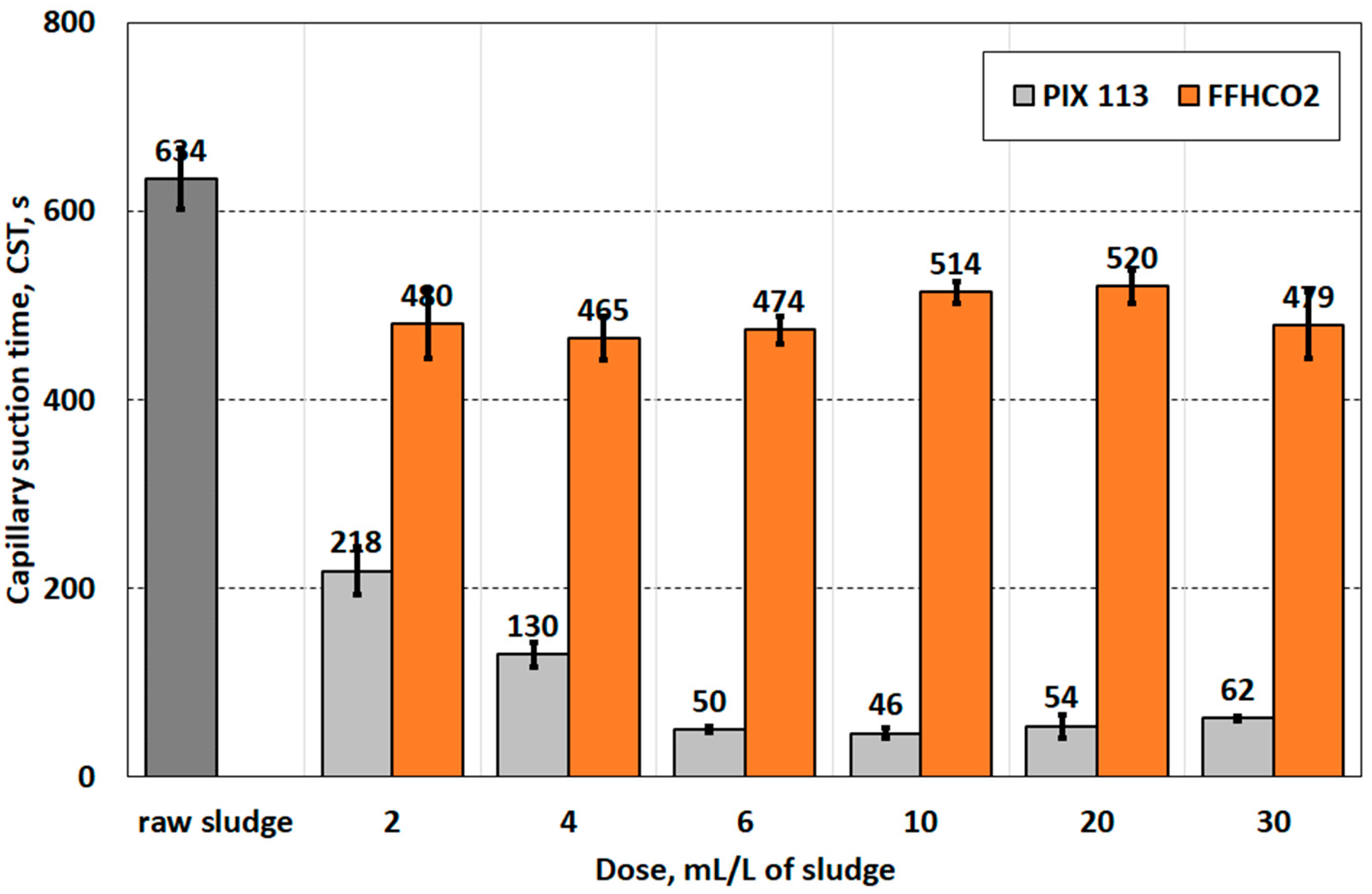
| pH | PIX 113 | 5.39 ± 0.06 | 4.87 ± 0.11 | 4.47 ± 0.11 | 3.03 ± 0.14 | 2.44 ± 0.06 | 2.27 ± 0.09 | a |
| FFHCO2 | 5.99 ± 0.06 | 6.0 ± 0.09 | 5.99 ± 0.11 | 6.01 ± 0.08 | 6.09 ± 0.19 | 6.06 ± 0.10 | b | |
| Turbidity NTU | PIX 113 | 216 ± 11 | 199 ± 10 | 187 ± 13 | 151 ± 15 | 144 ± 9 | 137 ± 3 | a |
| FFHCO2 | 487 ± 36 | 506 ± 29 | 531 ± 26 | 536 ± 24 | 473 ± 24 | 529 ± 27 | b | |
| Conductance uS/cm | PIX 113 | 169 ± 11 | 150 ± 7 | 140 ± 10 | 104 ± 8 | 77 ± 11 | 57 ± 9 | a |
| FFHCO2 | 1191 ± 28 | 1569 ± 33 | 2239 ± 43 | 2275 ± 28 | 2038 ± 58 | 2005 ± 68 | b | |
| Combination | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Initial pH | 8.80 | 8.80 | 8.80 | 8.80 | 8.80 | 8.80 |
| pH after inoculation | - | - | 7.40 ± 0.06 | 7.39 ± 0.09 | 7.37 ± 0.05 | 7.38 ± 0.10 |
| pH after adding FeSO4 | - | - | - | 6.96 ± 0.10 | - | 7.06 ± 0.09 |
| Amount of sulfuric acid, pH correction, mL/L | - | 16.8 ± 0.3 | 8.00 ± 0.2 | 7.8 ± 0.1 | 8.0 ± 0.2 | 7.8 ± 0.3 |
| Final pH | 8.80 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Combination | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| CSK, s | 11 ± 5 | 16 ± 2 | 20 ± 5 | 17 ± 5 | 15 ± 4 | 25 ± 3 |
| r, 10E12 m/kg | 2.61 ± 0.57 | 3.71 ± 0.81 | 4.49 ± 0.98 | 3.85 ± 0.73 | 3.35 ± 0,73 | 4.92 ± 0.72 |
| Turbidity (filtrate), NTU | 12.9 ± 1.3 | 6.92 ± 0.7 | 11.3 ± 3.2 | 10.3 ± 2.0 | 31.5 ± 7.6 | 26.5 ± 3.1 |
| Conductance (filtrate), mS/cm | 0.316 ± 0.09 | 0.879 ± 0.15 | 0.710 ± 0.42 | 0.483 ± 0.11 | 1.709 ± 0.26 | 1.642 ± 0.33 |
| Combination | LCK 153 | LCK 321 | LCK 654 |
|---|---|---|---|
| mg/L SO4 | mg/L Fe | mg/L SO3 | |
| A | 67.5 ± 5 | 6.4 ± 0.4 | 2.8 ± 0.3 |
| B | 669 ± 49 | 160.3 ± 1.2 | 123.7 ± 1.9 |
| C | 7280 ± 35 | 174.6 ± 8.6 | 80.3 ± 2.7 |
| D | 8970 ± 61 | 214.5 ± 5.2 | 126.4 ± 3.4 |
| E | 8030 ± 85 | 194.0 ± 7.0 | 108.6 ± 1.9 |
| F | 10,510 ± 66 | 250.1 ± 14.6 | 135.0 ± 5.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamizela, T.; Kowalczyk, M.; Worwąg, M.; Wystalska, K.; Zabochnicka, M.; Kępa, U. Possibilities of Managing Waste Iron Sorbent FFH after CO2 Capture as an Element of a Circular Economy. Materials 2024, 17, 2725. https://doi.org/10.3390/ma17112725
Kamizela T, Kowalczyk M, Worwąg M, Wystalska K, Zabochnicka M, Kępa U. Possibilities of Managing Waste Iron Sorbent FFH after CO2 Capture as an Element of a Circular Economy. Materials. 2024; 17(11):2725. https://doi.org/10.3390/ma17112725
Chicago/Turabian StyleKamizela, Tomasz, Mariusz Kowalczyk, Małgorzata Worwąg, Katarzyna Wystalska, Magdalena Zabochnicka, and Urszula Kępa. 2024. "Possibilities of Managing Waste Iron Sorbent FFH after CO2 Capture as an Element of a Circular Economy" Materials 17, no. 11: 2725. https://doi.org/10.3390/ma17112725





