Design of Environmental-Friendly Carbon-Based Catalysts for Efficient Advanced Oxidation Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Co/SiO2
2.3. Synthesis of Co-C/SiO2
2.4. Characterizations
2.5. Catalytic Activity
2.6. A Modified Kinetic Model (k-Value)
3. Results and Discussion
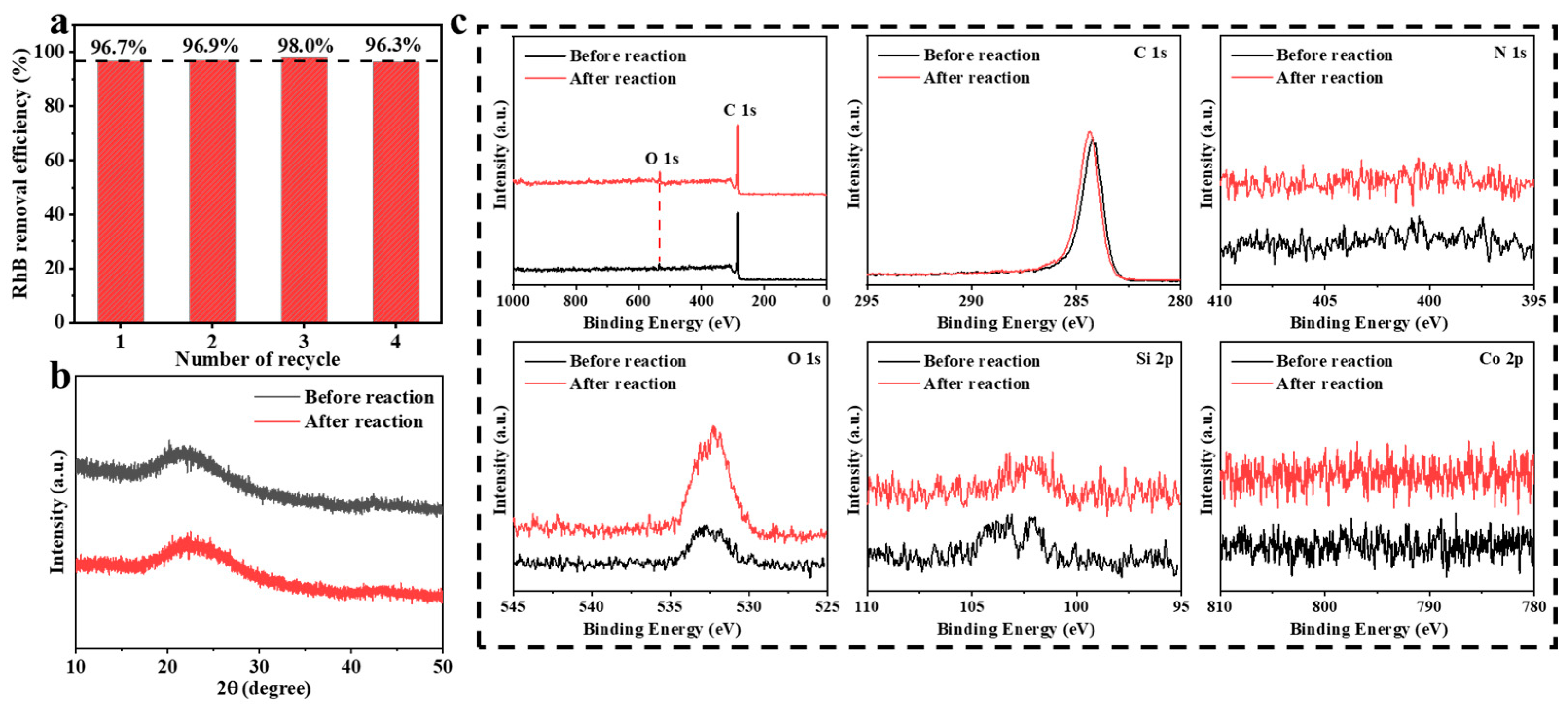
3.1. Synthesis and Characterization of Co-C/SiO2 Catalyst
3.2. Catalytic Performance of Co-C/SiO2 in AOPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, Y.; Lu, P.; Qu, G.; Ning, P.; Ren, N.; Wang, J.; Wu, F.; Chen, X.; Wang, Z.; Zhang, T.; et al. Study on the mechanism of rapid degradation of Rhodamine B with Fe/Cu@antimony tailing nano catalytic particle electrode in a three dimensional electrochemical reactor. Water Res. 2023, 244, 120487. [Google Scholar] [CrossRef]
- Dileep Kumar, V.G.; Kumari, S.; Balaji, K.R.; Ahmad Khan, A.; Ravikumar, C.R.; Basavaraja, B.M.; Santosh, M.S.; Rtimi, S. Singlet Oxygen Driven Enhanced Photocatalytic Degradation of 1,3,7-Trimethylpurine-2,6-dione using Surfactant Mediated PVA-CuO Nanocomposites: Combining physical adsorption and photocatalysis. Chem. Eng. J. 2023, 462, 142187. [Google Scholar] [CrossRef]
- Nayak, S.; Prasad, S.R.; Mandal, D.; Das, P. Carbon dot cross-linked polyvinylpyrrolidone hybrid hydrogel for simultaneous dye adsorption, photodegradation and bacterial elimination from waste water. J. Hazard. Mater. 2020, 392, 122287. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, Q.; Xue, C.; Chen, Z. Mechanism for removing 17beta-estradiol by bio-nanocomposite FJ1@rGO based on integration of adsorption and biodegradation. Chem. Eng. J. 2023, 473, 145317. [Google Scholar] [CrossRef]
- Jain, M.; Khan, S.A.; Sharma, K.; Jadhao, P.R.; Pant, K.K.; Ziora, Z.M.; Blaskovich, M.A.T. Current perspective of innovative strategies for bioremediation of organic pollutants from wastewater. Bioresour. Technol. 2021, 344, 126305. [Google Scholar] [CrossRef] [PubMed]
- Sai Preethi, P.; Hariharan, N.M.; Vickram, S.; Rameshpathy, M.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Yadav, V.; Ravindran, B.; Chang, S.W.; et al. Advances in bioremediation of emerging contaminants from industrial wastewater by oxidoreductase enzymes. Bioresour. Technol. 2022, 359, 127444. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Lu, J.; Zhou, Y.; Zhou, Y. In-situ production and activation of H2O2 for enhanced degradation of roxarsone by FeS2 decorated resorcinol-formaldehyde resins. J. Hazard. Mater. 2021, 424, 127650. [Google Scholar] [CrossRef]
- Li, X.; Hu, R.; Liu, Y.; Guo, X.; Cheng, J.; Hu, Y.; Chen, Y. Co-construction of oxygen doping and van der walls heterojunction in O-CB/ZnIn2S4 promoting photocatalytic production and activation of H2O2 for the degradation of antibiotics. J. Hazard. Mater. 2023, 459, 132187. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, D.; Zhang, R.; Ding, Y.; Ren, Z.; Fu, M.; Cao, X.; Zeng, G. Singlet oxygen-dominated activation of peroxymonosulfate by passion fruit shell derived biochar for catalytic degradation of tetracycline through a non-radical oxidation pathway. J. Hazard. Mater. 2021, 419, 126495. [Google Scholar] [CrossRef]
- Ma, C.; Guo, Y.; Zhang, D.; Wang, Y.; Li, N.; Ma, D.; Ji, Q.; Xu, Z. Metal-nitrogen-carbon catalysts for peroxymonosulfate activation to degrade aquatic organic contaminants: Rational design, size-effect description, applications and mechanisms. Chem. Eng. J. 2022, 454, 140216. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Q.; Wang, J.; Wei, X.; Zhang, R.; Wang, S.; Liu, P.; Chen, Z. Effects of foreign metal doping on the step-by-step oxidation process in M-OMS-2 catalyzed activation of PMS. J. Hazard. Mater. 2022, 434, 128773. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yan, J.; Wang, M.; Zhu, H.; Li, X.; Wu, L. Insights into hydrangea-like NiCo2S4 activating peroxymonosulfate for efficient degradation of atrazine. Chem. Eng. J. 2023, 477, 146876. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Huang, S.; Xu, J.; Liu, L.; Li, J.; Jing, J.; Zhu, Y. Electron-enriched supramolecular PDI-SiO2 promoting PDS activation for enhanced photocatalytic advanced oxidation. Appl. Catal. B Environ. 2023, 340, 123262. [Google Scholar] [CrossRef]
- Lin, M.; Liu, M.; Wu, J.; Owens, G.; Chen, Z. Green synthesis of Mn2O3 activated PDS to degrade estriol in medical wastewater and its degradation pathway. Chem. Eng. J. 2024, 484, 149713. [Google Scholar] [CrossRef]
- Rao, Z.; Zhu, N.; Wei, X.; Li, F.; Wu, P.; Dang, Z.; Cui, B. Efficient peroxydisulfate activation with nZVI/CuO@BC nanocomposite derived from wastes for degradation of tetrabromobisphenol A in alkaline environment. J. Hazard. Mater. 2021, 417, 126029. [Google Scholar] [CrossRef]
- Ren, W.; Huang, X.; Wang, L.; Liu, X.; Zhou, Z.; Wang, Y.; Lin, C.; He, M.; Wei, O. Degradation of simazine by heat-activated peroxydisulfate process: A coherent study on kinetics, radicals and models. Chem. Eng. J. 2021, 426, 131876. [Google Scholar] [CrossRef]
- Yi, X.; Ji, H.; Wang, C.; Li, Y.; Li, Y.; Zhao, C.; Wang, A.; Fu, H.; Wang, P.; Zhao, X.; et al. Photocatalysis-activated SR-AOP over PDINH/MIL-88A(Fe) composites for boosted chloroquine phosphate degradation: Performance, mechanism, pathway and DFT calculations. Appl. Catal. B Environ. 2021, 293, 120229. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Zhang, T.; Xu, Z.; Li, Y.; Li, B.; Tian, S. UV facilitated synergistic effects of polymetals in ore catalyst on peroxymonosulfate activation: Implication for the degradation of bisphenol S. Chem. Eng. J. 2021, 431, 133989. [Google Scholar] [CrossRef]
- Xu, D.; Ma, H. Degradation of rhodamine B in water by ultrasound-assisted TiO2 photocatalysis. J. Clean. Prod. 2021, 313, 127758. [Google Scholar] [CrossRef]
- Ahn, Y.-Y.; Choi, J.; Kim, M.; Kim, M.S.; Lee, D.; Bang, W.H.; Yun, E.-T.; Lee, H.; Lee, J.-H.; Lee, C.; et al. Chloride-Mediated Enhancement in Heat-Induced Activation of Peroxymonosulfate: New Reaction Pathways for Oxidizing Radical Production. Environ. Sci. Technol. 2021, 55, 5382–5392. [Google Scholar] [CrossRef]
- Liangdy, A.; Lee, W.J.; Tonanon, P.; Webster, R.D.; Snyder, S.A.; Lim, T.-T. Unravelling the synergism of catalytic oxidation and filtration in Co-Mn-oxide impregnated ceramic membrane for intensified degradation of recalcitrant micropollutant with peroxymonosulfate. Chem. Eng. J. 2022, 454, 140075. [Google Scholar] [CrossRef]
- Yu, T.; Chen, H.; Hu, T.; Feng, J.; Xing, W.; Tang, L.; Tang, W. Recent advances in the applications of encapsulated transition-metal nanoparticles in advanced oxidation processes for degradation of organic pollutants: A critical review. Appl. Catal. B Environ. 2023, 342, 123401. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Kang, S.; Hwang, J. CoMn2O4 embedded hollow activated carbon nanofibers as a novel peroxymonosulfate activator. Chem. Eng. J. 2020, 406, 127158. [Google Scholar] [CrossRef]
- Li, Y.; Yang, T.; Qiu, S.; Lin, W.; Yan, J.; Fan, S.; Zhou, Q. Uniform N-coordinated Single-Atomic Iron Sites Dispersed in Porous Carbon Framework to Activate PMS for Efficient BPA Degradation via High-Valent Iron-Oxo Species. Chem. Eng. J. 2020, 489, 124382. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, G.; Wang, Q.; Sun, Y.; Liu, M.; Wang, P. Facile synthesis of novel Co3O4-Bi2O3 catalysts and their catalytic activity on bisphenol A by peroxymonosulfate activation. Chem. Eng. J. 2017, 326, 1095–1104. [Google Scholar] [CrossRef]
- Liu, W.; Lu, Y.; Dong, Y.; Jin, Q.; Lin, H. A critical review on reliability of quenching experiment in advanced oxidation processes. Chem. Eng. J. 2023, 466, 143161. [Google Scholar] [CrossRef]
- Xie, M.; Dai, F.; Wang, Y.; Lv, W.; Zhang, Z.; Lu, X. Electronic Metal-Support Interaction Directing the Design of Fe(III)-Based Catalysts for Efficient Advanced Oxidation Processes by Dual Reaction Paths. Small 2022, 18, 2203269. [Google Scholar] [CrossRef]
- Miao, X.; Chen, X.; Wu, W.; Lin, D.; Yang, K. Intrinsic defects enhanced biochar/peroxydisulfate oxidation capacity through electron-transfer regime. Chem. Eng. J. 2022, 438, 135606. [Google Scholar] [CrossRef]
- Yu, C.; Zhao, Z.; Zong, Y.; Xu, L.; Zhang, B.; Wu, D. Electric field-enhanced coupled with metal-free peroxymonosulfate activactor: The selective oxidation of nonradical species-dominated system. Water Res. 2022, 227, 119323. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; Ge, J.; Yin, Y. Permeable silica shell through surface-protected etching. Nano Lett. 2008, 8, 2867–2871. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Q.; Liu, J.; Joo, J.; Lee, A.; Gan, Y.; Yin, Y. Sol-gel coating of inorganic nanostructures with resorcinol-formaldehyde resin. Chem. Commun. 2013, 49, 5135. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, S.; Li, S.; Tang, J.; Hua, T.; Li, F. Mechanism of the application of single-atom catalyst-activated PMS/PDS to the degradation of organic pollutants in water environment: A review. J. Clean. Prod. 2023, 397, 136468. [Google Scholar] [CrossRef]
- Ghanbari, F.; Zirrahi, F.; Olfati, D.; Gohari, F.; Hassani, A. TiO2 nanoparticles removal by electrocoagulation using iron electrodes: Catalytic activity of electrochemical sludge for the degradation of emerging pollutant. J. Mol. Liq. 2020, 310, 113217. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2015, 181, 103–117. [Google Scholar] [CrossRef]
- Chao, H.; Liu, H.; Sun, C.; Wang, P.; Tian, Z.; Cheng, H.; Huang, S.; Yang, X.; Wang, M.; Liu, Z. Peroxymonosulfate activation by graphene oxide-supported 3D-MoS2/FeCo2O4 sponge for highly efficient organic pollutants degradation. Environ. Pollut. 2023, 325, 121391. [Google Scholar]
- Gatou, M.-A.; Fiorentis, E.; Lagopati, N.; Pavlatou, E.A. Photodegradation of Rhodamine B and Phenol Using TiO2/SiO2 Composite Nanoparticles: A Comparative Study. Water 2023, 15, 2773. [Google Scholar] [CrossRef]
- Zhu, S.-R.; Qi, Q.; Zhao, W.-N.; Wu, M.-K.; Fang, Y.; Tao, K.; Yi, F.-Y.; Han, L. Hierarchical core-shell SiO2@PDA@BiOBr microspheres with enhanced visible-light-driven photocatalytic performance. Dalton Trans. 2017, 46, 11451–11458. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yang, G.; Li, J.; Zhang, S.; Fang, Y.; Peng, F.; Zhang, S.; Qiu, R. Efficient purification of tetracycline wastewater by activated persulfate with heterogeneous Co-V bimetallic oxides. J. Colloid Interface Sci. 2022, 619, 188–197. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Gong, M.; Wang, W.; Mu, Y.; Hu, Z.-H. α-MnO2/Palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate (PMS) for the degradation of Rhodamine B. Sep. Purif. Technol. 2019, 230, 115877. [Google Scholar] [CrossRef]
- Shanhao, L.; Xueer, N.; Pingyu, H.; Yali, C.; Jing, X.; Jindou, H.; Zhenjiang, L.; Aize, H. Defect-rich MoS2 piezocatalyst: Efficient boosting piezocatalytic activation of PMS activity towards degradation organic pollutant. Dyes Pigm. 2022, 206, 110678. [Google Scholar]
- Liu, D.; Chen, D.; Hao, Z.; Tang, Y.; Jiang, L.; Li, T.; Tian, B.; Yan, C.; Luo, Y.; Jia, B. Efficient degradation of Rhodamine B in water by CoFe2O4/H2O2 and CoFe2O4/PMS systems: A comparative study. Chemosphere 2022, 307, 135935. [Google Scholar] [CrossRef]
- Wang, M.; Wang, F.; Wang, P.; Chu, H.; Fu, H.; Zhao, C.; Wang, C.-C.; Zhao, J. Highly efficient and selective organic pollutants degradation via peroxymonosulfate activation over micron-sized Co-MOF: Nearly 100% singlet oxygen mechanism. Sep. Purif. Technol. 2023, 326, 124806. [Google Scholar] [CrossRef]
- Wu, D.; Ye, P.; Wang, M.; Wei, Y.; Li, X.; Xu, A. Cobalt nanoparticles encapsulated in nitrogen-rich carbon nanotubes as efficient catalysts for organic pollutants degradation via sulfite activation. J. Hazard. Mater. 2018, 352, 148–156. [Google Scholar] [CrossRef]
- Li, X.; Huang, S.; Xiao, H.; Deng, Y.; Wang, Z.; Liu, Z.-Q. Molybdenum phosphide (MoP) with dual active sites for the degradation of diclofenac in Fenton-like system. Chin. Chem. Lett. 2021, 33, 1321–1324. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, Z.; Deng, L. Enhanced heterogeneous degradation of sulfamethoxazole via peroxymonosulfate activation with novel magnetic MnFe2O4/GCNS nanocomposite. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 621, 126531. [Google Scholar] [CrossRef]
- Wei, L.; Li, J.; Zhou, C.; Song, B.; Qin, F.; Wang, W.; Luo, H.; Qin, D.; Huang, C.; Zhang, C.; et al. Design of copper oxide and oxygen codoped graphitic carbon nitride activator for efficient radical and nonradical activation of peroxymonosulfate. Chin. Chem. Lett. 2022, 34, 107893. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Z.; Zhou, Y.; Zhang, Z.; Lu, G.; Jiang, Z. Theoretical guidance for the construction of electron-rich reaction microcenters on C–O–Fe bridges for enhanced Fenton-like degradation of tetracycline hydrochloride. Chem. Eng. J. 2021, 411, 128535. [Google Scholar] [CrossRef]
- Yang, L.; Yang, H.; Yin, S.; Wang, X.; Xu, M.; Lu, G.; Liu, Z.; Sun, H. Fe Single-Atom Catalyst for Efficient and Rapid Fenton-Like Degradation of Organics and Disinfection against Bacteria. Small 2022, 18, 2104941. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Z.; Wang, J.; Guo, W.; Wu, X.; Jiang, Z. High-capacity NCNT-encapsulated metal NP catalysts on carbonised loofah with dual-reaction centres over C–M bond bridges for Fenton-like degradation of antibiotics. Appl. Catal. B Environ. 2022, 307, 121205. [Google Scholar] [CrossRef]
- Li, Y.; Lin, D.; Li, Y.; Jiang, P.; Fang, X.; Yu, B. Nonradical-dominated peroxymonosulfate activation through bimetallic Fe/Mn-loaded hydroxyl-rich biochar for efficient degradation of tetracycline. Nano Res. 2022, 16, 155–165. [Google Scholar] [CrossRef]
- Zhen, J.; Sun, J.; Xu, X.; Wu, Z.; Song, W.; Ying, Y.; Liang, S.; Miao, L.; Cao, J.; Lv, W.; et al. M-N3 Configuration on Boron Nitride Boosts Singlet Oxygen Generation via Peroxymonosulfate Activation for Selective Oxidation. Angew. Chem. Int. Ed. 2024, e202402669. [Google Scholar] [CrossRef]
- Li, W.; Zhang, B.; Li, X.; Zhang, H.; Zhang, Q. Preparation and characterization of novel immobilized Fe3O4@SiO2@mSiO2–Pd(0) catalyst with large pore-size mesoporous for Suzuki coupling reaction. Appl. Catal. A Gen. 2013, 459, 65–72. [Google Scholar] [CrossRef]
- Ma, W.; Du, Y.; Wang, N.; Miao, P. ZIF-8 derived nitrogen-doped porous carbon as metal-free catalyst of peroxymonosulfate activation. Environ. Sci. Pollut. Res. 2017, 24, 16276–16288. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Ding, D.; Cai, T. From rice straw to magnetically recoverable nitrogen doped biochar: Efficient activation of peroxymonosulfate for the degradation of metolachlor. Appl. Catal. B Environ. 2019, 254, 312–320. [Google Scholar] [CrossRef]
- Liu, P.; Xing, L.; Lin, H.; Wang, H.; Zhou, Z.; Su, Z. Construction of porous covalent organic polymer as photocatalysts for RhB degradation under visible light. Sci. Bull. 2017, 62, 931–937. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Zhou, X.; Zhang, C.; Chen, L.; Yan, H.; Qin, L.; Huang, D.; Ye, H.; Chen, W.; et al. Peroxydisulfate activation by sulfur-doped ordered mesoporous carbon: Insight into the intrinsic relationship between defects and 1O2 generation. Water Res. 2022, 221, 118797. [Google Scholar] [CrossRef]
- Bi, G.; Ding, R.; Song, J.; Luo, M.; Zhang, H.; Liu, M.; Huang, D.; Mu, Y. Discriminating the Active Ru Species Towards the Selective Generation of Singlet Oxygen from Peroxymonosulfate: Nanoparticles Surpass Single-Atom Catalysts. Angew. Chem. Int. Ed. 2024, 63, e202401551. [Google Scholar] [CrossRef]

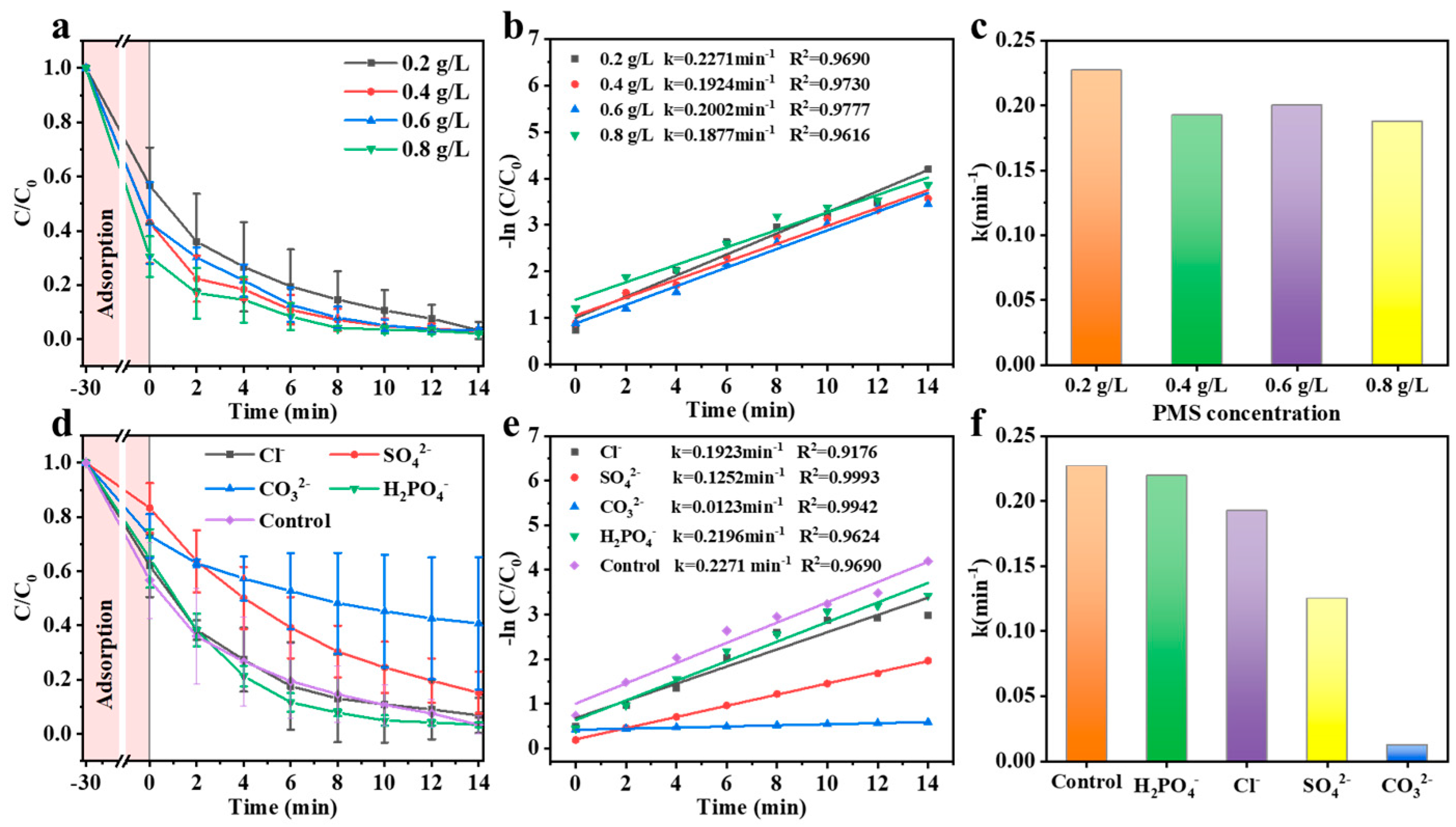
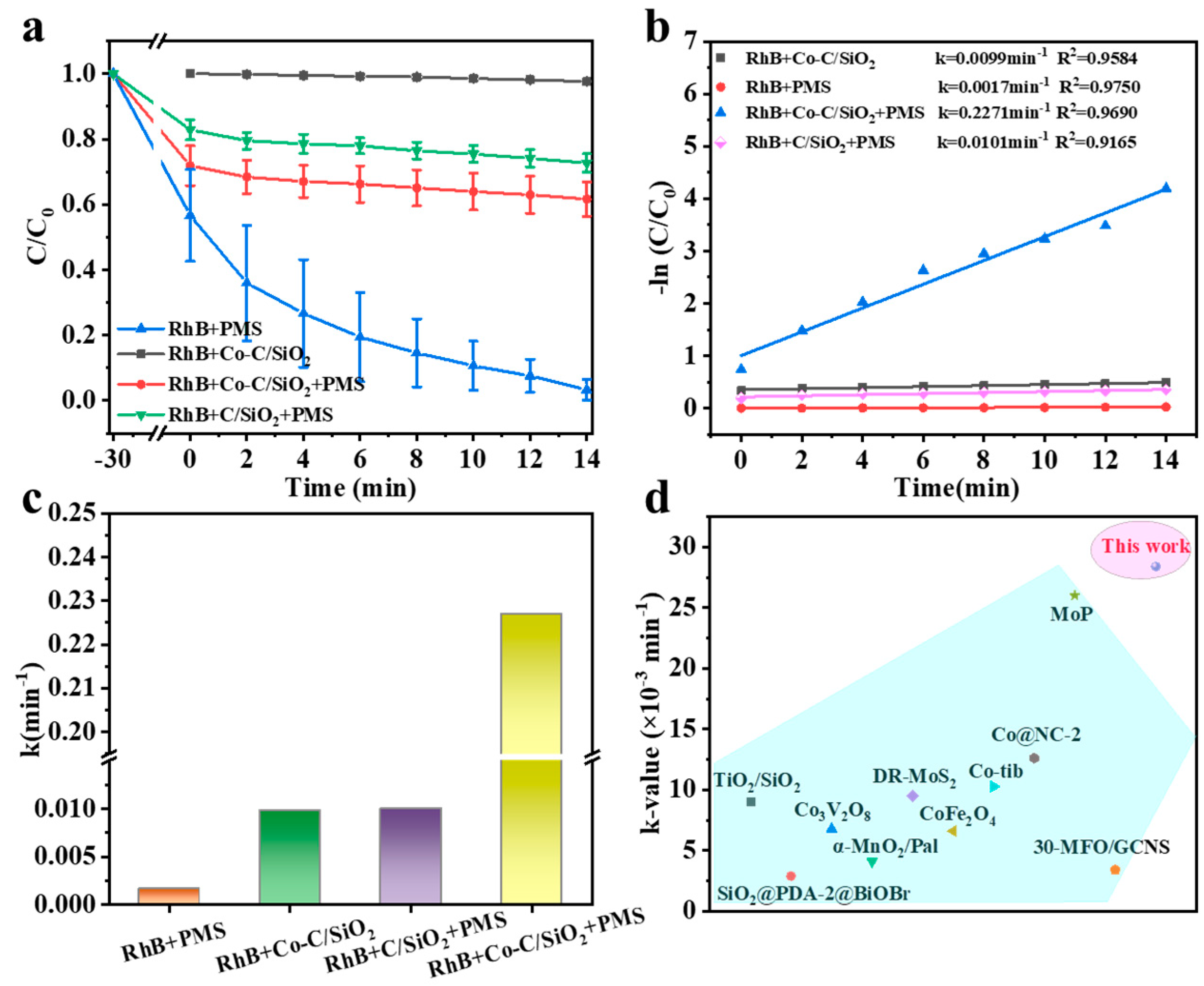
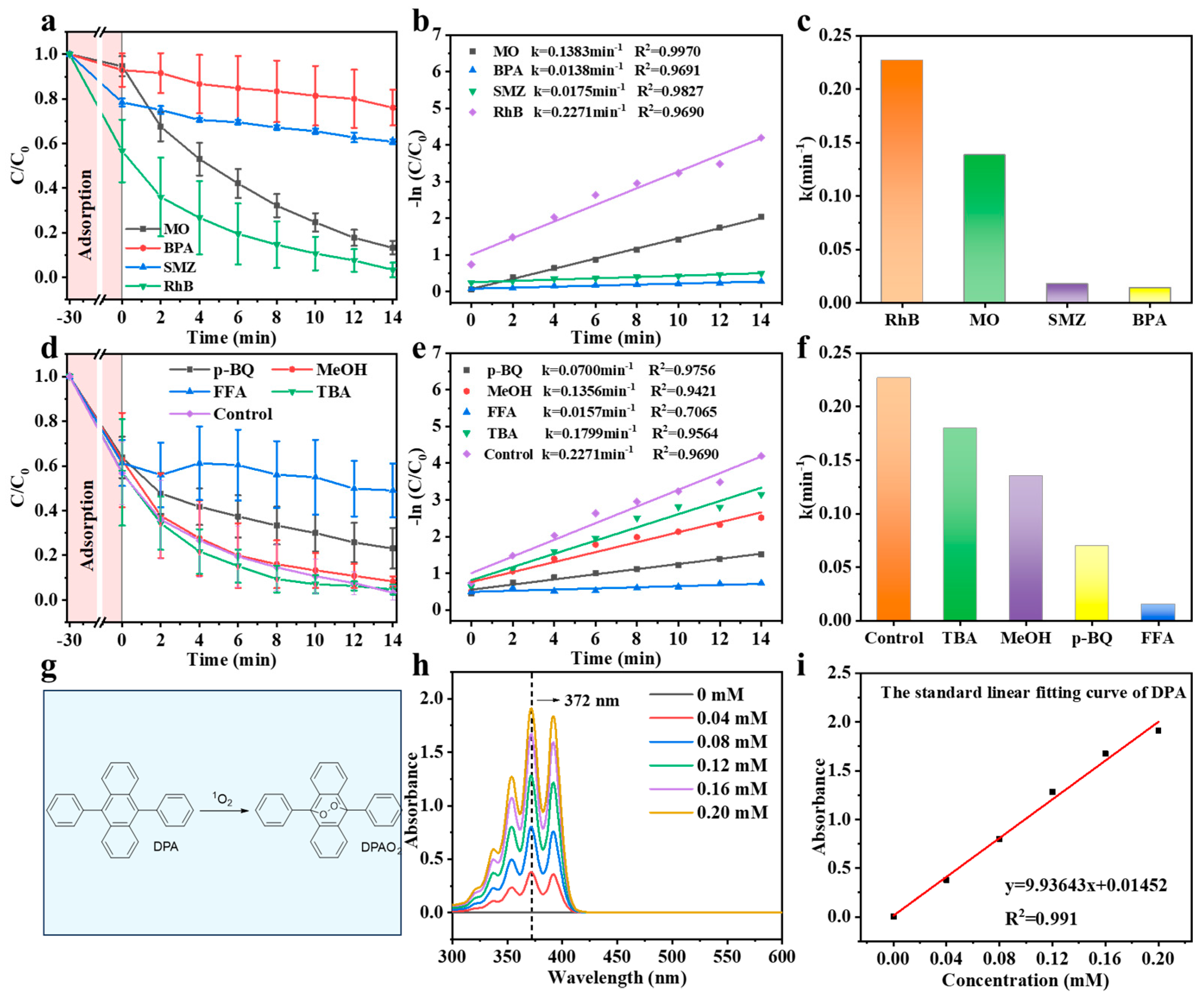
| Catalyst (g/L) | PMS (g/L) | Pollutant (mg/L) | Removal Efficiency | k (min−1) | k-Value (×10−3 min−1) | Ref. |
|---|---|---|---|---|---|---|
| TiO2/SiO2 (0.02) | visible-light irradiation | RhB (10) | 100% | 0.018 | 9 | [37] |
| SiO2@PDA-2@BiOBr (0.25) | visible-light irradiation | RhB (15) | 96% | 0.0487 | 2.9 | [38] |
| Co3V2O8 (0.2) | PS (25 mM) | TC (50) | 87.1% | 0.0271 | 6.775 | [39] |
| α-MnO2/Pal (0.1) | PMS (0.1) | RhB (20) | 100% | 0.02041 | 4.1 | [40] |
| DR-MoS2 (0.1) | PMS (3.25 mM) | RhB (10) | 92.1% | 0.09533 | 9.5 | [41] |
| CoFe2O4 (0.3) | PMS (7 mM) | RhB (20) | 100% | 0.0986 | 6.6 | [42] |
| Co-tib (0.2) | PMS (0.15 mM) | RhB (10) | 100% | 0.2053 | 10.27 | [43] |
| Co@NC-2 (0.4) | SO32− (5 mM) | MO (20) | 100% | 0.25232 | 12.62 | [44] |
| MoP (0.1) | H2O2 (2.0 mM) | DCF (20) | 100% | 0.13 | 26 | [45] |
| 30-MFO/GCNS (0.2) | PMS (2) | SMX (5) | 100% | 0.1363 | 3.41 | [46] |
| Co-C/SiO2 (0.2) | PMS (0.2) | RhB (25) | 96.7% | 0.2271 | 28.4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Kuang, G.; Jiang, X.; Wei, S.; Wang, H.; Zhang, Z. Design of Environmental-Friendly Carbon-Based Catalysts for Efficient Advanced Oxidation Processes. Materials 2024, 17, 2750. https://doi.org/10.3390/ma17112750
Xu X, Kuang G, Jiang X, Wei S, Wang H, Zhang Z. Design of Environmental-Friendly Carbon-Based Catalysts for Efficient Advanced Oxidation Processes. Materials. 2024; 17(11):2750. https://doi.org/10.3390/ma17112750
Chicago/Turabian StyleXu, Xinru, Guochen Kuang, Xiao Jiang, Shuoming Wei, Haiyuan Wang, and Zhen Zhang. 2024. "Design of Environmental-Friendly Carbon-Based Catalysts for Efficient Advanced Oxidation Processes" Materials 17, no. 11: 2750. https://doi.org/10.3390/ma17112750
APA StyleXu, X., Kuang, G., Jiang, X., Wei, S., Wang, H., & Zhang, Z. (2024). Design of Environmental-Friendly Carbon-Based Catalysts for Efficient Advanced Oxidation Processes. Materials, 17(11), 2750. https://doi.org/10.3390/ma17112750






