Effect of Secondary Foaming on the Structural Properties of Polyurethane Polishing Pad
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Sample
2.2.1. Preparation of Polyurethane Prepolymers
2.2.2. Preparation of Polyurethane Polishing Pad
2.3. Test and Characterization
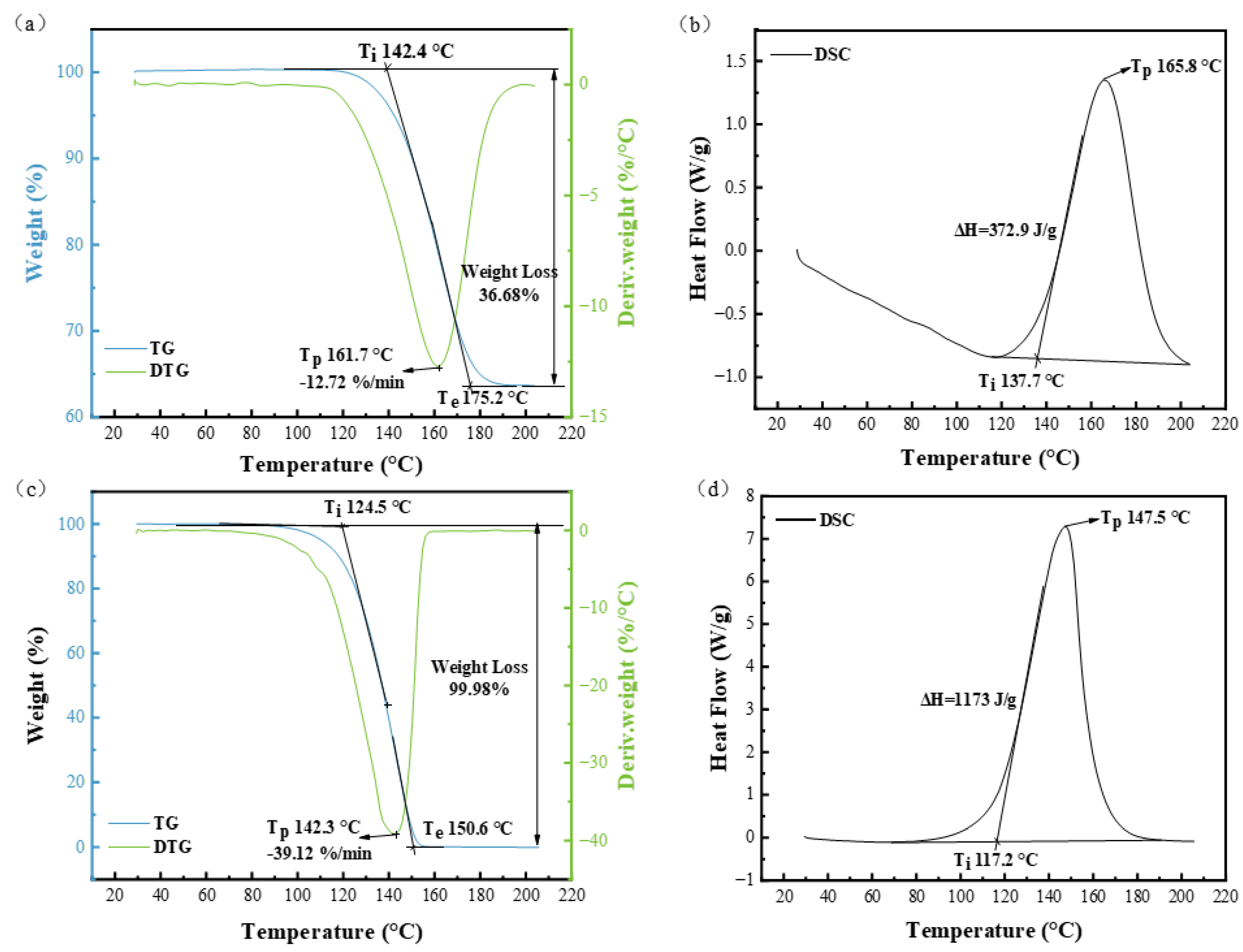
- Pyrolysis test: The decomposition temperatures of sodium bicarbonate and ammonium bicarbonate were determined using synchronous thermal analysis TG-DSC (STA 449 F3, German Netzsch Company, Selb, Germany). The test range was 30–200 °C, and the temperature increase rate was 10 °C/min.
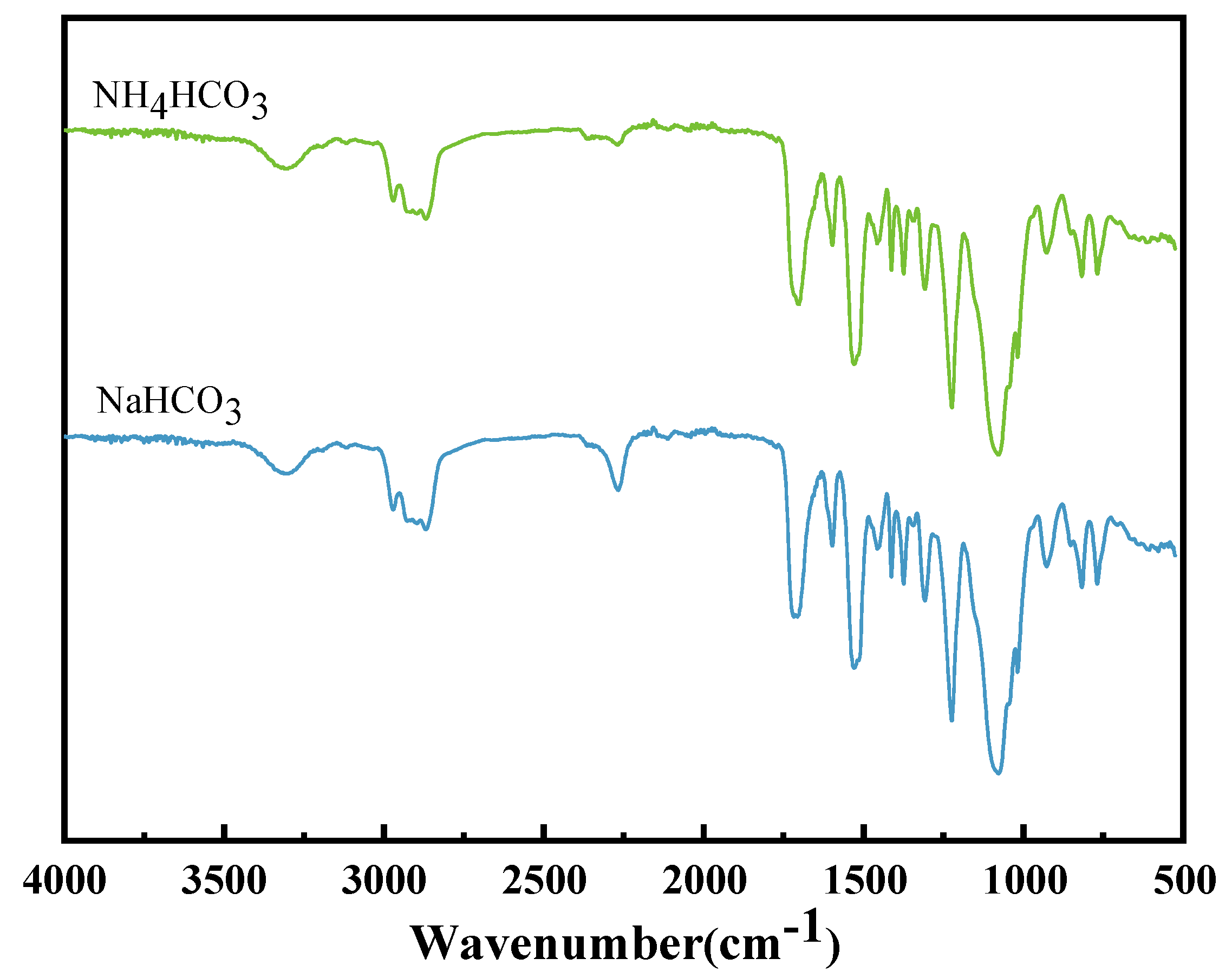
- Fourier infrared spectroscopy test: Samples were tested with Fourier transform infrared spectrometer (Type 8700, American Nicolet Company, Madison, WI, USA). The scanning wavelength range of the instrument was 4000~400 cm−1, and air was selected as the background.
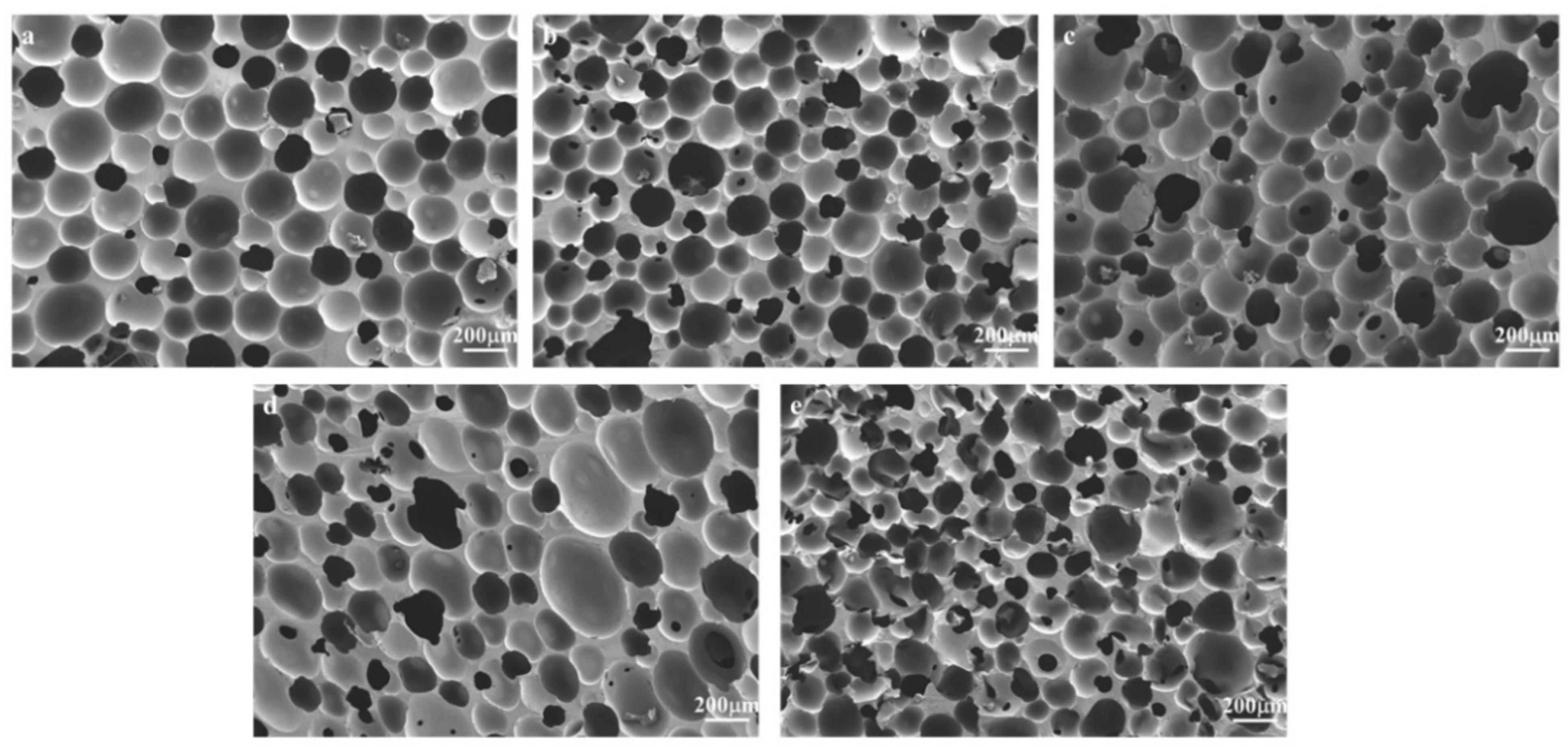
- Observation of bubble structure: The sample was cut into thin slices and dried in an oven for 24 h. The surface of the sample was treated with uniform gold spraying. The morphology of the sample surface was obtained by scanning electron microscopy (GeminiSEM 300, German ZEISS Company, Oberkochen, Germany).
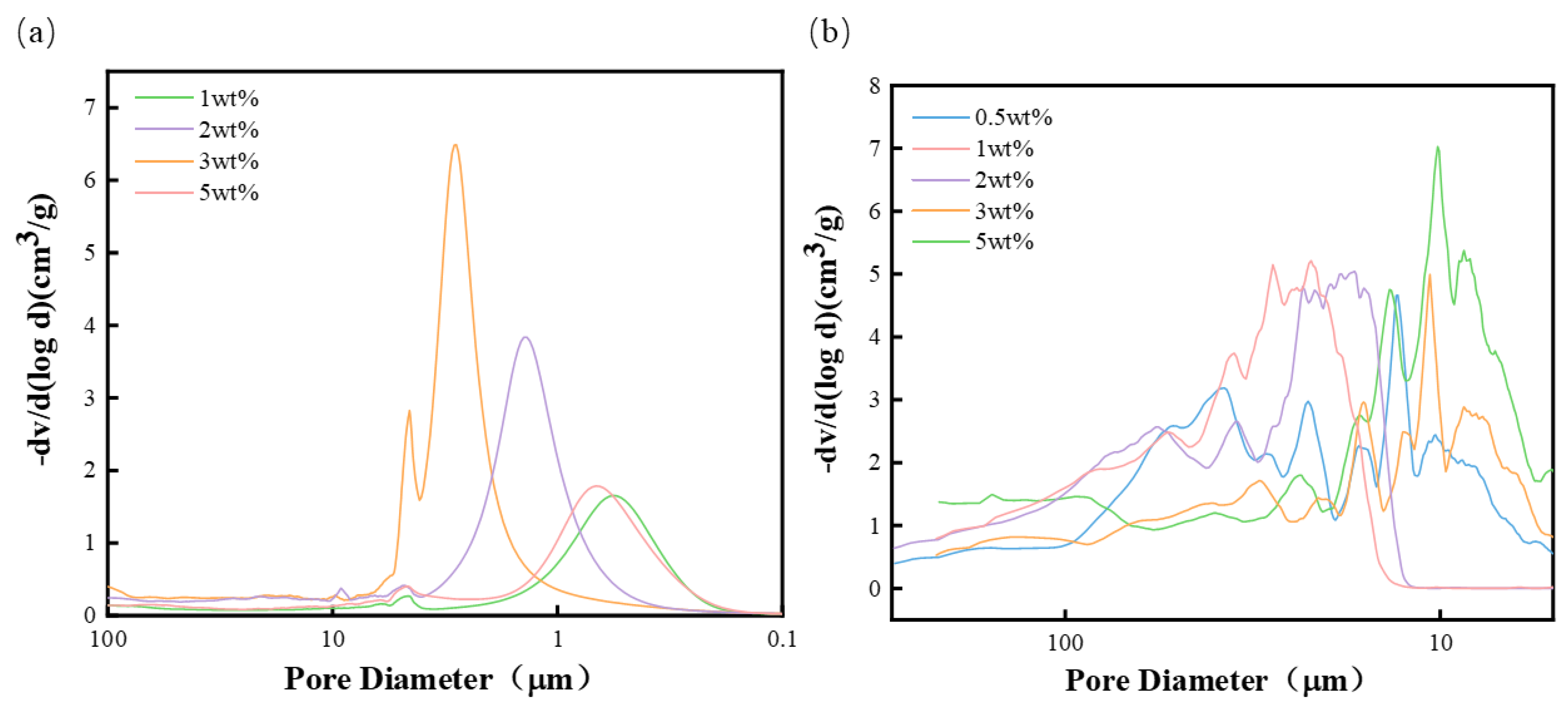
- Aperture distribution test: The sample was cut into 10 mm × 10 mm × 10 mm cubes with a mass of 0.1–0.4 g and dried. The pore diameter of the sample was tested using a mercury injection instrument (PoreMaster60, Austria Anton Paar Company, Graz, Austria).
- Density measurement: The sample was cut into 30 mm × 30 mm × 30 mm cubes, and the length, width, and height of the sample were measured and averaged from three different positions. The mass and volume were measured, and the density was calculated by Equation (1). Each group of samples was measured 5 times and averaged.
- 6.
- Measurement of volumetric water absorption: The sample was cut into 100 mm × 100 mm × 20 mm cubes with a weight of m1. The sample was immersed in a container about 20 mm away from the liquid level. After 96 h, the water on the surface was removed and weighed, m2.
- 7.
- Porosity test: Porosity referred to the percentage of the pore volume inside the foam to the total foam volume and was determined by the density of the foam and the matrix density of the polymer.
- 8.
- Hardness determination: The Shore C hardness of the sample was measured. Five points were calculated for each group of samples and averaged.
- 9.
- Compression strength test: The sample was made into a cube of 30 mm × 30 mm × 30 mm, and the compression mode of the material universal testing machine (AGS-X, Japan Shimadzu Company, Kyoto, Japan) was used to compress the sample by 6 mm at a compression speed of 3 mm/min and a compression rate of 20%. For the compression Poisson ratio test, the sample was made into a cube of 30 mm × 30 mm × 30 mm, a straight line was drawn perpendicular to the direction of compression on the sample as a test point, and then vernier calipers were used to measure the original length of the sample, the original thickness, and its dimensional changes under different strains; then, using the Poisson ratio formula, the foam Poisson ratio was calculated. An average value was taken after three measurements.
- 10.
- Test of polishing performance: Polishing performance was tested on the SK-380A polishing machine. The polishing workpiece was K9 glass with a diameter of 100 mm and a thickness of 0.5 mm. The concentration of silicon dioxide (130 nm), polishing fluid flow rate, polishing head, polishing disc, and polishing time were, respectively, set at 20%, 30 mL/min, 50 rpm, 50 rpm, and 20 min. The material removal rate (MRR) was calculated.
3. Result and Discussion
3.1. Thermal Decomposition Analysis
3.2. Structural Characterization
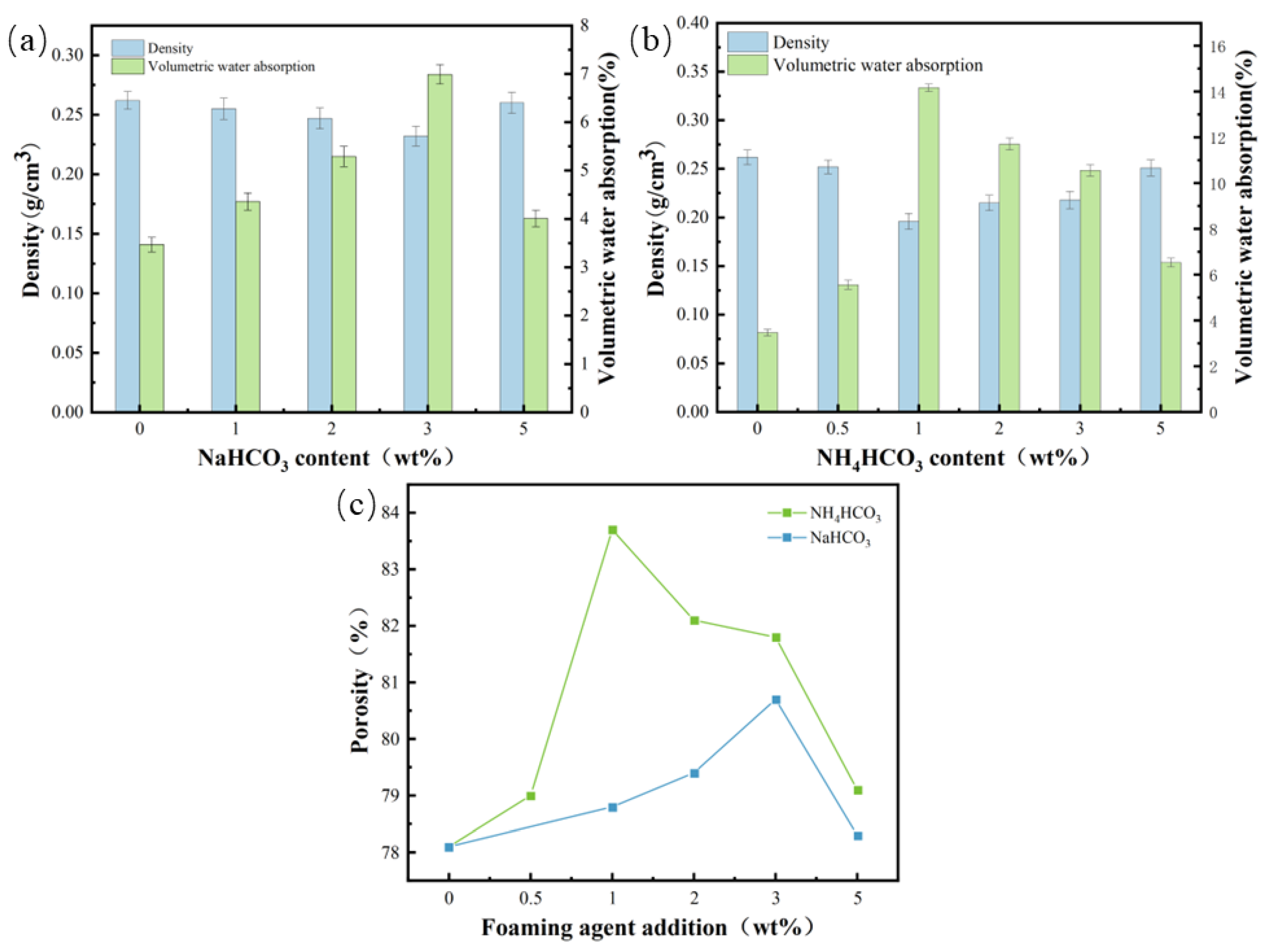
3.3. Physical Performance
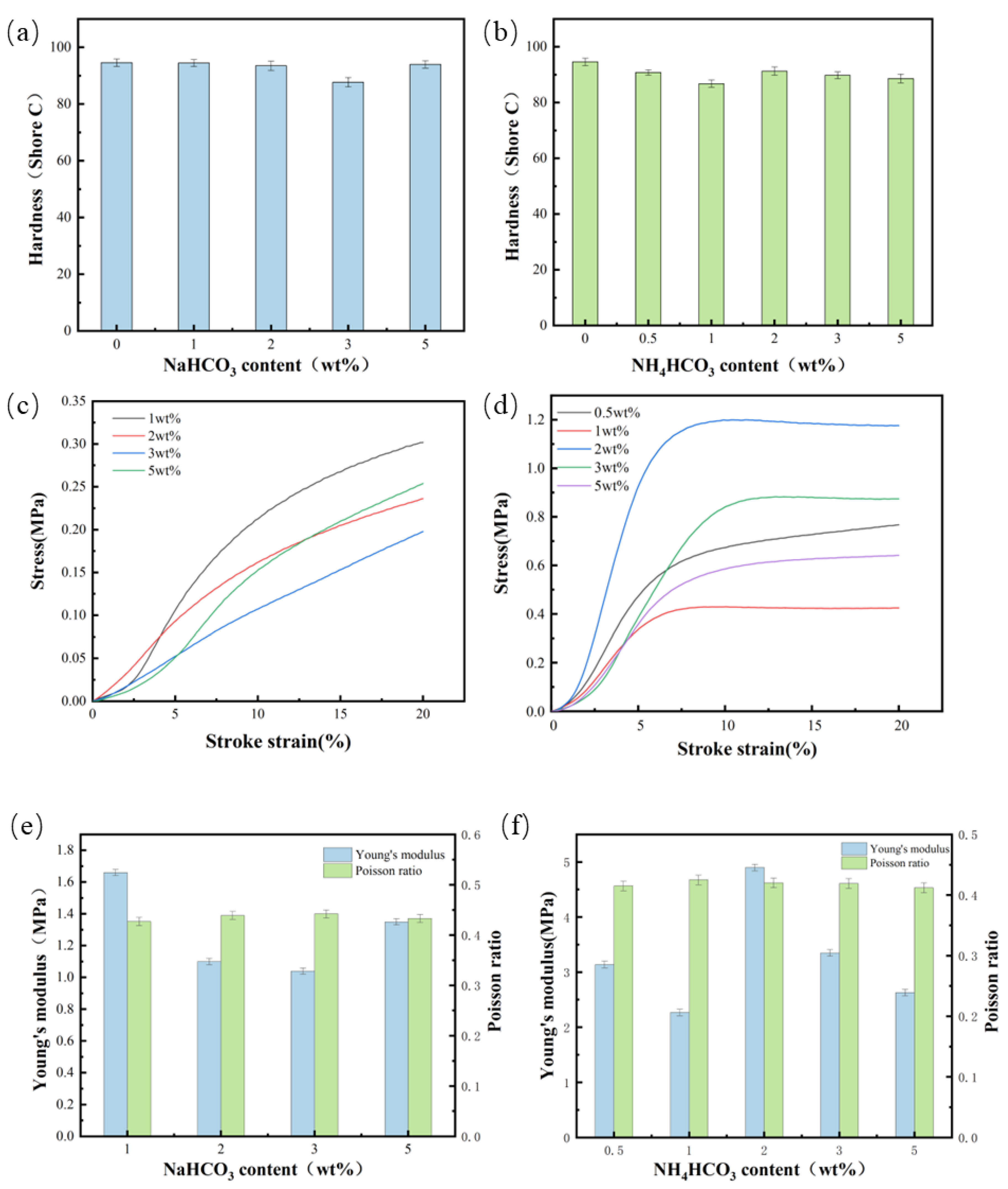
3.4. Mechanical Performance
3.5. Material Removal Rate
4. Conclusions
- The polyurethane foam with NH4CO3 has a more open pore structure and larger aperture than that with NaHCO3. The open pore structure increases with the increase in foaming agent added, but adding too much will cause bubble collapse and lead to the deterioration of various properties of polyurethane foam polishing pad.
- The polyurethane foam polishing pad added with NaHCO3 and NH4CO3 has a change in physical properties compared with the non-added polishing pad, the relative density decreases, and the porosity and volume water absorption increase significantly. With the increase in the addition of NaHCO3 and NH4CO3, the density will first decrease and then increase due to factors such as pore rupture, and the porosity and volume water absorption will first increase and then decrease.
- The hardness of the two groups of polyurethane foam polishing pads added with NaHCO3 and NH4CO3 can be maintained at about 90 Shore C, meeting the basic hardness required for the polishing process. The appropriate addition of NaHCO3 and NH4CO3 can enhance the mechanical properties of the polyurethane foam polishing pad. Nevertheless, excessive dosage will decrease the mechanical properties of the material.
- Compared with the polishing pad without the auxiliary foaming of NaHCO3 and NH4CO3, the material removal rate of the polishing pad prepared by the secondary foaming method was significantly improved, and the highest MRRs of NaHCO3 and NH4CO3 were 89.45 nm/min and 98.78 nm/min, respectively. Increasing the porosity while keeping the hardness basically unchanged can improve the MRR.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Wang, Z.; Liang, Y.; Meng, F.; Cui, Z.; Chen, T.; Yang, Y.; Fan, C.; Yu, T.; Zhao, J. Modelling of polyurethane polishing pad surface topography and fixed-point polished surface profile. Tribol. Int. 2024, 195, 109646. [Google Scholar] [CrossRef]
- Xie, J.; Fan, L.; Yao, D.; Su, F.; Mu, Z.; Zheng, Y. Ultra-robust, self-healable and recyclable polyurethane elastomer via a combination of hydrogen bonds, dynamic chemistry, and microphase separation. Mater. Today Chem. 2022, 23, 100708. [Google Scholar] [CrossRef]
- Zhuang, W.; Bi, Y.; Liu, B.; Hou, D.; Jing, S.; Lu, X.; Sun, M. Mechanical Properties of Polyurethane Mixture and Load Response Behaviour of Polyurethane Composite Pavement. Polymers 2023, 15, 417. [Google Scholar] [CrossRef] [PubMed]
- Belkhir, N.; Bouzid, D.; Herold, V. Morphological behavior and wear of polyurethane pads used in glass polishing process. Precis. Eng. 2012, 36, 641–649. [Google Scholar] [CrossRef]
- Lu, H.; Fookes, B.; Obeng, Y.; Machinski, S.; Richardson, K.A. Quantitative analysis of physical and chemical changes in CMP polyurethane pad surfaces. Mater. Charact. 2002, 49, 35–44. [Google Scholar] [CrossRef]
- Fuensanta, M.; Martín-Martínez, J. Structural and Viscoelastic Properties of Thermoplastic Polyurethanes Containing Mixed Soft Segments with Potential Application as Pressure Sensitive. Adhesives. Polym. 2021, 13, 3097. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chen, C.C.A. Development of modeling to investigate polyurethane pad hardness in chemical mechanical planarization/polishing (CMP) process. Jpn. J. Appl. Phys. 2022, 61, SJ1002. [Google Scholar]
- Prasad, A.; Fotou, G.; Li, S. The effect of polymer hardness, pore size, and porosity on the performance of thermoplastic polyurethane-based chemical mechanical polishing pads. Mater. Res. 2013, 28, 2380–2393. [Google Scholar] [CrossRef]
- Lorusso, C.; Vergaro, V.; Conciauro, F.; Ciccarella, G.; Congedo, P. Thermal and mechanical performance of rigid polyurethane foam added with commercial nanoparticles. Nanomater. Nanotechnol. 2017, 7, 184798041668411. [Google Scholar] [CrossRef]
- Hou, L.; Li, J.; Lu, Z.; Niu, Y.; Jiang, J.; Li, T. Effect of nanoparticles on foaming agent and the foamed concrete. Constr. Build. Mater. 2019, 227, 116698. [Google Scholar] [CrossRef]
- Mogre, C.; Thakurdesai, A.U.; Van Ommen, J.R.; Salameh, S.J. Long-term fluidization of titania nanoparticle agglomerates. Powder Technol. 2017, 316, 441–445. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, G.; Wang, Y.; Li, S.; Xu, Z.; Wang, Z.; Zhao, G. An efficient pre-foamed method to fabricate low density poly(ether-block-amide) foams. J. CO2 Util. 2024, 82, 102740. [Google Scholar] [CrossRef]
- Deng, F.; Jin, H.; Zhang, L.; He, Y. Higher tear strength of EPDM/SBR/TPR composites foam based on double foaming system. J. Elastomers Plast. 2020, 53, 373–385. [Google Scholar] [CrossRef]
- Coste, G.; Negrell, C.; Caillol, S. From gas release to foam synthesis, the second breath of blowing agents. Eur. Polym. J. 2020, 140, 110029. [Google Scholar] [CrossRef]
- Yakushin, V.; Cabulis, U.; Fridrihsone, V.; Kravchenko, S.; Pauliks, R. Properties of polyurethane foam with fourth-generation blowing agent. e-Polymers 2021, 21, 763–769. [Google Scholar] [CrossRef]
- Andersons, J.; Modniks, J.; Kirpluks, M.; Transfer, M. Modelling the effect of morphology on thermal aging of low-density closed-cell PU foams. Int. Commun. Heat Mass Transf. 2022, 139, 106432. [Google Scholar] [CrossRef]
- Greco, A.; Masselli, C. Reduction of the Greenhouse Gasses Emissions in Refrigeration. Tec. Ital.-Ital. J. Eng. Sci. 2020, 64, 30–38. [Google Scholar] [CrossRef]
- Kamińska, K.; Barczewski, M.; Kurańska, M.; Malewska, E.; Polaczek, K.; Prociak, A.J.M. The Effect of a Chemical Foaming Agent and the Isocyanate Index on the Properties of Open-Cell Polyurethane Foams. Materials 2022, 15, 6087. [Google Scholar] [CrossRef]
- Li, X.C.; Bi, Y.S.; Bi, G. An application performance study of monoisopropanolamine carbonate in refrigerator insulation materials. AIP Adv. 2024, 14, 015220. [Google Scholar] [CrossRef]
- Hasan, M.R.M.; You, Z.; Yin, H.; You, L.; Zhang, R.J.C.; Materials, B. Characterizations of foamed asphalt binders prepared using combinations of physical and chemical foaming agents. Constr. Build. Mater. 2019, 204, 94–104. [Google Scholar] [CrossRef]
- Lobos, J.; Thirumuruganandham, S.P.; Rodríguez-Pérez, M.A. Density Gradients, Cellular Structure and Thermal Conductivity of High-Density Polyethylene Foams by Different Amounts of Chemical Blowing Agent. Polymers 2022, 14, 4082. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.S.; Leng, T.P.; Rahmat, A.R.; Zainuddin, F.; Keat, Y.C.; Suppiah, K.; Alsagayar, Z.S. The effect of sodium bicarbonate as blowing agent on the mechanical properties of epoxy. Mater. Today Proc. 2019, 16, 1622–1629. [Google Scholar] [CrossRef]
- Fawzi, T.; Yu, L.; Badri, K.; Sajuri, Z.; Al-Talib, A.A.M.; Noum, S.Y.E. Sodium hydrogen bicarbonate and water as blowing agent in palm kernel oil based polyol polyurethane foam. Mater. Today Proc. 2021, 39, 993–998. [Google Scholar] [CrossRef]
- Yu, H.; Guo, C.; Ye, X.; Pan, Y.; Tu, J.; Wu, Z.; Chen, Z.; Liu, X.; Huang, J.; Ren, Q.; et al. Wide-Range Flexible Capacitive Pressure Sensors Based on Dielectrics with Various Porosity. Micromachines 2022, 13, 1588. [Google Scholar] [CrossRef] [PubMed]
- Fasihi, M.; Targhi, A.A.; Bayat, H. The simultaneous effect of nucleating and blowing agents on the cellular structure of polypropylene foamed via the extrusion process. e-Polymers 2016, 16, 235–241. [Google Scholar] [CrossRef]
- Wu, S.; Xu, W.; Zhang, F.; Wu, H. Effect of Polyurethane on High- and Low-Temperature Performance of Graphene Oxide-Modified Asphalt and Analysis of the Mechanism Based on Infrared Spectrum. Coatings 2022, 12, 590. [Google Scholar] [CrossRef]
- Zheng, B.; Li, Y.; Ma, H.; Chen, G.; Gu, Y.; Wang, C. Pyrolysis characteristics, kinetics and products of flexible polyurethane foam in luxury cruise ship via TG, FTIR, GC-MS and Shuffled Complex Evolution. J. Anal. Appl. Pyrolysis 2024, 179, 106497. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Lv, Y.; Liu, M. Research on lignin-modified flexible polyurethane foam and its application in sound absorption. J. Ind. Eng. Chem. 2024, 3, 19. [Google Scholar] [CrossRef]
- Rijo, B.; Fernando, E.; Ramos, M.; Dias, A.P.S. Biodiesel production over sodium carbonate and bicarbonate catalysts. Fuel 2022, 323, 124383. [Google Scholar] [CrossRef]
- Zhang, H.Q. Relations between crystallographic characteristics and crystal structure and the properties for ammonium bicarbonate. Acta Phys. Sin. 1984, 33, 391–398. [Google Scholar] [CrossRef]
- Uy Lan, D.N.; Fauzi, M.S.; Viet, C.X.; Raps, D.; Altstädt, V. Viscoelastic epoxy foams by an aqueous emulsion foaming process. J. Cell. Plast. 2019, 56, 105–118. [Google Scholar] [CrossRef]
- Majid, N.A.; Rehman, A.; Mohd Sani, N.F.; Hayeemasae, N.; Ismail, H.; Masraff, M.S.; Shuib, R.K. Facial fabrication of self-healing natural rubber foam based on zinc thiolate ionic networks. J. Appl. Polym. Sci. 2024, 141, 55280. [Google Scholar] [CrossRef]
- Bhagavathula, K.B.; Meredith, C.S.; Ouellet, S.; Satapathy, S.S.; Romanyk, D.L.; Hogan, J.D. Density, Microstructure, and Strain-Rate Effects on the Compressive Response of Polyurethane Foams. Exp. Mech. 2021, 62, 505–519. [Google Scholar] [CrossRef]
- House, J.E. A TG study of the kinetics of decomposition of ammonium carbonate and ammonium bibarbonate. Thermochim. Acta 1980, 40, 225–233. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Shih, S.-M. Intrinsic kinetics of the thermal decomposition of sodium bicarbonate. Thermochim. Acta 1993, 223, 177–186. [Google Scholar] [CrossRef]
- Abedini, N.H.Z.; Nourani, A.; Mohseni, M.; Hosseini, N.; Norouzi, S.; Bakhshayesh, P.R. Effects of geometrical and processing parameters on mechanical properties of auxetic polyurethane foams. SN Appl. Sci. 2022, 4, 162. [Google Scholar] [CrossRef]
- Gholami, M.S.; Doutres, O.; Atalla, N. Effect of microstructure closed-pore content on the mechanical properties of flexible polyurethane foam. Int. J. Solids Struct. 2017, 112, 97–105. [Google Scholar] [CrossRef]
- Uneda, M.; Kubo, N.; Hatatani, M.; Hotta, K.; Morinaga, H. Analysis of the material removal mechanism in chemical mechanical polishing with in-situ macroscale nonwoven pad contact interface observation using an evanescent field. Precis. Eng. 2023, 82, 281–289. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Jiang, Z.; Zhu, M.; Wang, B.; Chen, J.; Wang, W. Effect of Secondary Foaming on the Structural Properties of Polyurethane Polishing Pad. Materials 2024, 17, 2759. https://doi.org/10.3390/ma17112759
Chen M, Jiang Z, Zhu M, Wang B, Chen J, Wang W. Effect of Secondary Foaming on the Structural Properties of Polyurethane Polishing Pad. Materials. 2024; 17(11):2759. https://doi.org/10.3390/ma17112759
Chicago/Turabian StyleChen, Minxuan, Zhenlin Jiang, Min Zhu, Baoxiu Wang, Jiapeng Chen, and Wenjun Wang. 2024. "Effect of Secondary Foaming on the Structural Properties of Polyurethane Polishing Pad" Materials 17, no. 11: 2759. https://doi.org/10.3390/ma17112759



