Hydrothermal Growth and Orientation of LaFeO3 Epitaxial Films
Abstract
1. Introduction
2. Materials and Method
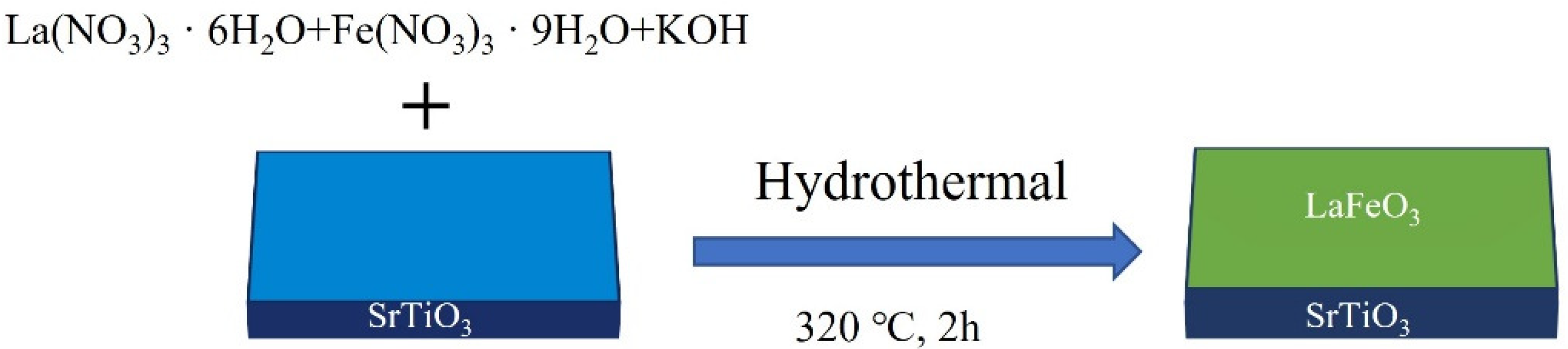
2.1. Synthesis of Bulk LaFeO3 Powders
2.2. Preparation of LaFeO3 Films
2.3. Material Characterization
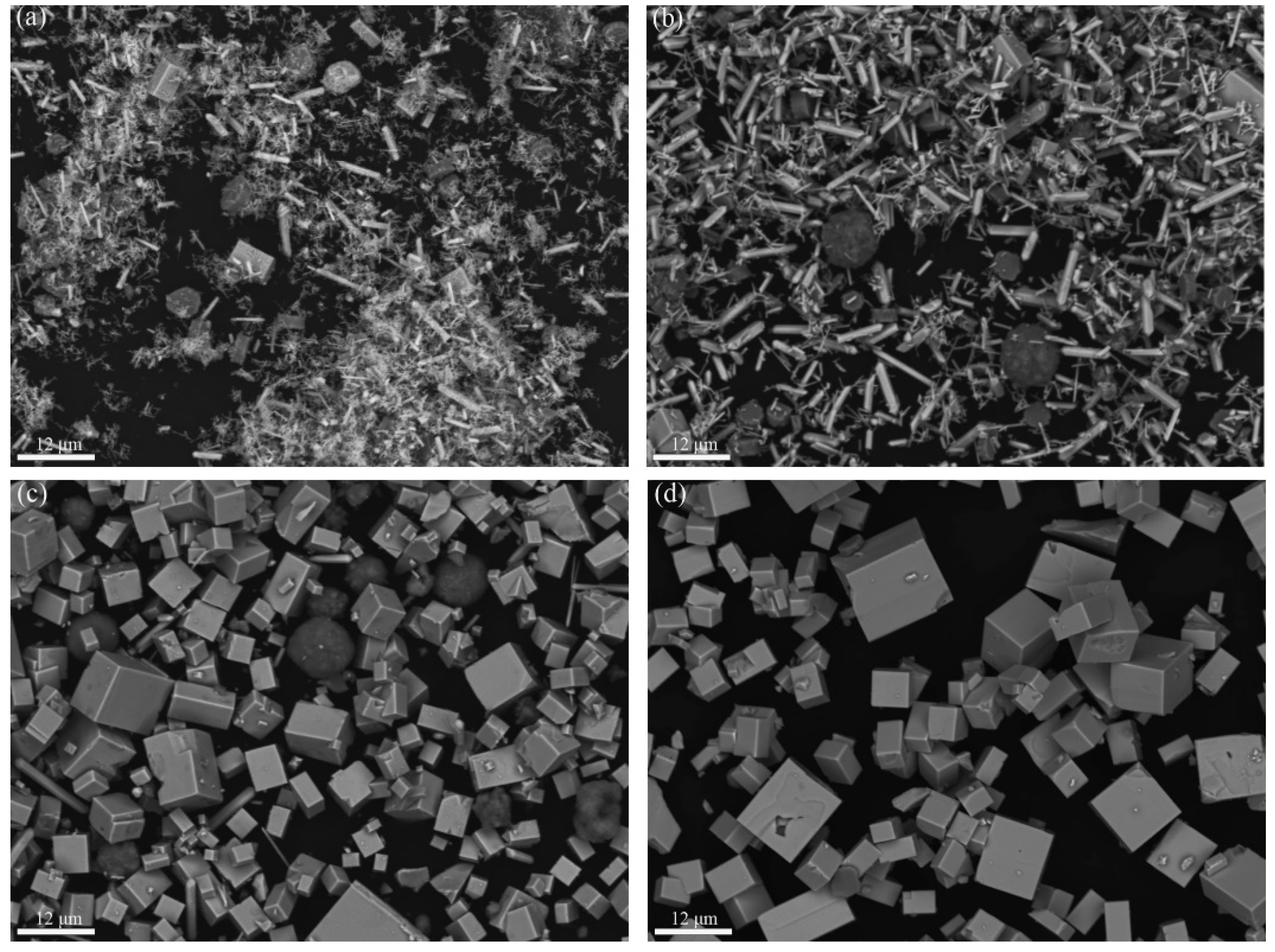
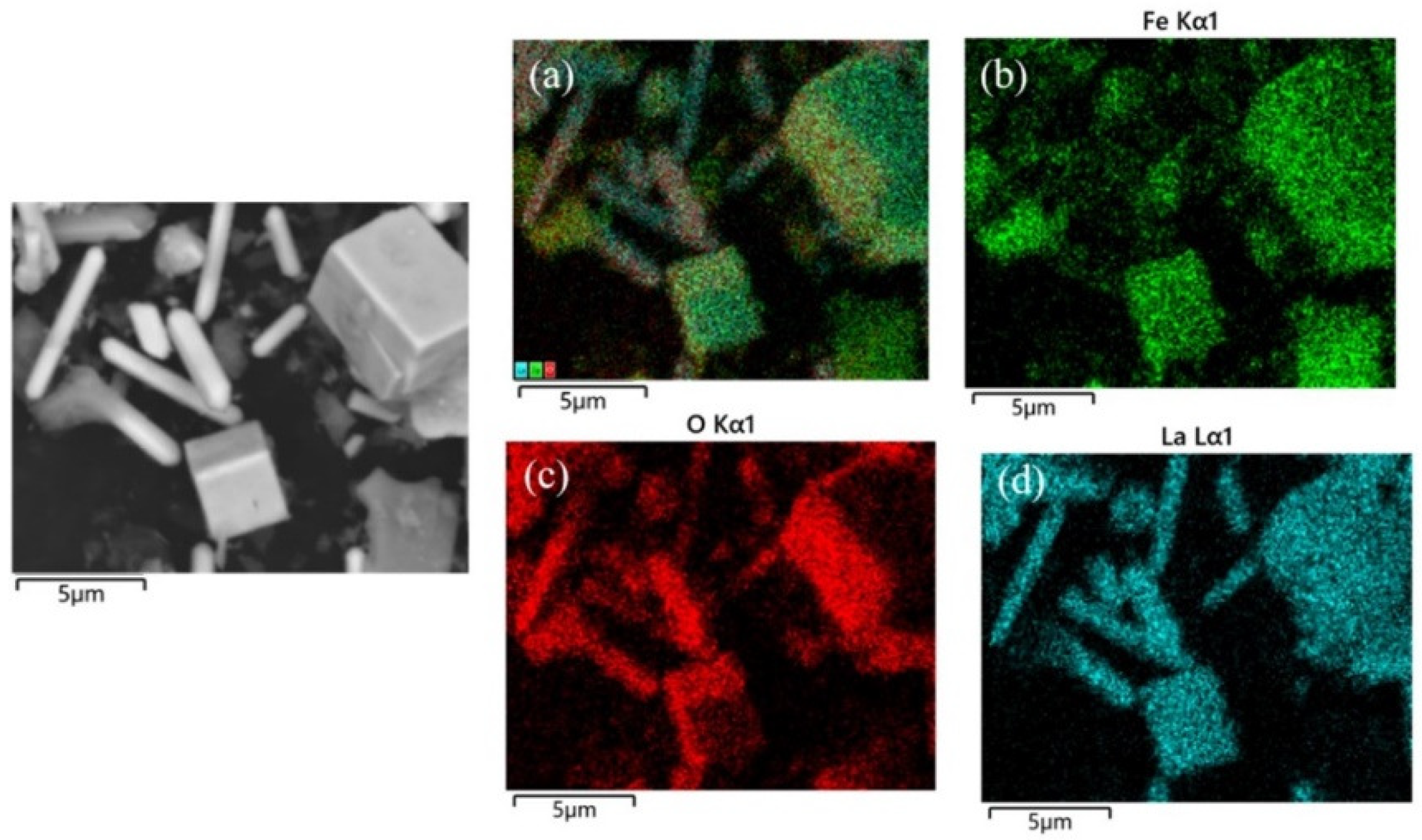
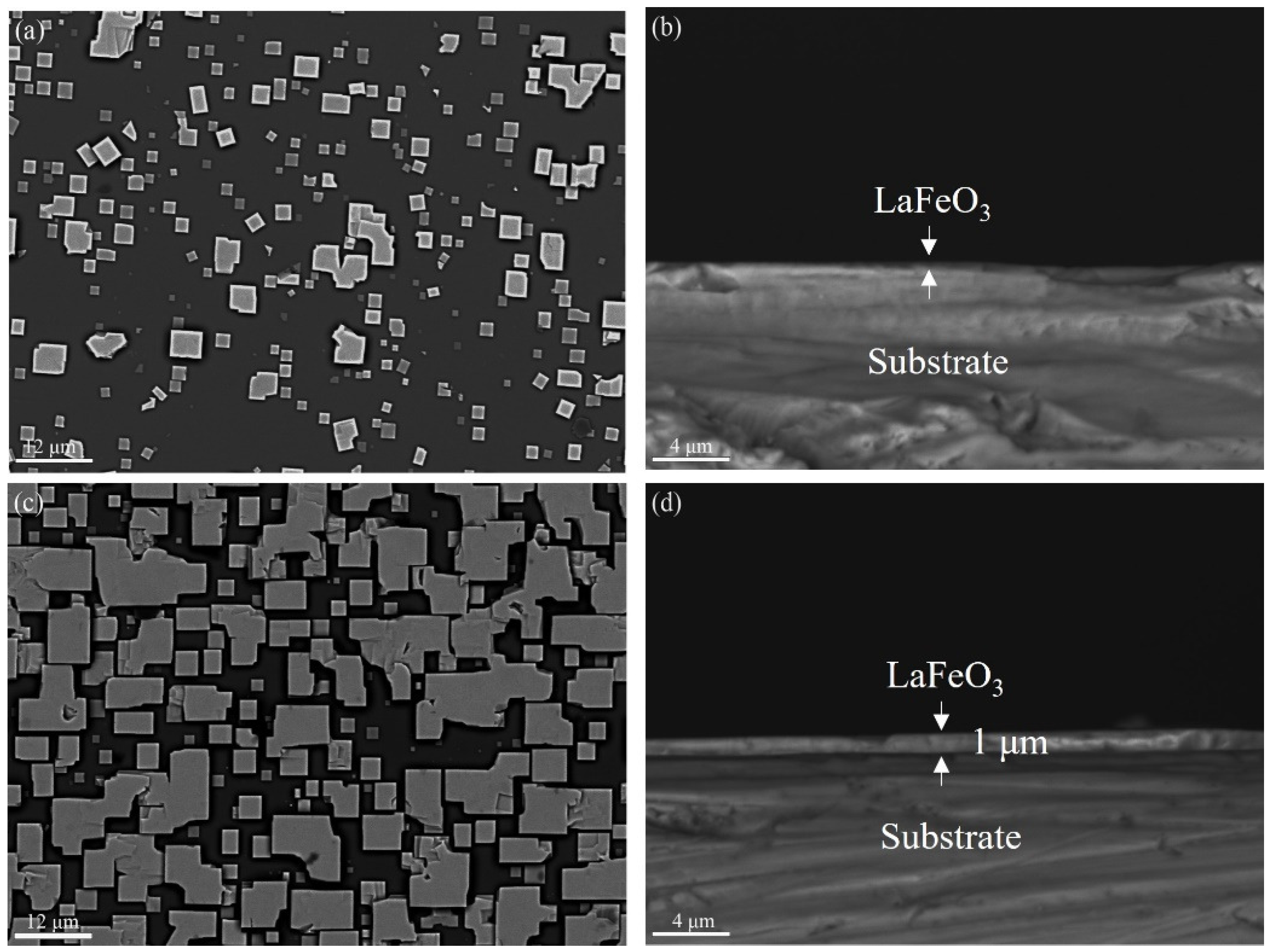
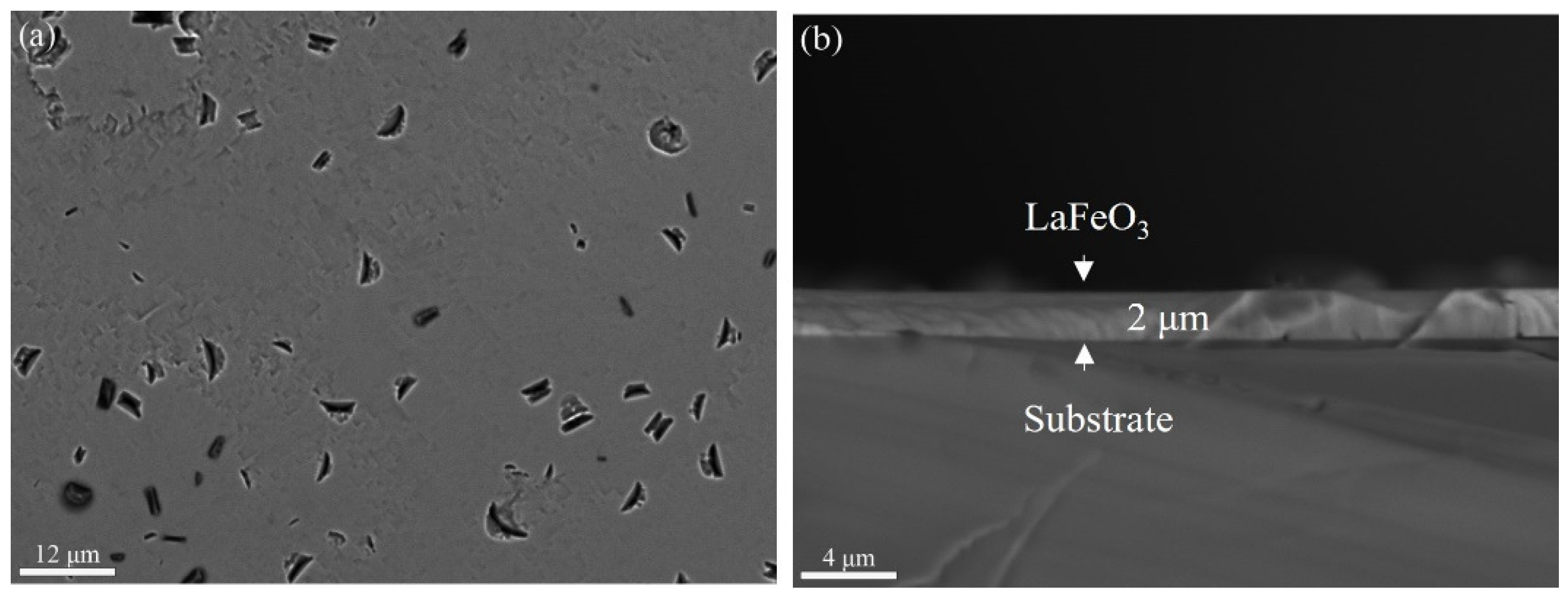
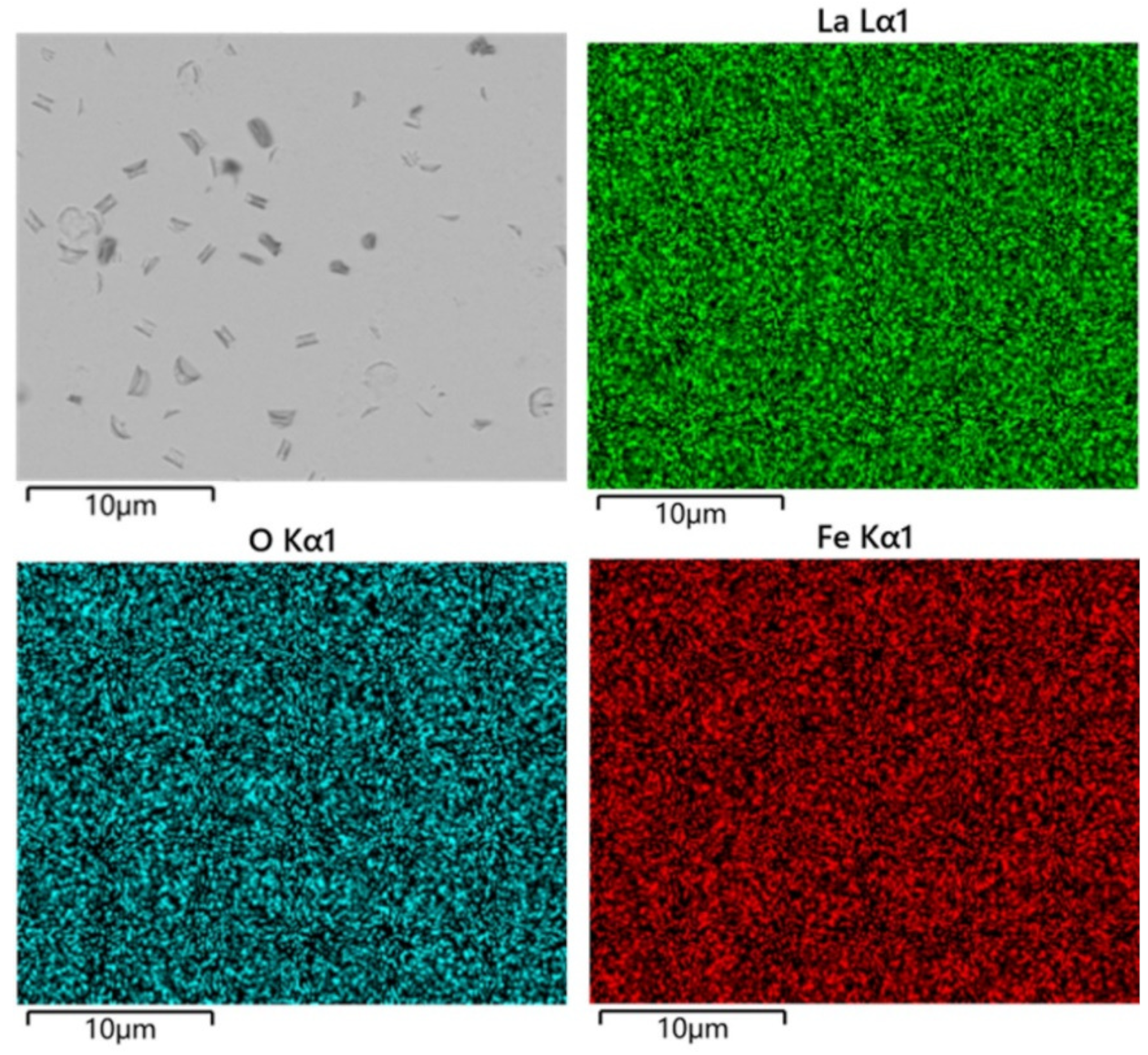
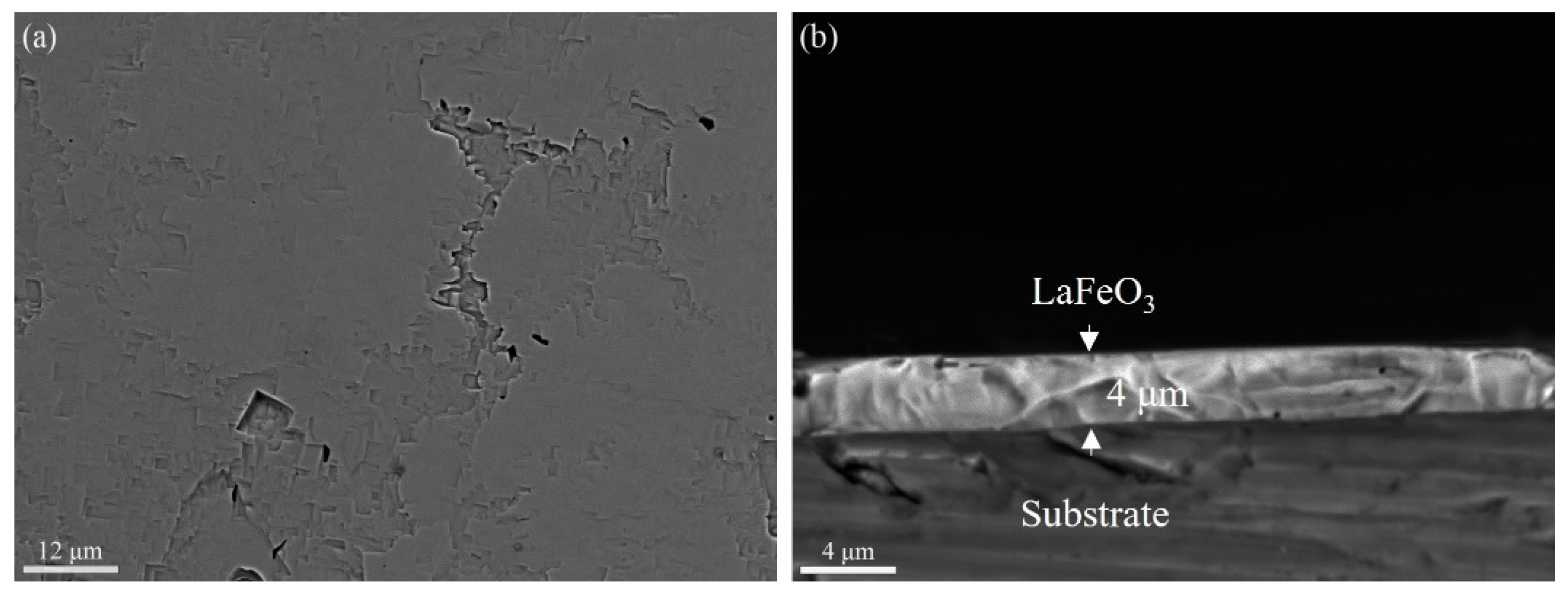
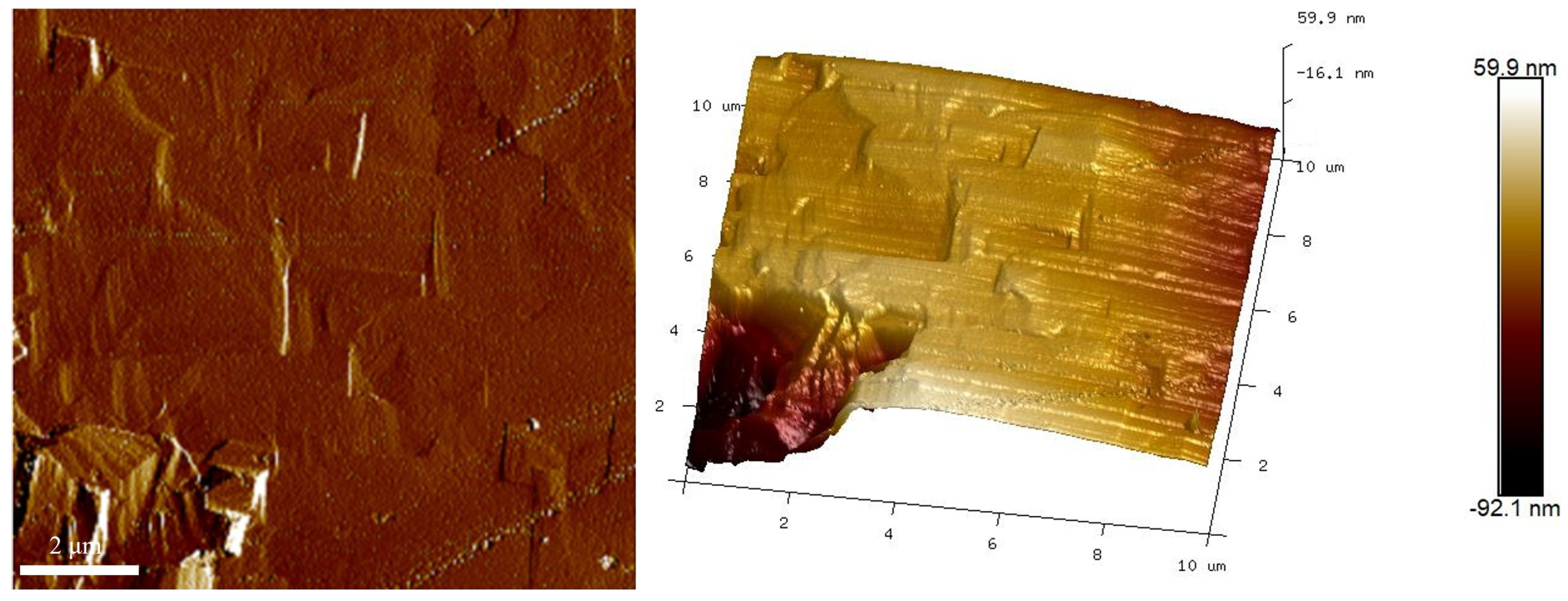
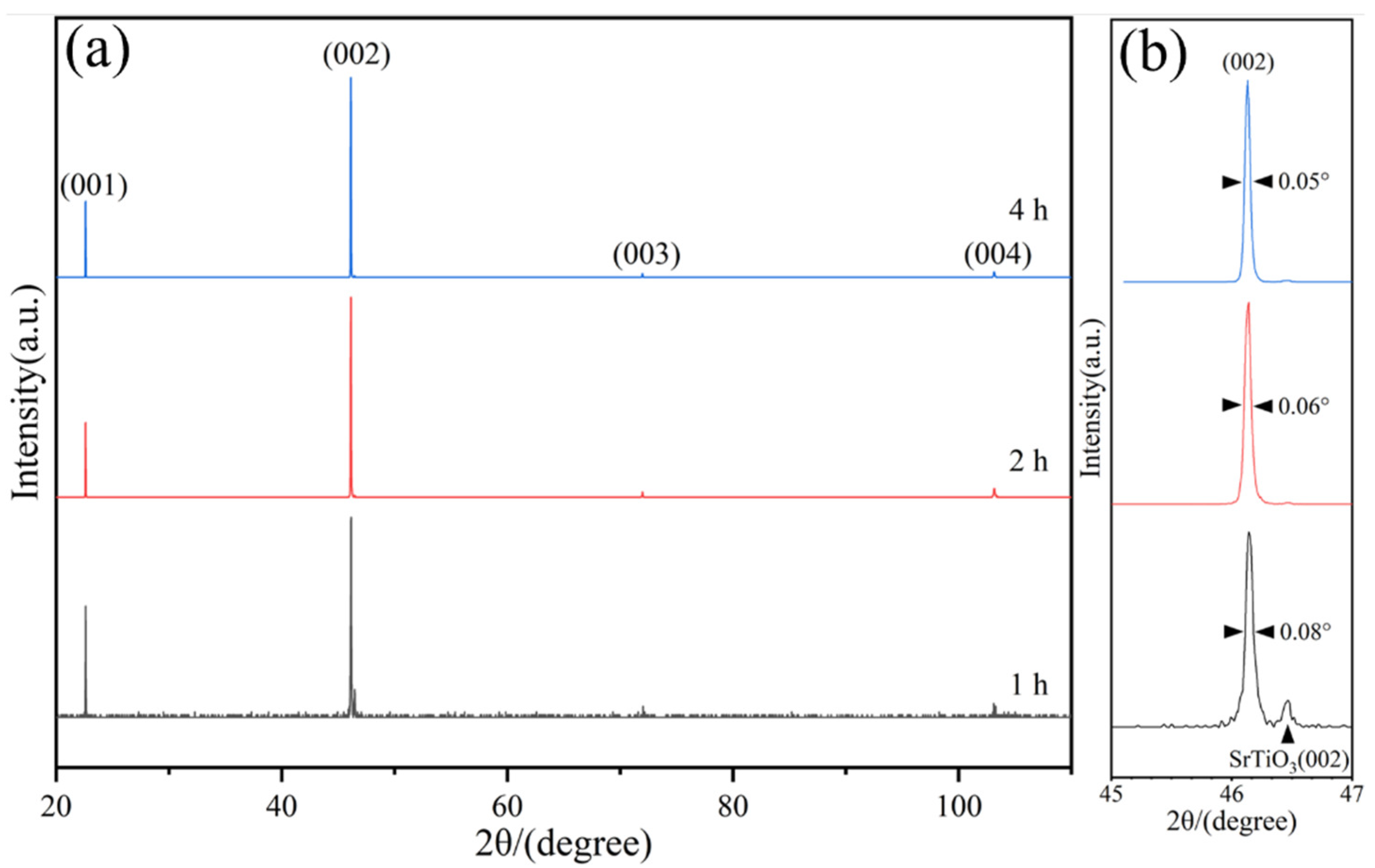
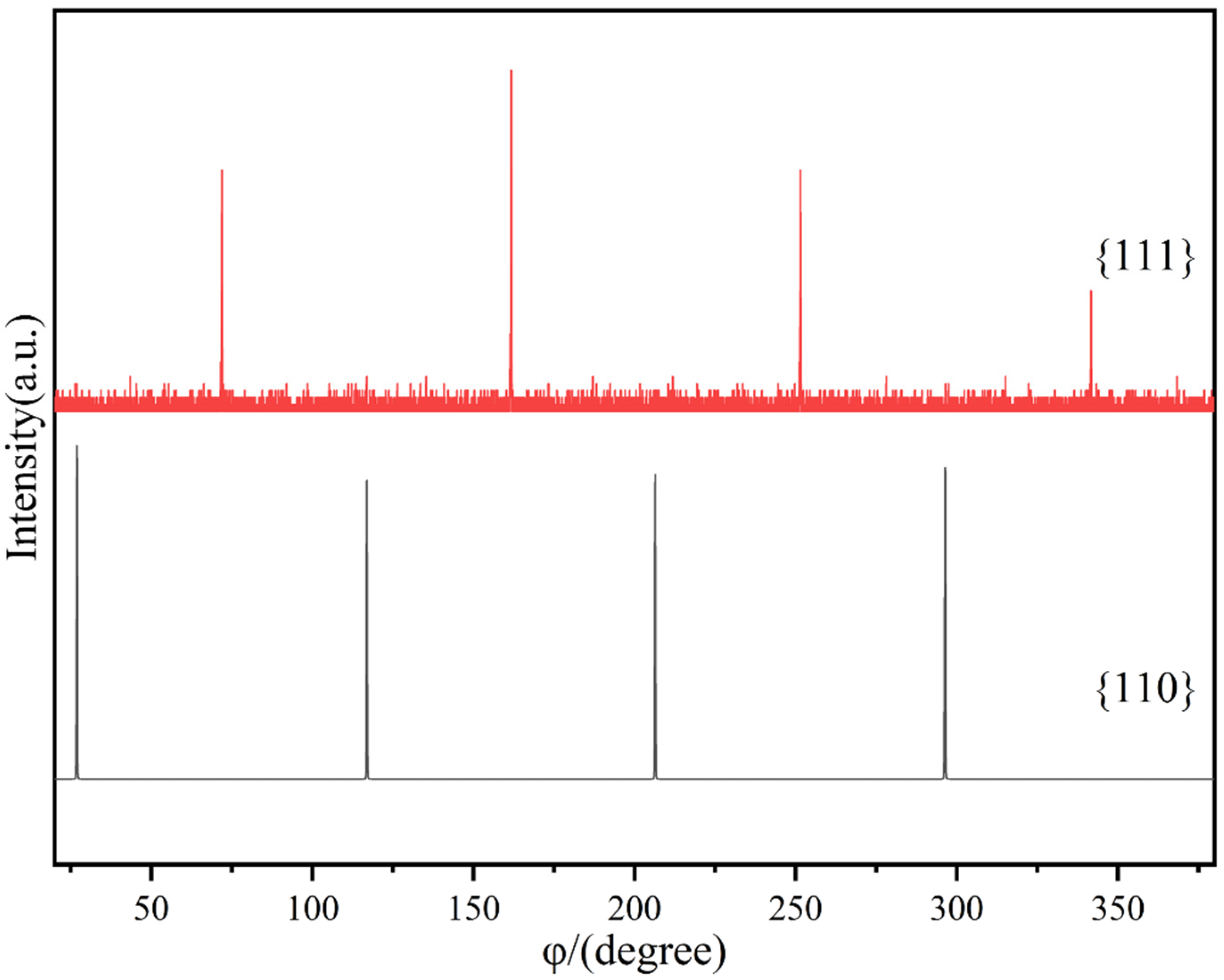
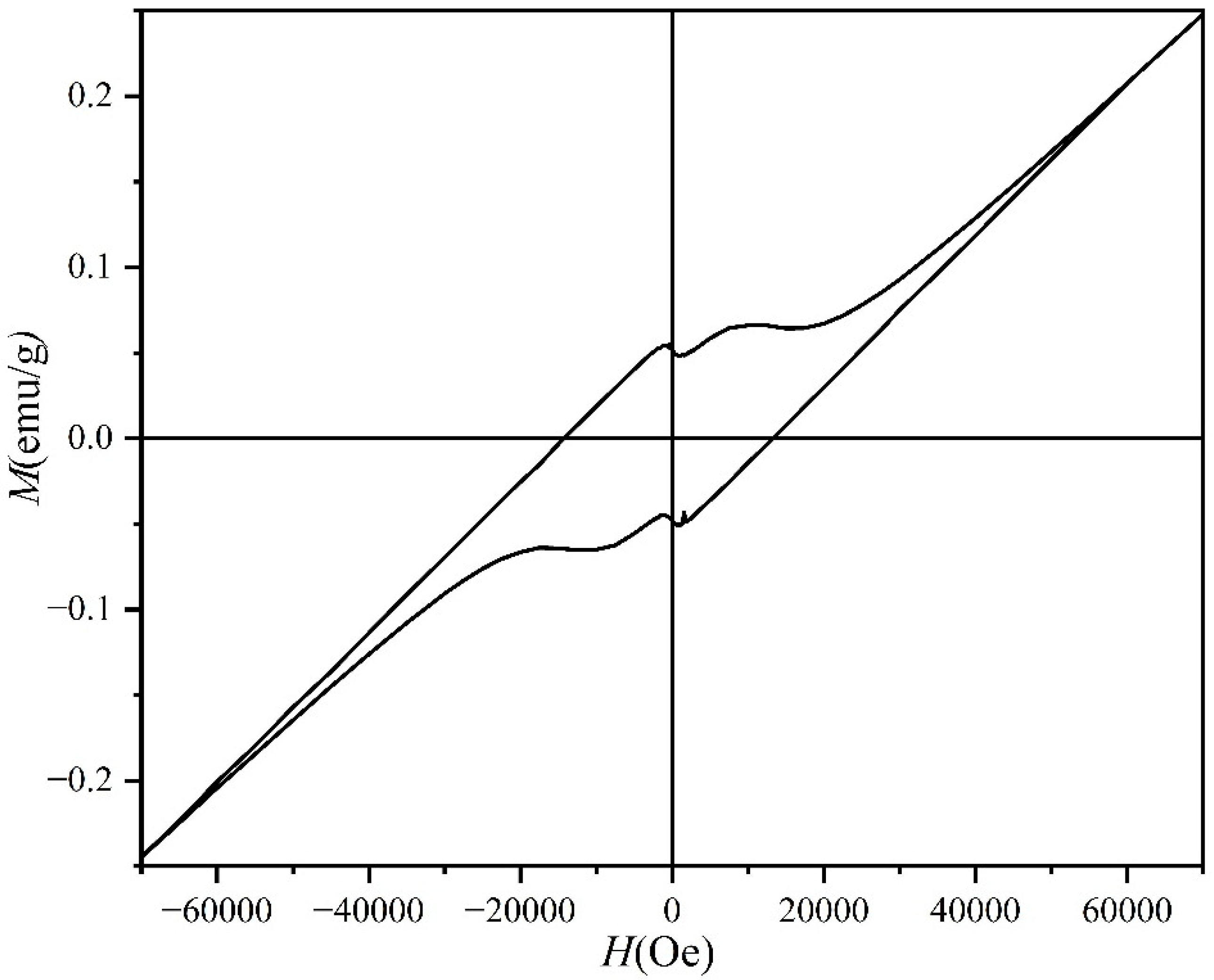
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Wang, C.; Chen, G.; Wang, S.; Yu, Z.; Wang, T.; Ke, W.; Fang, G. A generic lanthanum doping strategy enabling efficient lead halide perovskite luminescence for backlights. Sci. Bull. 2023, 68, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Sakthipandi, K.; Rajendran, V.; Jayakumar, T.; Raj, B.; Kulandivelu, P. Synthesis and on-line ultrasonic characterisation of bulk and nanocrystalline La0.68Sr0.32MnO3 perovskite manganite. J. Alloys Compd. 2011, 509, 3457–3467. [Google Scholar] [CrossRef]
- Choura-Maatar, S.; M’nassri, R.; Cheikhrouhou-Koubaa, W.; Koubaa, M.; Cheikhrouhou, A.; Hlil, E.K. Role of lanthanum vacancy on the structural, magnetic and magnetocaloric properties in the lacunar perovskite manganites La0.8−x□xNa0.2MnO3 (0 ≤ x ≤ 0.15). R. Soc. Chem. Adv. 2017, 7, 50347–50357. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, X.; Tian, Y.; Wu, X.; Zhang, J.; Zuo, W. Niobium doped lanthanum calcium ferrite perovskite as a novel electrode material for symmetrical solid oxide fuel cells. J. Power Sources 2016, 326, 35–42. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.; Luo, W. Perovskite-type lanthanum ferrite based photocatalysts: Preparation, properties, and applications. J. Energy Chem. 2022, 66, 314–338. [Google Scholar] [CrossRef]
- Bhargav, K.K.; Ram, S.; Majumder, S.B. Physics of the multi-functionality of lanthanum ferrite ceramics. J. Appl. Phys. 2014, 115, 4109. [Google Scholar] [CrossRef]
- Fan, H.; Keane, M.; Singh, P.; Han, M. Electrochemical performance and stability of lanthanum strontium cobalt ferrite oxygen electrode with gadolinia doped ceria barrier layer for reversible solid oxide fuel cell. J. Power Sources 2014, 268, 634–639. [Google Scholar] [CrossRef]
- Jun, B.M.; Elanchezhiyan, S.S.; Yoon, Y.; Wang, D.; Kim, S.; Muthu Prabhu, S.; Park, C.M. Accelerated photocatalytic degradation of organic pollutants over carbonate-rich lanthanum-substituted zinc spinel ferrite assembled reduced graphene oxide by ultraviolet (UV)-activated persulfate. Chem. Eng. J. 2020, 393, 124733. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Lin, Y.T.; Chen, J.L.; Zhang, J.; Zhu, Z.Q.; Liu, Q.J. A high sensitivity gas sensor for formaldehyde based on silver doped lanthanum ferrite. Sens. Actuators B Chem. 2014, 190, 171–176. [Google Scholar] [CrossRef]
- Gholizadeh, A. The effects of A/B-site substitution on structural, redox and catalytic properties of lanthanum ferrite nanoparticles. J. Mater. Res. Technol. 2019, 8, 457–466. [Google Scholar] [CrossRef]
- Wang, B.; Yu, Q.; Zhang, S.; Wang, T.; Sun, P.; Chuai, X.; Lu, G. Gas sensing with yolk-shell LaFeO3 microspheres prepared by facile hydrothermal synthesis. Sens. Actuators B Chem. 2018, 258, 1215–1222. [Google Scholar] [CrossRef]
- Roseno, K.T.C.; Brackmann, R.; da Silva, M.A.; Schmal, M. Investigation of LaCoO3, LaFeO3 and LaCo0.5Fe0.5O3 perovskites as catalyst precursors for syngas production by partial oxidation of methane. Int. J. Hydrogen Energy 2016, 41, 18178–18192. [Google Scholar] [CrossRef]
- Rai, A.; Sharma, A.L.; Thakur, A.K. Evaluation of aluminium doped lanthanum ferrite based electrodes for supercapacitor design. Solid State Ion. 2014, 262, 230–233. [Google Scholar] [CrossRef]
- Cao, E.; Wang, H.; Wang, X.; Yang, Y.; Hao, W.; Sun, L.; Zhang, Y. Enhanced ethanol sensing performance for chlorine doped nanocrystalline LaFeO3-δ powders by citric sol-gel method. Sens. Actuators B Chem. 2017, 251, 885–893. [Google Scholar] [CrossRef]
- Gao, R.L.; Fu, C.L.; Cai, W.; Chen, G.; Deng, X.L.; Yang, H.W.; Sun, J.R.; Zhao, Y.G.; Shen, B.G. Mechanism of ferroelectric resistive switching in Bi0.9La0.1FeO3 thin films. Thin Solid Film. 2015, 583, 13–18. [Google Scholar] [CrossRef]
- Kakizaki, K.; Taguchi, H.; Hiratsuka, N. Magnetic properties of La-Co substituted barium ferrite thin films with large magnetic anisotropy. J. Magn. Magn. Mater. 2004, 272, 2241–2243. [Google Scholar] [CrossRef]
- Kim, M.; Kim, D.H.; Han, G.D.; Choi, H.J.; Choi, H.R.; Shim, J.H. Lanthanum strontium cobaltite-infiltrated lanthanum strontium cobalt ferrite cathodes fabricated by inkjet printing for high-performance solid oxide fuel cells. J. Alloys Compd. 2020, 843, 155806. [Google Scholar] [CrossRef]
- Park, K.-I.; Xu, S.; Liu, Y.; Hwang, G.-T.; Kang, S.-J.L.; Wang, Z.L.; Lee, K.J. Piezoelectric BaTiO3 Thin Film Nanogenerator on Plastic Substrates. Nano Lett. 2010, 10, 4939–4943. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Lippmaa, M.; Yamamoto, T.; Meguro, S.; Koinuma, H. Improved stoichiometry and misfit control in perovskite thin film formation at a critical fluence by pulsed laser deposition. Appl. Phys. Lett. 2005, 87, 241919. [Google Scholar] [CrossRef]
- Burton, A.R.; Paudel, R.; Matthews, B.; Sassi, M.; Spurgeon, S.R.; Farnum, B.H.; Comes, R.B. Thickness dependent OER electrocatalysis of epitaxial LaFeO3 thin films. J. Mater. Chem. A 2022, 10, 1909–1918. [Google Scholar] [CrossRef]
- Flynn, B.T.; Zhang, K.H.L.; Shutthanandan, V.; Varga, T.; Colby, R.J.; Oleksak, R.P.; Manandhar, S.; Engelhard, M.H.; Chambers, S.A.; Henderson, M.A.; et al. Growth and surface modification of LaFeO3 thin films induced by reductive annealing. Appl. Surf. Sci. 2015, 330, 309–315. [Google Scholar] [CrossRef]
- Moser, T.; Kothandaraman, R.; Yang, S.; Walter, A.; Siegrist, S.; Lai, H.; Gilshtein, E.; Tiwari, A.N.; Fu, F. Understanding the Formation Process of Perovskite Layers Grown by Chemical Vapour Deposition. Front. Energy Res. 2022, 10, 883882. [Google Scholar] [CrossRef]
- Tan, C.K.; Goh, G.K.L.; Cheah, W.L. Dielectric properties of hydrothermally epitaxied I-V perovskite thin films. Thin Solid Film. 2007, 515, 6577–6581. [Google Scholar] [CrossRef]
- Rout, D.; Han, S.H.; Moon, K.S.; Kim, H.G.; Cheon, C.I.; Kang, S.-J.L. Low temperature hydrothermal epitaxy and Raman study of heteroepitaxial BiFeO3 film. Appl. Phys. Lett. 2009, 95, 122509. [Google Scholar] [CrossRef]
- Bassiri-Gharb, N.; Bastani, Y.; Bernal, A. Chemical solution growth of ferroelectric oxide thin films and nanostructures. Chem. Soc. Rev. 2014, 43, 2125–2140. [Google Scholar] [CrossRef]
- Huang, A.; Handoko, A.D.; Goh, G.K.L.; Pallathadka, P.K.; Shannigrahi, S. Hydrothermal synthesis of (00l) epitaxial BiFeO3 films on SrTiO3 substrate. CrystEngComm 2010, 12, 3806–3814. [Google Scholar] [CrossRef]
- Guo, K.; Tao, Y.; Liu, Y.; Lyu, Y.; Pan, Z. One-Stage Hydrothermal Growth and Characterization of Epitaxial LaMnO3 Films on SrTiO3 Substrate. Materials 2022, 15, 5928. [Google Scholar] [CrossRef]
- Goh, G.K.L.; Levi, C.G.; Hwan Choi, J.; Lange, F.F. Hydrothermal epitaxy of KNbO3 thin films and nanostructures. J. Cryst. Growth 2006, 286, 457–464. [Google Scholar] [CrossRef]
- Tong, F.; Zhao, Y.; Wang, M.-H. Cube-like LaFeO3 microstructures synthesised by a hydrothermal method and their optical properties. Micro Nano Lett. 2019, 14, 259–262. [Google Scholar] [CrossRef]
- Ahn, S.; Jung, W.; Choi, S.-K. Size dependence of initial polarization direction in nanosized epitaxial PbTiO3 islands fabricated by hydrothermal epitaxy below Curie temperature. Appl. Phys. Lett. 2005, 86, 172901. [Google Scholar] [CrossRef]
- Bi, L.; Kim, H.-S.; Dionne, G.F.; Ross, C.A.; Paik, H.; Park, Y.C. Orientation control and self-assembled nanopyramid structure of LaFeO3 films epitaxially grown on SrTiO3(001) substrates. Appl. Phys. Lett. 2009, 95, 121908. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Yun, H.J.; Yoon, J.-W. Characterization and magnetic properties of LaFeO3 nanofibers synthesized by electrospinning. J. Alloys Compd. 2014, 583, 320–324. [Google Scholar] [CrossRef]
- Acharya, S.; Mondal, J.; Ghosh, S.; Roy, S.K.; Chakrabarti, P.K. Multiferroic behavior of lanthanum orthoferrite (LaFeO3). Mater. Lett. 2010, 64, 415–418. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xian, G.; Zheng, T.; Tao, Y.; Pan, Z. Hydrothermal Growth and Orientation of LaFeO3 Epitaxial Films. Materials 2024, 17, 2758. https://doi.org/10.3390/ma17112758
Xian G, Zheng T, Tao Y, Pan Z. Hydrothermal Growth and Orientation of LaFeO3 Epitaxial Films. Materials. 2024; 17(11):2758. https://doi.org/10.3390/ma17112758
Chicago/Turabian StyleXian, Guang, Tongxin Zheng, Yaqiu Tao, and Zhigang Pan. 2024. "Hydrothermal Growth and Orientation of LaFeO3 Epitaxial Films" Materials 17, no. 11: 2758. https://doi.org/10.3390/ma17112758
APA StyleXian, G., Zheng, T., Tao, Y., & Pan, Z. (2024). Hydrothermal Growth and Orientation of LaFeO3 Epitaxial Films. Materials, 17(11), 2758. https://doi.org/10.3390/ma17112758





