Long-Term Thermal Stabilization of Poly(Lactic Acid)
Abstract
1. Introduction
2. Materials and Methods
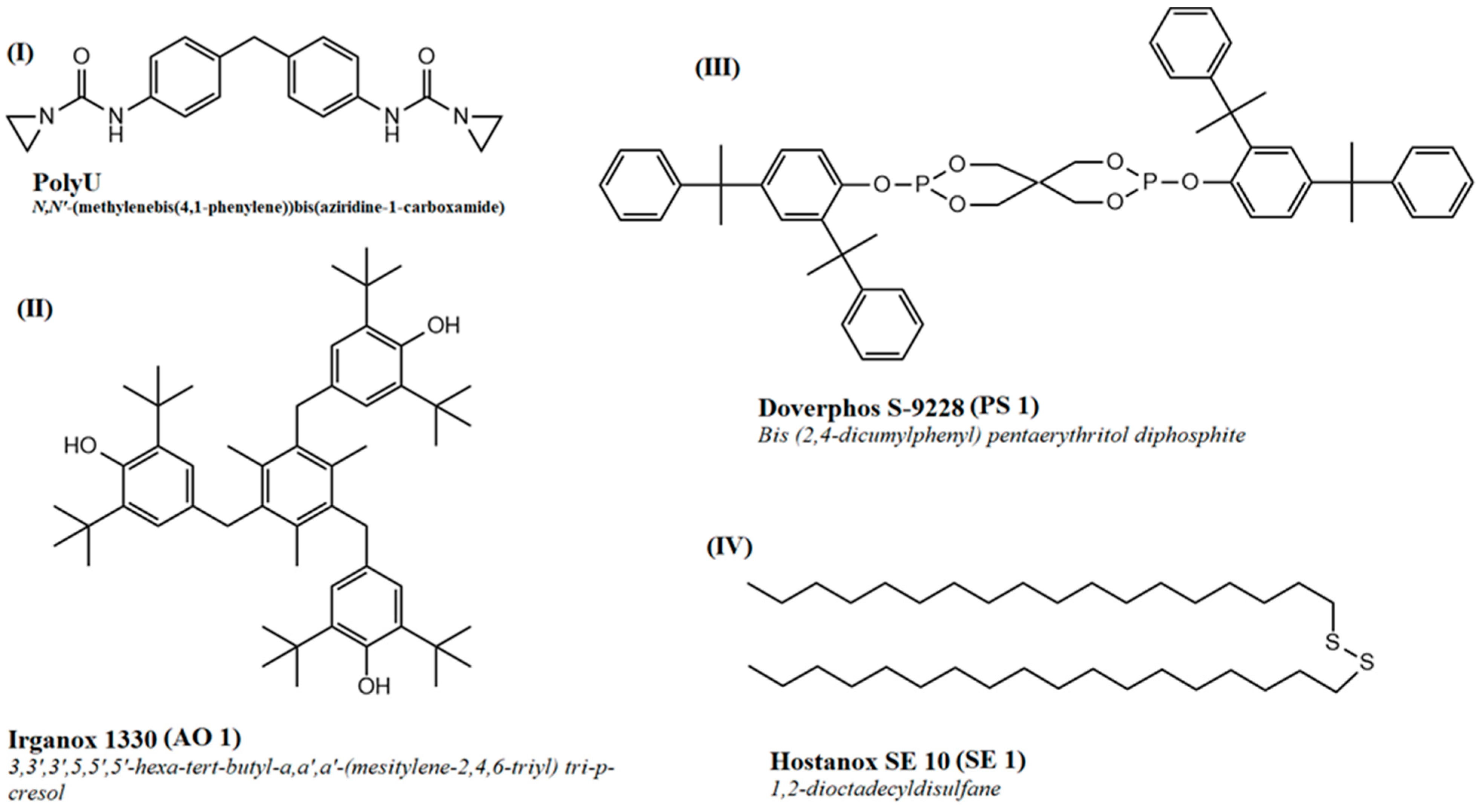
2.1. Materials
2.2. Compounding
2.3. Aging Tests
2.4. Characterization
3. Results
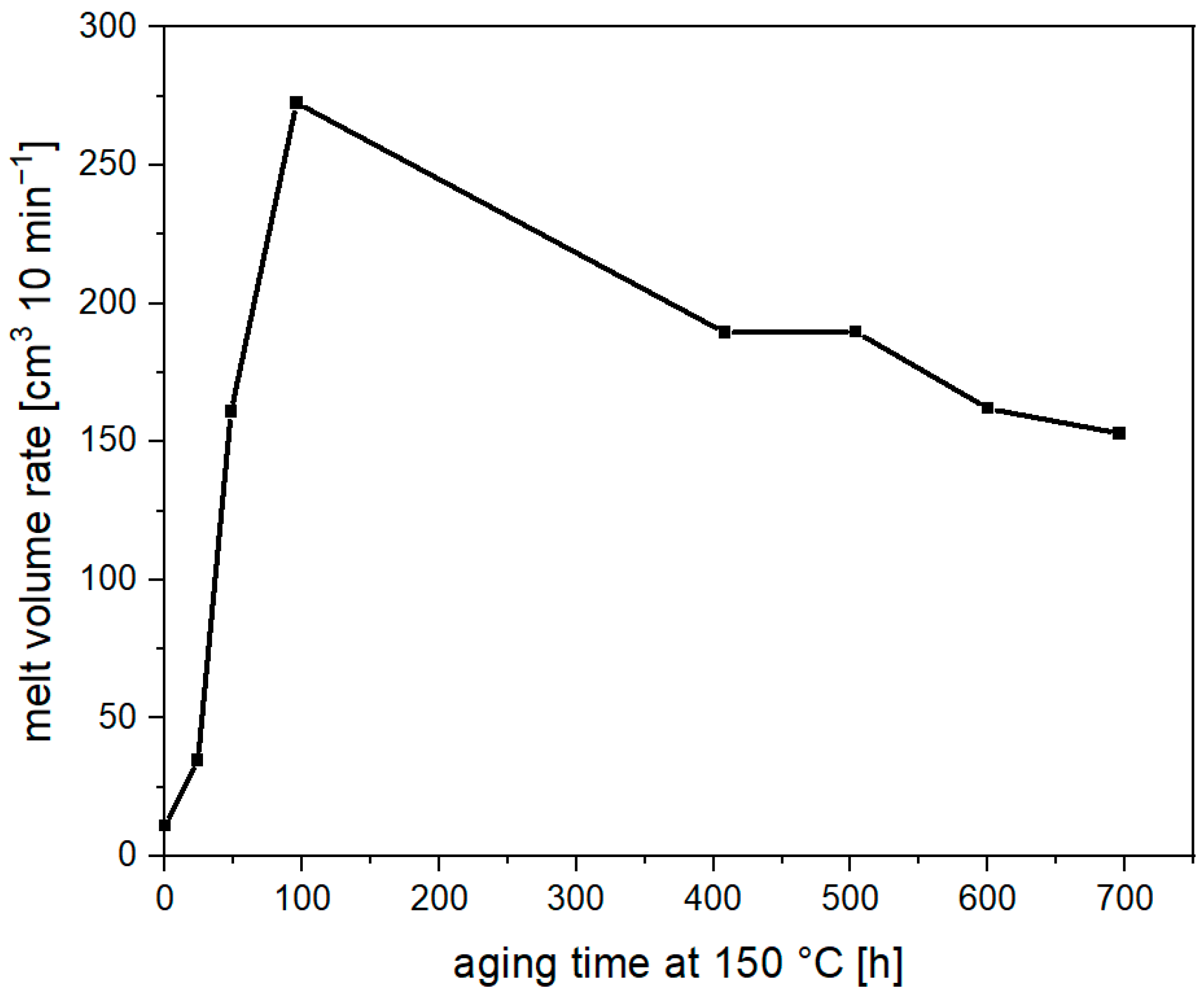
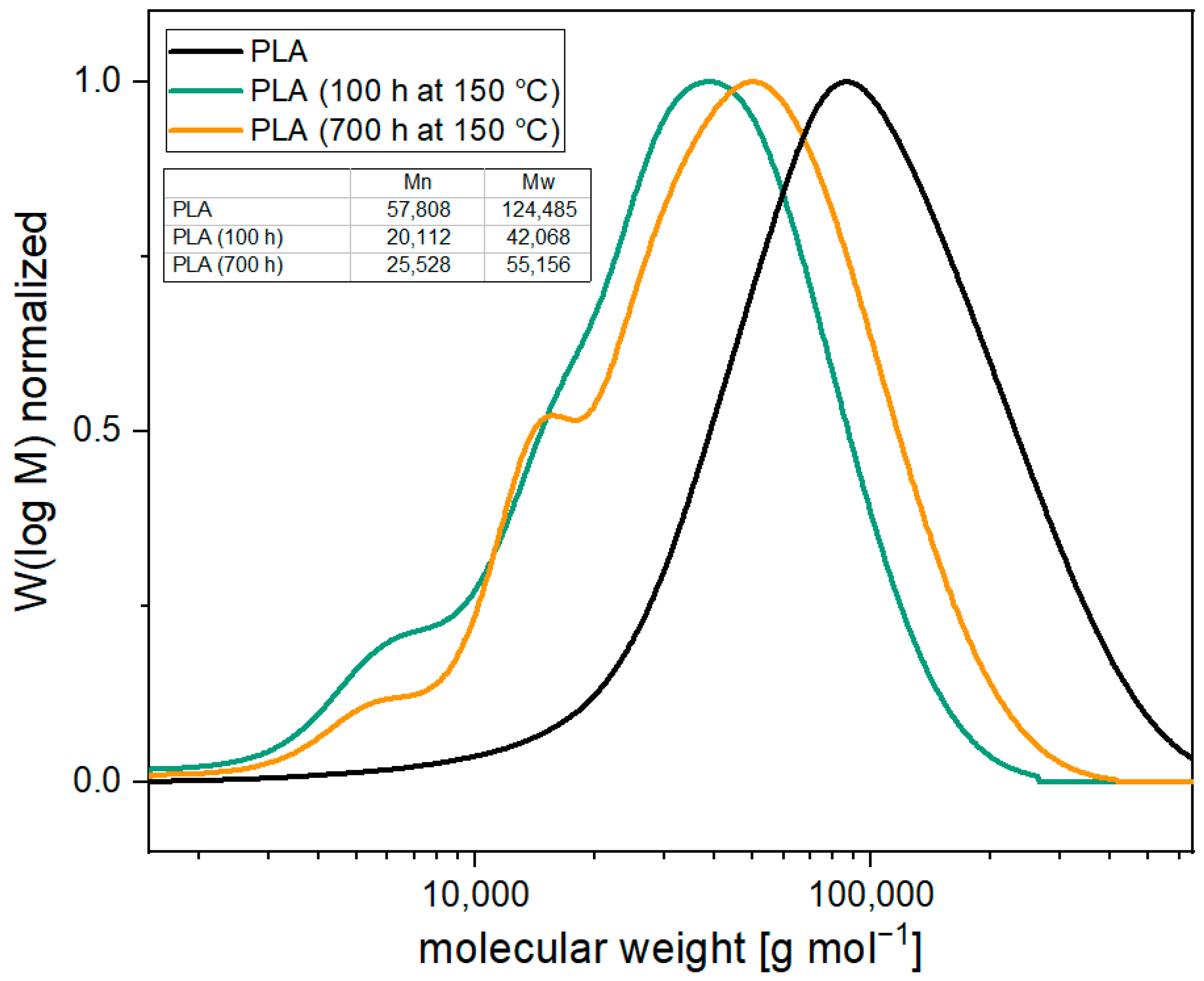
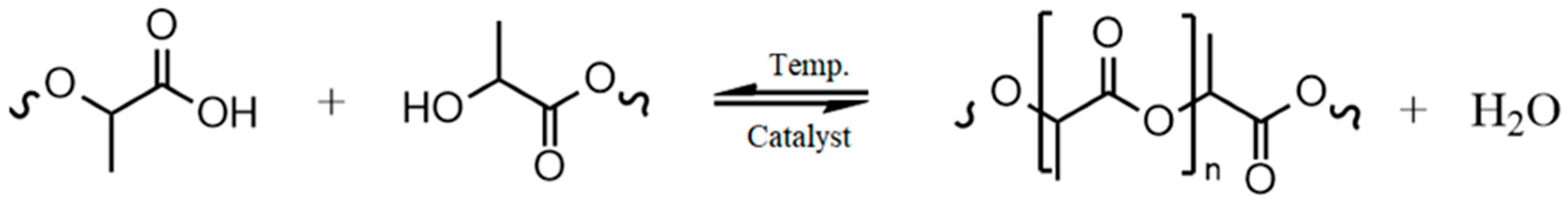
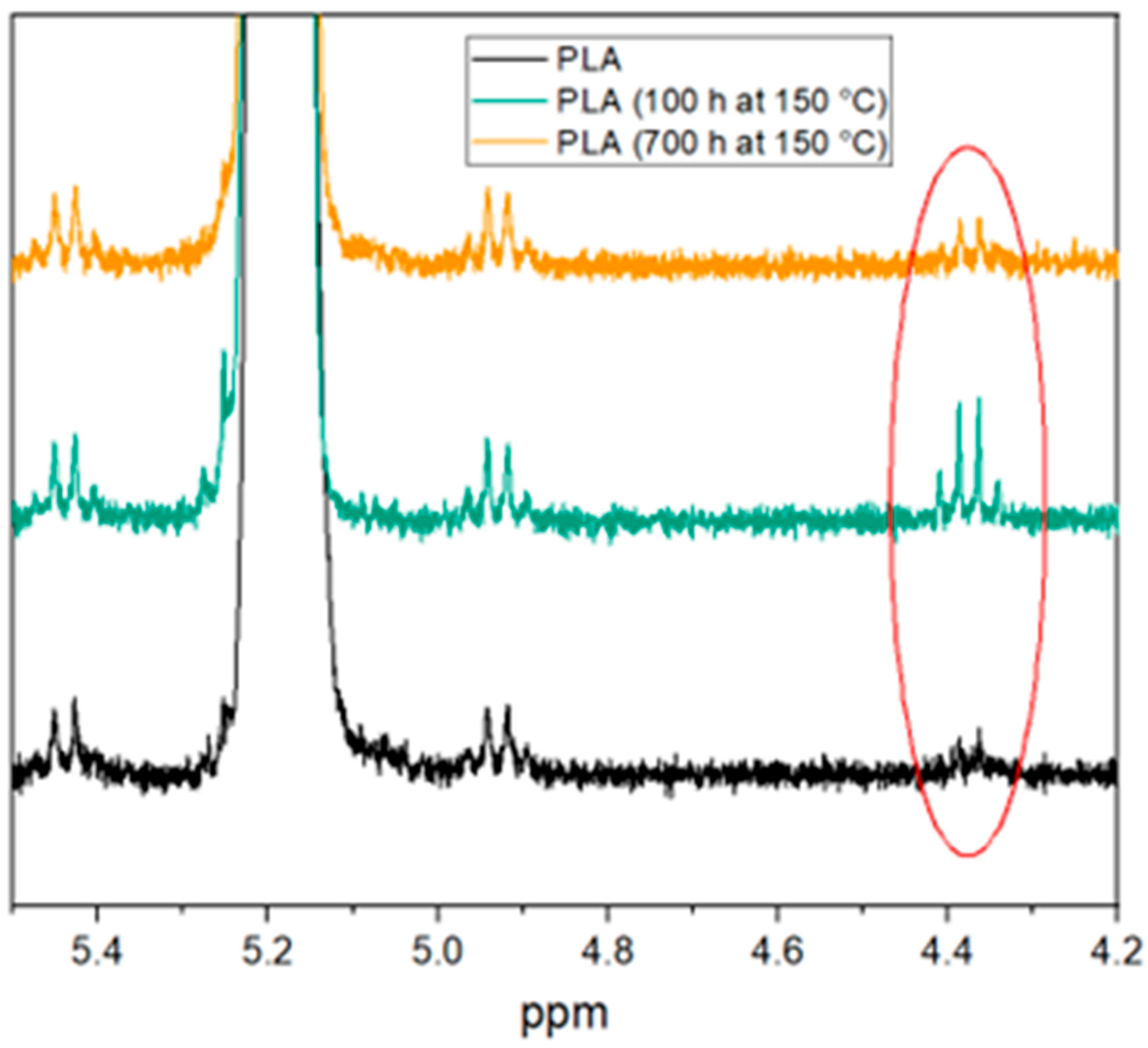
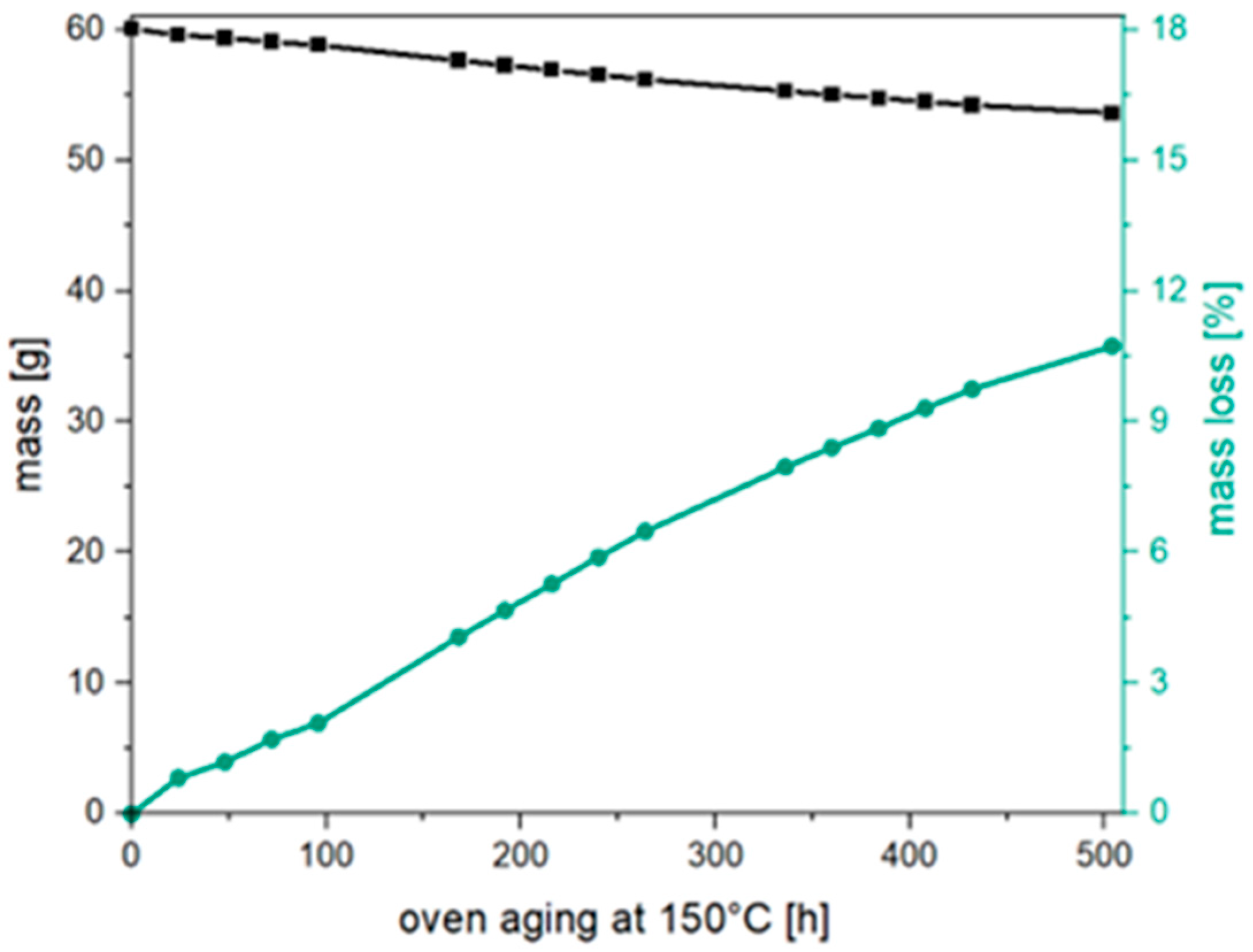
3.1. Thermal Aging of PLA and Investigation of the Degradation Mechnism
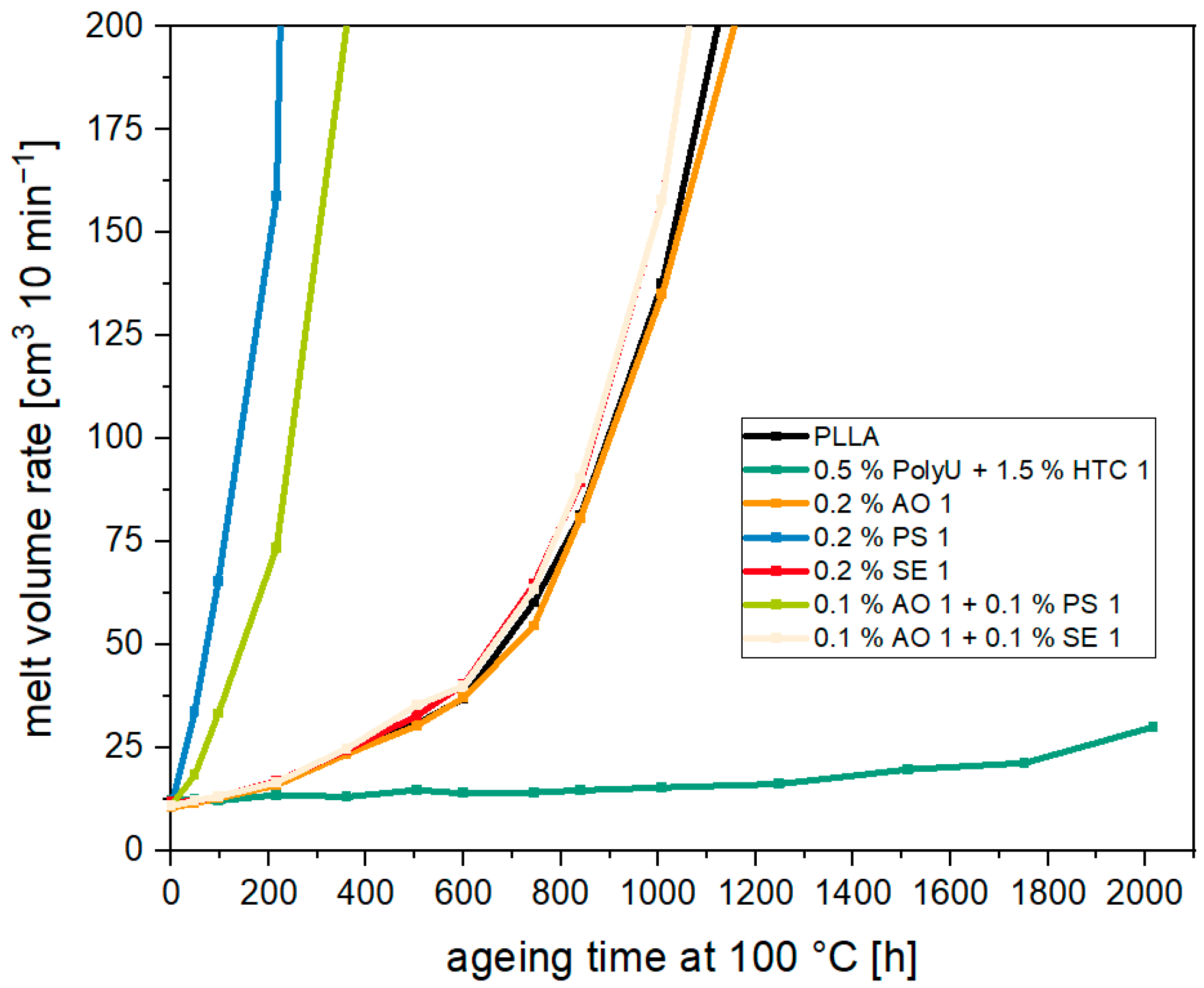
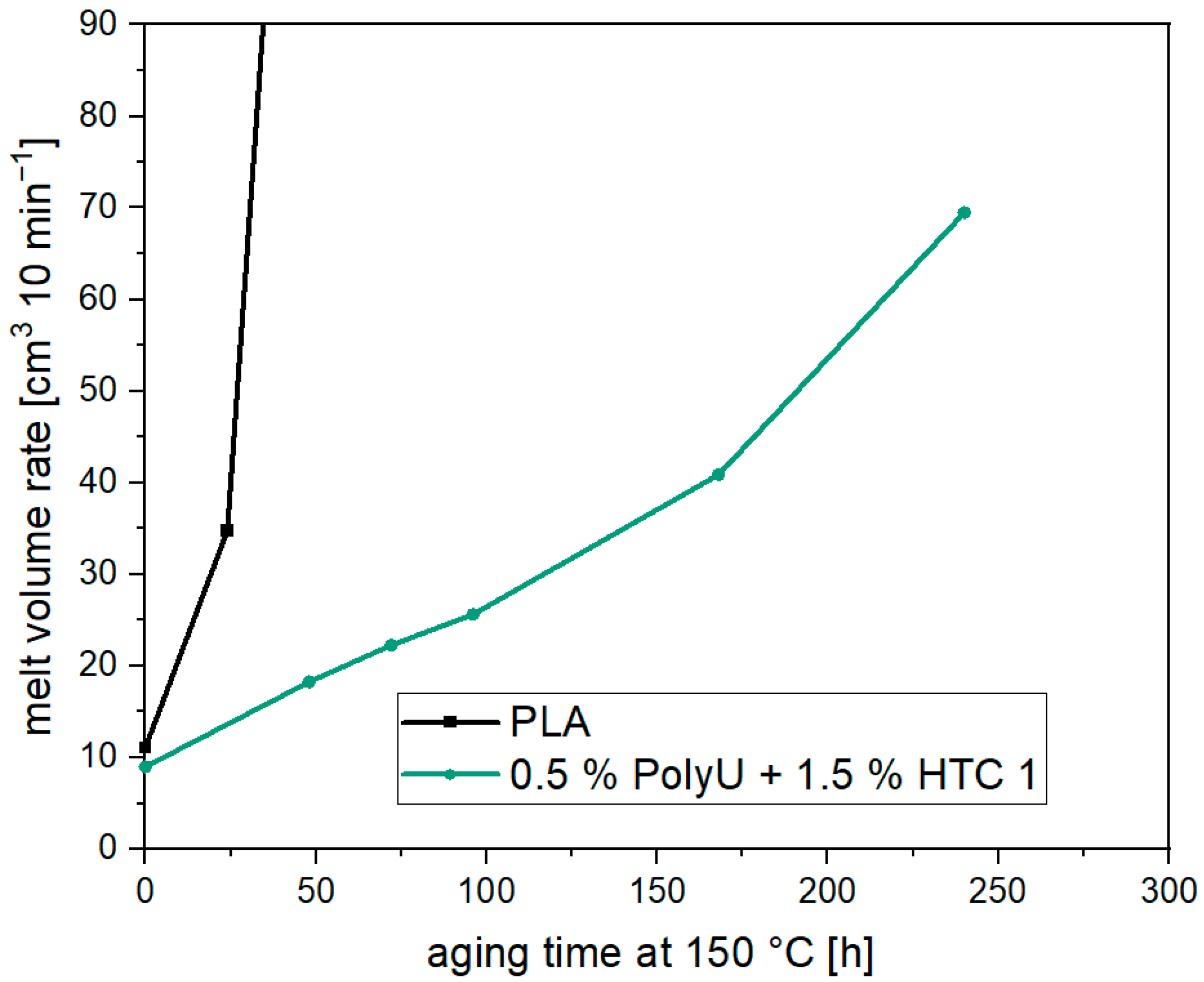
3.2. Thermal Aging of PLA and Investigation of Possible Stabilizers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auras, R.; Lim, L.-T.; Selke, S.E.M.; Tsuji, H. Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-0-470-29366-9. [Google Scholar]
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Li, S. Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. J. Biomed. Mater. Res. 1999, 48, 342–353. [Google Scholar] [CrossRef]
- Xu, L.; Crawford, K.; Gorman, C.B. Effects of Temperature and pH on the Degradation of Poly(lactic acid) Brushes. Macromolecules 2011, 44, 4777–4782. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: Assessment of structural modification and kinetic parameters. Polym. Degrad. Stab. 2013, 98, 1006–1014. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, M.E.C.; Stares, S.L.; Barra, G.M.d.O.; Hotza, D. Effects of accelerated weathering on properties of 3D-printed PLA scaffolds. Mater. Today Commun. 2022, 33, 104821. [Google Scholar] [CrossRef]
- Zaidi, L.; Kaci, M.; Bruzaud, S.; Bourmaud, A.; Grohens, Y. Effect of natural weather on the structure and properties of polylactide/Cloisite 30B nanocomposites. Polym. Degrad. Stab. 2010, 95, 1751–1758. [Google Scholar] [CrossRef]
- Kopinke, F.-D.; Mackenzie, K. Mechanistic aspects of the thermal degradation of poly(lactic acid) and poly(β-hydroxybutyric acid). J. Anal. Appl. Pyrolysis 1997, 40–41, 43–53. [Google Scholar] [CrossRef]
- Abe, H.; Takahashi, N.; Kim, K.J.; Mochizuki, M.; Doi, Y. Thermal degradation processes of end-capped poly(L-lactide)s in the presence and absence of residual zinc catalyst. Biomacromolecules 2004, 5, 1606–1614. [Google Scholar] [CrossRef]
- Amorin, N.S.Q.S.; Rosa, G.; Alves, J.F.; Gonçalves, S.P.C.; Franchetti, S.M.M.; Fechine, G.J.M. Study of thermodegradation and thermostabilization of poly(lactide acid) using subsequent extrusion cycles. J. Appl. Polym. Sci. 2014, 131, 40023. [Google Scholar] [CrossRef]
- Rasselet, D.; Ruellan, A.; Guinault, A.; Miquelard-Garnier, G.; Sollogoub, C.; Fayolle, B. Oxidative degradation of polylactide (PLA) and its effects on physical and mechanical properties. Eur. Polym. J. 2014, 50, 109–116. [Google Scholar] [CrossRef]
- Anakabe, J.; Santamaria-Echart, A.; Eceiza, A.; Arbelaiz, A.; Zaldua Huici, A.M. Evolution of the mechanical properties and estimation of the useful lifespan of poly(lactic acid) based compounds. Polym. Int. 2018, 67, 761–769. [Google Scholar] [CrossRef]
- Zweifel, H.; Maier, R.D.; Schiller, M. Plastics Additives Handbook, 6th ed.; Hanser: Munich, Germany, 2009; ISBN 978-3-446-40801-2. [Google Scholar]
- Pelzl, B.; Wolf, R.; Kaul, B.L. Plastics, Additives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; pp. 1–57. ISBN 9783527306732. [Google Scholar]
- Zweifel, H. Effect of Stabilization of Polypropylene during Processing and Its Influence on Long-Term Behavior under Thermal Stress. In Polymer Durability; Clough, R.L., Billingham, N.C., Gillen, K.T., Eds.; American Chemical Society: Washington, DC, USA, 1996; pp. 375–396. ISBN 0-8412-3134-6. [Google Scholar]
- Neureiter, N.P.; Bown, D.E. Synergism between Phenols and Sulfides in the Stabilization of Polyolefins to Oxidation. I&EC Prod. Res. Dev. 1962, 1, 236–241. [Google Scholar] [CrossRef]
- Al-Malaika, S. Mechanisms of antioxidant action and stabilisation technology—The Aston experience. Polym. Degrad. Stab. 1991, 34, 1–36. [Google Scholar] [CrossRef]
- Schwetlick, K.; Pionteck, J.; Winkler, A.; Hähner, U.; Kroschwitz, H.; Habicher, W.D. Organophosphorus antioxidants: Part X—Mechanism of antioxidant action of aryl phosphites and phosphonites at higher temperatures. Polym. Degrad. Stab. 1991, 31, 219–228. [Google Scholar] [CrossRef]
- Oliveira, M.; Santos, E.; Araújo, A.; Fechine, G.J.; Machado, A.V.; Botelho, G. The role of shear and stabilizer on PLA degradation. Polym. Test. 2016, 51, 109–116. [Google Scholar] [CrossRef]
- Cicero, J.A.; Dorgan, J.R.; Dec, S.F.; Knauss, D.M. Phosphite stabilization effects on two-step melt-spun fibers of polylactide. Polym. Degrad. Stab. 2002, 78, 95–105. [Google Scholar] [CrossRef]
- Sirisinha, K.; Samana, K. Improvement of melt stability and degradation efficiency of poly (lactic acid) by using phosphite. J. Appl. Polym. Sci. 2021, 138, 49951. [Google Scholar] [CrossRef]
- Li, C.; Gong, W.; Cao, Q.; Yao, Z.; Meng, X.; Xin, Z. Enhancement of cardanol-loaded halloysite for the thermo-oxidative stability and crystallization property of polylactic acid. Appl. Clay Sci. 2022, 216, 106357. [Google Scholar] [CrossRef]
- Pérez Amaro, L.; Cicogna, F.; Passaglia, E.; Morici, E.; Oberhauser, W.; Al-Malaika, S.; Dintcheva, N.T.; Coiai, S. Thermo-oxidative stabilization of poly(lactic acid) with antioxidant intercalated layered double hydroxides. Polym. Degrad. Stab. 2016, 133, 92–100. [Google Scholar] [CrossRef]
- Polidar, M.; Metzsch-Zilligen, E.; Pfaendner, R. Controlled and Accelerated Hydrolysis of Polylactide (PLA) through Pentaerythritol Phosphites with Acid Scavengers. Polymers 2022, 14, 4237. [Google Scholar] [CrossRef] [PubMed]
- DIN EN ISO 1133-2; Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics—Part 2: Method for Materials Sensitive to Time-Temperature History and/or Moisture (ISO 1133-2:2011). Beuth Verlag GmbH: Berlin, Germany, 2011.
- Vouyiouka, S.; Theodoulou, P.; Symeonidou, A.; Papaspyrides, C.D.; Pfaendner, R. Solid state polymerization of poly(lactic acid): Some fundamental parameters. Polym. Degrad. Stab. 2013, 98, 2473–2481. [Google Scholar] [CrossRef]
- Moon, S.-I.; Lee, C.-W.; Taniguchi, I.; Miyamoto, M.; Kimura, Y. Melt/solid polycondensation of l-lactic acid: An alternative route to poly(l-lactic acid) with high molecular weight. Polymer 2001, 42, 5059–5062. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Climent-Pascual, E.; de La Orden, M.U.; Martínez Urreaga, J. Effect of solid-state polymerization on the structure and properties of mechanically recycled poly(lactic acid). Polym. Degrad. Stab. 2020, 171, 109045. [Google Scholar] [CrossRef]
- Kucharczyk, P.; Poljansek, I.; Sedlarik, V. The Effect of Various Catalytic Systems on Solid-State Polymerization of Poly-(L-lactic acid). J. Macromol. Sci. Part A 2012, 49, 795–805. [Google Scholar] [CrossRef]
- Porfyris, A.; Vasilakos, S.; Zotiadis, C.; Papaspyrides, C.; Moser, K.; van der Schueren, L.; Buyle, G.; Pavlidou, S.; Vouyiouka, S. Accelerated ageing and hydrolytic stabilization of poly(lactic acid) (PLA) under humidity and temperature conditioning. Polym. Test. 2018, 68, 315–332. [Google Scholar] [CrossRef]
- Espartero, J.L.; Rashkov, I.; Li, S.M.; Manolova, N.; Vert, M. NMR Analysis of Low Molecular Weight Poly(lactic acid)s. Macromolecules 1996, 29, 3535–3539. [Google Scholar] [CrossRef]
- Limsukon, W.; Auras, R.; Selke, S. Hydrolytic degradation and lifetime prediction of poly(lactic acid) modified with a multifunctional epoxy-based chain extender. Polym. Test. 2019, 80, 106108. [Google Scholar] [CrossRef]
- Shih, C. Chain-end scission in acid catalyzed hydrolysis of poly (d,l-lactide) in solution. J. Control. Release 1995, 34, 9–15. [Google Scholar] [CrossRef]
- Gleadall, A.; Pan, J.; Kruft, M.-A.; Kellomäki, M. Degradation mechanisms of bioresorbable polyesters. Part 1. Effects of random scission, end scission and autocatalysis. Acta Biomater. 2014, 10, 2223–2232. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Alfaro, P.; Nerín, C. Migration of oligomers from a food contact biopolymer based on polylactic acid (PLA) and polyester. Anal. Bioanal. Chem. 2019, 411, 3521–3532. [Google Scholar] [CrossRef] [PubMed]
- Mutsuga, M.; Kawamura, Y.; Tanamoto, K. Migration of lactic acid, lactide and oligomers from polylactide food-contact materials. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Gardette, M.; Thérias, S.; Gardette, J.-L.; Murariu, M.; Dubois, P. Photooxidation of polylactide/calcium sulphate composites. Polym. Degrad. Stab. 2011, 96, 616–623. [Google Scholar] [CrossRef]
- Hallstein, J.; Metzsch-Zilligen, E.; Pfaendner, R. Enhancing the Hydrolytic Stability of Poly(lactic acid) Using Novel Stabilizer Combinations. Polymers 2024, 16, 506. [Google Scholar] [CrossRef] [PubMed]
- Valero, L.; Gainche, M.; Esparcieux, C.; Delor-Jestin, F.; Askanian, H. Vegetal Polyphenol Extracts as Antioxidants for the Stabilization of PLA: Toward Fully Biobased Polymer Formulation. ACS Omega 2024, 9, 7725–7736. [Google Scholar] [CrossRef]
- Ara, M.; Watanabe, M.; Imai, Y. Effect of blending calcium compounds on hydrolytic degradation of poly(dl-lactic acid-coglycolic acid). Biomaterials 2002, 23, 2479–2483. [Google Scholar] [CrossRef]
| Conditions | Moisture Content/ppm |
|---|---|
| PLA batch | 2601 |
| 100 °C thermal aging | 110 |
| 60 °C water bath | 11,080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallstein, J.; Metzsch-Zilligen, E.; Pfaendner, R. Long-Term Thermal Stabilization of Poly(Lactic Acid). Materials 2024, 17, 2761. https://doi.org/10.3390/ma17112761
Hallstein J, Metzsch-Zilligen E, Pfaendner R. Long-Term Thermal Stabilization of Poly(Lactic Acid). Materials. 2024; 17(11):2761. https://doi.org/10.3390/ma17112761
Chicago/Turabian StyleHallstein, Jannik, Elke Metzsch-Zilligen, and Rudolf Pfaendner. 2024. "Long-Term Thermal Stabilization of Poly(Lactic Acid)" Materials 17, no. 11: 2761. https://doi.org/10.3390/ma17112761
APA StyleHallstein, J., Metzsch-Zilligen, E., & Pfaendner, R. (2024). Long-Term Thermal Stabilization of Poly(Lactic Acid). Materials, 17(11), 2761. https://doi.org/10.3390/ma17112761






