A Hydration Model to Evaluate the Properties of Cement–Quartz Powder Hybrid Concrete
Abstract
:1. Introduction
2. Hydration Model and Evaluation of Properties
2.1. Experimental Outline
2.2. Hydration Model
2.3. Property Evaluations
2.3.1. Evaluation of Long-Term Hydration Heat
2.3.2. Evaluation of Compressive Strength Development
2.3.3. Evaluation of Ultrasonic Pulse Velocity Development
2.3.4. Evaluation of Surface Electrical Resistivity Development
3. Summary of Hydration Model
4. Comparison with Previous Models and Limitations of Proposed Model
4.1. Comparison with Previous Models
4.2. Limitations of Proposed Model
5. Conclusions
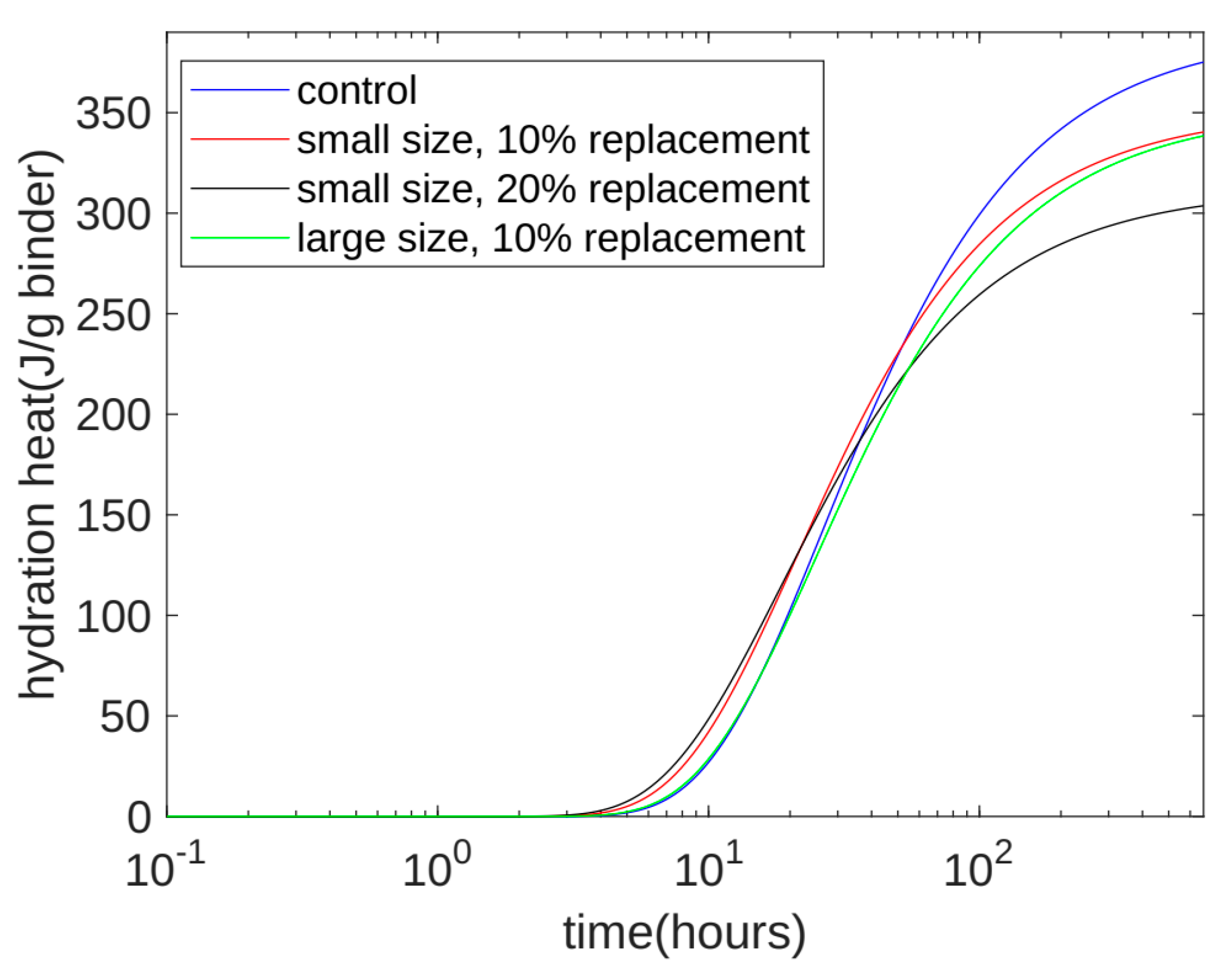
- Based on the seven-day accumulated hydration heat, the parameters of the three-parameter hydration model were obtained through regression. We found that for different quartz powder substitution rates and quartz powder particle fineness, the ultimate hydration heat release value per gram of cement was the same at 390.145 J/g cement, and the shape index of the hydration model was also the same at −1.003. For Mix 1, Mix 2, Mix 3, and Mix 4, the hydration rate parameters were 26.95, 21.39, 18.78, and 25.30, respectively. As the substitution amount increased or as the particle size of quartz powder decreased, the hydration rate parameter decreased, and the hydration rate of the cement accelerated.
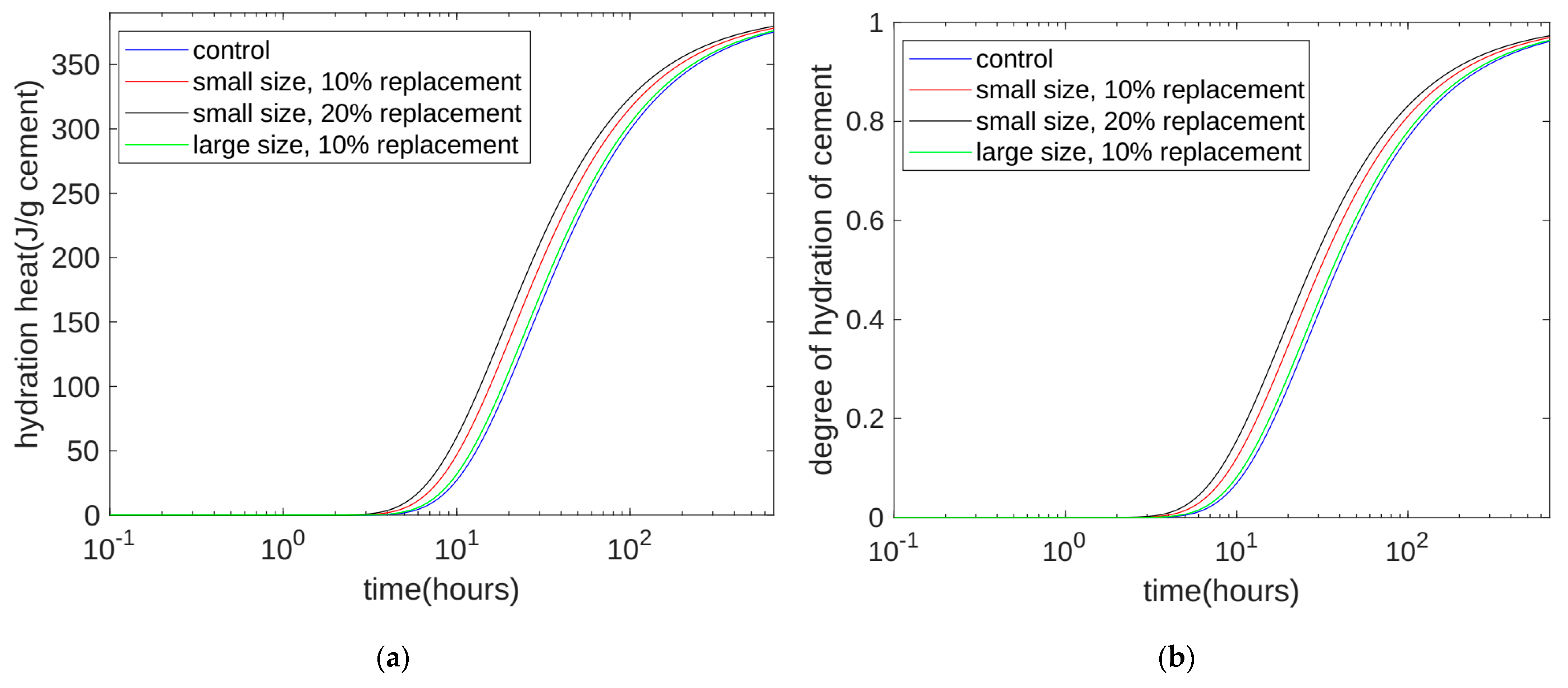
- Currently, using isothermal hydration heat can only measure the hydration heat from the beginning of stirring to seven days of age. However, our hydration model can calculate the heat and level of hydration over longer periods of time. After the model’s calculation, we found that at 28 days of the curing period, for all the specimens, the reactivity of the cement was similar, with both at 0.963. The values of the cumulative heat of hydration were also similar, with both at 375.5 J/g cement. This shows that the quartz powder mainly plays a physical role by changing the rate of the hydration reaction, but it does not change the ultimate extent of the hydration reaction.
- The calculation results show that, at approximately 30 h (in the early stage of hydration), the cumulative heat of hydration value for the specimens with fine quartz powder was higher than that for the control specimens. This could be because the nucleation effect of quartz powder played a dominant role. For the coarse quartz, the value of the accumulated hydration heat of the powder specimen was similar to that of the control specimen, which may be due to the acceleration of the nucleation effect and the deceleration of the dilution effect canceling each other out.
- The calculation results show that, in the late stage of hydration (28 days), as the substitution amount of quartz powder increased, the value of the cumulative heat of hydration decreased. This is because the dilution effect of the quartz powder played a dominant role. For quartz powders of different fineness, when the substitution amount was the same, the cumulative heat of hydration over 28 days was similar. This shows that the fineness of the quartz powder mainly affects early hydration and has a limited influence on later hydration.
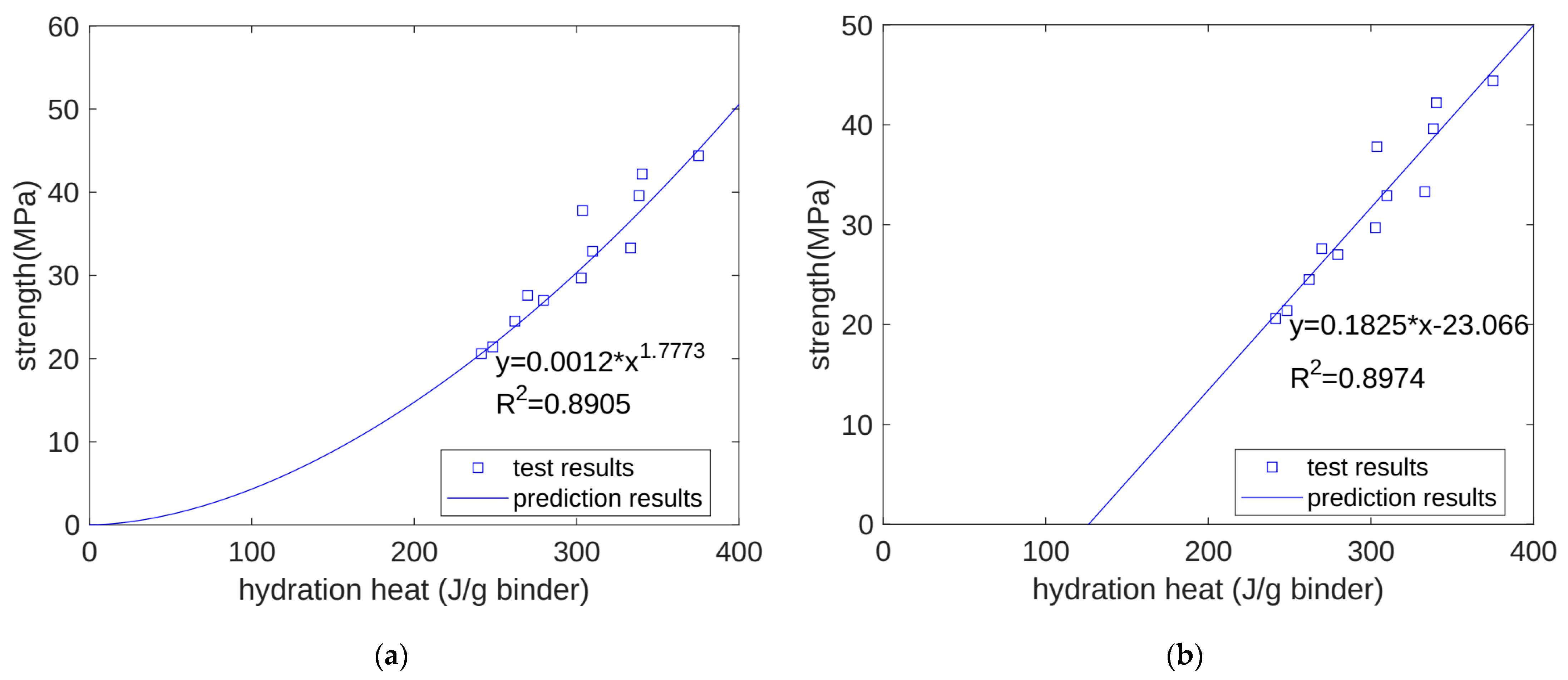
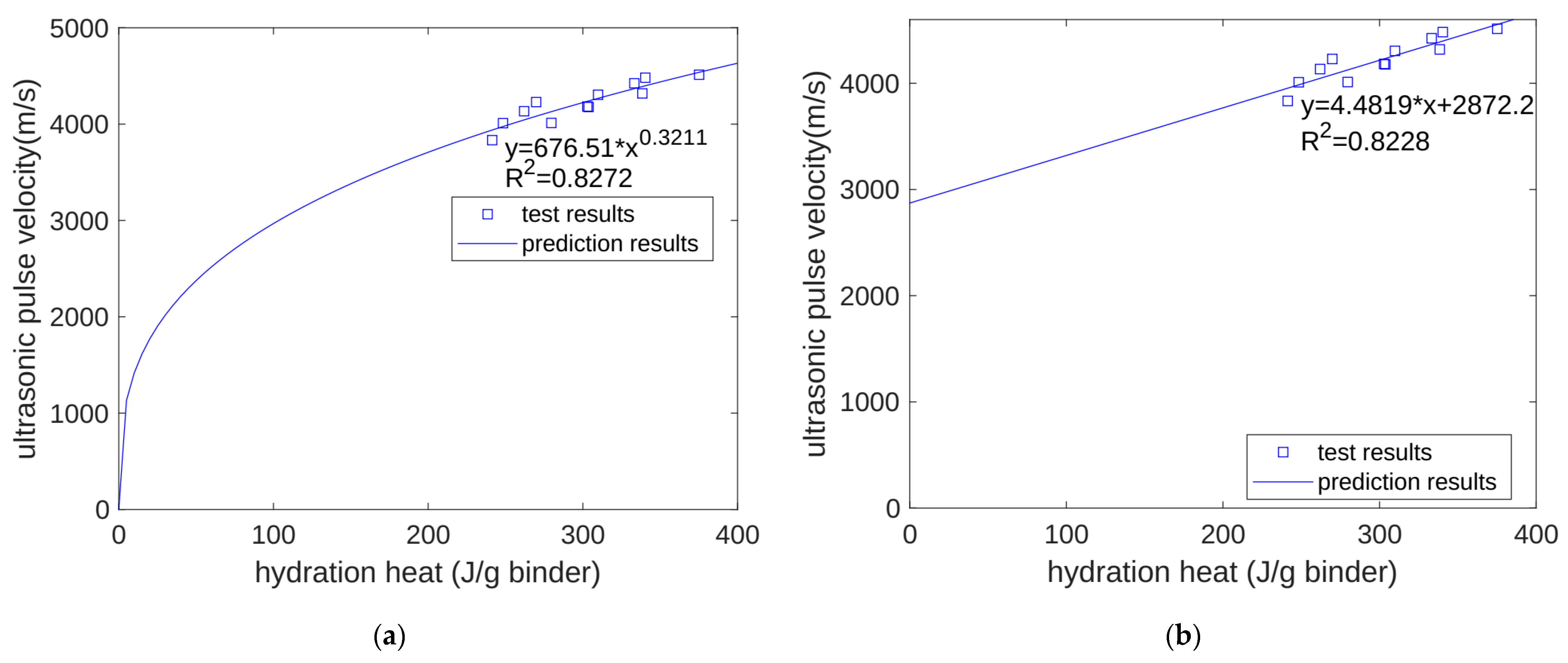
- We predicted the properties of concrete at different ages based on the heat of hydration per gram of cementitious material. For compressive strength, when using a power function and a linear function for regression, the correlation coefficients were 0.8905 and 0.8974, respectively. For ultrasonic pulse velocity, when using a power function and a linear function for regression, the correlation coefficients were 0.8272 and 0.8228, respectively. For surface electrical resistivity, when using a power function and a linear function for regression, the correlation coefficients were 0.9157 and 0.9117, respectively. Overall, the predictive performance of the power and linear functions was similar, with no significant difference being found.
6. Recommendations for Future Work
- First, the effects of other reactive admixtures, such as silica fume, slag, and fly ash, should be considered in the hydration model. These reactive admixtures are more widely used in the concrete industry than quartz powder.
- Second, the effect of temperature in the hydration model and the strength prediction model should be considered, such as the effect of temperature on the hydration reaction rate and compressive strength. This is important, as the development of cement-based material properties is different in hot weather and cold weather.
- Third, in terms of durability prediction, methods to predict various durability properties based on the hydration degree, such as carbonation, chloride ion penetration, and steel corrosion, should be considered. The proposed model only evaluates properties before 28 days; durability beyond 28 days should be studied in the future.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tavares, L.R.C.; Junior, J.F.T.; Costa, L.M.; Bezerra, A.C.d.S.; Cetlin, P.R.; Aguilar, M.T.P. Influence of quartz powder and silica fume on the performance of Portland cement. Sci. Rep. 2020, 10, 21461. [Google Scholar] [CrossRef]
- Kang, S.-H.; Jeong, Y.; Tana, K.H.; Moon, J. The use of limestone to replace physical filler of quartz powder in UHPFRC. Cem. Concr. Compos. 2018, 94, 238–247. [Google Scholar] [CrossRef]
- Park, K.-B.; Noguchi, T.; Plawsky, J. Modeling of hydration reactions using neural networks to predict the average properties of cement paste. Cem. Concr. Res. 2005, 35, 1676–1684. [Google Scholar] [CrossRef]
- Park, K.-B.; Jee, N.-Y.; Yoon, I.-S.; Lee, H.-S. Prediction of temperature distribution in high-strength concrete using hydration model. ACI Mater. J. 2008, 105, 180–186. [Google Scholar]
- Maruyama, I.; Matsushita, T.; Noguchi, T. Numerical Modeling of Portland Cement Hydration. In Proceedings of the International RILEM Symopsium on Concrete Modelling-CONMOD’08, RILEM, Delft, The Netherlands, 26–28 May 2008. [Google Scholar]
- Van Breugel, K. Numerical simulation of hydration and microstructural development in hardening cement-based materials (I) theory. Cem. Concr. Res. 1995, 25, 319–331. [Google Scholar] [CrossRef]
- Ye, G.; Van Breugel, K.; Fraaij, A.L.A. Three-dimensional microstructure analysis of numerically simulated cementitious materials. Cem. Concr. Res. 2003, 33, 215–222. [Google Scholar] [CrossRef]
- Van Breugel, K. Numerical simulation of hydration and microstructural development in hardening cement-based materials: (II) applications. Cem. Concr. Res. 1995, 25, 522–530. [Google Scholar] [CrossRef]
- Dolado, J.S.; Van Breugel, K. Recent advances in modeling for cementitious materials. Cem. Concr. Res. 2011, 41, 711–726. [Google Scholar] [CrossRef]
- Lin, F.; Meyer, C. Hydration kinetics modeling of Portland cement considering the effects of curing temperature and applied pressure. Cem. Concr. Res. 2009, 39, 255–265. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Huang, L.; Yan, P. A new hydration kinetics model of composite cementitious materials, part 1: Hydration kinetic model of Portland cement. J. Am. Ceram. Soc. 2020, 103, 1477–2271. [Google Scholar] [CrossRef]
- Schutter, G.D. Hydration and temperature development of concrete made with blast-furnace slag cement. Cem. Concr. Res. 1999, 29, 143–149. [Google Scholar] [CrossRef]
- Jiang, W.; Schutter, G.D.; Yuan, Y. Degree of hydration based prediction of early age basic creep and creep recovery of blended concrete. Cem. Concr. Compos. 2014, 48, 83–90. [Google Scholar] [CrossRef]
- Schutter, G.D. Finite element simulation of thermal cracking in massive hardening concrete elements using degree of hydration based material laws. Comput. Struct. 2002, 80, 2035–2042. [Google Scholar] [CrossRef]
- Schutter, G.D.; Taerwe, L. Degree of hydration-based description of mechanical properties of early age concrete. Mater. Struct. 1996, 29, 335–344. [Google Scholar] [CrossRef]
- Bentz, D.P.; Coveney, P.V.; Garboczi, E.J.; Kleyn, M.F.; Stutzman, P.E. Cellular automaton simulations of cement hydration and microstructure development. Model. Simul. Mater. Sci. Eng. 1994, 2, 783. [Google Scholar] [CrossRef]
- Bentz, D.P.; Waller, V.; de Larrard, F. Prediction of Adiabatic Temperature Rise in Conventional and High-Performance Concretes Using a 3-D Microstructural Model. Cem. Concr. Res. 1998, 28, 285–297. [Google Scholar] [CrossRef]
- Bentz, D.P. Modeling the influence of limestone filler on cement hydration using CEMHYD3D. Cem. Concr. Compos. 2006, 28, 124–129. [Google Scholar] [CrossRef]
- Bentz, D.P.; Jensen, O.M.; Coats, A.M.; Glasser, F.P. Influence of silica fume on diffusivity in cement-based materials: I. Experimental and computer modeling studies on cement pastes. Cem. Concr. Res. 2000, 30, 953–962. [Google Scholar] [CrossRef]
- Papadakis, V.G. Experimental investigation and theoretical modeling of silica fume activity in concrete. Cem. Concr. Res. 1999, 29, 79–86. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of fly ash on Portland cement systems: Part I. Low-calcium fly ash. Cem. Concr. Res. 1999, 29, 1727–1736. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of fly ash on Portland cement systems: Part II. High-calcium fly ash. Cem. Concr. Res. 2000, 30, 1647–1654. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of supplementary cementing materials on concrete resistance against carbonation and chloride ingress. Cem. Concr. Res. 2000, 30, 291–299. [Google Scholar] [CrossRef]
- Demis, S.; Efstathiou, M.P.; Papadakis, V.G. Computer-aided modeling of concrete service life. Cem. Concr. Compos. 2014, 47, 9–18. [Google Scholar] [CrossRef]
- Rezagholilou, A.; Papadakis, V.G.; Nikraz, H. Rate of carbonation in cement modified base course material. Constr. Build. Mater. 2017, 150, 646–652. [Google Scholar] [CrossRef]
- Maekawa, K.; Ishida, T.; Kishi, T. Multi-Scale Modeling of Structural Concrete; CRC Press: London, UK, 2009; p. 672. [Google Scholar]
- Maekawa, K.; Ishida, T. Modeling of structural performances under coupled environmental and weather actions. Mater. Struct. 2002, 35, 591–602. [Google Scholar] [CrossRef]
- Ishida, T.; Maekawa, K.; Kishi, T. Enhanced modeling of moisture equilibrium and transport in cementitious materials under arbitrary temperature and relative humidity history. Cem. Concr. Res. 2007, 37, 565–578. [Google Scholar] [CrossRef]
- Iwama, K.; Higuchi, K.; Maekawa, K. Thermo-Mechanistic Multi-Scale Modeling of Structural Concrete at High Temperature. J. Adv. Concr. Technol. 2020, 18, 272–293. [Google Scholar] [CrossRef]
- Li, P.; Jiang, Z.; An, X.; Maekawa, K.; Du, S. Time-dependent retardation effect of epoxy latexes on cement hydration: Experiments and multi-component hydration model. Constr. Build. Mater. 2022, 320, 126282. [Google Scholar] [CrossRef]
- Lothenbach, B.; Winnefeld, F. Thermodynamic modelling of the hydration of Portland cement. Cem. Concr. Res. 2006, 36, 209–226. [Google Scholar] [CrossRef]
- Lothenbach, B.; Matschei, T.; Möschner, G.; Glasser, F.P. Thermodynamic modelling of the effect of temperature on the hydration and porosity of Portland cement. Cem. Concr. Res. 2008, 38, 1–18. [Google Scholar] [CrossRef]
- Lothenbach, B.; Saout, G.L.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Loser, R.; Lothenbach, B.; Leemann, A.; Tuchschmid, M. Chloride resistance of concrete and its binding capacity—Comparison between experimental results and thermodynamic modeling. Cem. Concr. Compos. 2010, 32, 34–42. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Lothenbach, B.; Belie, N.D.; Gruyaert, E.; Skibsted, J.; Snellings, R.; Vollpracht, A. TC 238-SCM: Hydration and microstructure of concrete with SCMs: State of the art on methods to determine degree of reaction of SCMs. Mater. Struct. 2015, 48, 835–862. [Google Scholar] [CrossRef]
- Lothenbach, B.; Zajac, M. Application of thermodynamic modelling to hydrated cements. Cem. Concr. Res. 2019, 123, 105779. [Google Scholar] [CrossRef]
- Bentz, D.P. Influence of water-to-cement ratio on hydration kinetics: Simple models based on spatial considerations. Cem. Concr. Res. 2006, 36, 238–244. [Google Scholar] [CrossRef]
- Xuan, M.-Y.; Guo, R.; Lin, R.-S.; Wang, X.; Wang, X.-Y. Mechanism of fineness and content of quartz powder on the improvement of carbonation curing efficiency. Cem. Concr. Compos. 2024, 150, 105570. [Google Scholar] [CrossRef]
- ASTM C1702; Standard Test Method for Measurement of Heat of Hydration of Hydraulic Cementitious Materials Using Isothermal Conduction Calorimetry. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM C109; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 50 mm [2 in.] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM C597; Standard Test Method for Ultrasonic Pulse Velocity through Concrete. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D3633; Standard Test Method for Electrical Resistivity of Membrane-Pavement Systems. ASTM International: West Conshohocken, PA, USA, 2023.
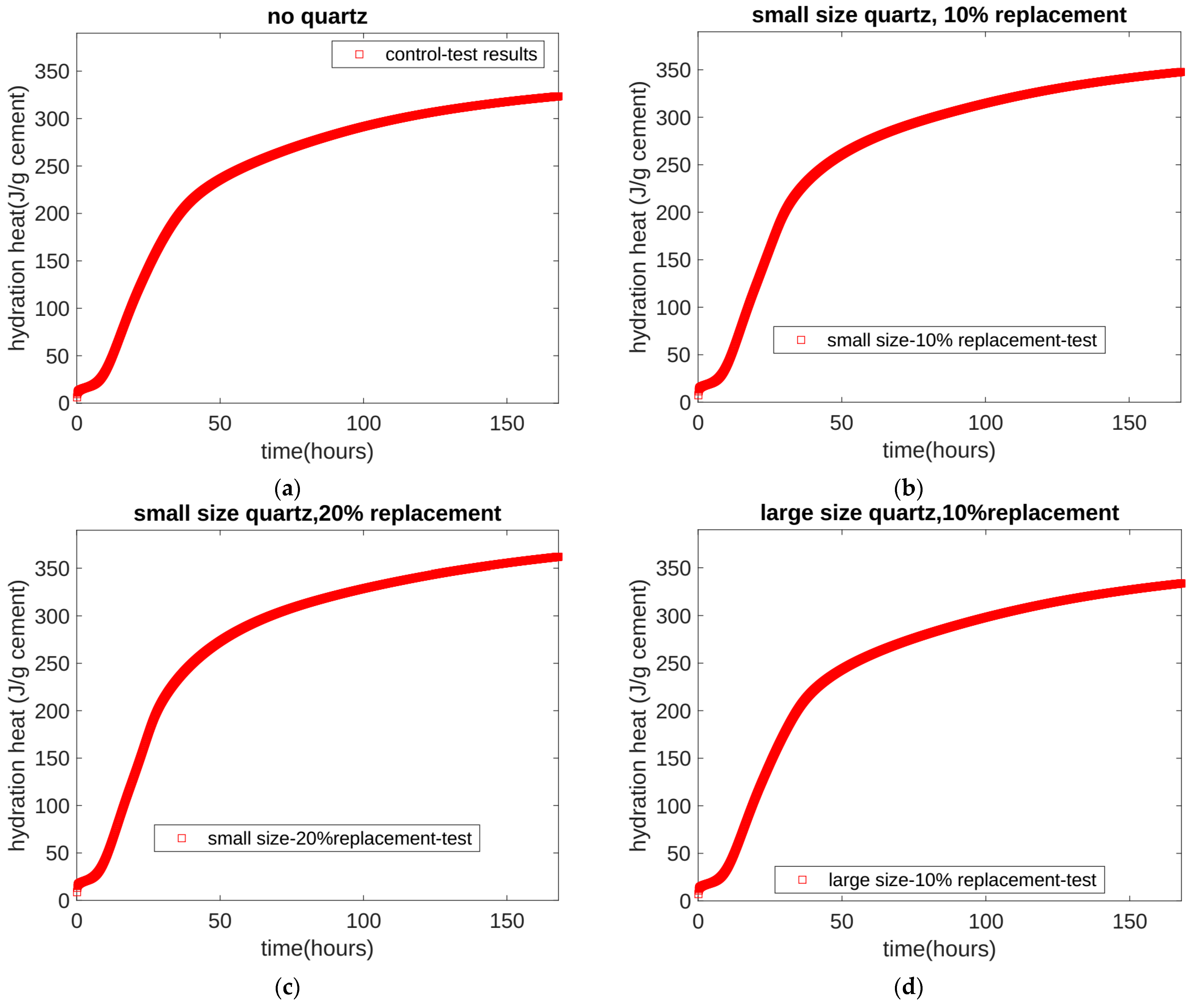
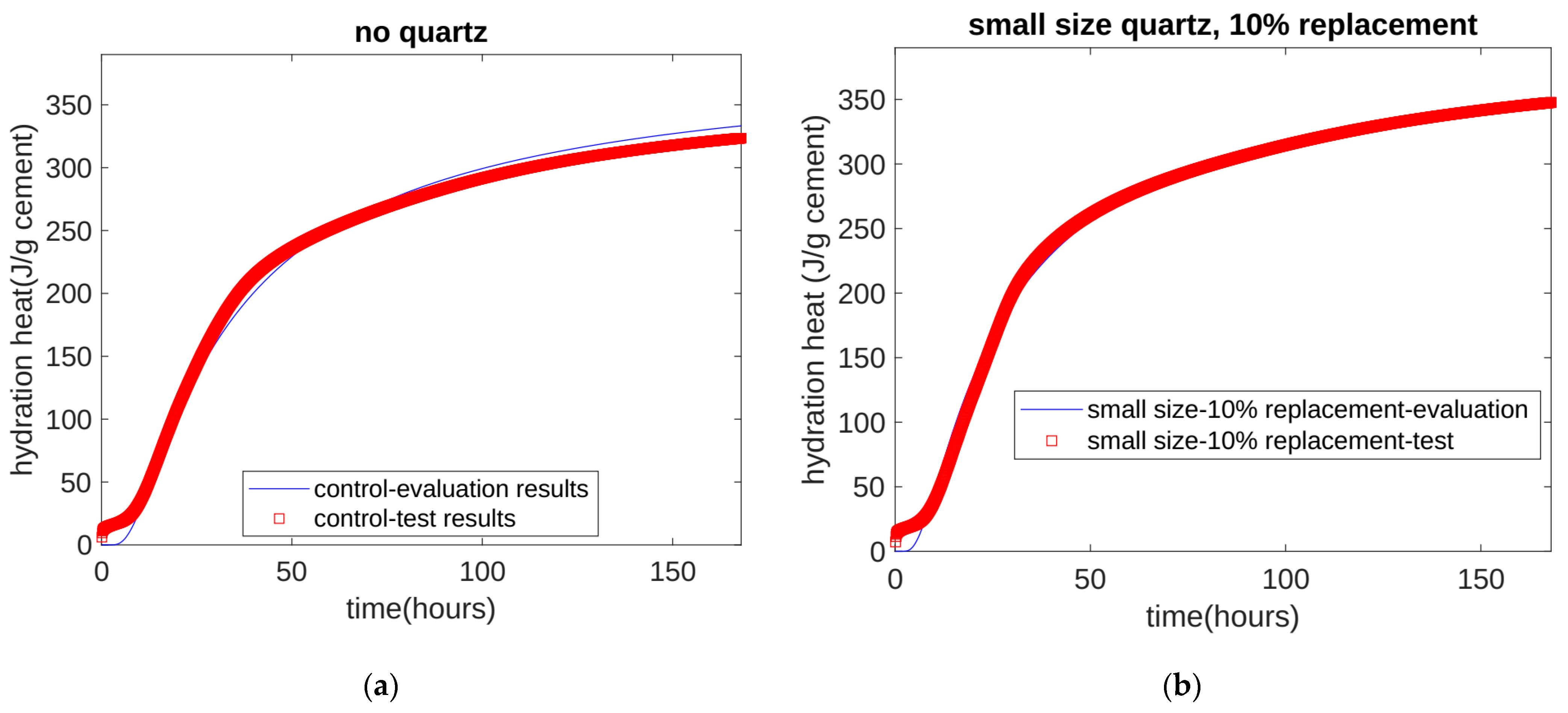
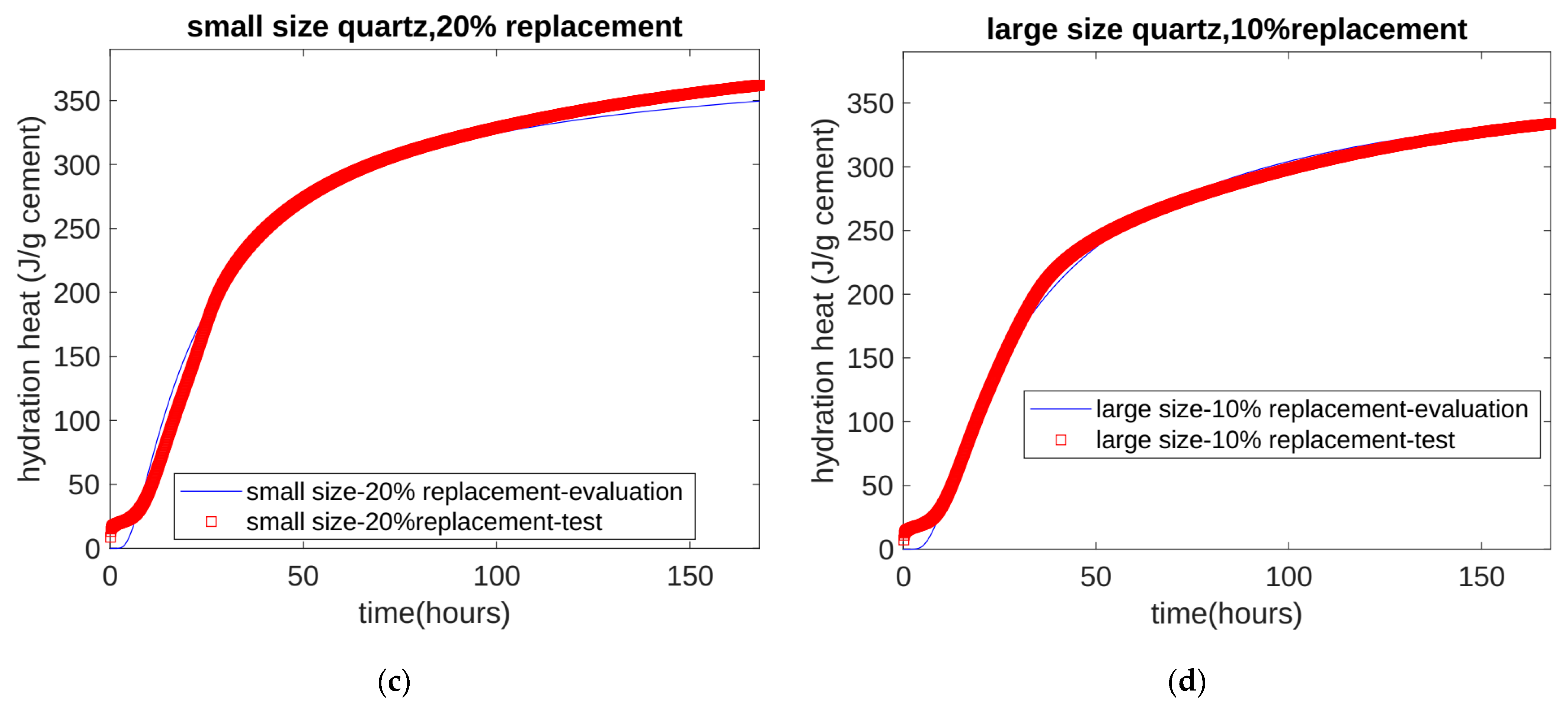

| Cement (%) | Quartz (%) | Water (%) | Sand (%) | |
|---|---|---|---|---|
| Mix 1 | 100 | 0 | 50 | 250 |
| Mix 2 (small size) | 90 | 10 (small size) | 50 | 250 |
| Mix 3 (small size) | 80 | 20 (small size) | 50 | 250 |
| Mix 4 (large size) | 90 | 10 (large size) | 50 | 250 |
| QCU | r1 (Mix 1) | r2 (Mix 2) | r3 (Mix 3) | r4 (Mix 4) | s |
|---|---|---|---|---|---|
| 390.145 | 26.95 | 21.39 | 18.78 | 25.30 | −1.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Liu, Y.; Wang, X.-Y. A Hydration Model to Evaluate the Properties of Cement–Quartz Powder Hybrid Concrete. Materials 2024, 17, 2769. https://doi.org/10.3390/ma17112769
Yang B, Liu Y, Wang X-Y. A Hydration Model to Evaluate the Properties of Cement–Quartz Powder Hybrid Concrete. Materials. 2024; 17(11):2769. https://doi.org/10.3390/ma17112769
Chicago/Turabian StyleYang, Bo, Yao Liu, and Xiao-Yong Wang. 2024. "A Hydration Model to Evaluate the Properties of Cement–Quartz Powder Hybrid Concrete" Materials 17, no. 11: 2769. https://doi.org/10.3390/ma17112769






