Thermoplastic Polyurethane-poly(N-isopropylacrylamide) Copolymer for Selective Uptake of Alcohol from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TPU-PNIPAM
2.3. Characterization of Materials
2.4. Physical Property Test
2.5. Maximum Uptake Capacity Test
2.6. Uptake Performance Test
2.7. Standard Deviation and Statistical Analysis
3. Results and Discussion
3.1. Characterization Analysis
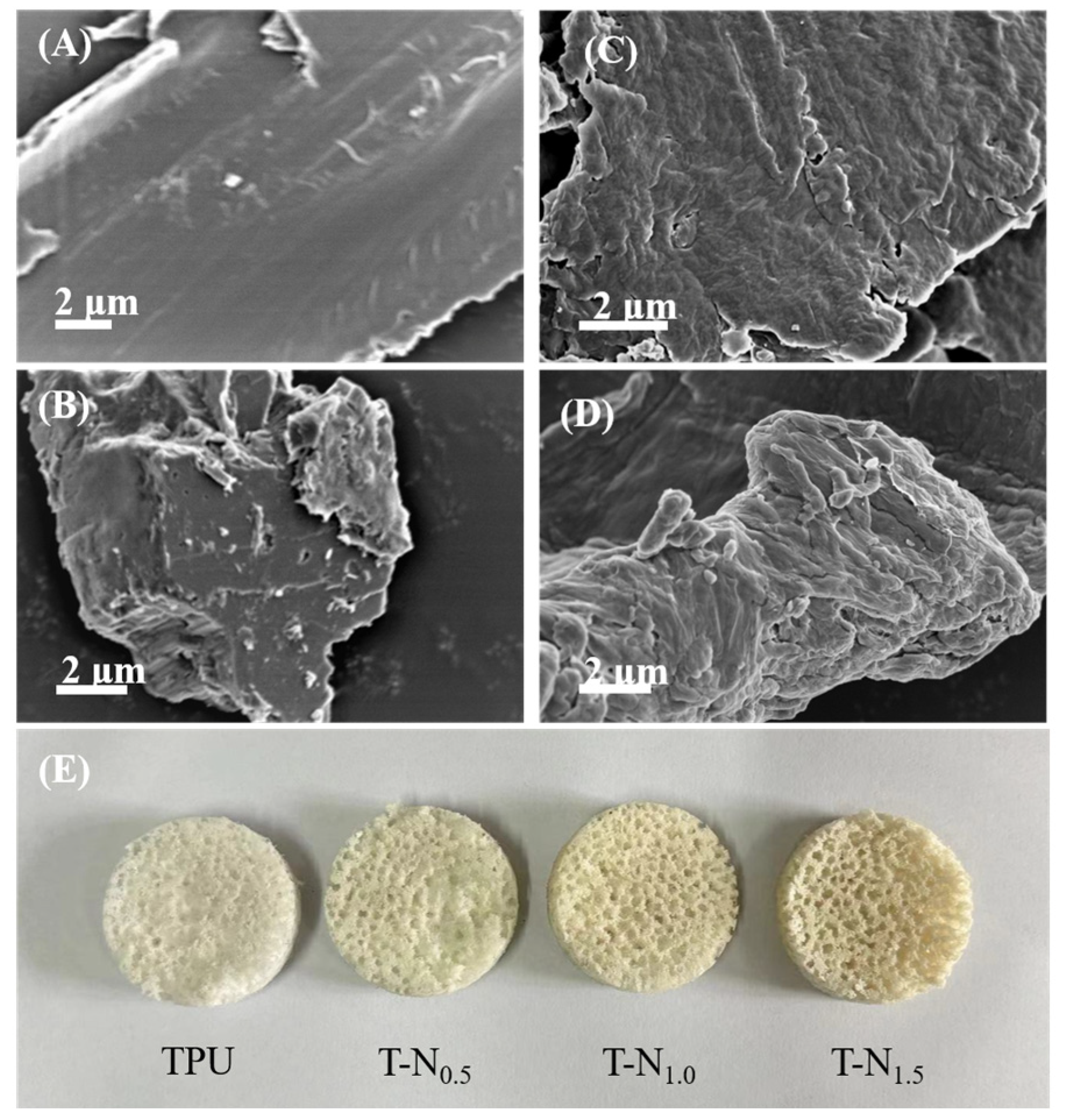
3.1.1. Morphology Analysis
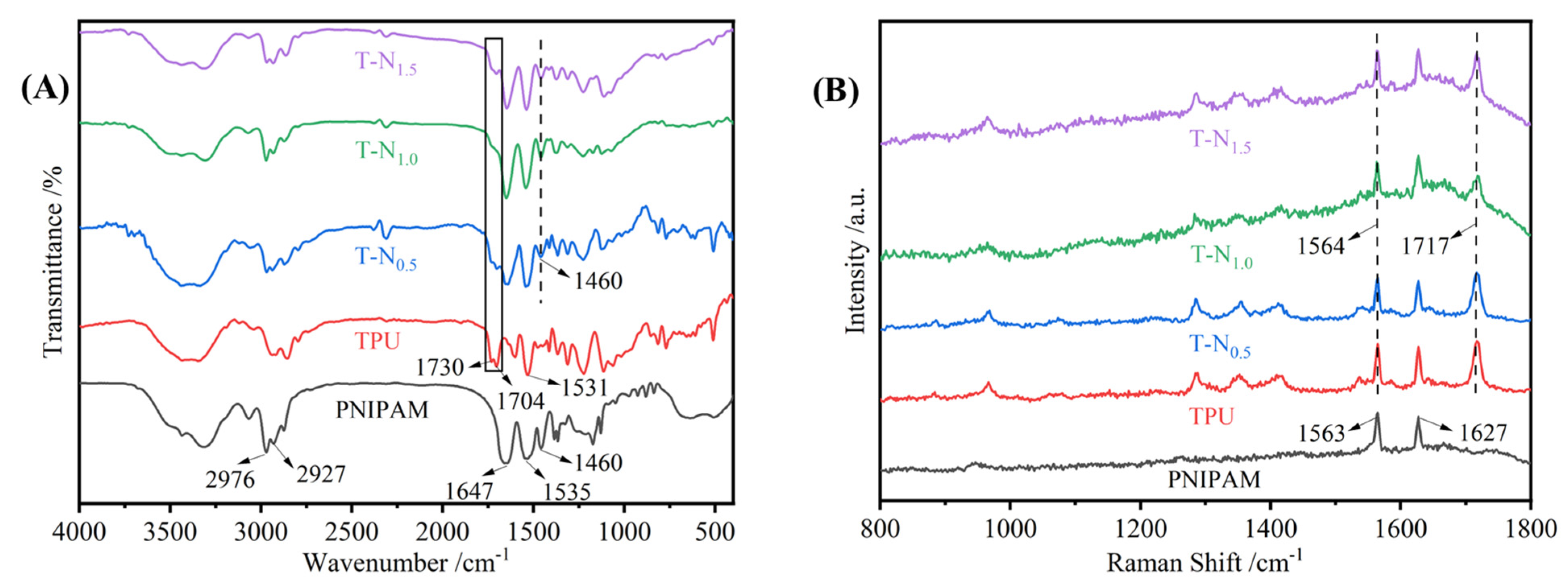
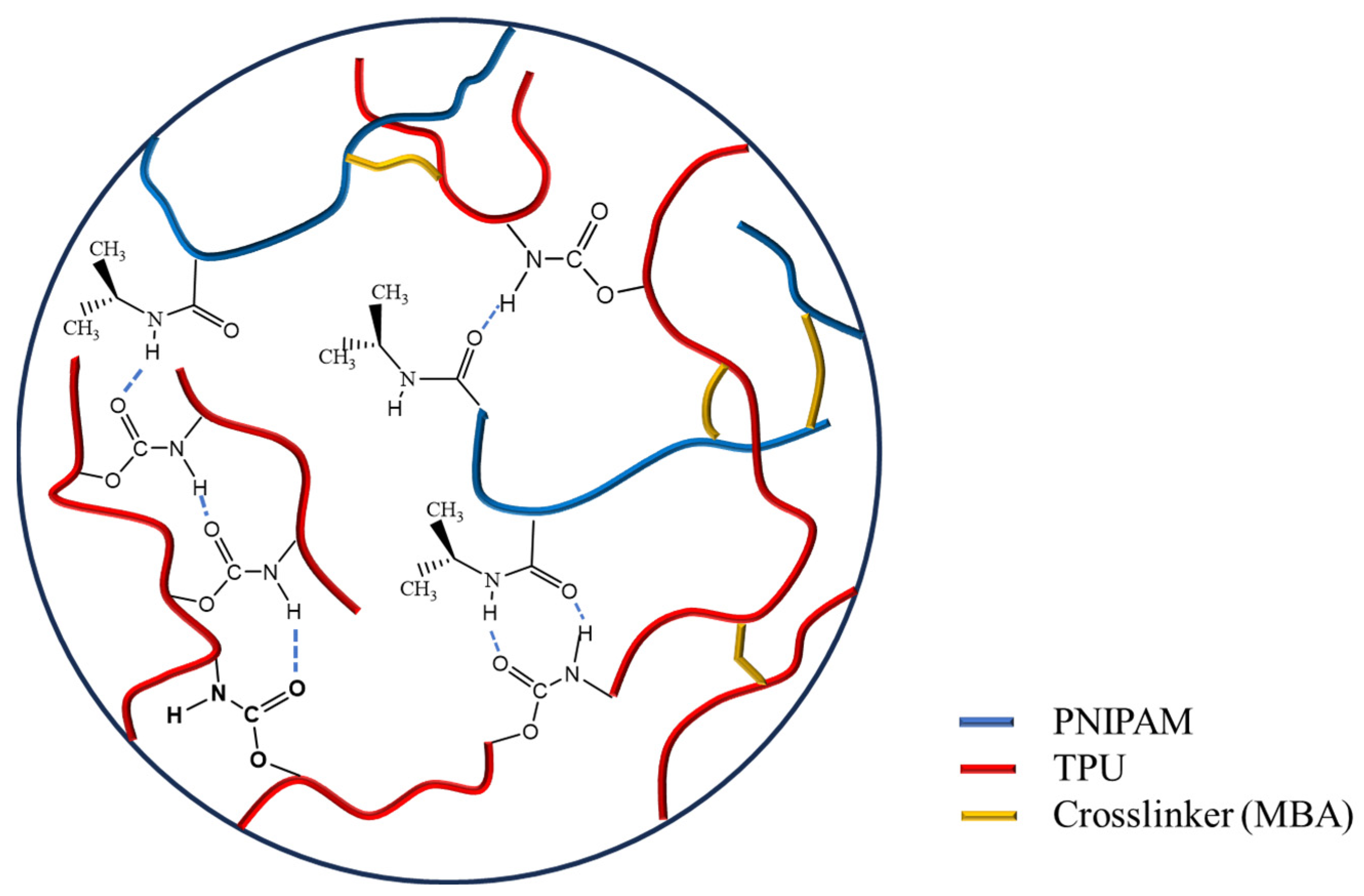
3.1.2. FT-IR
3.1.3. Raman
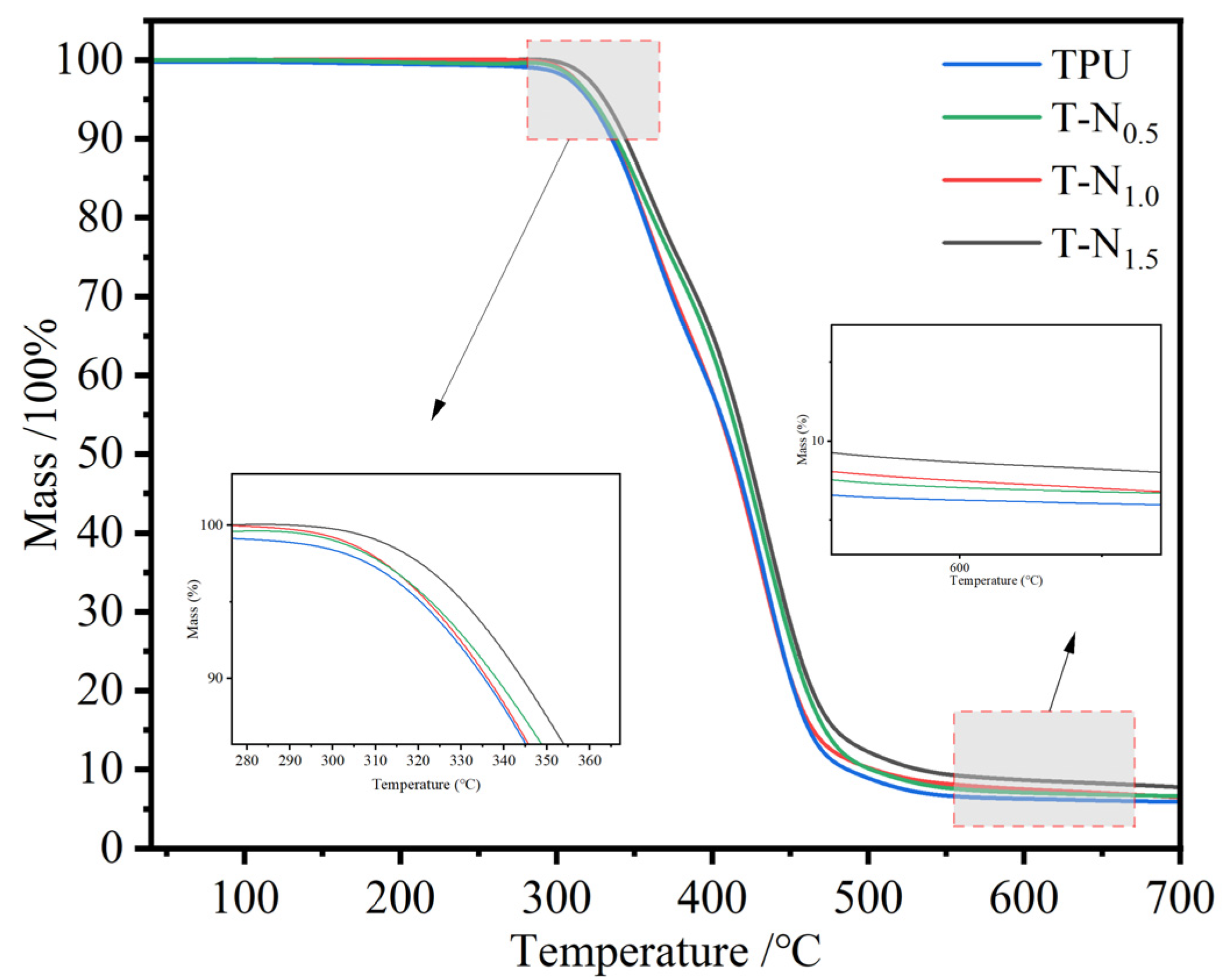
3.1.4. TG
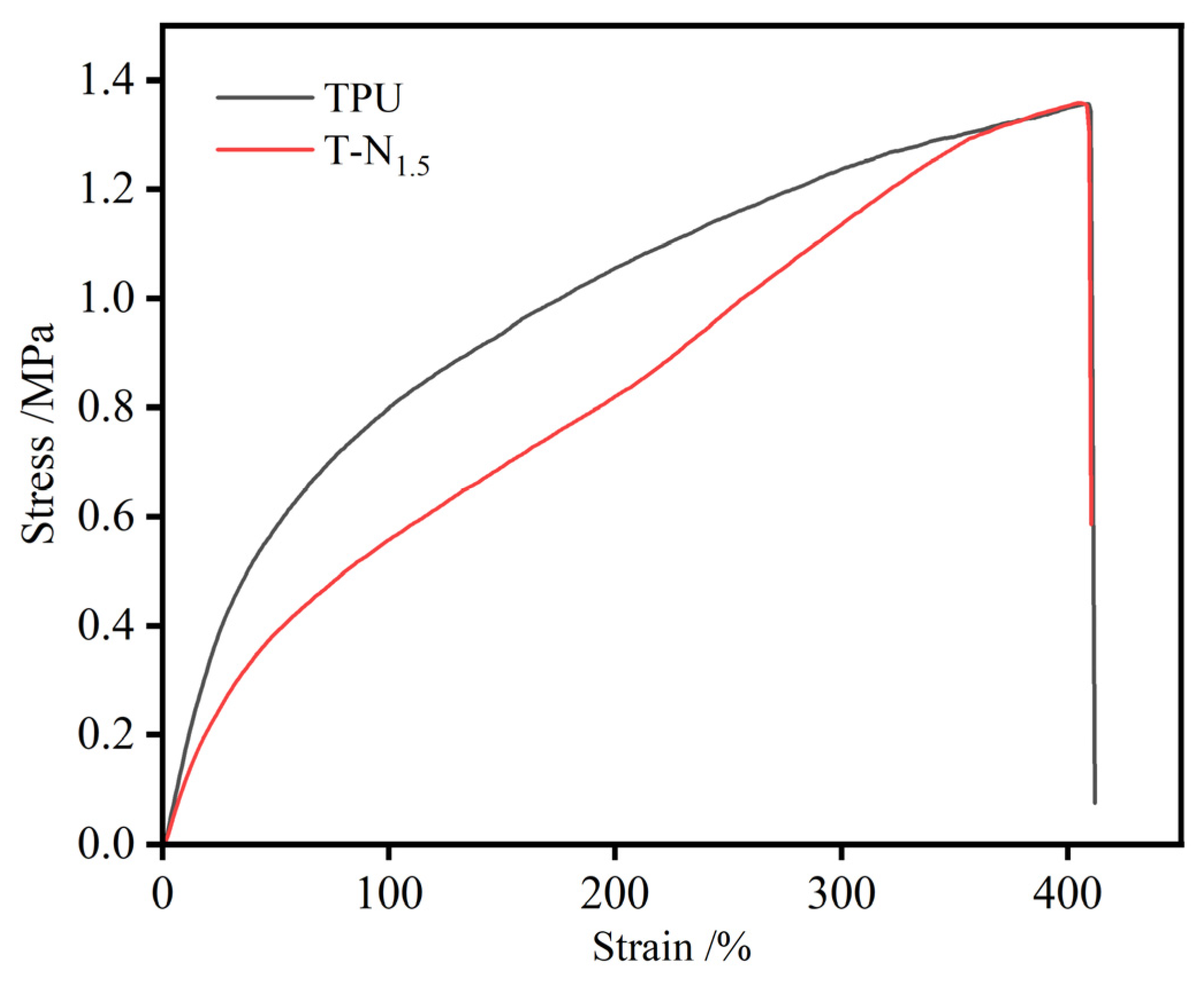
3.2. Physical Property Test
3.3. Uptake Property Analysis
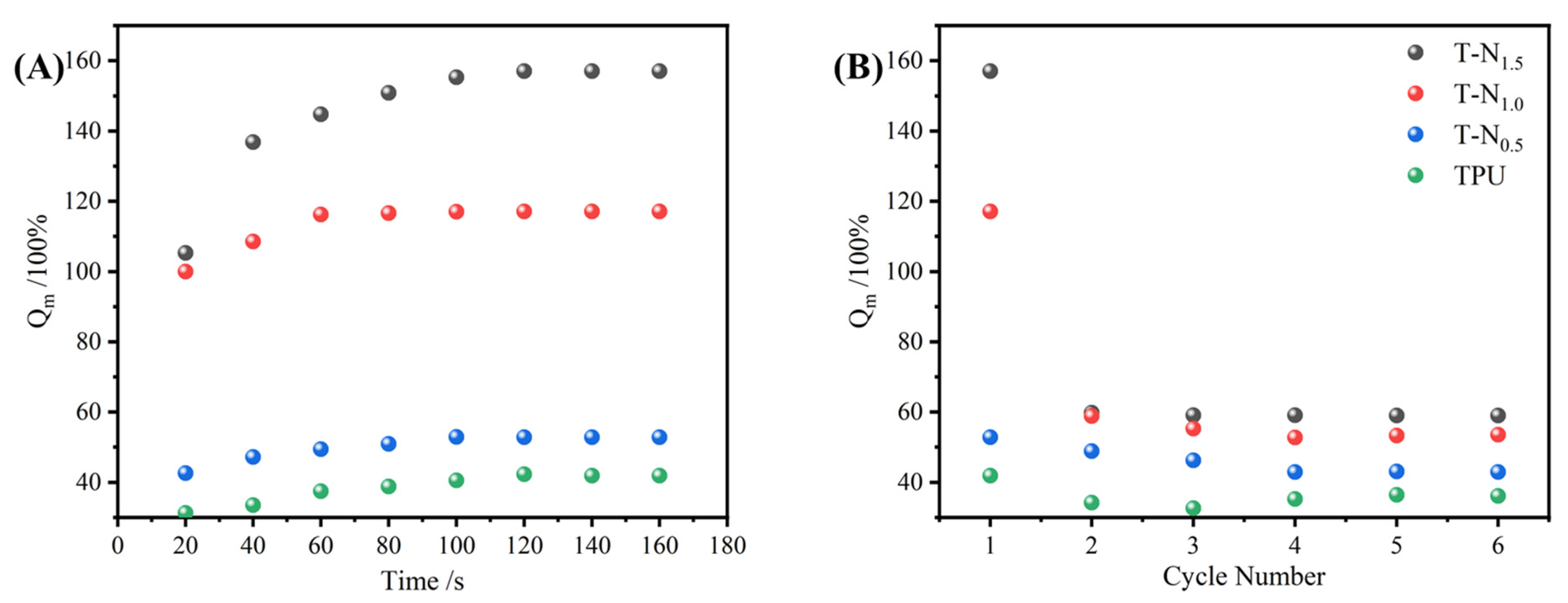
3.3.1. Maximum Uptake Capacity
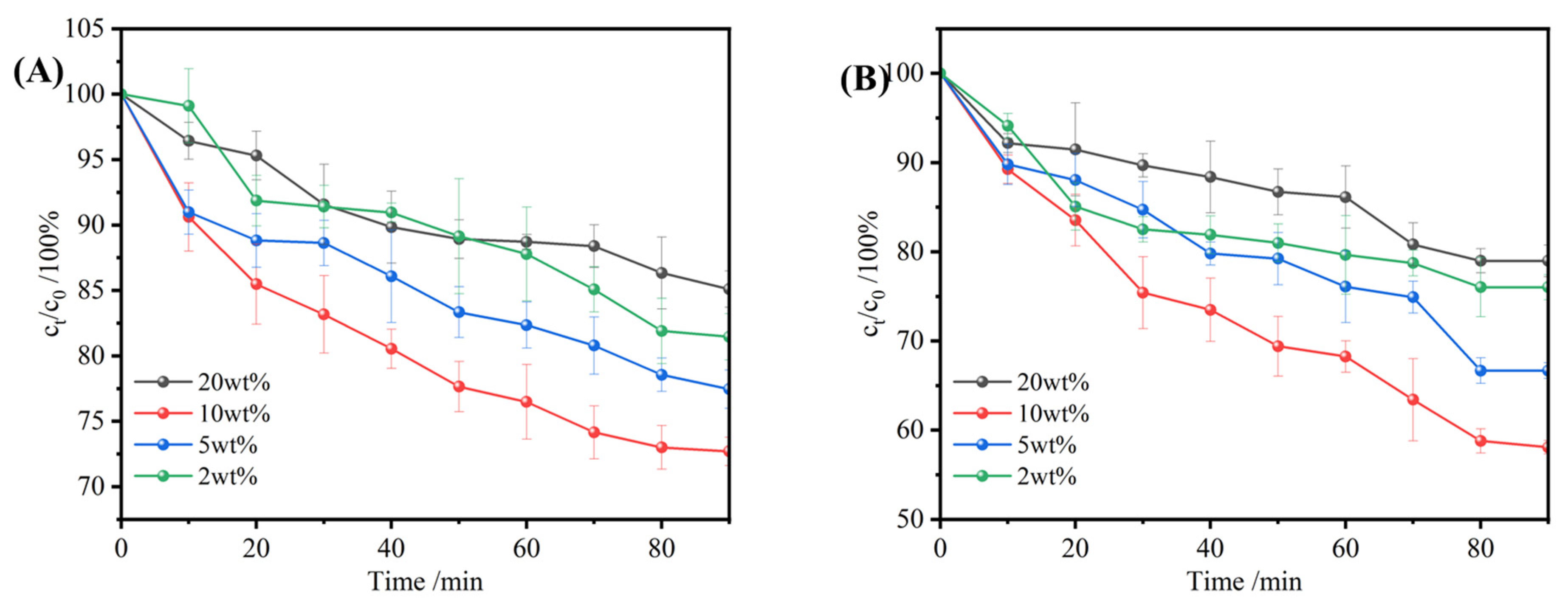
3.3.2. The Impact of PNIPAM Loading on the Uptake Performance
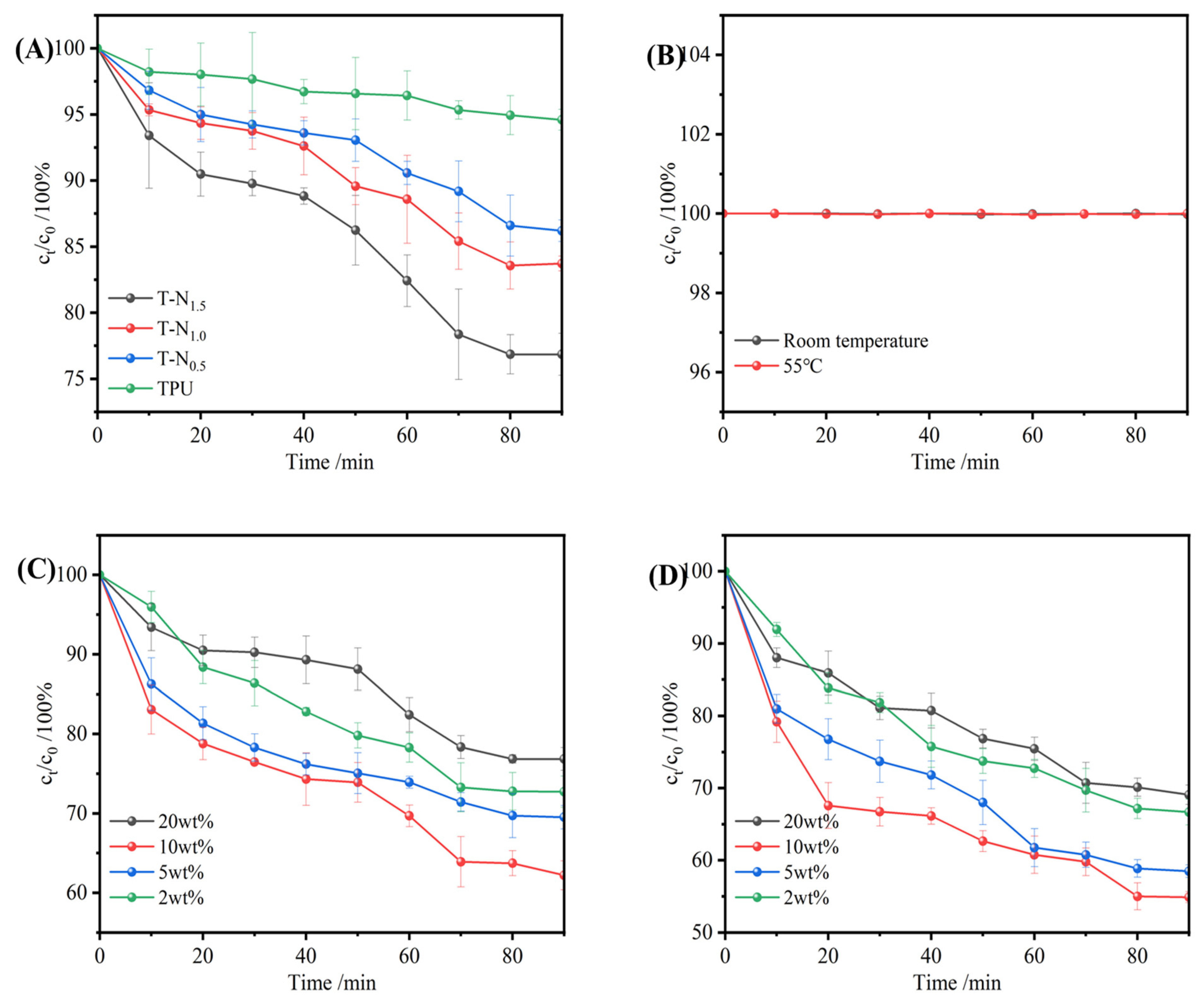
3.3.3. Effect of Initial Ethanol Concentration on Uptake Performance
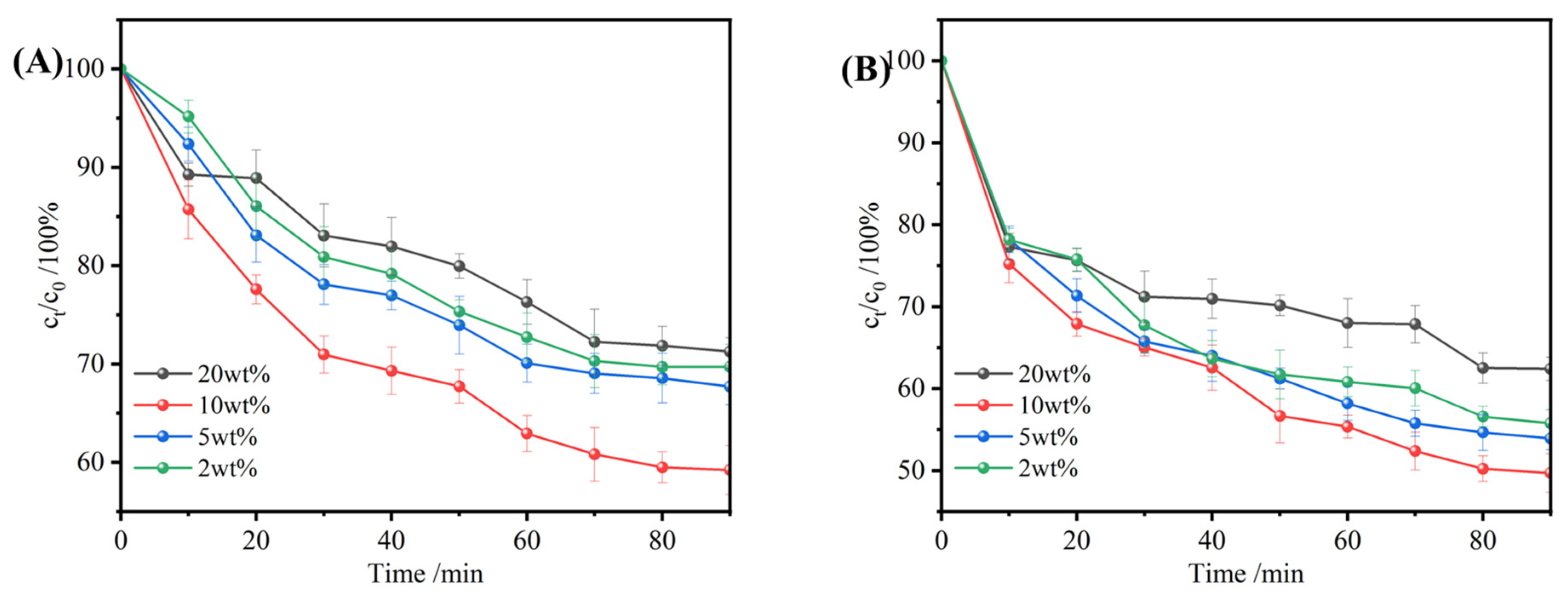
3.3.4. The Effect of Temperature on the Uptake Performance of TPU-PNIPAM
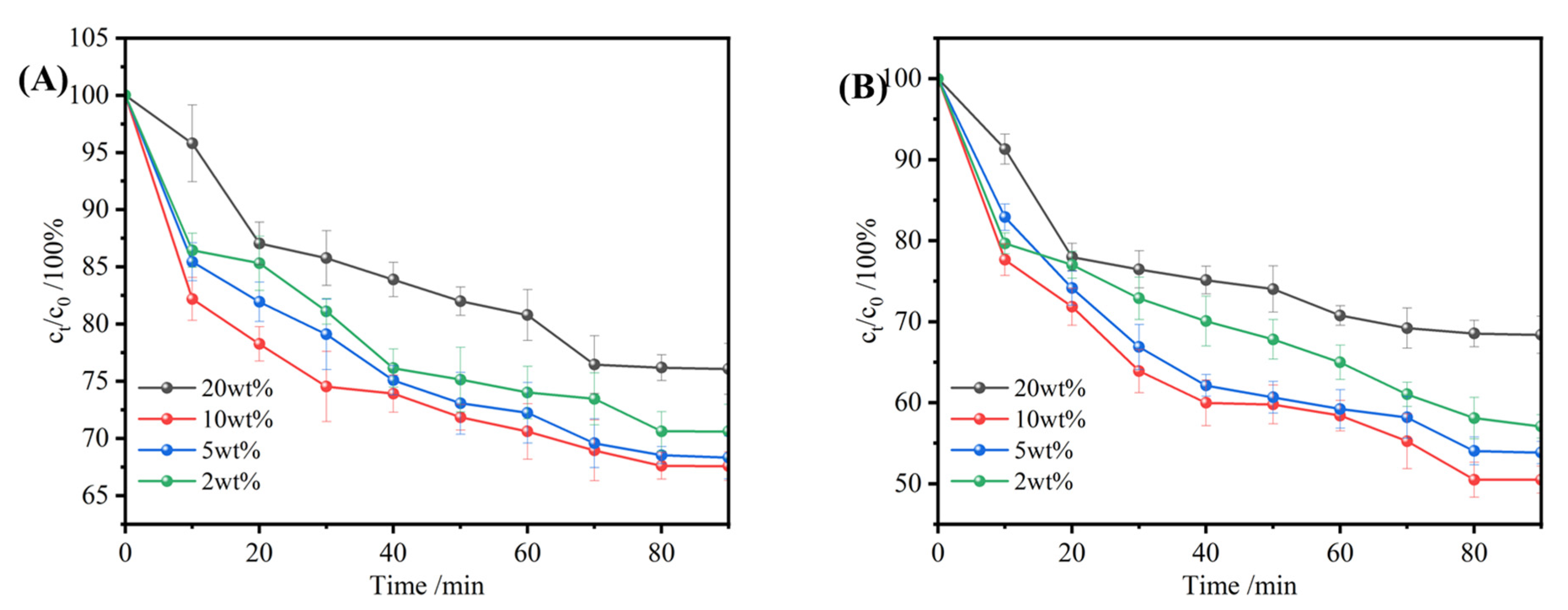
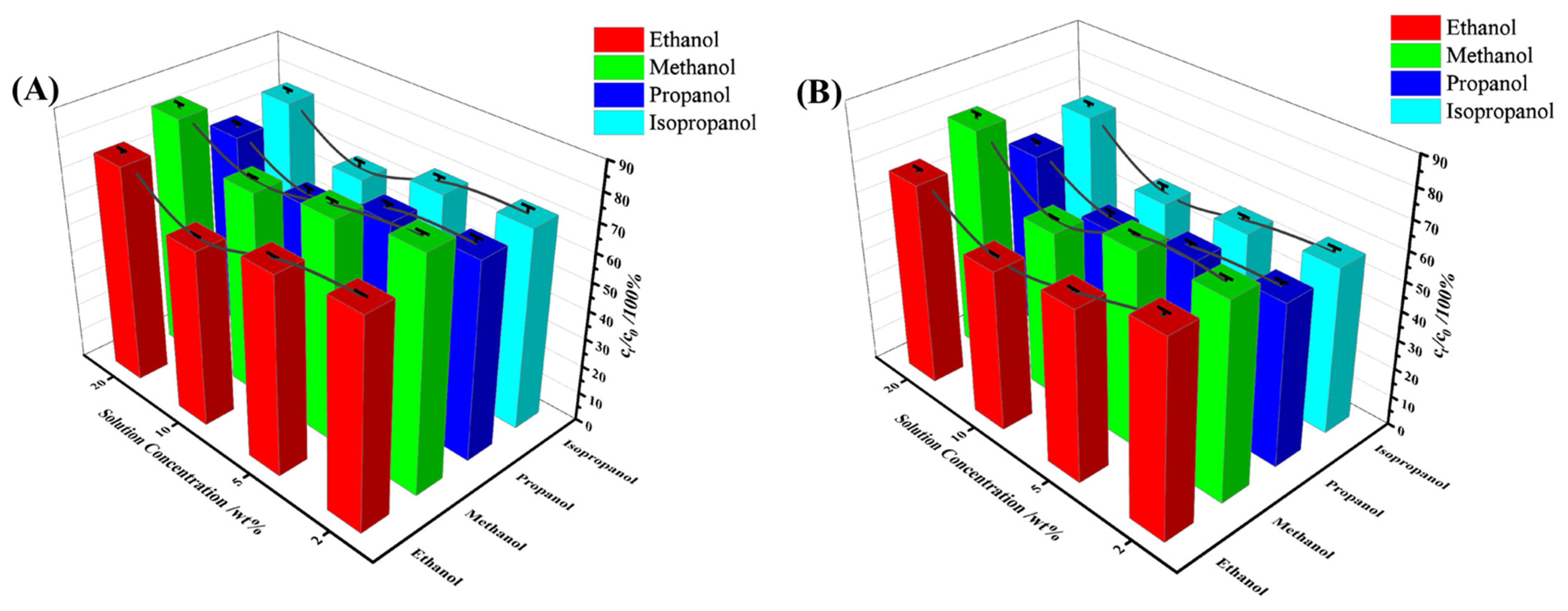
3.3.5. Effect of Different Alcohols on Selective Uptake Performance
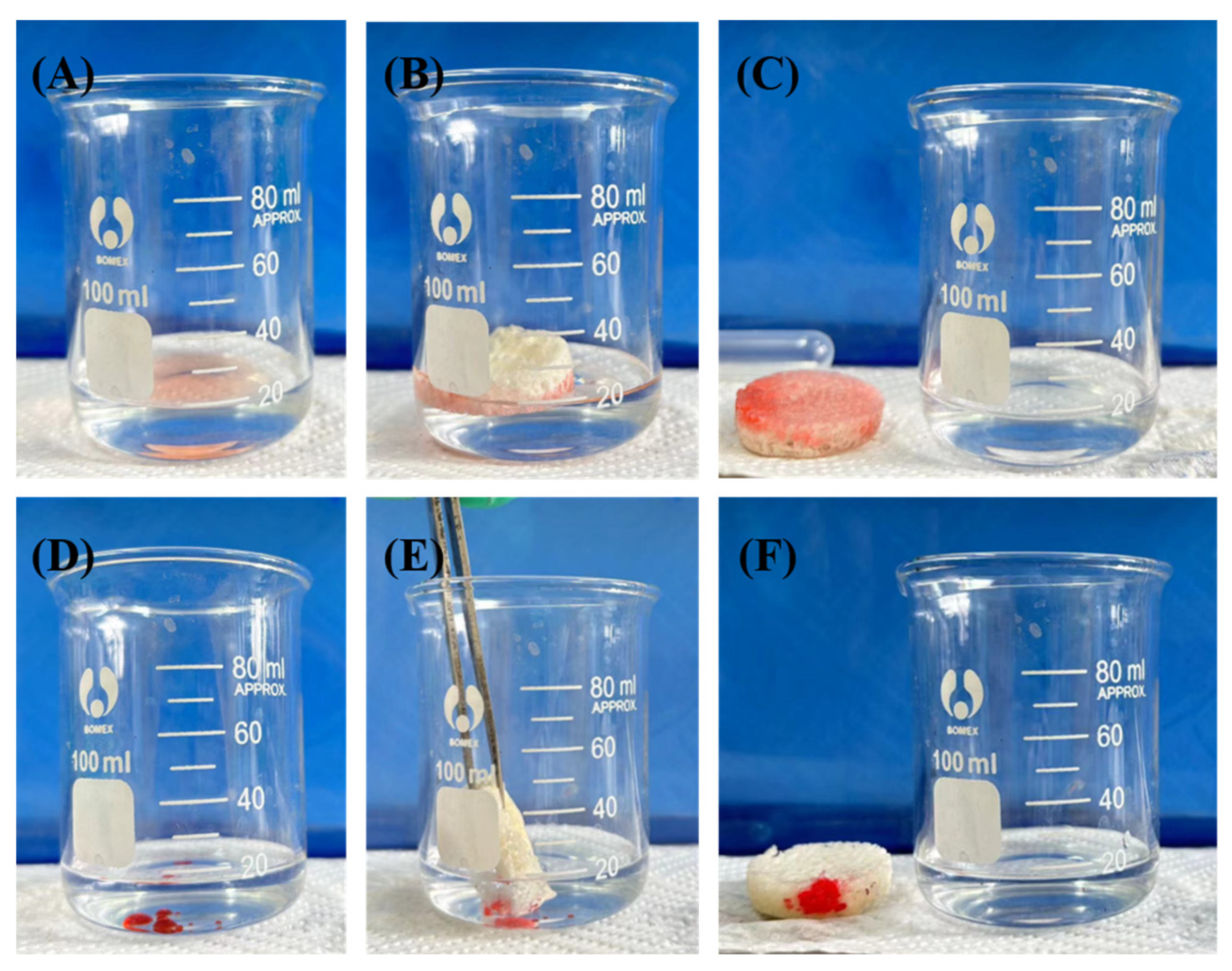
3.3.6. Selective Uptake of Other Pollutants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ramanathan, K.; Koch, C.K.; Oh, S.H. Kinetic modeling of hydrocarbon adsorbers for gasoline and ethanol fuels. Chem. Eng. J. 2012, 207–208, 175–194. [Google Scholar] [CrossRef]
- Bondy, S.C.; Guo, S.X. Effect of Ethanol Treatment on Indices of Cumulative Oxidative Stress. Eur. J. Pharmacol. 1994, 270, 349–355. [Google Scholar] [CrossRef]
- Faisal, M.; Khan, S.B.; Rahman, M.M.; Jamal, A.; Umar, A. Ethanol Chemi-Sensor: Evaluation of Structural, Optical and Sensing Properties of CuO Nanosheets. Mater. Lett. 2011, 65, 1400–1403. [Google Scholar] [CrossRef]
- Wang, L.; Huang, H.; Chang, Y.; Zhong, C. Integrated High Water Affinity and Size Exclusion Effect on Robust Cu-Based Metal-Organic Framework for Efficient Ethanol—Water Separation. ACS Sustain. Chem. Eng. 2021, 9, 3195–3202. [Google Scholar] [CrossRef]
- Lively, R.P.; Dose, M.E.; Thompson, J.A.; McCool, B.A.; Chance, R.R.; Koros, W.J. Ethanol and water adsorption in methanol-derived ZIF-71. Chem. Commun. 2011, 47, 8667–8669. [Google Scholar] [CrossRef]
- He, X.; Wang, T.; Huang, J.; Chen, J.; Li, J. Fabrication and characterization of superhydrophobic PDMS composite membranes for efficient ethanol recovery via pervaporation. Sep. Purif. Technol. 2020, 241, 116675. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, B.; Huang, A. Polydopamine-based superhydrophobic membranes for biofuel recovery. J. Mater. Chem. A 2013, 1, 11970–11974. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, T.; Xia, Q.; Yu, X.; Wang, Y. Constructing superhydrophobic ZIF-8 layer with bud-like surface morphology on PDMS composite membrane for highly efficient ethanol/water separation. J. Environ. Chem. Eng. 2021, 9, 104977. [Google Scholar] [CrossRef]
- Nalaparaju, A.; Zhao, X.; Jiang, J. Biofuel purification by pervaporation and vapor permeation in metal-organic frameworks: A computational study. Energy Environ. Sci. 2011, 4, 2107–2116. [Google Scholar] [CrossRef]
- Krishna, R.; van Baten, J.M. Water/Alcohol Mixture Adsorption in Hydrophobic Materials: Enhanced Water Ingress Caused by Hydrogen Bonding. ACS Omega 2020, 5, 28393–28402. [Google Scholar] [CrossRef]
- Chovau, S.; Gaykawad, S.; Straathof AJ, J.; Van der Bruggen, B. Influence of fermentation by-products on the purification of ethanol from water using pervaporation. Bioresour. Technol. 2011, 102, 1669–1674. [Google Scholar] [CrossRef]
- Ahmed, I.; Pa, N.F.C.; Nawawi, M.G.M.; Rahman, W.A.W.A. Modified Polydimethylsiloxane/Polystyrene Blended IPN Pervaporation Membrane for Ethanol/Water Separation. J. Appl. Polym. Sci. 2011, 122, 2666–2679. [Google Scholar] [CrossRef]
- Xu, C.; Lu, X.; Wang, Z. Effects of sodium ions on the separation performance of pure-silica MFI zeolite membranes. J. Membr. Sci. 2017, 524, 124–131. [Google Scholar] [CrossRef]
- Kommu, A.; Singh, J.K. Separation of Ethanol and Water Using Graphene and Hexagonal Boron Nitride Slit Pores: A Molecular Dynamics Study. J. Phys. Chem. C 2017, 121, 7867–7880. [Google Scholar] [CrossRef]
- Pitt, W.W., Jr.; Haag, G.L.; Lee, D.D. Recovery of ethanol from fermentation broths using selective sorption-desorption. Biotechnol. Bioeng. 1983, 25, 123–131. [Google Scholar] [CrossRef]
- DeJaco, R.F.; Loprete, K.; Pennisi, K.; Majumdar, S.; Siepmann, J.I.; Daoutidis, P.; Murnen, H.; Tsapatsis, M. Modeling and simulation of gas separations with spiral-wound membranes. AIChE J. 2020, 66, e16274. [Google Scholar] [CrossRef]
- Cousin-Saint-Remi, J.; Denayer, J.F.M. Applying the wave theory to fixed-bed dynamics of Metal-Organic Frameworks exhibiting stepped adsorption isotherms: Water/ethanol separation on ZIF-8. Chem. Eng. J. 2017, 324, 313–323. [Google Scholar] [CrossRef]
- Nalaparaju, A.; Zhao, X.S.; Jiang, J.W. Molecular Understanding for the Adsorption of Water and Alcohols in Hydrophilic and Hydrophobic Zeolitic Metal-Organic Frameworks. J. Phys. Chem. C 2010, 114, 11542–11550. [Google Scholar] [CrossRef]
- Delgado, J.A.; Agueda, V.I.; Uguina, M.A.; Sotelo, J.L.; Garcia, A.; Brea, P.; Garcia-Sanz, A. Separation of ethanol-water liquid mixtures by on a polymeric resin Sepabeads 207®. Chem. Eng. J. 2013, 220, 89–97. [Google Scholar] [CrossRef]
- de Lima, G.F.; Mavrandonakis, A.; de Abreu, H.A.; Duarte, H.A.; Heine, T. Mechanism of Alcohol-Water Separation in Metal-Organic Frameworks. J. Phys. Chem. C 2013, 117, 4124–4130. [Google Scholar] [CrossRef]
- Liang, F.; Wang, H.; Liu, G.; Zhao, J.; Jin, W. Designing highly selective and stable water transport channel through graphene oxide membranes functionalized with polyhedral oligomeric silsesquioxane for ethanol dehydration. J. Membr. Sci. 2021, 638, 119675. [Google Scholar] [CrossRef]
- Cao, X.; Zhou, Y.; Wei, X.; Zhai, W.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. Lightweight mechanical robust foam with a herringbone-like porous structure for oil/water separation and filtering. Polym. Testing 2018, 72, 86–93. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Hong, D.; Javni, I.; Erina, N.; Zhang, F.; Ilavský, J. Phase structure in segmented polyurethanes having fatty acid-based soft segments. Polymer. 2013, 54, 372–380. [Google Scholar] [CrossRef]
- Wu, T.; Chen, B. Facile Fabrication of Porous Conductive Thermoplastic Polyurethane Nanocomposite Films via Solution Casting. Sci. Rep. 2017, 7, 17470. [Google Scholar] [CrossRef]
- Muñoz-Chilito, J.; Lara-Ramos, J.A.; Marín, L.; Machuca-Martínez, F.; Correa-Aguirre, J.P.; Hidalgo-Salazar, M.A.; García-Navarro, S.; Roca-Blay, L.; Rodríguez, L.A.; Mosquera-Vargas, E.; et al. Morphological Electrical and Hardness Characterization of Carbon Nanotube-Reinforced Thermoplastic Polyurethane (TPU) Nanocomposite Plates. Molecules 2023, 28, 3598. [Google Scholar] [CrossRef]
- Karamikamkar, S.; Abidli, A.; Tafreshi, O.A.; Ghaffari-Mosanenzadeh, S.; Buahom, P.; Naguib, H.E.; Park, C.B. Nanocomposite Aerogel Network Featuring High Surface Area and Superinsulation Properties. Chem. Mater. 2024, 36, 642–656. [Google Scholar] [CrossRef]
- Liu, C.; Shi, Y.; Ye, H.; He, J.; Lin, Y.; Li, Z.; Lu, J.; Tang, Y.; Wang, Y.; Chen, L. Functionalizing MXene with hypophosphite for highly fire safe thermoplastic polyurethane composites. Compos. Part A Appl. Sci. Manuf. 2023, 168, 107486. [Google Scholar] [CrossRef]
- Cong, H.P.; Qiu, J.H.; Yu, S.H. Thermoresponsive Poly(N-isopropylacrylamide)/Graphene/Au Nanocomposite Hydrogel for Water Treatment by a Laser-Assisted Approach. Small 2015, 11, 1165–1170. [Google Scholar] [CrossRef]
- Backes, S.; Krause, P.; Tabaka, W.; Witt, M.U.; von Klitzing, R. Combined Cononsolvency and Temperature Effects on Adsorbed PNIPAM Microgels. Langmuir. 2017, 33, 14269–14277. [Google Scholar] [CrossRef]
- Wang, N.; Ru, G.; Wang, L.; Feng, J. 1H MAS NMR Studies of the Phase Separation of Poly (N-isopropylacrylamide) Gel in Binary Solvents. Langmuir. 2009, 25, 5898–5902. [Google Scholar] [CrossRef]
- Ou, R.; Wei, J.; Jiang, L.; Simon, G.P.; Wang, H. Robust Thermoresponsive Polymer Composite Membrane with Switchable Superhydrophilicity and Superhydrophobicity for Efficient Oil-Water Separation. Environ. Sci. Technol. 2016, 50, 906–914. [Google Scholar] [CrossRef]
- Shang, J.; Gao, R.; Su, F.; Wang, H.; Zhu, D. Colloidal Probes of PNIPAM-Grafted SiO2 in Studying the Microrheology of Thermally Sensitive Microgel Suspensions. Adv. Polym. Technol. 2020, 2020, 3971953. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Yan, K. Research on the performance and mechanism of asphalt modified by thermoplastic polyurethane with different chemical structures. Constr. Build. Mater. 2023, 409, 133814. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Chase, D.B.; Akins, R.E., Jr.; Rabolt, J.F. Characterization of electrospun poly(N-isopropyl acrylamide) fibers. Polymer 2008, 49, 4025–4032. [Google Scholar] [CrossRef]
- Hu, W.-J.; Li, Y.-M.; Li, Y.-R.; Wang, D.-Y. Highly efficient intumescent flame retardant of dopamine-modified ammonium polyphosphate for the thermoplastic polyurethane elastomer. J. Therm. Anal. Calorim. 2023, 128, 1841–1851. [Google Scholar] [CrossRef]
- Zeng, K.; Fang, Y.; Zheng, S. Organic–inorganic hybrid hydrogels involving poly(N-isopropylacrylamide) and polyhedral oligomeric silsesquioxane: Preparation and rapid thermoresponsive properties. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 504–516. [Google Scholar] [CrossRef]
- Wang, C.-H.; Bai, P.; Siepmann, J.I.; Clark, A.E. Deconstructing Hydrogen-Bond Networks in Confined Nanoporous Materials: Implications for Alcohol-Water Separation. Phys. Chem. C 2014, 118, 19723–19732. [Google Scholar] [CrossRef]
- Kojima, H.; Tanaka, F. Reentrant volume phase transition of cross-linked poly(N-isopropylacrylamide) gels in mixed solvents of water/methanol. Soft Matter 2012, 8, 3010–3020. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Ru, G.; Liu, C.; Feng, J. Phase Transition and Preferential Alcohol Adsorption of Poly(N,N-diethylacrylamide) Gel in Water/Alcohol Mixtures. Macromolecules 2015, 48, 1126–1133. [Google Scholar] [CrossRef]
- Ito, Y.; Ito, T.; Takaba, H.; Nakao, S.-I. Development of gating membranes that are sensitive to the concentration of ethanol. J. Membr. Sci. 2005, 261, 145–151. [Google Scholar] [CrossRef]
- Xie, R.; Song, X.-L.; Luo, F.; Liu, Z.; Wang, W.; Ju, X.-J.; Chu, L.-Y. Ethanol-Responsive Poly(Vinylidene Difluoride) Membranes with Nanogels as Functional Gates. Chem. Eng. Technol. 2016, 39, 841–848. [Google Scholar] [CrossRef]


| T-N0.5 | T-N1.0 | T-N1.5 | |
|---|---|---|---|
| FT-IR -C(CH3)2 | 2.16% | 2.93% | 3.37% |
| Raman -NH2- | 9.44% | 8.93% | 7.24% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Cheng, T.; Zhou, G. Thermoplastic Polyurethane-poly(N-isopropylacrylamide) Copolymer for Selective Uptake of Alcohol from Aqueous Solution. Materials 2024, 17, 2795. https://doi.org/10.3390/ma17122795
Wang F, Cheng T, Zhou G. Thermoplastic Polyurethane-poly(N-isopropylacrylamide) Copolymer for Selective Uptake of Alcohol from Aqueous Solution. Materials. 2024; 17(12):2795. https://doi.org/10.3390/ma17122795
Chicago/Turabian StyleWang, Fei, Tiexin Cheng, and Guangdong Zhou. 2024. "Thermoplastic Polyurethane-poly(N-isopropylacrylamide) Copolymer for Selective Uptake of Alcohol from Aqueous Solution" Materials 17, no. 12: 2795. https://doi.org/10.3390/ma17122795
APA StyleWang, F., Cheng, T., & Zhou, G. (2024). Thermoplastic Polyurethane-poly(N-isopropylacrylamide) Copolymer for Selective Uptake of Alcohol from Aqueous Solution. Materials, 17(12), 2795. https://doi.org/10.3390/ma17122795





