Post-Processing Effect on the Corrosion Resistance of Super Duplex Stainless Steel Produced by Laser Powder Bed Fusion
Abstract
1. Introduction
2. Materials and Methods
- As-printed, without any post-processing heat treatment (AS);
- Solution annealing of as-printed samples at 1100 °C for 15 min, fast cooling in water (SA);
- Stress relieving of as-printed samples at 300 °C for 5 h, slow cooling with the furnace (SR 300/5 h);
- Stress relieving of as-printed samples at 400 °C for 1 h, slow cooling with the furnace (SR 400/1 h);
- Stress relieving of as-printed samples at 550 °C for 5 h, slow cooling with the furnace (SR 550/5 h).
3. Results and Discussion
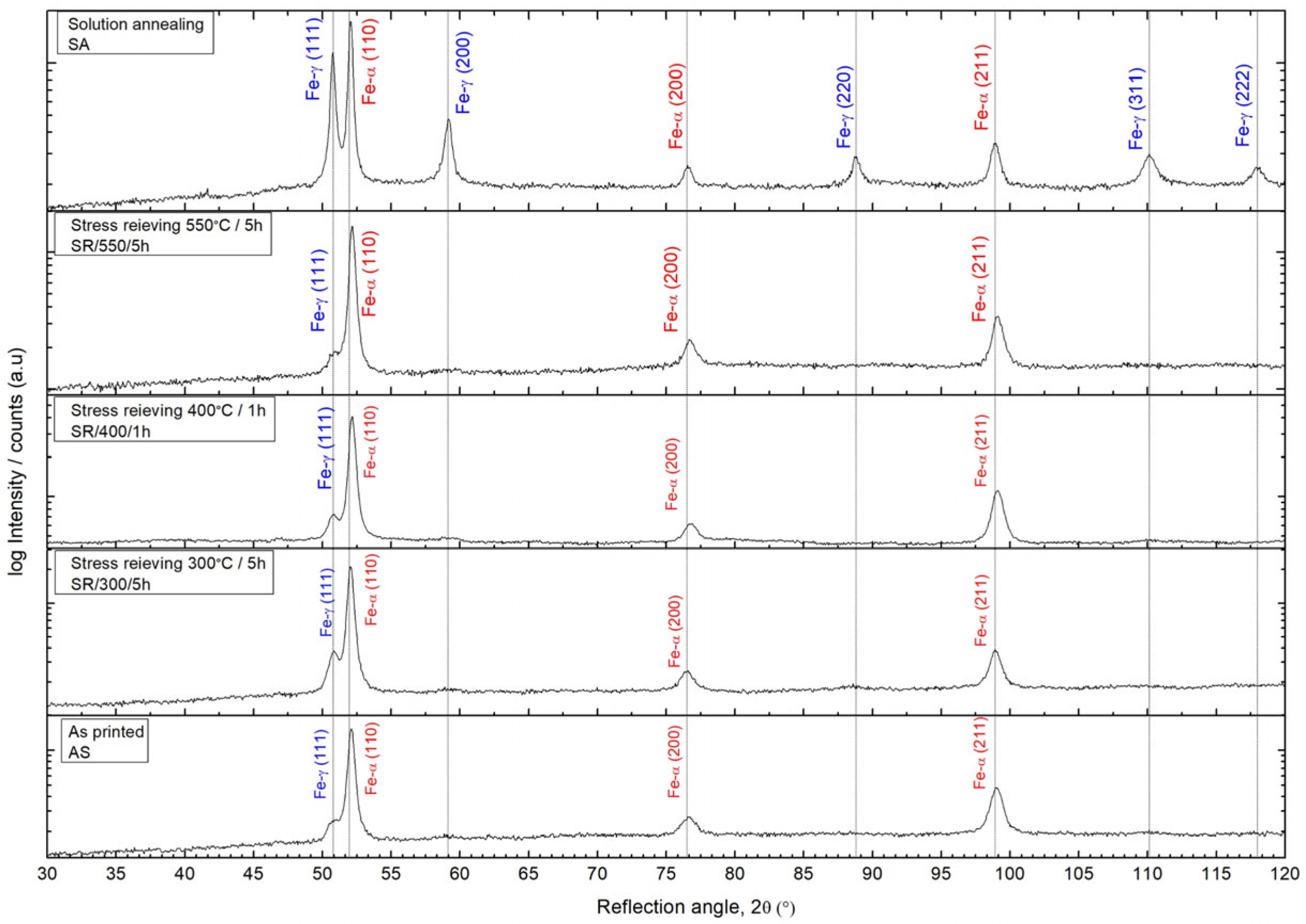
3.1. Microstructural Analysis
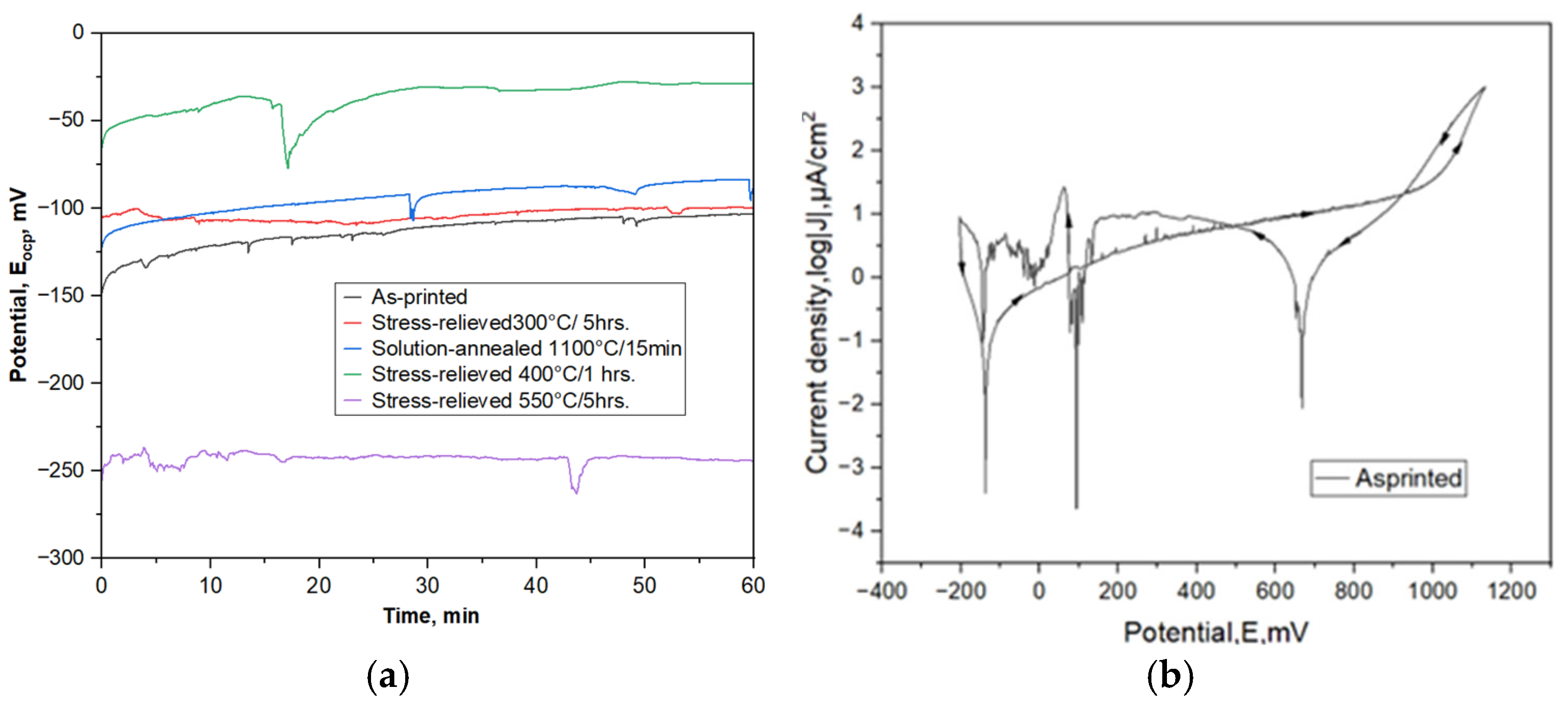
3.2. Potentiodynamic Polarization Test
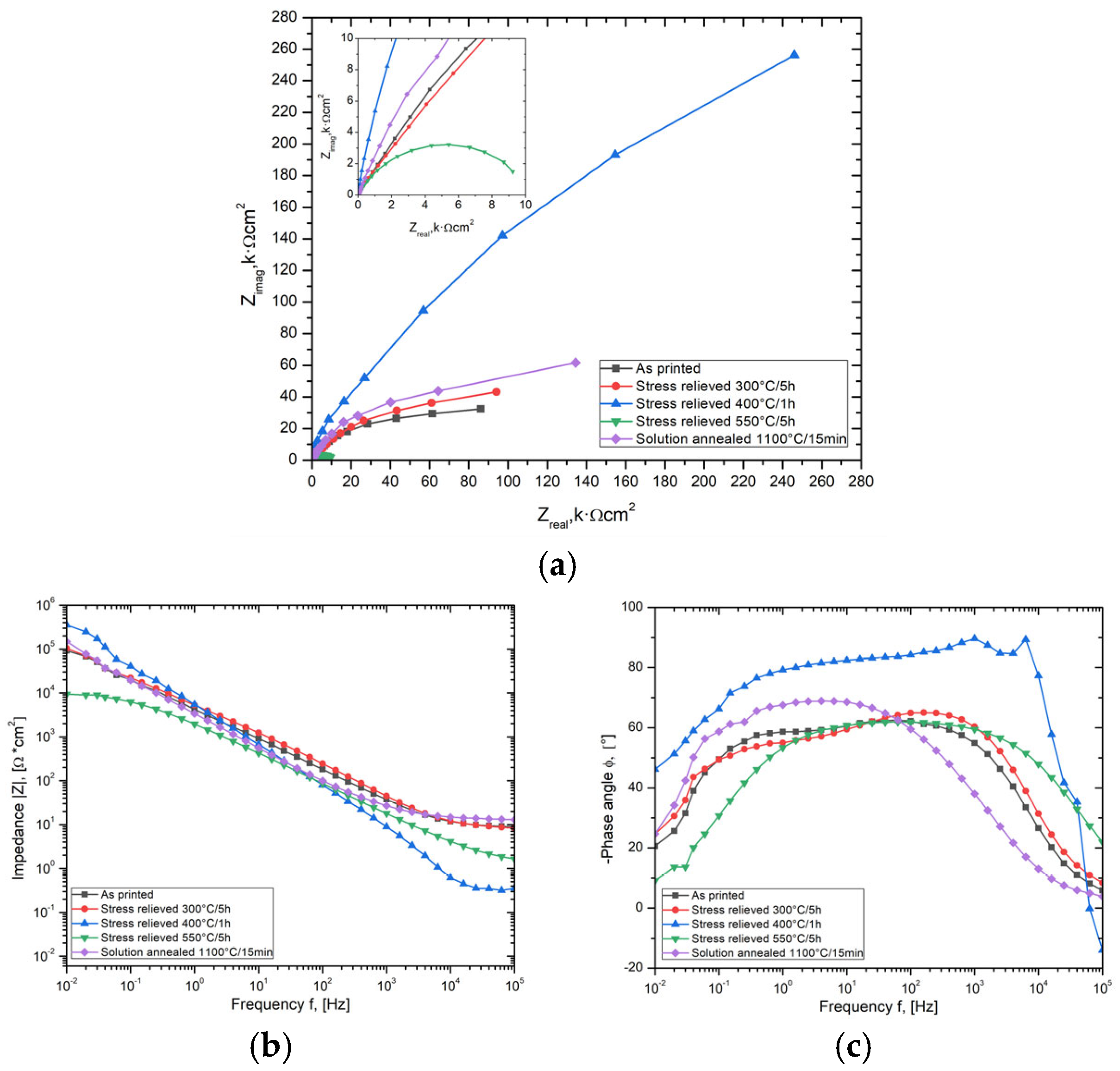
3.3. Electrochemical Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Nilsson, J.-O. Super Duplex Stainless Steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Gunn, R. Duplex Stainless Steels: Microstructure, Properties and Applications; Woodhead Publishing: Sawston, UK, 1997. [Google Scholar]
- Pohl, M.; Storz, O.; Glogowski, T. Effect of Intermetallic Precipitations on the Properties of Duplex Stainless Steel. Mater Charact 2007, 58, 65–71. [Google Scholar] [CrossRef]
- Fritz, J. Practical Guide to Using Duplex Stainless Steels (10044), A Guide to the Use of Nickel-Containing Alloys No 10044; Nickel Institute: Toronto, ON, Canada, 2020; Available online: www.nickelinstitute.org (accessed on 5 June 2024).
- Mulhi, A.; Dehgahi, S.; Waghmare, P.; Qureshi, A.J. Process Parameter Optimization of 2507 Super Duplex Stainless Steel Additively Manufactured by the Laser Powder Bed Fusion Technique. Metals 2023, 13, 725. [Google Scholar] [CrossRef]
- Xiang, H.; Chen, G.; Zhao, W.; Wu, C. Densification Behavior and Build Quality of Duplex Stainless Steel Fabricated by Laser Powder Bed Fusion. Metals 2023, 13, 741. [Google Scholar] [CrossRef]
- Fang, Y.; Kim, M.-K.; Zhang, Y.; Kim, T.; No, J.; Suhr, J. A New Grain Refinement Route for Duplex Stainless Steels: Micro-Duplex Stainless Steel Matrix Composites Processed by Laser Powder Bed Fusion. Mater. Sci. Eng. A 2023, 881, 145351. [Google Scholar] [CrossRef]
- Haghdadi, N.; Chen, H.; Chen, Z.; Babu, S.S.; Liao, X.; Ringer, S.P.; Primig, S. Intergranular Precipitation and Chemical Fluctuations in an Additively Manufactured 2205 Duplex Stainless Steel. Scr. Mater. 2022, 219, 114894. [Google Scholar] [CrossRef]
- Gargalis, L.; Karavias, L.; Graff, J.S.; Diplas, S.; Koumoulos, E.P.; Karaxi, E.K. Novel Powder Feedstock towards Microstructure Engineering in Laser Powder Bed Fusion: A Case Study on Duplex/Super Duplex and Austenitic Stainless-Steel Alloys. Metals 2023, 13, 1546. [Google Scholar] [CrossRef]
- Laleh, M.; Haghdadi, N.; Hughes, A.E.; Primig, S.; Tan, M.Y.J. Enhancing the Repassivation Ability and Localised Corrosion Resistance of an Additively Manufactured Duplex Stainless Steel by Post-Processing Heat Treatment. Corros. Sci. 2022, 198, 110106. [Google Scholar] [CrossRef]
- Haghdadi, N.; Ledermueller, C.; Chen, H.; Chen, Z.; Liu, Q.; Li, X.; Rohrer, G.; Liao, X.; Ringer, S.; Primig, S. Evolution of Microstructure and Mechanical Properties in 2205 Duplex Stainless Steels during Additive Manufacturing and Heat Treatment. Mater. Sci. Eng. A 2022, 835, 142695. [Google Scholar] [CrossRef]
- Köhler, M.L.; Kunz, J.; Herzog, S.; Kaletsch, A.; Broeckmann, C. Microstructure Analysis of Novel LPBF-Processed Duplex Stainless Steels Correlated to Their Mechanical and Corrosion Properties. Mater. Sci. Eng. A 2021, 801, 140432. [Google Scholar] [CrossRef]
- Shang, F.; Chen, X.; Wang, Z.; Ji, Z.; Ming, F.; Ren, S.; Qu, X. The Microstructure, Mechanical Properties, and Corrosion Resistance of UNS S32707 Hyper-Duplex Stainless Steel Processed by Selective Laser Melting. Metals 2019, 9, 1012. [Google Scholar] [CrossRef]
- Cui, C.; Becker, L.; Gärtner, E.; Boes, J.; Lentz, J.; Uhlenwinkel, V.; Steinbacher, M.; Weber, S.; Fechte-Heinen, R. Laser Additive Manufacturing of Duplex Stainless Steel via Powder Mixture. J. Manuf. Mater. Process. 2022, 6, 72. [Google Scholar] [CrossRef]
- Papula, S.; Song, M.; Pateras, A.; Chen, X.-B.; Brandt, M.; Easton, M.; Yagodzinskyy, Y.; Virkkunen, I.; Hänninen, H. Selective Laser Melting of Duplex Stainless Steel 2205: Effect of Post-Processing Heat Treatment on Microstructure, Mechanical Properties, and Corrosion Resistance. Materials 2019, 12, 2468. [Google Scholar] [CrossRef]
- Xiang, H.; Zhao, W.; Lu, Y. Effect of Solution Temperature on Microstructure and Mechanical Properties of Selective Laser Melted Fe–22Cr–5Ni-0.26N Duplex Stainless Steel. J. Mater. Res. Technol. 2022, 19, 1379–1389. [Google Scholar] [CrossRef]
- Kaščák, Ľ.; Varga, J.; Bidulská, J.; Bidulský, R.; Grande, M.A. Simulation tool for material behaviour prediction in additive manufacturing. Acta Metall. Slovaca 2023, 29, 113–118. [Google Scholar] [CrossRef]
- Stornelli, G.; Gaggia, D.; Rallini, M.; Di Schino, A. Heat Treatment Effect on Maraging Steel Manufactured by Laser Powder Bed Fusion Technology: Microstructure and Mechanical Properties. Acta Metall. Slovaca 2021, 27, 122–126. [Google Scholar] [CrossRef]
- Bidulsky, R.; Gobber, F.S.; Bidulska, J.; Ceroni, M.; Kvackaj, T.; Grande, M.A. Coated Metal Powders for Laser Powder Bed Fusion (L-PBF) Processing: A Review. Metals 2021, 11, 1831. [Google Scholar] [CrossRef]
- Da Fonseca, G.S.; Barbosa, L.O.R.; Ferreira, E.A.; Xavier, C.R.; De Castro, J.A. Microstructural, Mechanical, and Electrochemical Analysis of Duplex and Superduplex Stainless Steels Welded with the Autogenous TIG Process Using Different Heat Input. Metals 2017, 7, 538. [Google Scholar] [CrossRef]
- Gargalis, L.; Karavias, L.; Graff, J.S.; Diplas, S.; Koumoulos, E.P.; Karaxi, E.K. A Comparative Investigation of Duplex and Super Duplex Stainless Steels Processed through Laser Powder Bed Fusion. Metals 2023, 13, 1897. [Google Scholar] [CrossRef]
- Outokumpu. Duplex Stainless Steels—Outokumpu Forta Range Datasheet; Outokumpu: Helsinki, Finland, 2022. [Google Scholar]
- PN EN ISO 17475; 2010 Corrosion of Metals and Alloys—Electrochemical Test Methods—Guidelines for Conducting Potentiostatic and Potentiodynamic Polarization Measurements. Polish Committee for Standardization. PKN: Warszawa, Poland, 2010.
- Mareci, D.; Nemtoi, G.; Aelenei, N.; Bocanu, C. The Electrochemical Behaviour of Various Non-Precious Ni and Co Based Alloys in Artificial Saliva. Eur. Cells Mater. 2005, 10, 1–7, discussion 1–7. [Google Scholar]
- Brytan, Z. Comparison of Vacuum Sintered and Selective Laser Melted Steel AISI 316L. Arch. Metall. Mater. 2017, 62, 2125–2131. [Google Scholar] [CrossRef][Green Version]
- Tański, T.; Brytan, Z.; Labisz, K. Fatigue Behaviour of Sintered Duplex Stainless Steel. Procedia Eng. 2014, 74, 421–428. [Google Scholar] [CrossRef]
- Rai, S.; Choudhary, B.K.; Jayakumar, T.; Rao, K.B.S.; Raj, B. Characterization of Low Cycle Fatigue Damage in 9Cr–1Mo Ferritic Steel Using X-Ray Diffraction Technique. Int. J. Press. Vessel. Pip. 1999, 76, 275–281. [Google Scholar] [CrossRef]
- Xie, C.; Li, B.; Liu, G.; Liu, J.; Ying, H.; Li, D.; Wang, S.; Wang, L. Study on the Effect of Solution Treatment on Mechanical and Corrosion Properties of SAF 2507DSS Produced by LPBF. J. Mater. Res. Technol. 2023, 26, 2070–2081. [Google Scholar] [CrossRef]
- Murkute, P.; Pasebani, S.; Burkan Isgor, O. Effects of Heat Treatment and Applied Stresses on the Corrosion Performance of Additively Manufactured Super Duplex Stainless Steel Clads. Materialia 2020, 14, 100878. [Google Scholar] [CrossRef]
- Knyazeva, M.; Pohl, M. Duplex Steels. Part II: Carbides and Nitrides. Metallogr. Microstruct. Anal. 2013, 2, 343–351. [Google Scholar] [CrossRef]
- Pettersson, N.; Pettersson, R.F.A.; Wessman, S. Precipitation of Chromium Nitrides in the Super Duplex Stainless Steel 2507. Met. Mater. Trans. A Phys. Met. Mater. Sci. 2015, 46, 1062–1072. [Google Scholar] [CrossRef]
- Finšgar, M.; Fassbender, S.; Hirth, S.; Milošev, I. Electrochemical and XPS study of polyethyleneimines of different molecular sizes as corrosion inhibitors for AISI 430 stainless steel in near-neutral chloride media. Mater. Chem. Phys. 2009, 116, 198–206. [Google Scholar] [CrossRef]
- Finšgar, M.; Fassbender, S.; Nicolini, F.; Milošev, I. Polyethyleneimine as a Corrosion Inhibitor for ASTM 420 Stainless Steel in Near-Neutral Saline Media. Corros. Sci. 2009, 51, 525–533. [Google Scholar] [CrossRef]
- Mengistu, D.; Brytan, Z.; Bidulská, J.; Bidulský, R. Corrosion evaluation of super duplex stainless steel made by LPBF. Acta Metall. Slovaca 2024, 30, 34–40. [Google Scholar] [CrossRef]
- Reimann, L.; Brytan, Z.; Jania, G. Influence of Filler Metal on Electrochemical Characteristics of a Laser-Welded CoCrMoW Alloy Used in Prosthodontics. Materials 2022, 15, 5721. [Google Scholar] [CrossRef] [PubMed]
- Jović, V.D. Calculation of a Pure Double Layer Capacitance from a Constant Phase Element in the Impedance Measurements. Mater. Prot. 2022, 63, 50–57. [Google Scholar] [CrossRef]
- Bio-Logic Science Instruments. Application Note #20 2010; Bio-Logic Science Instruments: Knoxsville, TN, USA, 2019; Available online: www.biologic.net (accessed on 5 June 2024).
- Okazaki, Y. Characterization of Oxide Film of Implantable Metals by Electrochemical Impedance Spectroscopy. Materials 2019, 12, 3466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, T.; Che, L.; Huang, G. Microstructure and Recrystallization Behavior of Heating Rate-Controlled Electrolytic Capacitor Aluminum Foil under Cold Forming and Annealing. Materials 2023, 16, 4128. [Google Scholar] [CrossRef] [PubMed]





| Element | Fe | Cr | Ni | Mo | Mn | Si | Cu | S | P | C | N | O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt. % | 65.01 | 25.2 | 6.3 | 3.8 | 0.4 | 0.3 | 0.15 | 0.001 | 0.024 | 0.0160 | 0.35 | 0.0170 |
| Condition | Parameter | Fe-α’ (110) | Fe-α’ (200) | Fe-α’ (200) | β, 2Θ° (med.) | D (nm) | δ × 10−3 (nm−2) | ε × 10−3 |
|---|---|---|---|---|---|---|---|---|
| SA 1100 °C/15 min | 2Θ° | 52.05109 | 76.58382 | 98.92256 | 0.631 | 20.44 | 2.78 | 3.46 |
| β, Θ° | 0.37954 | 0.75392 | 0.75971 | |||||
| SR 550 °C/5 h | 2Θ° | 52.16993 | 76.75633 | 99.11614 | 0.762 | 14.47 | 5.36 | 4.31 |
| β, Θ° | 0.45318 | 1.01012 | 0.82259 | |||||
| SR 400 °C/1 h | 2Θ° | 52.16231 | 76.81057 | 99.11002 | 0.825 | 13.47 | 6.14 | 4.62 |
| β, Θ° | 0.51106 | 1.07759 | 0.8878 | |||||
| SR 300 °C/5 h | 2Θ° | 52.04914 | 76.51321 | 98.94692 | 0.854 | 13.06 | 7.07 | 4.94 |
| β, Θ° | 0.49392 | 1.19726 | 0.87041 | |||||
| AS | 2Θ° | 52.10395 | 76.64378 | 99.02586 | 0.903 | 12.16 | 7.85 | 5.21 |
| β, Θ° | 0.50937 | 1.24371 | 0.95526 |
| Sample Designation | EOCP | Ebr | Erp | Erp–Ecorr | Esec | Jcorr | βa | βc | Ecorr | Rp |
|---|---|---|---|---|---|---|---|---|---|---|
| mV | mV | mV | mV | mV | µA/cm2 | mV | mV | mV | kΩ·cm2 | |
| AS | −103 | 999 | 1084 | 1231 | 649 | 140.63 | 195.73 | 51.98 | −147.17 | 126.82 |
| SR 300 °C/ 5 h | −99.7 | 996 | 1083 | 1225 | 634 | 166.60 | 255.88 | 59.11 | −142.86 | 125.14 |
| SR 400 °C/1 h | −28.6 | 1055 | 1127 | 1210 | 368 | 59.06 | 225.50 | 50.05 | −83.81 | 301.10 |
| SR 550 °C/5 h | −244 | −96.3 | 130 | 391 | −195 | 1159.0 | 117.99 | 78.67 | −261.03 | 17.68 |
| SA 1100 °C/15 min | −88.2 | 1011 | 1102 | 1238 | 701 | 131.89 | 295.36 | 53.86 | −136.35 | 149.35 |
| Sample Designation | RS | Ydl µF·s(n−1)·cm−2 | n | Cdl | Rct |
|---|---|---|---|---|---|
| Ω·cm2 | µF·cm−2 | kΩ·cm2 | |||
| AS | 7.980 | 83.61 | 0.70 | 9.691 | 78.34 |
| SR 300 °C/ 5 h | 6.950 | 49.57 | 0.70 | 4.983 | 94.80 |
| SR 400 °C/1 h | 0.370 | 51.24 | 0.85 | 23.59 | 240.65 |
| SR 550 °C/5 h | 1.210 | 126.40 | 0.77 | 16.83 | 9.260 |
| SA 1100 °C/15 min | 13.55 | 70.25 | 0.77 | 16.93 | 121.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brytan, Z.; Dagnaw, M.; Bidulská, J.; Bidulský, R.; Muhamad, M.R. Post-Processing Effect on the Corrosion Resistance of Super Duplex Stainless Steel Produced by Laser Powder Bed Fusion. Materials 2024, 17, 2807. https://doi.org/10.3390/ma17122807
Brytan Z, Dagnaw M, Bidulská J, Bidulský R, Muhamad MR. Post-Processing Effect on the Corrosion Resistance of Super Duplex Stainless Steel Produced by Laser Powder Bed Fusion. Materials. 2024; 17(12):2807. https://doi.org/10.3390/ma17122807
Chicago/Turabian StyleBrytan, Zbigniew, Mengistu Dagnaw, Jana Bidulská, Róbert Bidulský, and Mohd Ridha Muhamad. 2024. "Post-Processing Effect on the Corrosion Resistance of Super Duplex Stainless Steel Produced by Laser Powder Bed Fusion" Materials 17, no. 12: 2807. https://doi.org/10.3390/ma17122807
APA StyleBrytan, Z., Dagnaw, M., Bidulská, J., Bidulský, R., & Muhamad, M. R. (2024). Post-Processing Effect on the Corrosion Resistance of Super Duplex Stainless Steel Produced by Laser Powder Bed Fusion. Materials, 17(12), 2807. https://doi.org/10.3390/ma17122807








