Evaluation of the Acousto-Optic Figure of Merit and the Maximum Value of the Elasto-Optic Constant of Liquids
Abstract
1. Introduction
2. Theory of the Elasto-Optic Effect in Liquids
2.1. Elasto-Optic Effect
2.2. Review of Permittivity Models
3. Comparison of Permittivity Models
4. AO Figure of Merit of Liquids
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Medium | n | (g/cm)3 | V (m/s) | p Exp | p Eykman | p Lorentz | (s3/kg) Exp | (s3/kg) Eykman | (s3/kg) Lorentz |
|---|---|---|---|---|---|---|---|---|---|
| 1,1,1-Trichloroethane | 1.438 [26] | 1.338 [26] | 943 [45] | 0.282 [26] | 0.313 | 0.339 | 625 | 771 | 903 |
| 1,1,2,2-Tetrachloroethane | 1.494 [26] | 1.595 [26] | 1170 [46] | 0.307 [26] | 0.316 | 0.349 | 411 | 435 | 530 |
| 1,2-Dichloroethane | 1.445 [26] | 1.253 [26] | 1216 [46] | 0.293 [26] | 0.314 | 0.340 | 347 | 397 | 467 |
| 1-Butanol | 1.399 [26] | 0.810 [26] | 1257 [46] | 0.302 [26] | 0.309 | 0.330 | 426 | 445 | 507 |
| 1-Chlorobutane | 1.402 [26] | 0.886 [26] | 1117 [46] | 0.296 [26] | 0.309 | 0.330 | 538 | 587 | 671 |
| 1-Chloropropane | 1.388 [26] | 0.891 [26] | 1091 [46] | 0.293 [26] | 0.307 | 0.327 | 530 | 582 | 659 |
| 1-Hexane | 1.388 [26] | 0.673 [26] | 1098 [46] | 0.305 [26] | 0.307 | 0.327 | 745 | 756 | 856 |
| 1-Nitropropane | 1.402 [26] | 1.001 [26] | 1252 [47] | 0.275 [26] | 0.309 | 0.330 | 292 | 368 | 421 |
| 2-Aminoethanol | 1.454 [26] | 1.016 [26] | 1741 [46] | 0.302 [26] | 0.314 | 0.342 | 161 | 174 | 206 |
| 2-Ethoxyethyl Acetate | 1.404 [26] | 0.973 [26] | 1129 [46] | 0.374 [26] | 0.309 | 0.331 | 768 | 525 | 601 |
| 2-Methyl-1-Propanol | 1.396 [26] | 0.802 [26] | 1188 [48] | 0.302 [26] | 0.308 | 0.329 | 502 | 523 | 595 |
| 2-Nitropropane | 1.394 [26] | 0.988 [26] | 1201 [47] | 0.272 [26] | 0.308 | 0.328 | 317 | 407 | 462 |
| 3-Methyl-1-Butanol | 1.407 [26] | 0.810 [26] | 1220 [46] | 0.289 [26] | 0.310 | 0.332 | 442 | 506 | 580 |
| a-Bromonaphthalene | 1.649 [15] | 1.479 [15] | 1376 [15] | 0.326 [15] | 0.312 | 0.366 | 553 | 507 | 698 |
| Acetaldehyde | 1.331 [26] | 0.778 [26] | 1137 [46] | 0.280 [26] | 0.295 | 0.309 | 381 | 424 | 465 |
| Acetic Acid | 1.372 [26] | 1.049 [26] | 1134 [46] | 0.264 [26] | 0.304 | 0.322 | 303 | 403 | 452 |
| Acetic Anhydride | 1.390 [26] | 1.081 [26] | 1249 [46] | 0.272 [26] | 0.307 | 0.327 | 254 | 324 | 368 |
| Acetone | 1.356 [15] | 0.785 [15] | 1170 [15] | 0.298 [15] | 0.301 | 0.317 | 438 | 448 | 498 |
| Acetonitrile | 1.344 [26] | 0.782 [26] | 1300 [46] | 0.268 [26] | 0.298 | 0.314 | 247 | 306 | 337 |
| a-Chloronaphthalene | 1.626 [15] | 1.188 [15] | 1454 [15] | 0.327 [15] | 0.313 | 0.364 | 541 | 497 | 671 |
| Acrolein | 1.402 [26] | 0.839 [26] | 1207 [49] | 0.300 [26] | 0.309 | 0.330 | 462 | 491 | 561 |
| Acrylonitrile | 1.392 [26] | 0.806 [26] | 1172 [50] | 0.292 [26] | 0.307 | 0.328 | 478 | 529 | 601 |
| Aniline | 1.579 [15] | 1.017 [15] | 1639 [15] | 0.332 [15] | 0.316 | 0.360 | 382 | 345 | 448 |
| Anisole | 1.517 [26] | 0.994 [26] | 1425 [46] | 0.306 [26] | 0.317 | 0.352 | 397 | 425 | 526 |
| Benzene | 1.495 [15] | 0.874 [15] | 1290 [15] | 0.329 [15] | 0.316 | 0.349 | 643 | 595 | 725 |
| Benzonitrile | 1.528 [26] | 1.005 [26] | 1603 [51] | 0.308 [26] | 0.317 | 0.354 | 291 | 308 | 385 |
| Bicyclohexyl | 1.480 [26] | 0.886 [26] | 1422 [46] | 0.336 [26] | 0.316 | 0.347 | 466 | 411 | 495 |
| Bromobenzene | 1.552 [15] | 1.488 [15] | 1160 [15] | 0.320 [15] | 0.316 | 0.357 | 616 | 602 | 766 |
| Bromoform | 1.591 [15] | 2.877 [15] | 921 [15] | 0.325 [15] | 0.315 | 0.361 | 764 | 717 | 940 |
| Butyl Acetate | 1.394 [26] | 0.881 [26] | 1226 [46] | 0.299 [26] | 0.308 | 0.328 | 403 | 428 | 487 |
| Butyric Acid | 1.398 [26] | 0.958 [26] | 1203 [46] | 0.271 [26] | 0.308 | 0.329 | 328 | 426 | 485 |
| Carbon Disulphide | 1.617 [15] | 1.256 [15] | 1144 [15] | 0.338 [15] | 0.314 | 0.363 | 1087 | 937 | 1255 |
| Carbon Tetrachloride | 1.460 [26] | 1.594 [26] | 936 [46] | 0.280 [26] | 0.315 | 0.343 | 583 | 734 | 873 |
| Carbon Tetrachloride | 1.456 [15] | 1.584 [15] | 922 [15] | 0.331 [15] | 0.314 | 0.342 | 841 | 758 | 899 |
| Chlorobenzene | 1.525 [26] | 1.106 [26] | 1289 [46] | 0.311 [26] | 0.317 | 0.353 | 513 | 532 | 662 |
| Chlorobenzene | 1.519 [15] | 1.101 [15] | 1270 [15] | 0.330 [15] | 0.317 | 0.353 | 593 | 546 | 677 |
| Chloroform | 1.446 [26] | 1.489 [26] | 1001 [46] | 0.314 [26] | 0.314 | 0.340 | 601 | 601 | 708 |
| Chloroform | 1.444 [15] | 1.480 [15] | 978 [15] | 0.305 [15] | 0.313 | 0.340 | 609 | 643 | 756 |
| Cinnamaldehyde | 1.611 [15] | 1.049 [15] | 1555 [15] | 0.341 [15] | 0.314 | 0.363 | 514 | 438 | 583 |
| Crotonaldehyde | 1.437 [26] | 0.852 [26] | 1344 [49] | 0.305 [26] | 0.313 | 0.338 | 397 | 417 | 489 |
| Cyclohexane | 1.426 [26] | 0.779 [26] | 1279 [46] | 0.307 [26] | 0.312 | 0.336 | 486 | 503 | 584 |
| Cyclohexylamine | 1.459 [26] | 0.867 [26] | 1430 [52] | 0.321 [26] | 0.315 | 0.343 | 393 | 377 | 448 |
| Decane | 1.412 [26] | 0.730 [26] | 1253 [46] | 0.309 [26] | 0.310 | 0.333 | 525 | 530 | 610 |
| Dichloromethane | 1.424 [26] | 1.326 [26] | 1092 [46] | 0.280 [26] | 0.312 | 0.336 | 380 | 469 | 545 |
| Dimethoxymethane | 1.353 [26] | 0.860 [26] | 1146 [46] | 0.269 [26] | 0.301 | 0.317 | 344 | 429 | 476 |
| Dodecane | 1.422 [26] | 0.749 [26] | 1298 [46] | 0.308 [26] | 0.311 | 0.335 | 478 | 489 | 566 |
| Ethanol | 1.361 [26] | 0.789 [26] | 1160 [46] | 0.287 [26] | 0.302 | 0.319 | 427 | 472 | 527 |
| Ethyl Acetate | 1.372 [26] | 0.901 [26] | 1162 [46] | 0.284 [26] | 0.304 | 0.322 | 382 | 438 | 491 |
| Ethyl Acetate | 1.370 [15] | 0.895 [15] | 1148 [15] | 0.281 [15] | 0.304 | 0.322 | 385 | 451 | 505 |
| Ethyl Alcohol | 1.359 [15] | 0.785 [15] | 1146 [15] | 0.280 [15] | 0.302 | 0.318 | 417 | 485 | 540 |
| Ethyl Butyrate | 1.393 [26] | 0.879 [26] | 1197 [46] | 0.292 [26] | 0.308 | 0.328 | 413 | 458 | 521 |
| Ethyl Formate | 1.360 [26] | 0.923 [26] | 1136 [53] | 0.248 [26] | 0.302 | 0.319 | 288 | 426 | 475 |
| Ethyl Malonate | 1.414 [26] | 1.055 [26] | 1267 [54] | 0.277 [26] | 0.310 | 0.333 | 286 | 358 | 413 |
| Ethyl Oxalate | 1.410 [26] | 1.079 [26] | 1276 [55] | 0.278 [26] | 0.310 | 0.332 | 272 | 337 | 388 |
| Ethyl Propionate | 1.384 [26] | 0.890 [26] | 1183 [46] | 0.277 [26] | 0.306 | 0.326 | 365 | 447 | 505 |
| Ethylenediamine | 1.457 [26] | 0.897 [26] | 1672 [56] | 0.414 [26] | 0.314 | 0.342 | 391 | 226 | 268 |
| Formic Acid | 1.371 [26] | 1.220 [26] | 1287 [46] | 0.290 [26] | 0.304 | 0.322 | 215 | 237 | 265 |
| iso-Butil Alcohol | 1.390 [15] | 0.798 [15] | 1194 [15] | 0.287 [15] | 0.307 | 0.327 | 436 | 501 | 569 |
| Methyl Benzoate | 1.517 [26] | 1.089 [26] | 1380 [57] | 0.300 [26] | 0.317 | 0.352 | 384 | 426 | 528 |
| Methyl Oleate | 1.452 [26] | 0.874 [26] | 1425 [58] | 0.299 [26] | 0.314 | 0.341 | 331 | 366 | 432 |
| Methylene Iodide | 1.732 [15] | 3.308 [15] | 962 [15] | 0.332 [15] | 0.305 | 0.370 | 1012 | 851 | 1257 |
| n-Butil Alcohol | 1.395 [15] | 0.806 [15] | 1245 [15] | 0.309 [15] | 0.308 | 0.329 | 451 | 449 | 512 |
| n-Heptane | 1.388 [26] | 0.684 [26] | 1152 [46] | 0.310 [26] | 0.307 | 0.327 | 656 | 643 | 729 |
| n-Hexane | 1.375 [26] | 0.659 [26] | 1098 [46] | 0.308 [26] | 0.305 | 0.323 | 735 | 719 | 808 |
| n-Hexane | 1.372 [15] | 0.655 [15] | 1090 [15] | 0.322 [15] | 0.304 | 0.322 | 816 | 728 | 817 |
| Nitrobenzene | 1.552 [26] | 1.203 [26] | 1475 [46] | 0.298 [26] | 0.316 | 0.357 | 323 | 363 | 461 |
| Nitrobenzene | 1.546 [15] | 1.198 [15] | 1448 [15] | 0.331 [15] | 0.316 | 0.356 | 412 | 376 | 476 |
| Nitroethane | 1.392 [26] | 1.051 [26] | 1272 [59] | 0.288 [26] | 0.308 | 0.328 | 280 | 318 | 362 |
| Nitromethane | 1.381 [26] | 1.138 [26] | 1338 [46] | 0.281 [26] | 0.306 | 0.325 | 202 | 238 | 269 |
| Nonane | 1.405 [26] | 0.718 [26] | 1297 [46] | 0.309 [26] | 0.309 | 0.331 | 470 | 471 | 540 |
| n-Pentane | 1.358 [26] | 0.626 [26] | 1030 [46] | 0.273 [26] | 0.301 | 0.318 | 681 | 830 | 924 |
| o-Dichlorobenzene | 1.552 [26] | 1.306 [26] | 1296 [46] | 0.309 [26] | 0.316 | 0.357 | 468 | 491 | 624 |
| Oleic Acid | 1.460 [26] | 0.891 [26] | 1400 [60] | 0.298 [26] | 0.315 | 0.343 | 352 | 392 | 466 |
| o-Toluidine | 1.565 [15] | 0.994 [15] | 1598 [15] | 0.326 [15] | 0.316 | 0.358 | 385 | 362 | 465 |
| Phenil hydrazine | 1.599 [15] | 1.094 [15] | 1716 [15] | 0.334 [15] | 0.315 | 0.362 | 337 | 300 | 396 |
| Phosphorus tribromide | 1.690 [15] | 2.861 [15] | 930 [15] | 0.331 [15] | 0.309 | 0.368 | 1107 | 964 | 1373 |
| Piperidine | 1.453 [26] | 0.860 [26] | 1400 [46] | 0.234 [26] | 0.314 | 0.342 | 218 | 393 | 464 |
| Propionaldehyde | 1.362 [26] | 0.797 [26] | 1379 [61] | 0.280 [26] | 0.302 | 0.319 | 240 | 279 | 311 |
| Propionic Acid | 1.387 [26] | 0.993 [26] | 1166 [62] | 0.266 [26] | 0.307 | 0.326 | 320 | 425 | 481 |
| Propionitrile | 1.366 [26] | 0.782 [26] | 1271 [46] | 0.267 [26] | 0.303 | 0.320 | 289 | 371 | 415 |
| Propyl Acetate | 1.384 [26] | 0.888 [26] | 1198 [46] | 0.276 [26] | 0.306 | 0.326 | 350 | 433 | 489 |
| Quinoline | 1.615 [15] | 1.092 [15] | 1567 [15] | 0.331 [15] | 0.314 | 0.363 | 464 | 416 | 557 |
| Styrene | 1.547 [26] | 0.906 [26] | 1354 [46] | 0.291 [26] | 0.316 | 0.356 | 516 | 610 | 773 |
| Tetrachloroethylene | 1.506 [26] | 1.623 [26] | 1053 [46] | 0.306 [26] | 0.316 | 0.351 | 577 | 616 | 756 |
| Trans-Decahydronaphthalene | 1.469 [26] | 0.870 [26] | 1398 [63] | 0.317 [26] | 0.315 | 0.345 | 426 | 421 | 503 |
| Trichloroethylene | 1.478 [26] | 1.468 [26] | 1049 [46] | 0.314 [26] | 0.316 | 0.346 | 606 | 611 | 736 |
| Trichloroethylene | 1.472 [15] | 1.460 [15] | 1021 [15] | 0.305 [15] | 0.315 | 0.345 | 608 | 651 | 780 |
| Trycresyl Phosphate | 1.549 [15] | 1.172 [15] | 1502 [15] | 0.321 [15] | 0.316 | 0.356 | 358 | 348 | 442 |
| Water | 1.330 [15] | 0.997 [15] | 1491 [15] | 0.273 [15] | 0.295 | 0.309 | 125 | 146 | 160 |
| Water | 1.333 [26] | 0.998 [26] | 1482 [46] | 0.360 [26] | 0.296 | 0.310 | 224 | 151 | 166 |
References
- Schrodel, Y.; Hartmann, C.; Zheng, J.; Lang, T.; Steudel, M.; Rutsch, M.; Salman, S.H.; Kellert, M.; Pergament, M.; Hahn-Jose, T.; et al. Acousto-optic modulation of gigawatt-scale laser pulses in ambient air. Nat. Photonics 2023, 18, 54–59. [Google Scholar] [CrossRef]
- Omidali, M.; Mardanshahi, A.; Särestoniemi, M.; Zhao, Z.; Myllyla, T. Acousto-Optics: Recent studies and medical applications. Biosensors 2023, 13, 186. [Google Scholar] [CrossRef]
- Pozhar, V.E.; Bulatov, M.F.; Machikhin, A.S.; Shakhnov, V.A. Technical implementation of acousto-optical instruments: Basic types. J. Phys. Conf. Ser. 2019, 1421, 012058. [Google Scholar] [CrossRef]
- Klein, W.; Cook, B. Unified Approach to Ultrasonic Light Diffraction. IEEE Trans. Sonics Ultrason. 1967, 14, 123–134. [Google Scholar] [CrossRef]
- Klein, W.; Fitts, W. Optical Diffraction by an Acoustically Oriented Liquid. IEEE Trans. Sonics Ultrason. 1974, 21, 204–208. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H. Accurate design of a TeO2 noncollinear acousto-optic tunable filter with refractive index correction. Opt. Lett. 2023, 48, 3395. [Google Scholar] [CrossRef]
- Sando, D.; Yang, Y.; Bousquet, E.; Carretero, C.; Garcia, V.; Fusil, S.; Dolfi, D.; Barthelemy, A.; Ghosez, P.; Bellaiche, L.; et al. Large elasto-optic effect and reversible electrochromism in multiferroic BiFeO3. Nat. Commun. 2016, 7, 10718. [Google Scholar] [CrossRef]
- Martienssen, W.; Warlimont, H. (Eds.) Springer Handbook of Condensed Matter and Materials Data; Springer: Berlin/Heidelberg, Germany, 2005; p. 1121. [Google Scholar] [CrossRef]
- Rinaldi, D.; Natali, P.P.; Montalto, L.; Davì, F. The refraction indices and Brewster law in stressed isotropic materials and cubic crystals. Crystals 2021, 11, 1104. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Chen, X.; Le, T.; Wei, H.; Li, Y. High-speed non-contact measurement of elasto-optic coefficient via laser-induced phonons. Appl. Phys. Lett. 2022, 121, 251102. [Google Scholar] [CrossRef]
- Uchida, N. Comment on “On the efficiency of liquids in acoustooptic devices”. Jpn. J. Appl. Phys. 1972, 11, 415–416. [Google Scholar] [CrossRef]
- Kragh, H. The Lorenz-Lorentz formula: Origin and early history. Substantia 2018, 2, 7–18. [Google Scholar] [CrossRef]
- Neumaier, L.; Schilling, J.; Bardow, A.; Gross, J. Dielectric constant of mixed solvents based on perturbation theory. Fluid Phase Equilibria 2022, 555, 113346. [Google Scholar] [CrossRef]
- Talebian, E.; Talebian, M. A general review on the derivation of Clausius–Mossotti relation. Optik 2013, 124, 2324–2326. [Google Scholar] [CrossRef]
- Uchida, N. Elastooptic coefficient of liquids determined by ultrasonic light diffraction method. Jpn. J. Appl. Phys. 1968, 7, 1259–1266. [Google Scholar] [CrossRef]
- Wahid, H. Molecular scattering of light in pure liquids. J. Opt. 1995, 26, 109–121. [Google Scholar] [CrossRef]
- Weiss, L.; Tazibt, A.; Tidu, A.; Aillerie, M. Water density and polarizability deduced from the refractive index determined by interferometric measurements up to 250 MPa. J. Chem. Phys. 2012, 136, 124201. [Google Scholar] [CrossRef]
- Onsager, L. Electric moments of molecules in liquids. J. Am. Chem. Soc. 1936, 58, 1486–1493. [Google Scholar] [CrossRef]
- Maribo-Mogensen, B.; Kontogeorgis, G.M.; Thomsen, K. Modeling of dielectric properties of complex fluids with an equation of state. J. Phys. Chem. B 2013, 117, 3389–3397. [Google Scholar] [CrossRef]
- Oster, G. The scattering of light and its applications to chemistry. Chem. Rev. 1948, 43, 319–365. [Google Scholar] [CrossRef]
- Abdel-Azim, A.A.A.; Munk, P. Light scattering of liquids and liquid mixtures. 1. Compressibility of pure liquids. J. Phys. Chem. 1987, 91, 3910–3914. [Google Scholar] [CrossRef]
- Mandel, M. A statistical approach to the Onsager and Kirkwood dielectric theory for liquids of rigid dipoles. Physica 1972, 57, 141–151. [Google Scholar] [CrossRef]
- Zhang, C.; Hutter, J.; Sprik, M. Computing the Kirkwood g-factor by combining constant Maxwell electric field and electric displacement simulations: Application to the dielectric constant of liquid water. J. Phys. Chem. Lett. 2016, 7, 2696–2701. [Google Scholar] [CrossRef]
- Niedrich, Z. Alternative optical equation for dielectric liquids. Phys. Rev. E 1999, 60, 4099–4104. [Google Scholar] [CrossRef]
- Proutiere, A. Agreement between molecular polarizability anisotropies (γ2) deduced from Rayleigh light scattering and static Kerr birefringence in liquids—Experimental and theoretical aspects. Mol. Phys. 1988, 65, 499–512. [Google Scholar] [CrossRef]
- Proutiere, A.; Megnassan, E.; Hucteau, H. Refractive index and density variations in pure liquids: A new theoretical relation. J. Phys. Chem. 1992, 96, 3485–3489. [Google Scholar] [CrossRef]
- Hucteau, H.; Proutiere, A. Refractive index and density variations in liquids. Progress in theoretical formulation. J. Mol. Liq. 1994, 62, 93–112. [Google Scholar] [CrossRef]
- Bot, A. Comment on “Refractive index variations in pure liquids: A new theoretical relation”. J. Phys. Chem. 1993, 97, 2804. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, L. Estimating scattering of pure water from density fluctuation of the refractive index. Opt. Express 2009, 17, 1671. [Google Scholar] [CrossRef]
- Evain, K.; Illien, B.; Chabanel, M.; Beignon, M. Comparison of Proutière and Carr-Zimm theories for static light-scattering molecular weight determination: Application to surfactants in solution. J. Phys. Chem. B 2007, 111, 1597–1603. [Google Scholar] [CrossRef]
- Dewaele, A.; Eggert, J.H.; Loubeyre, P.; Le Toullec, R. Measurement of refractive index and equation of state in dense He, H2, H2O, and Ne under high pressure in a diamond anvil cell. Phys. Rev. B 2003, 67, 094112. [Google Scholar] [CrossRef]
- Meeten, G.H. Empirical relation for the isothermal density derivative of the optical dielectric constant. Nature 1968, 218, 761. [Google Scholar] [CrossRef]
- Vedam, K.; Limsuwan, P. Piezo- and elasto-optic properties of liquids under high pressure. II. Refractive index vs density. J. Chem. Phys. 1978, 69, 4772–4778. [Google Scholar] [CrossRef]
- Ramesh, K. Photoelasticity. In Springer Handbooks; Springer: New York, NY, USA, 2008; pp. 701–742. [Google Scholar] [CrossRef]
- Sapozhnikov, O.A.; Maxwell, A.D.; Bailey, M.R. Modeling of photoelastic imaging of mechanical stresses in transparent solids mimicking kidney stones. J. Acoust. Soc. Am. 2020, 147, 3819–3829. [Google Scholar] [CrossRef]
- Technical Devices Inc. Liquid Sound Speed Chart. 2024. Available online: https://tdi-pm.com/images/tdi/PDF/Resources-Brochures/Liquid-Sound-Speed-Charts.xls (accessed on 29 January 2024).
- Aziz, R.A.; Bowman, D.H.; Lim, C.C. An Examination of the Relationship between Sound Velocity and Density in Liquids. Can. J. Phys. 1972, 50, 646–654. [Google Scholar] [CrossRef]
- Kerl, K.; Varchmin, H. Refractive index dispersion (RID) of some liquids in the UV/VIS between 20 °C and 60 °C. J. Mol. Struct. 1995, 349, 257–260. [Google Scholar] [CrossRef]
- Moutzouris, K.; Papamichael, M.; Betsis, S.C.; Stavrakas, I.; Hloupis, G.; Triantis, D. Refractive, dispersive and thermo-optic properties of twelve organic solvents in the visible and near-infrared. Appl. Phys. B 2013, 116, 617–622. [Google Scholar] [CrossRef]
- Grace, E.; Butcher, A.; Monroe, J.; Nikkel, J.A. Index of refraction, Rayleigh scattering length, and Sellmeier coefficients in solid and liquid argon and xenon. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2017, 867, 204–208. [Google Scholar] [CrossRef]
- Donadio, D.; Bernasconi, M.; Tassone, F. Photoelasticity of sodium silicate glass from first principles. Phys. Rev. B 2004, 70, 214205. [Google Scholar] [CrossRef][Green Version]
- Liang, X.; Ismail-Beigi, S. Degree of locality of elasto-optic response in solids. Phys. Rev. B 2019, 100, 245204. [Google Scholar] [CrossRef]
- Imai, T.; Kawamura, S.; Oka, S. Space charge assisted evaluation method of the elasto-optic coefficients of electrooptic crystals. Opt. Mater. Express 2020, 10, 2181. [Google Scholar] [CrossRef]
- Laskar, J.M.; Shravan Kumar, P.; Herminghaus, S.; Daniels, K.E.; Schröter, M. High refractive index immersion liquid for superresolution 3D imaging using sapphire-based aplanatic numerical aperture increasing lens optics. Appl. Opt. 2016, 55, 3165. [Google Scholar] [CrossRef]
- Renuka Kumari, S.; Venkateswarlu, P.; Prabhakar, G. Excess volumes and speeds of sound of mixtures of 1,2-dibromoethane with chlorinated ethanes and ethenes at 303.15 K. J. Chem. Thermodyn. 2005, 37, 737–742. [Google Scholar] [CrossRef]
- Springer Nature. SpringerMaterials. Available online: https://materials.springer.com/ (accessed on 7 February 2024).
- Mehta, S.; Chauhan, R. Volume and compressibility of mixtures of γ-butyrolactam (n = 5) with nitro-compounds. Fluid Phase Equilibria 2001, 187–188, 209–220. [Google Scholar] [CrossRef]
- Langa, E.; Mainar, A.M.; Pardo, J.I.; Urieta, J.S. Excess enthalpy, density, and speed of sound for the mixtures β-pinene + 2-methyl-1-propanol or 2-methyl-2-propanol at several semperatures. J. Chem. Eng. Data 2007, 52, 2182–2187. [Google Scholar] [CrossRef]
- SensoTech GmbH. Density Measurement in Liquids. Available online: https://www.sensotech.com/en/density-measurement-in-liquids (accessed on 7 February 2024).
- Oswal, S.L.; Patel, N.B. Speeds of sound, isentropic compressibilities, and excess volumes of binary mixtures of acrylonitrile with organic solvents. J. Chem. Eng. Data 2000, 45, 225–230. [Google Scholar] [CrossRef]
- Shukla, R.; Kumar, A.; Awasthi, N.; Srivastava, U.; Srivastava, K. Speed of sound and isentropic compressibility of benzonitrile, chlorobenzene, benzyl chloride and benzyl alcohol with benzene from various models at temperature range 298.15–313.15 K. Arab. J. Chem. 2017, 10, 895–905. [Google Scholar] [CrossRef]
- Burakowski, A.; Gliński, J. Dimerization constants from acoustic measurements: Solutions of benzene, cyclohexylamine and aniline in cyclohexane. J. Solut. Chem. 2017, 46, 1501–1513. [Google Scholar] [CrossRef][Green Version]
- Tabuchi, D. Dispersion and absorption of sound in ethyl formate and study of the rotational isomers. J. Chem. Phys. 1958, 28, 1014–1021. [Google Scholar] [CrossRef]
- Baluja, S.; Pandaya, N.; Kachhadia, N.; Solanki, A.; Inamdar, P. Thermodynamic and acoustical studies of binary mixtures of diethyl malonate at 308.15 K. Phys. Chem. Liq. 2005, 43, 309–316. [Google Scholar] [CrossRef]
- Nayak, J.N.; Aralaguppi, M.I.; Aminabhavi, T.M. Density, viscosity, refractive index, and speed of sound in the binary mixtures of 1,4-dioxane + ethyl acetoacetate, + diethyl oxalate, + diethyl phthalate, or + dioctyl phthalate at 298.15, 303.15, and 308.15 K. J. Chem. Eng. Data 2003, 48, 1489–1494. [Google Scholar] [CrossRef]
- Dubey, G.P.; Kumar, K. Densities, viscosities, and speeds of sound of binary liquid mixtures of ethylenediamine with alcohols at T = (293.15 to 313.15) K. J. Chem. Eng. Data 2011, 56, 2995–3003. [Google Scholar] [CrossRef]
- Rathnam, M.V.; Mankumare, S.; Kumar, M.S.S. Density, viscosity, and speed of sound of (methyl benzoate + cyclohexane), (methyl benzoate + n-hexane), (methyl benzoate + heptane), and (methyl benzoate + octane) at temperatures of (303.15, 308.15, and 313.15) K. J. Chem. Eng. Data 2009, 55, 1354–1358. [Google Scholar] [CrossRef]
- Ott, L.S.; Huber, M.L.; Bruno, T.J. Density and speed of sound measurements on five fatty acid methyl esters at 83 kPa and temperatures from (278.15 to 338.15) K. J. Chem. Eng. Data 2008, 53, 2412–2416. [Google Scholar] [CrossRef]
- Roy, R.; Mondal, S.; Ghosh, S.; Jengathe, S. Acoustic data on molecular interactions in mixtures of nitromethane and nitroethane in acetone at 303–318 K. Russ. J. Phys. Chem. A 2018, 92, 2606–2611. [Google Scholar] [CrossRef]
- Rostocki, A.J.; Siegoczyński, R.M.; Kiełczyński, P.; Szalewski, M.; Balcerzak, A.; Zduniak, M. Employment of a novel ultrasonic method to investigate high pressure phase transitions in oleic acid. High Press. Res. 2011, 31, 334–338. [Google Scholar] [CrossRef]
- Groot, M.S.d. Ultrasonic Relaxation Due to Rotational Isomerism. Ph.D. Thesis, Universiteit Leiden, Leiden, The Netherlands, 1958. Available online: https://www.lorentz.leidenuniv.nl/history/proefschriften/sources/DeGroot_1958.pdf (accessed on 5 June 2024).
- Bahadur, I.; Deenadayalu, N.; Naidoo, P.; Ramjugernath, D. Density, speed of sound, and refractive index measurements for the binary systems (butanoic acid+propanoic acid, or 2-methyl-propanoic acid) at T = (293.15 to 313.15) K. J. Chem. Thermodyn. 2013, 57, 203–211. [Google Scholar] [CrossRef]
- Luning Prak, D.J.; Lee, B.G. Density, viscosity, speed of sound, bulk modulus, surface tension, and flash point of binary mixtures of 1,2,3,4-tetrahydronaphthalene and trans-decahydronaphthalene. J. Chem. Eng. Data 2016, 61, 2371–2379. [Google Scholar] [CrossRef]
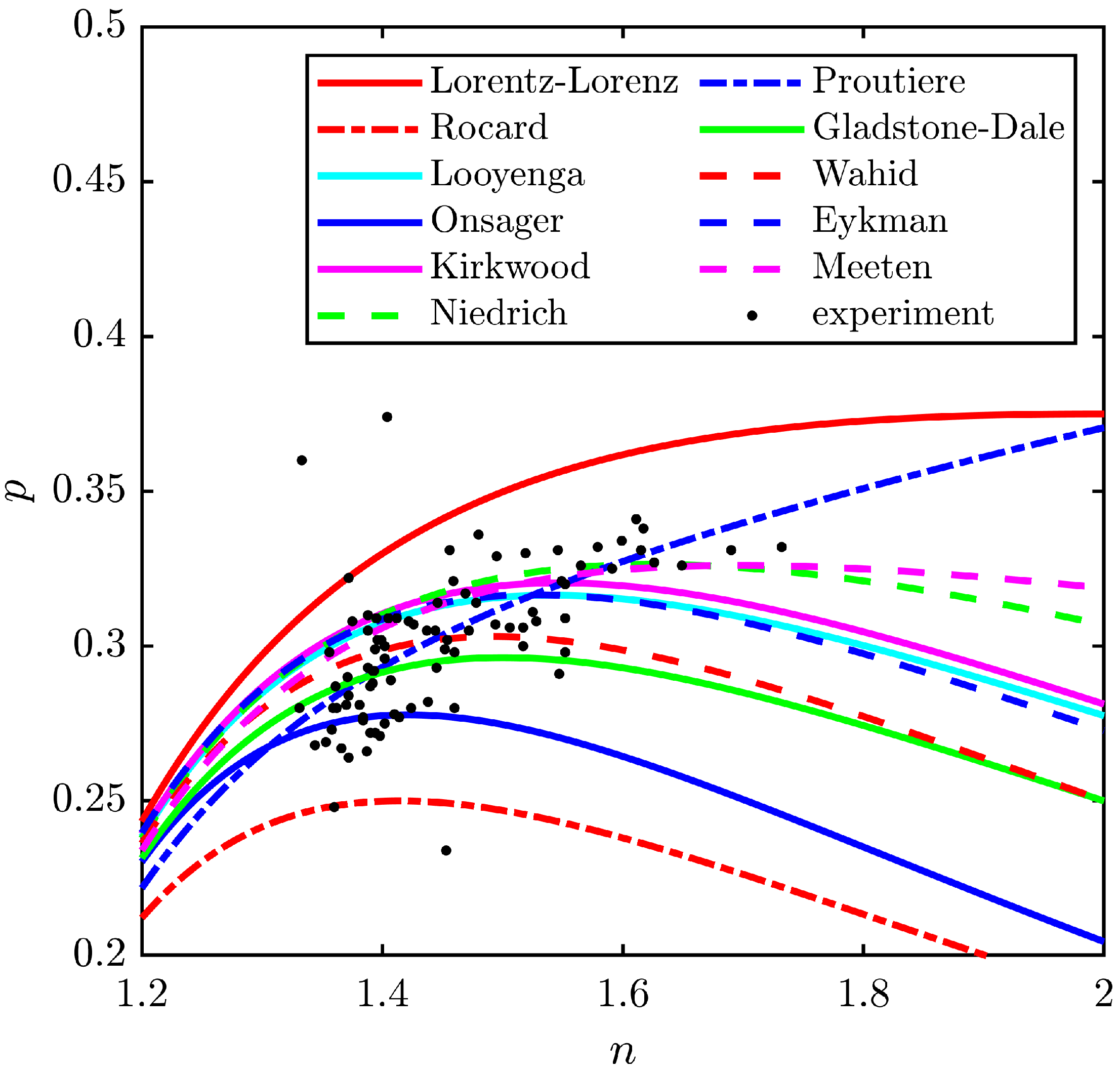
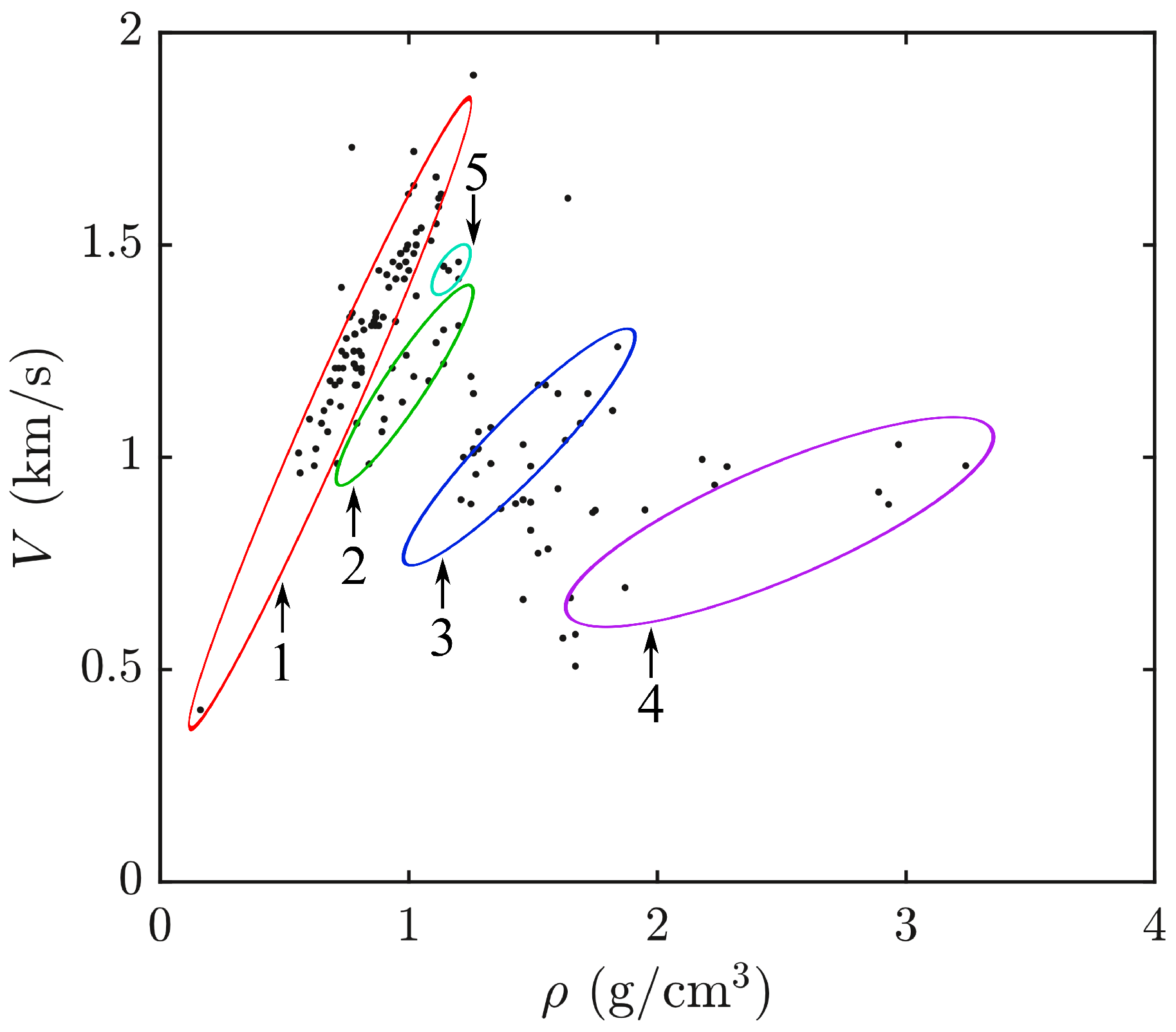

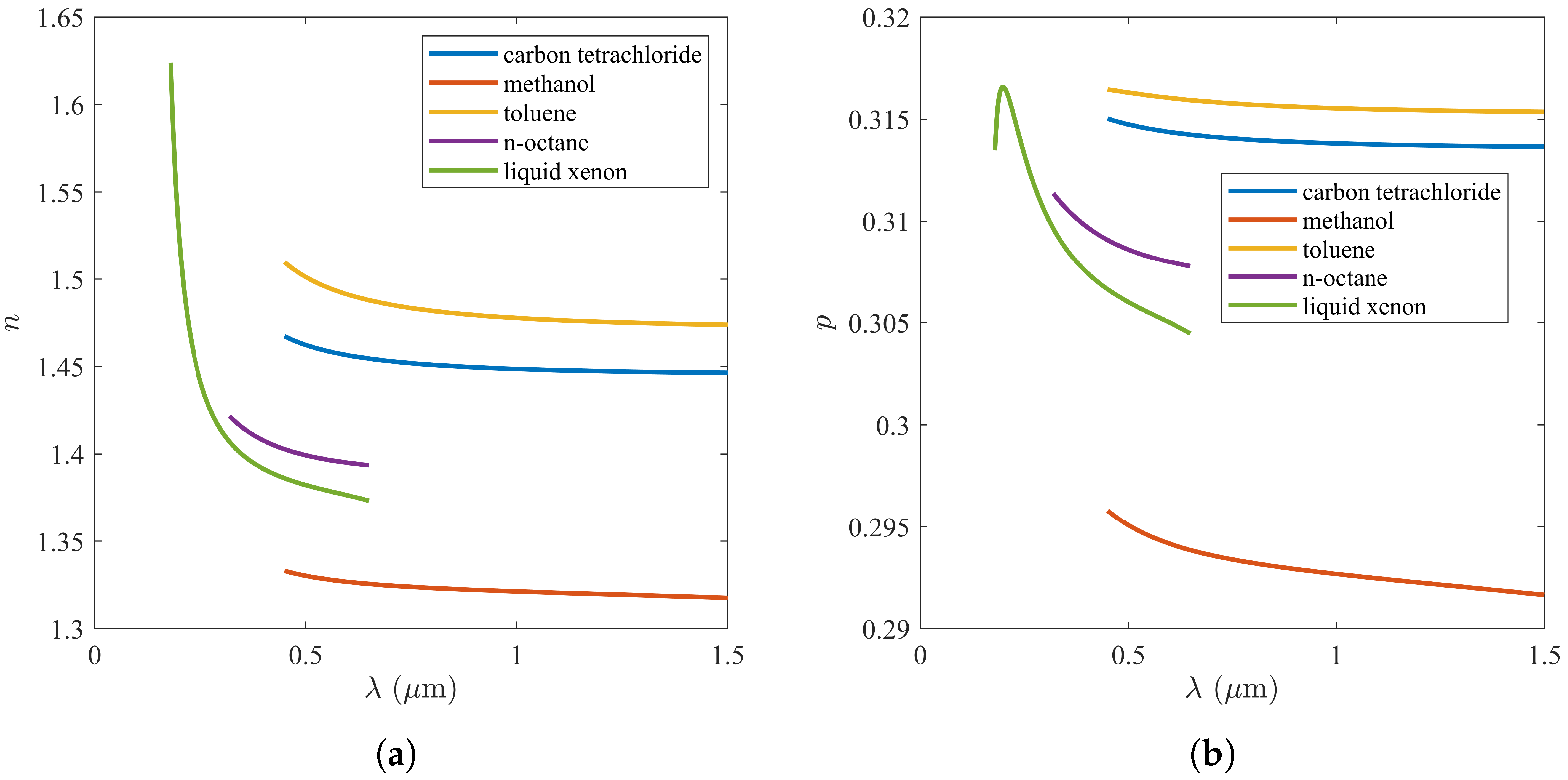
| Lorentz | Rocard | Looyenga | Onsager | Kirkwood | Niedrich | Gladstone | Eykman | Wahid | Meeten | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2.000 | 1.414 | 1.540 | 1.421 | 1.547 | 1.625 | 1.500 | 1.581 | 1.492 | 1.696 | |
| max(p) | 0.375 | 0.250 | 0.316 | 0.278 | 0.320 | 0.326 | 0.296 | 0.316 | 0.303 | 0.326 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikitin, P.A.; Pozhar, V.E. Evaluation of the Acousto-Optic Figure of Merit and the Maximum Value of the Elasto-Optic Constant of Liquids. Materials 2024, 17, 2810. https://doi.org/10.3390/ma17122810
Nikitin PA, Pozhar VE. Evaluation of the Acousto-Optic Figure of Merit and the Maximum Value of the Elasto-Optic Constant of Liquids. Materials. 2024; 17(12):2810. https://doi.org/10.3390/ma17122810
Chicago/Turabian StyleNikitin, Pavel A., and Vitold E. Pozhar. 2024. "Evaluation of the Acousto-Optic Figure of Merit and the Maximum Value of the Elasto-Optic Constant of Liquids" Materials 17, no. 12: 2810. https://doi.org/10.3390/ma17122810
APA StyleNikitin, P. A., & Pozhar, V. E. (2024). Evaluation of the Acousto-Optic Figure of Merit and the Maximum Value of the Elasto-Optic Constant of Liquids. Materials, 17(12), 2810. https://doi.org/10.3390/ma17122810






