The Use of Microfiltration for the Pretreatment of Backwash Water from Sand Filters
Abstract
:1. Introduction
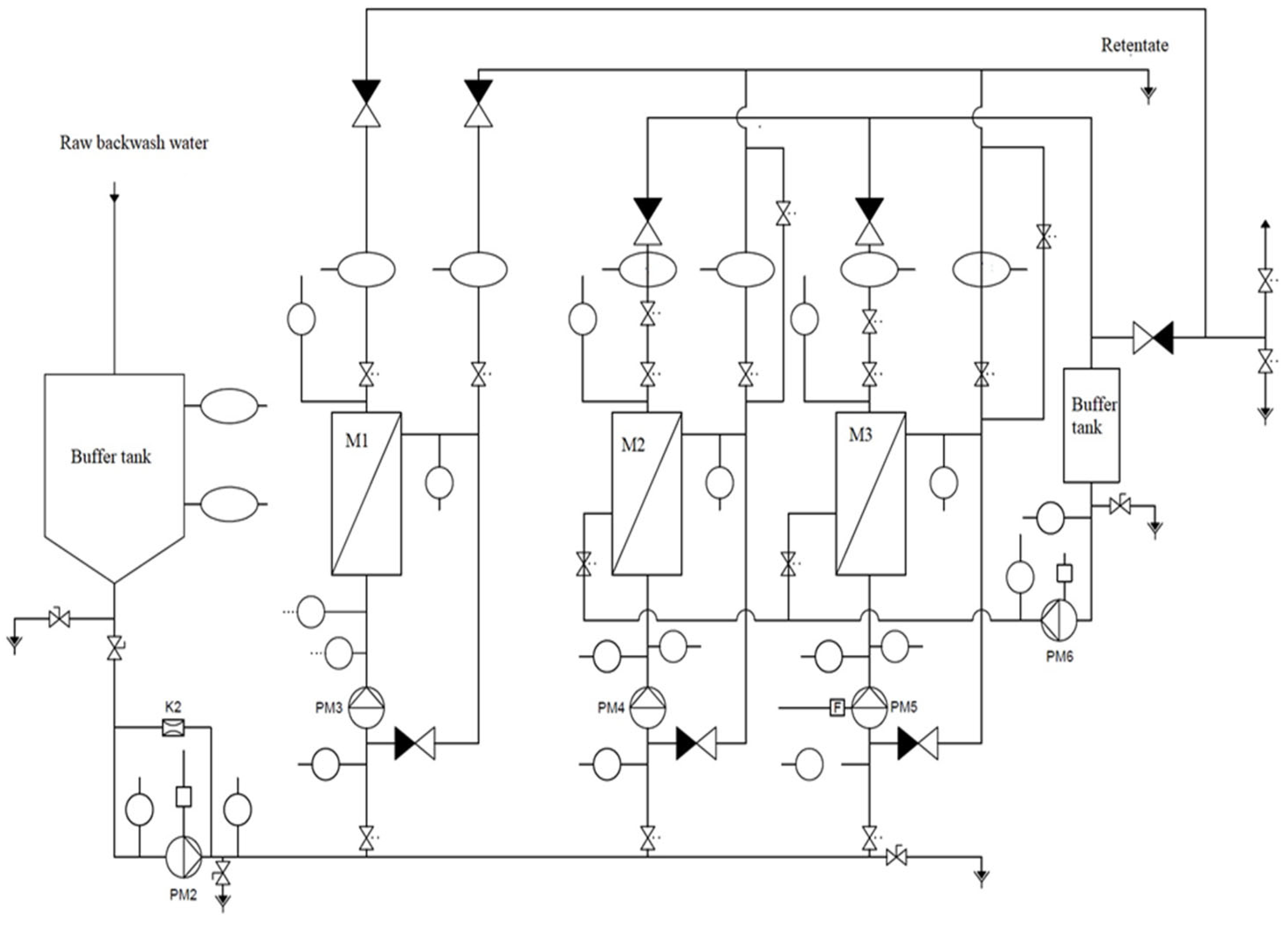
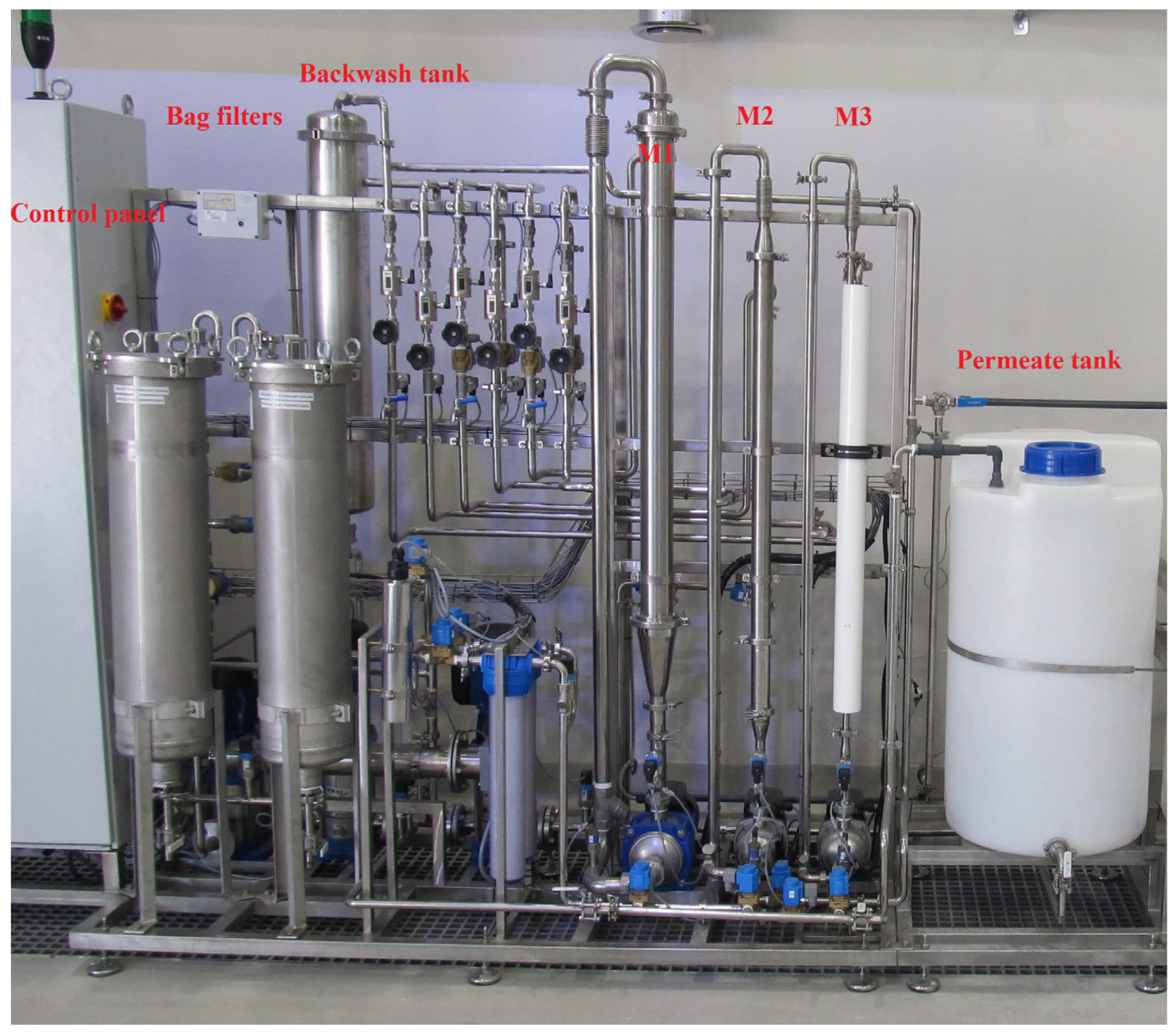
2. Materials and Methods
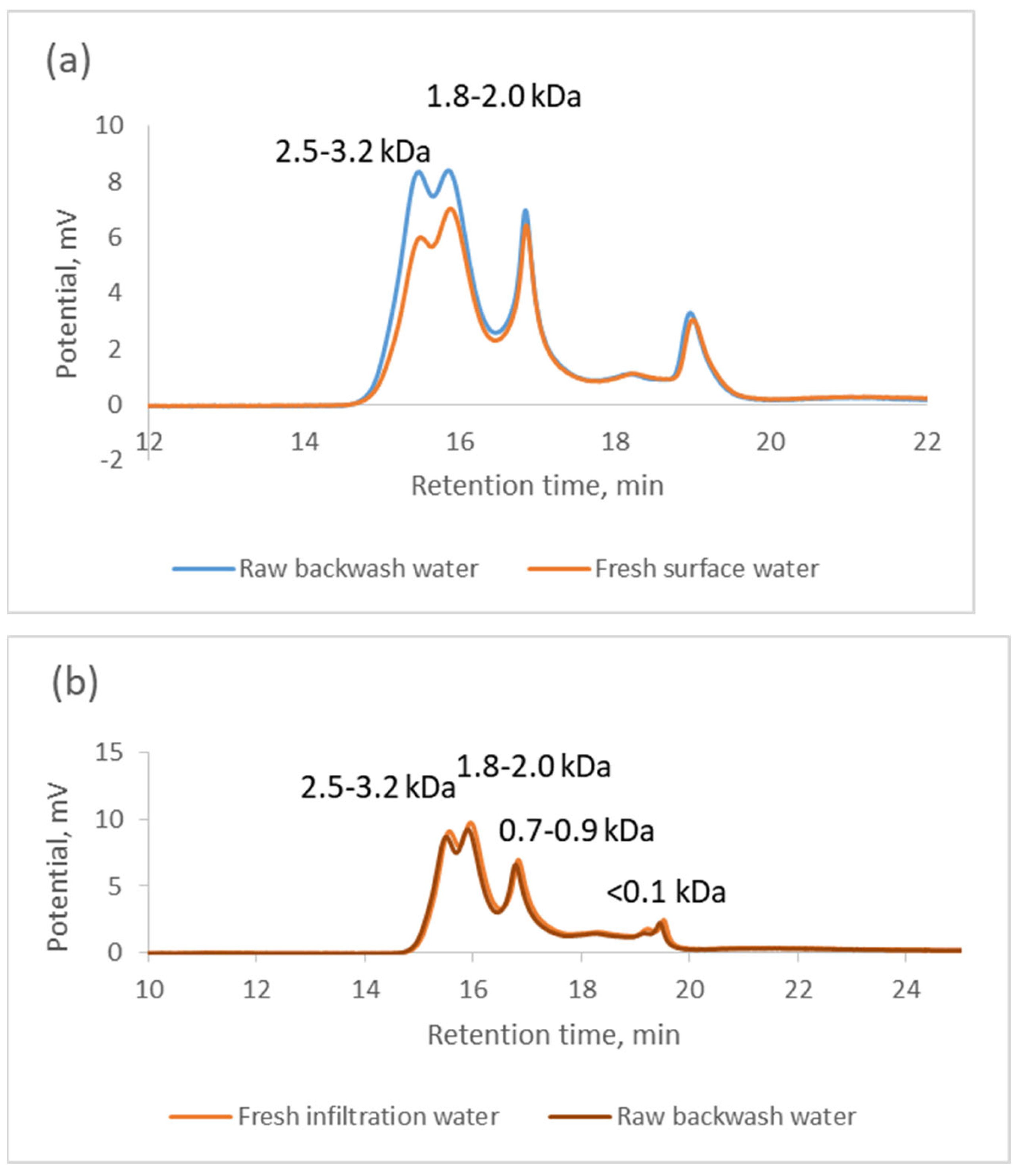
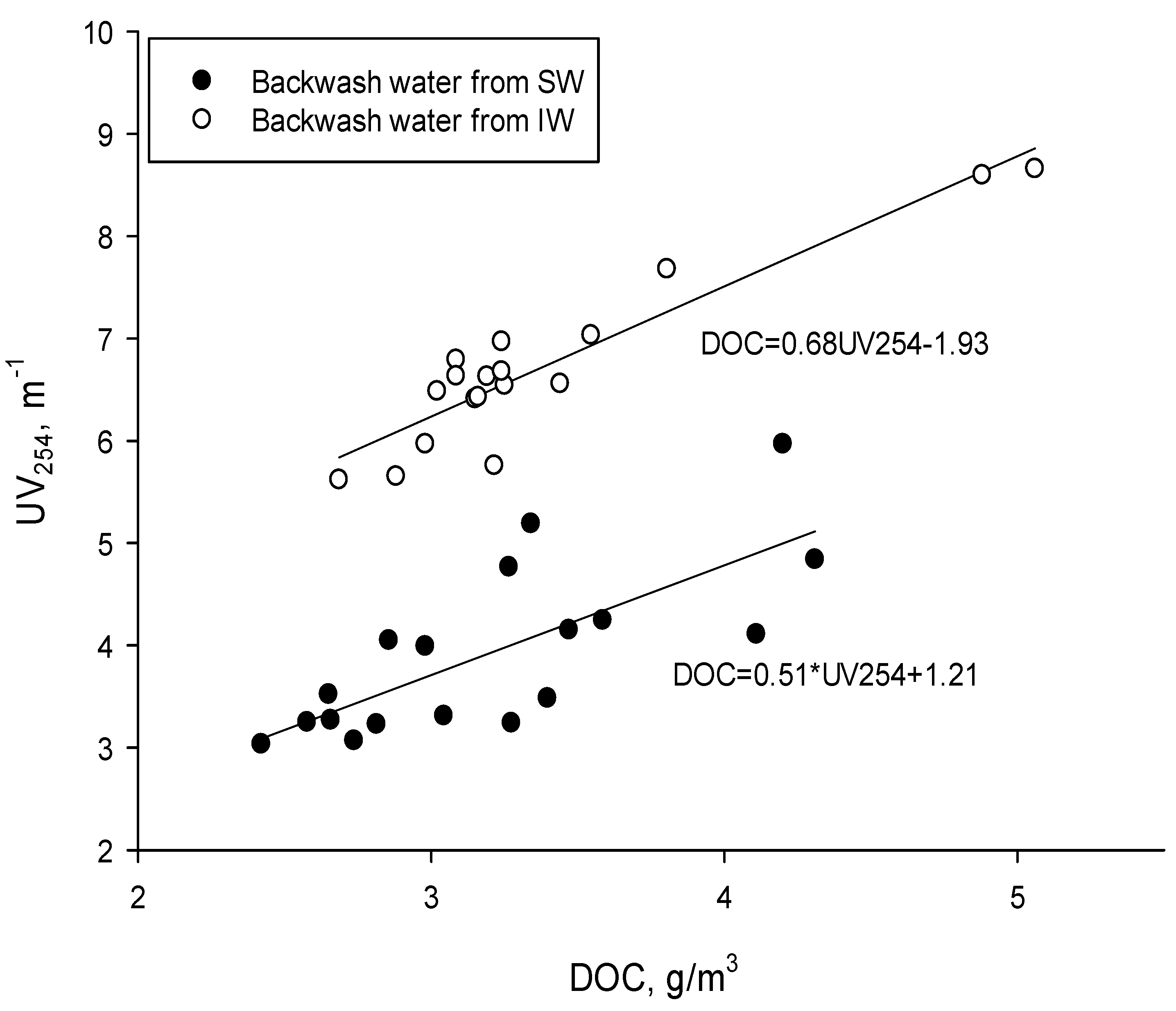
3. Results and Discussion
4. Conclusions
- The composition of the backwash water subjected to microfiltration has the greatest effect on the pretreatment efficiency;
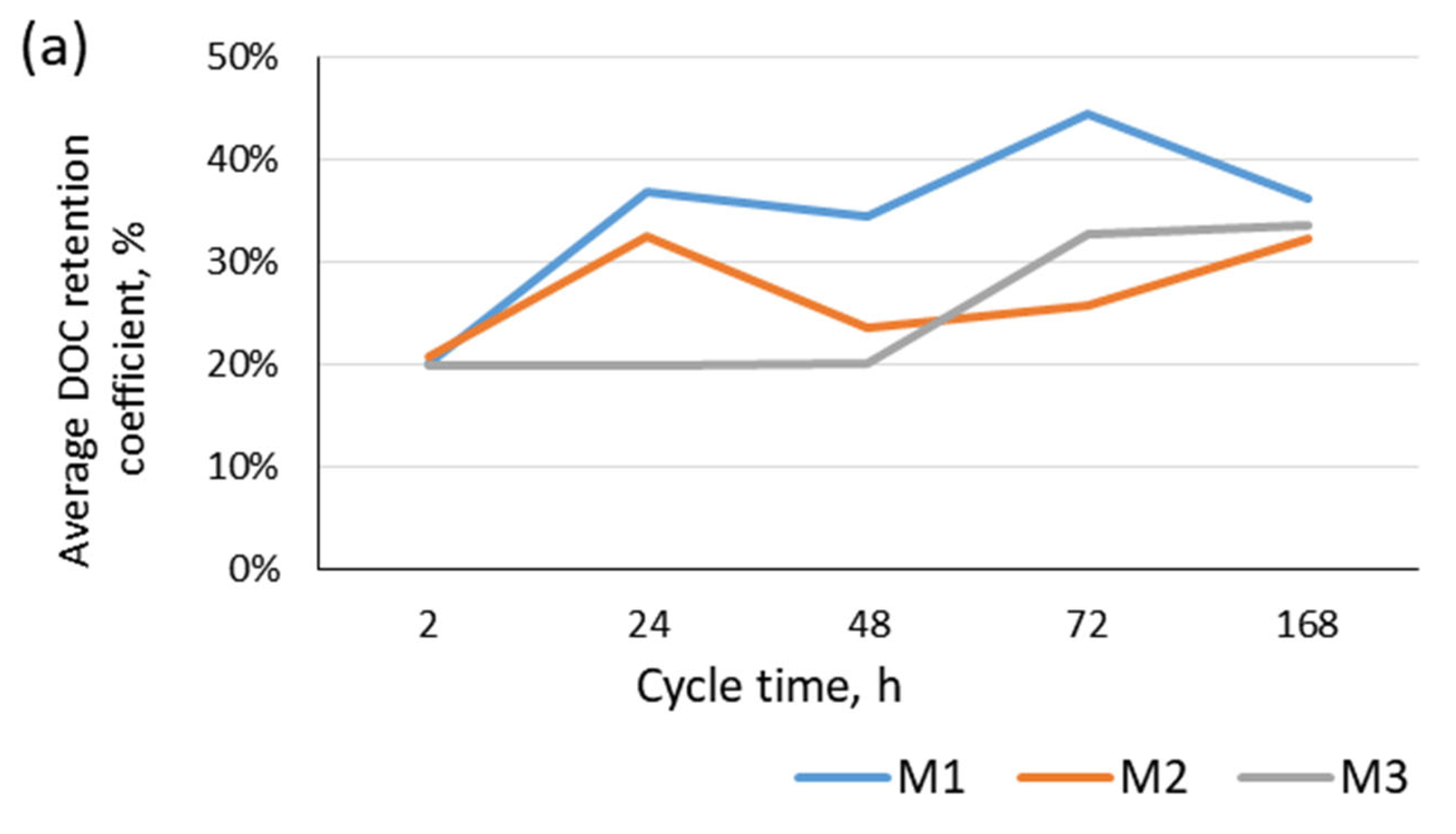
- All the types of microfiltration membranes used allowed for the effective and sufficient returning of treated backwash water to the main water treatment line and the removal of organic substances in ranges from 19.9% to 44.5% and from 7.2% to 53.9% from backwash water from the treatments of surface water and infiltration water;
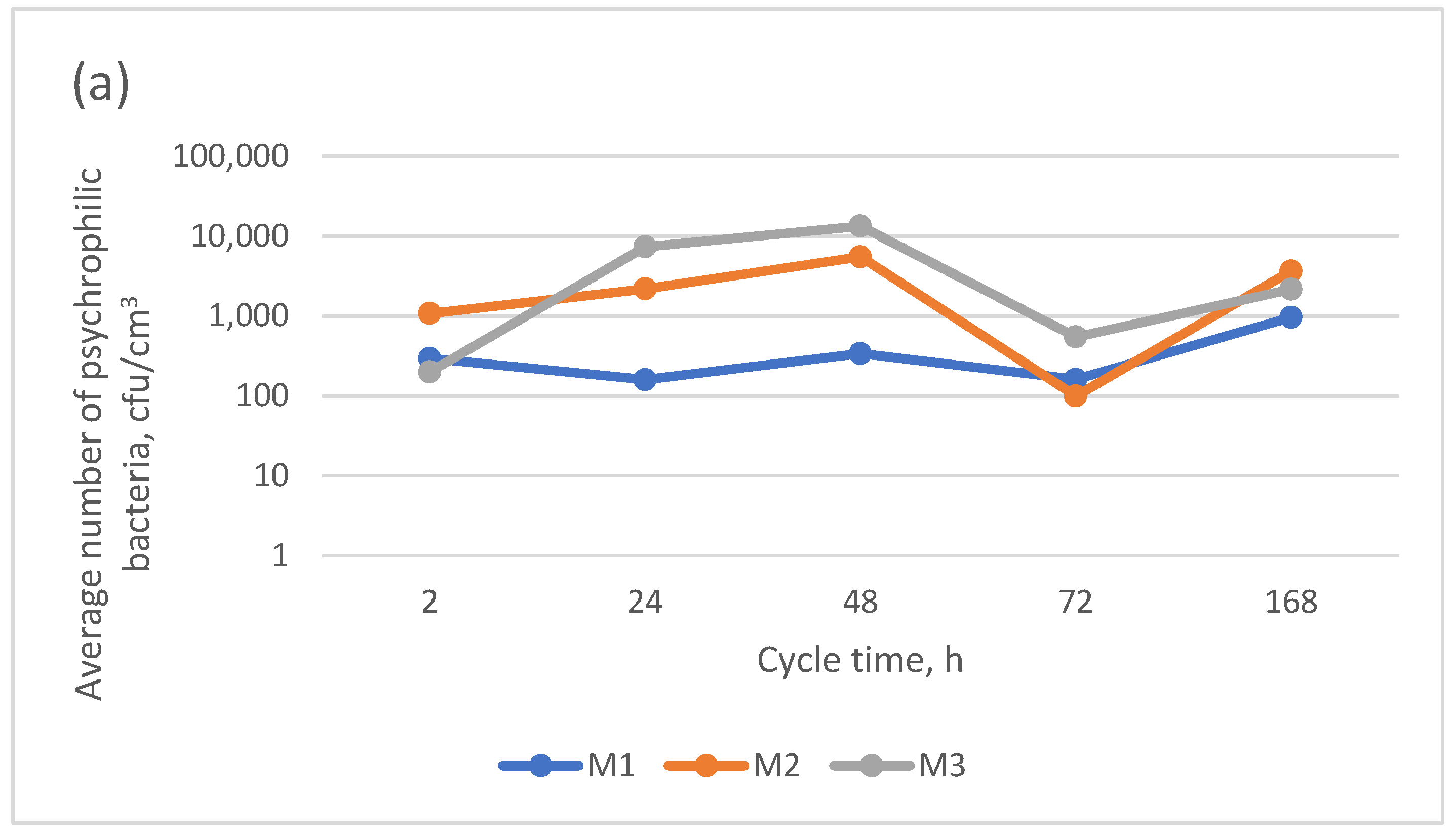
- Regardless of the type, none of the microfiltration membranes used ensured the biological stability of the pretreated backwash water;
- The average numbers of psychrophilic bacteria in the permeate were 384, 3625, and 2563 from M1, M2, and M3, respectively; the PVDF microfiltration membrane had the highest psychrotrophic bacterial retention rate;
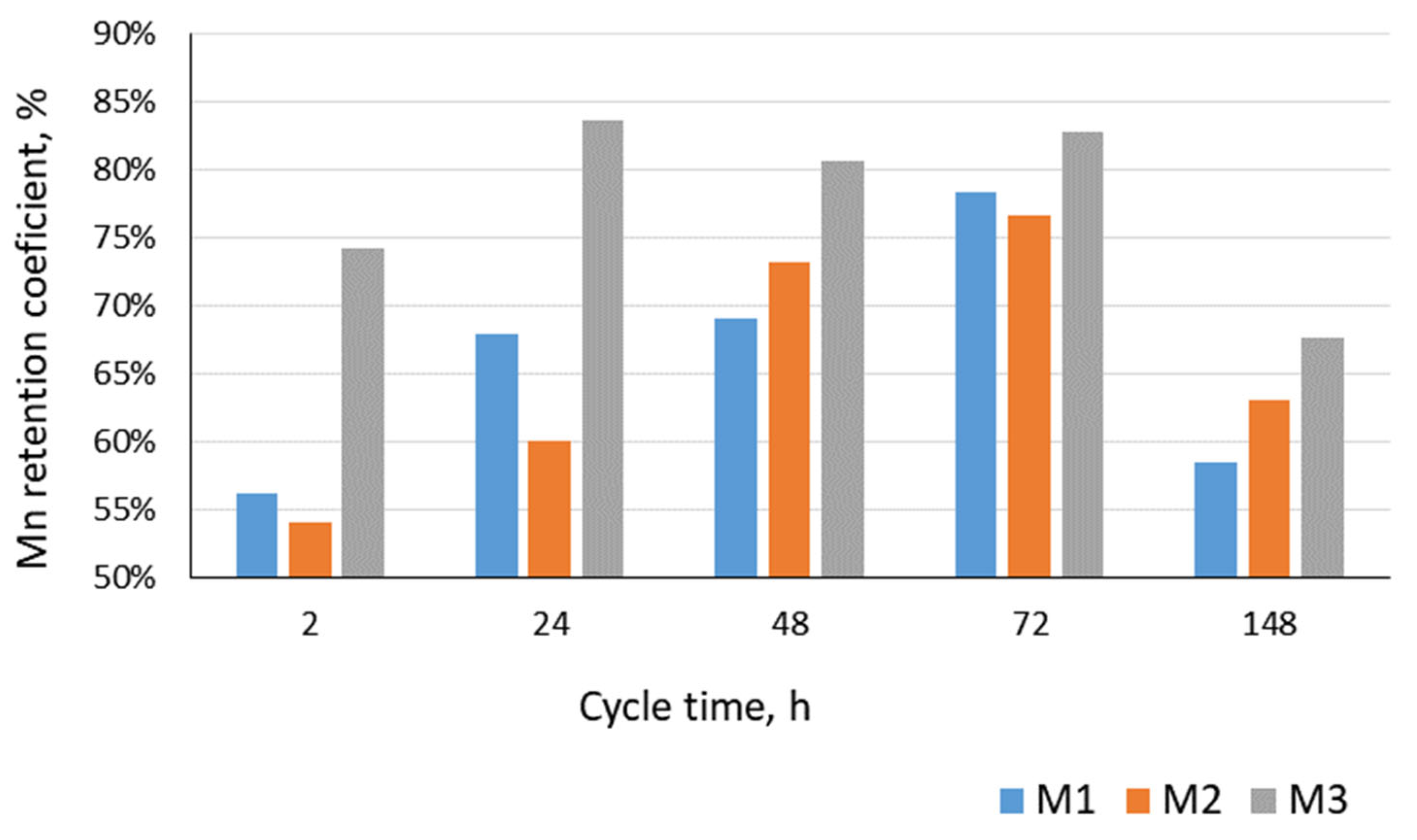
- None of the microfiltration membranes used ensured the removal of manganese compounds to the levels found in the infiltration water. Their lowest concentrations were found in permeate from M3 (average: 125 µg/dm3);
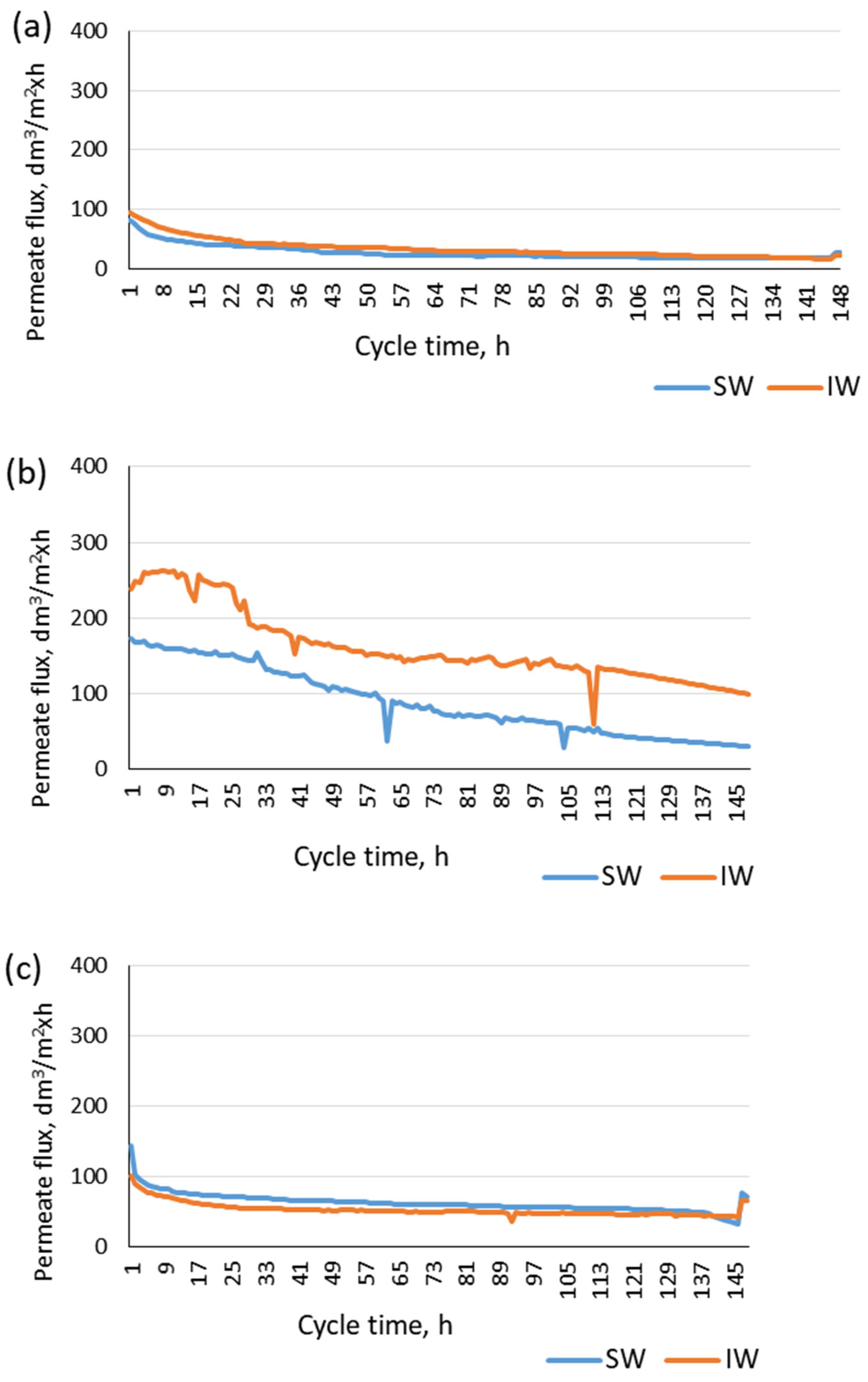
- The PVDF membrane showed the highest permeate flux stability, whereas the PP membrane was characterized by the lowest stability;
- Microfiltration cannot be used as the sole process for the pretreatment of back-wash water before it is returned to the water treatment system, regardless of its origin.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanham, D.; Alfieri, L.; Flörke, M.; Grimaldi, S.; Lorini, V.; de Roo, A.; Feyen, L. The number of people exposed to water stress in relation to how much water is reserved for the environment: A global modelling study. Lancet Planet. Health 2021, 5, e766–e774. [Google Scholar] [CrossRef]
- Transforming Our World: The 2030 Agenda for Sustainable Development. UN Resolution Adopted by the General Assembly on 25 September 2015. A/RES/70/1. Available online: https://undocs.org/en/A/RES/70/1 (accessed on 19 January 2024).
- Zielina, M.; Dąbrowski, W. Energy and water savings during backwashing of rapid filter plants. Energies 2021, 14, 3782. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Salem, M.A.; El-Khateeb, M.A.; Mansour, M.S.; Abdel-Shafy, N.H. Innovative System for Recycling of Backwashing Water in Drinking Water Plant. Egypt. J. Chem. 2020, 63, 885–895. [Google Scholar] [CrossRef]
- Mazari, L.; Abdessemed, D. Feasibility of Reuse Filter Backwash Water as Primary/Aid Coagulant in Coagulation–Sedimentation Process for Tertiary Wastewater Treatment. Arab. J. Sci. Eng. 2020, 45, 7409–7417. [Google Scholar] [CrossRef]
- Turan, M. Backwashing of granular media filters and membranes for water treatment: A review. AQUA-Water Infrastruct. Ecosyst. Soc. 2023, 72, 274–298. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, J.; Lin, H.; Chen, J.; Qi, J.; Bai, Y.; Qu, J. Impacts of backwashing on micropollutant removal and associated microbial assembly processes in sand filters. Front. Environ. Sci. Eng. 2023, 17, 34. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ebrahimi, A.; Azarpira, H.; Tashauoei, H.R.; Mahvi, H.A. Dataset on the spentfilter backwash watertreatment by sedimentation, coagulationand ultrafiltration. Data Brief 2017, 15, 916–921. [Google Scholar] [CrossRef]
- Wiercik, P. The Research on Treatment of Filter Backwash Water Originating during Backwashing of Iron and Manganese Removal Filters. Ph.D. Thesis, Wroclaw University of Life Sciences, Wroclaw, Poland, 2011. Available online: https://www.dbc.wroc.pl/dlibra/publication/15214/edition/21037/content (accessed on 1 January 2024). (in Polish).
- Cheng, L.H.; Xiong, Z.Z.; Cai, S.; Li, D.W.; Xu, X.H. Aeration-manganese sand filter-ultrafiltration to remove iron and manganese from water: Oxidation effect and fouling behavior of manganese sand coated film. J. Water Process Eng. 2020, 38, 101621. [Google Scholar] [CrossRef]
- Welling, C.M.; Sasidaran, S.; Kachoria, P.; Hennessy, S.; Lynch, B.J.; Teleski, S.; Hawkins, B.T. Field testing of a household-scale onsite blackwater treatment system in Coimbatore. India. Sci. Total Environ. 2020, 713, 136706. [Google Scholar] [CrossRef]
- Alhussaini, M.A.; Binger, Z.M.; Souza-Chaves, B.M.; Amusat, O.O.; Park, J.; Bartholomew, T.V.; Achilli, A. Analysis of backwash settings to maximize net water production in an engineering-scale ultrafiltration system for water reuse. J. Water Process Eng. 2023, 53, 103761. [Google Scholar] [CrossRef]
- Cogan, N.G.; Ozturk, D.; Ishida, K.; Safarik, J.; Chellam, S. Membrane aging effects on water recovery during full-scale potable reuse: Mathematical optimization of backwashing frequency for constant-flux microfiltration. Sep. Purif. Technol. 2022, 286, 120294. [Google Scholar] [CrossRef]
- Silva, F.; Geraldes, A.M.; Albuquerque, A. Water reuse in a municipal sports center. In Proceedings of the 1st International FibEnTech Congress—New Opportunities for Fibrous Materials in the Ecological Transition, Covilhã, Portugal, 9–10 December 2022. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Konieczny, K.; Wszelaka-Rylik, M.; Macherzyński, B. Membrane processes innovation in environmental protection: Review. Arch. Environ. Protect. 2019, 45, 20–29. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Microfiltration membrane processes: A review of research trends over the past decade. J. Water Process Eng. 2019, 32, 100941. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, M.; Yao, M.; Qiu, Z.; Hong, Y.; Lan, W.; Xia, H.; Jin, X. Membrane Fouling and Performance of Flat Ceramic Membranes in the Application of Drinking Water Purification. Water 2019, 11, 2606. [Google Scholar] [CrossRef]
- Wolska, M.; Urbańska-Kozłowska, H. Assessing the Possibilities of Backwash Water Reuse Filters in the Water Treatment System—Case Analysis. Water 2023, 15, 2452. [Google Scholar] [CrossRef]
- Erickson, H.P. Size and Shape of Protein Molecules at the Nanometer Level Determined by Sedimentation, Gel Filtration, and Electron Microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Bodzek, M.; Płatowska, A. The Fouling of Semi-Permeable Membranes during Water Treatment by Use of UF/MF Proces—Review. Eng. Environ. Prot. 2009, 12, 5–24. [Google Scholar]
- Deng, L.; Ngo, H.H.; Guo, W.; Zhang, H. Pre-coagulation coupled with sponge-membrane filtration for organic matter removal and membrane fouling control during drinking water treatment. Water Res. 2019, 157, 155–166. [Google Scholar] [CrossRef]
- Gray, S.R.; Ritchie, C.B.; Tran, T.; Bolto, B.A. Effect of NOM characteristics and membrane type on microfiltration performance. Water Res. 2007, 41, 3833–3841. [Google Scholar] [CrossRef]
- Campinas, M.; Viegas, R.M.; Coelho, R.; Lucas, H.; Rosa, M.J. Adsorption/coagulation/ceramic microfiltration for treating challenging waters for drinking water production. Membranes 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Laurell, P.; Poutanen, H.; Hesampour, M.; Tuutijärvi, T.; Vahala, R. Feasibility and Environmental Impact of NOM Reduction by Microfiltration at a Finnish Surface Water Treatment Plant. Water 2023, 15, 1822. [Google Scholar] [CrossRef]
- Wakeman, R. The influence of particle properties on filtration. Sep. Purif. Technol. 2007, 58, 234–241. [Google Scholar] [CrossRef]
- Lin, J.L.; Huang, C.; Pan, J.R.; Wang, Y.S. Fouling mitigation of a dead-end microfiltration by mixing-enhanced preoxidation for Fe and Mn removal from groundwater. Colloids Surf. A Physicochem. Eng. Asp. 2013, 419, 87–93. [Google Scholar] [CrossRef]
- Chen, M.; Shen, S.; Zhang, F.; Zhang, C.; Xiong, J. Biodegradable Dissolved Organic Carbon (BDOC) Removal from Micro-Polluted Water Source Using Ultrafiltration: Comparison with Conventional Processes, Operation Conditions and Membrane Fouling Control. Polymers 2022, 14, 4689. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, A.; Shepard, A.D.; Hardiman, K.; Walsh, M.E. Impact of recycling filter backwash water on organic removal in coagulation–sedimentation processes. Water Res. 2008, 42, 4683–4691. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wen, G.; Li, S.; Chang, H.; Shao, S.; Huang, T.; Li, G.; Liang, H. Effect of Pre-oxidation on Low Pressure Membrane (LPM) For water and Wastewater Treatment: A Review. Chemosphere 2019, 231, 278–300. [Google Scholar] [CrossRef]
- Hakami, M.W.; Alkhudhiri, A.; Al-Batty, S.; Zacharof, M.P.; Maddy, J.; Hilal, N. Ceramic microfiltration membranes in wastewater treatment: Filtration behavior, fouling and prevention. Membranes 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, L.; Wang, Z. Preparation of PVDF/GOSiO2 hybrid microfiltration membrane towards enhanced perm-selectivity and anti-fouling property. J. Taiwan Inst. Chem. Eng. 2016, 78, 500–509. [Google Scholar] [CrossRef]
- Jepsen, K.L.; Bram, M.V.; Pedersen, S.; Yang, Z. Membrane Fouling for Produced Water Treatment: A Review Study from a Process Control Perspective. Water 2018, 10, 847. [Google Scholar] [CrossRef]
- Woo, S.T.; Yun, T.; Kwak, S.Y. Fouling-resistant microfiltration membrane modified with magnetite nanoparticles by reversible conjunction. Sep. Purif. Technol. 2018, 202, 299–306. [Google Scholar] [CrossRef]


| Technical Data | Membrane Module | ||
|---|---|---|---|
| M1 | M2 | M3 | |
| Manufacturer/supplier | Polymem Tech | ||
| Module type | spiral | capillary | capillary |
| Membrane material | Poly vinylidene fluoride (PVDF) | Polypropylene (PP) | α Alumina |
| Maximum permissible temperature (°C): | 45 | 45 | 140 |
| Maximum allowable pressure (MPa): | 1.0 | 0.3 | 0.8 |
| Membrane (pore size) (μm): | 0.2 | 0.2 | 0.2 |
| Membrane (m2): | 1.8 | 0.5 | 1.26 |
| Parameter | Unit | Surface Water | Infiltration Water | ||
|---|---|---|---|---|---|
| Fresh Water | Raw Backwash Water | Fresh Water | Raw Backwash Water | ||
| Turbidity | NTU | 1.3–22 | 1.2–3.9 | 4.2–37 | 59–92 |
| Color | g/m3 | 1–38 | 3–5 | 6.0–28 | 7–10 |
| pH | 7.1–8.2 | 7.2–8.7 | 6.8–7.8 | 7.4–7.6 | |
| UV254 | m−1 | 6.3–27.2 | 3.99–7.49 | 6.3–11.4 | 6.60–9.77 |
| SUVA | m2/g | 0.93–1.66 | 1.47–2.16 | ||
| DOC | g/m3 | 3.07–10.2 | 3.32–5.20 | 2.91–5.9 | 3.45–6.62 |
| Enterococci | cfu/100 cm3 | 0–100 | 0–28 | 0–15 | 0–3 |
| Clostridium perfringens | cfu/100 cm3 | 7–290 | 1–10 | 0 | 0 |
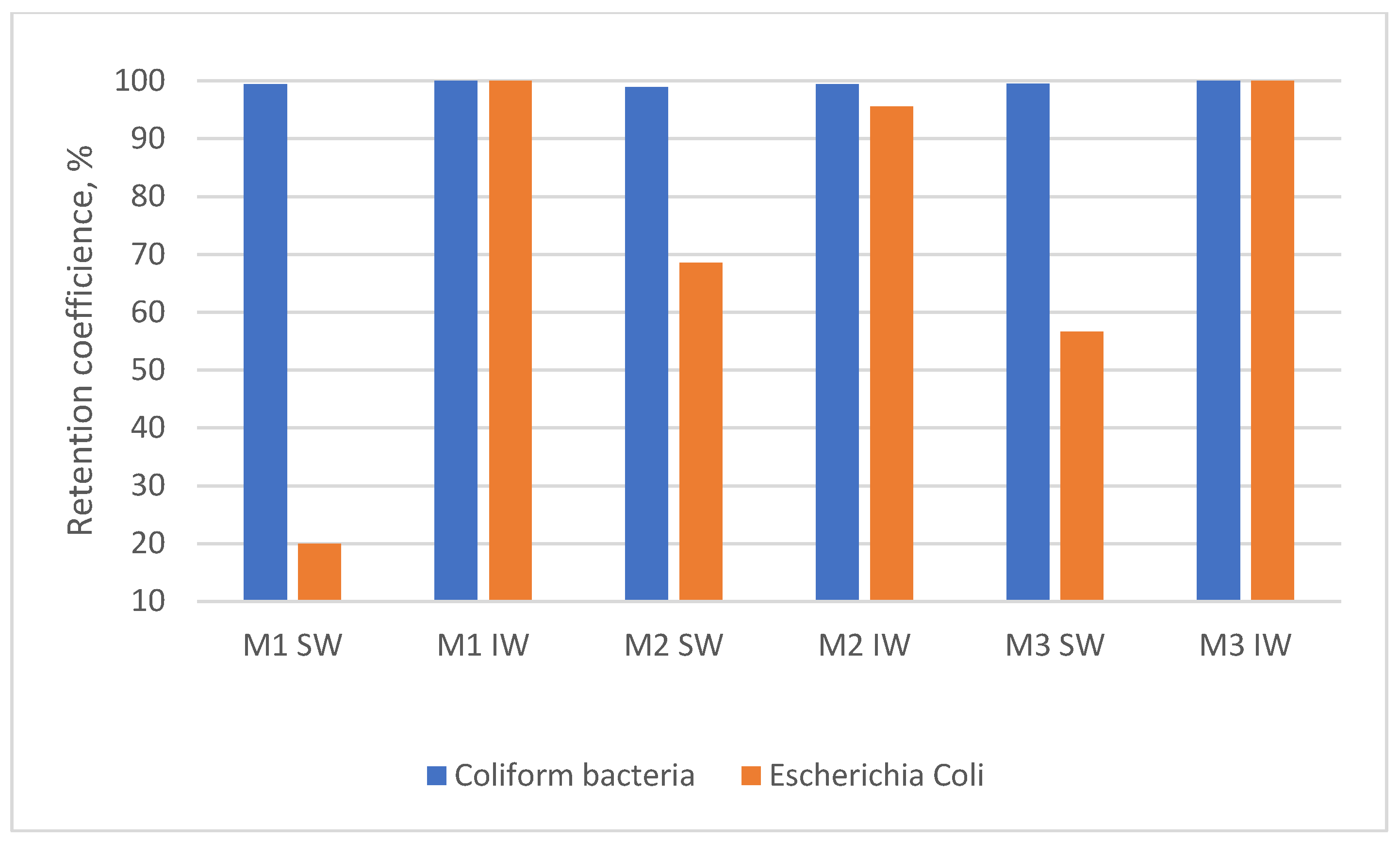
| Coliform bacteria | cfu/100 cm3 | 2–9200 | 55–2420 | 0–201 | 8–276 |
| Escherichia Coli | cfu/100 cm3 | 1–790 | 1–5 | 0 | 2–5 |
| Psychrophilic bacteria | cfu/1 cm3 | 490–170,000 | 6200–210,000 | 11–550 | 4700–18,000 |
| Fe | g/m3 | 0.06–0.64 | 0.02–0.52 | 0.61–5.59 | 6.35–12.11 |
| Mn | g/m3 | 0.01–0.29 | 0.02–0.25 | 0.25–0.55 | 0.35–0.58 |
| Parameter | Unit | Surface Water | Infiltration Water | ||||
|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M1 | M2 | M3 | ||
| Turbidity | NTU | 0.3–0.32 | 0.3–0.36 | 0.3–1.1 | 0.3 | 0.3–1.7 | 0.3 |
| Color | g/m3 | 2–4 | 2–6 | 2–4 | 7–8 | 7–11 | 5–8 |
| pH | 7.8–8.7 | 7.9–8.4 | 6.8–8.6 | 7.3–7.7 | 7.5–7.7 | 7.5–7.7 | |
| UV254 | m−1 | 2.89–3.45 | 2.91–5.58 | 2.62–4.69 | 6.36–7.20 | 6.21–13.3 | 4.91–6.17 |
| SUVA | m2/g | 0.82–1.38 | 1.16–1.51 | 0.91–1.43 | 1.99–2.28 | 1.29–2.08 | 1.62–2.35 |
| DOC | g/m3 | 2.22–3.65 | 2.35–4.8 | 2.50–4.25 | 3.01–3.20 | 3.05–5.31 | 2.46–3.22 |
| Enterococci | cfu/100 cm3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Clostridium perfringens | cfu/100 cm3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coliform bacteria | cfu/100 cm3 | 1 | 1 | 0–1 | 0 | 0–1 | 0 |
| Escherichia Coli | cfu/100 cm3 | 1 | 1–5 | 0–1 | 0 | 0–2 | 0 |
| Psychrophilic bacteria | cfu/1 cm3 | 4–1600 | 200–5900 | 2–40,000 | 8–230 | 8–8000 | 39–13,000 |
| Fe | g/m3 | 0.02 | 0.02–0.05 | 0.02–0.21 | 0.02–0.06 | 0.02–0.63 | 0.02 |
| Mn | g/m3 | 0.005 | 0.01–0.02 | 0.01–0.12 | 0.07–0.30 | 0.04–0.25 | 0.06–0.23 |
| Parameter | Surface Water | Infiltration Water | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M1 | M2 | M3 | |||||||
| min. | max. | min. | max. | min. | max. | min. | max. | min. | max. | min. | max. | |
| Turbidity | 83.7% | 87.1% | 88.5% | 96.2% | 74.5% | 95.7% | 99.5% | 99.5% | 98.4% | 99.6% | 99.6% | 99.6% |
| Color | 0.0% | 31.7% | 0.0% | 22.5% | 8.3% | 33.3% | 0.0% | 12.5% | 5.0% | 30.0% | 5.6% | 27.0% |
| UV254 | 19.3% | 27.2% | 10.3% | 32.5% | 14.1% | 35.1% | 9.2% | 16.4% | 0.7% | 36.4% | 8.1% | 20.4% |
| DOC | 20.1% | 44.5% | 20.7% | 32.5% | 19.9% | 33.5% | 14.7% | 18.9% | 7.8% | 53.9% | 9.2% | 24.4% |
| Psychrophilic bacteria | 90.8% | 98.6% | 10.0% | 99.9% | 23.7% | 98.1% | 98.5% | 99.3% | 77.1% | 99.9% | 59.1% | 71.6% |
| Fe | 71.0% | 74.2% | 31.0% | 87.5% | 4.8% | 33.7% | 99.3% | 99.7% | 96.5% | 99.8% | 99.8% | 99.8% |
| Mn | 66.9% | 74.8% | 79.6% | 84.0% | 56.4% | 77.8% | 56.1% | 78.4% | 43.5% | 76.7% | 67.6% | 83.7% |
| Module | Initial Permeate Flux, dm3/m2h | Relative Membrane Permeability (after 7 Days of Use) | ||
|---|---|---|---|---|
| Surface Water | Infiltration Water | Surface Water | Infiltration Water | |
| M1 | 82.30 | 95.15 | 0.33 | 0.25 |
| M2 | 173.06 | 237.25 | 0.17 | 0.41 |
| M3 | 143.34 | 100.50 | 0.49 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolska, M.; Kabsch-Korbutowicz, M.; Rosińska, A.; Solipiwko-Pieścik, A.; Urbańska-Kozłowska, H. The Use of Microfiltration for the Pretreatment of Backwash Water from Sand Filters. Materials 2024, 17, 2819. https://doi.org/10.3390/ma17122819
Wolska M, Kabsch-Korbutowicz M, Rosińska A, Solipiwko-Pieścik A, Urbańska-Kozłowska H. The Use of Microfiltration for the Pretreatment of Backwash Water from Sand Filters. Materials. 2024; 17(12):2819. https://doi.org/10.3390/ma17122819
Chicago/Turabian StyleWolska, Małgorzata, Małgorzata Kabsch-Korbutowicz, Agata Rosińska, Anna Solipiwko-Pieścik, and Halina Urbańska-Kozłowska. 2024. "The Use of Microfiltration for the Pretreatment of Backwash Water from Sand Filters" Materials 17, no. 12: 2819. https://doi.org/10.3390/ma17122819






