Effect of Water-Soluble Polymers on the Rheology and Microstructure of Polymer-Modified Geopolymer Glass-Ceramics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Geopolymer Composite Plaques
2.3. Characterization of Geopolymer Composite Plaques
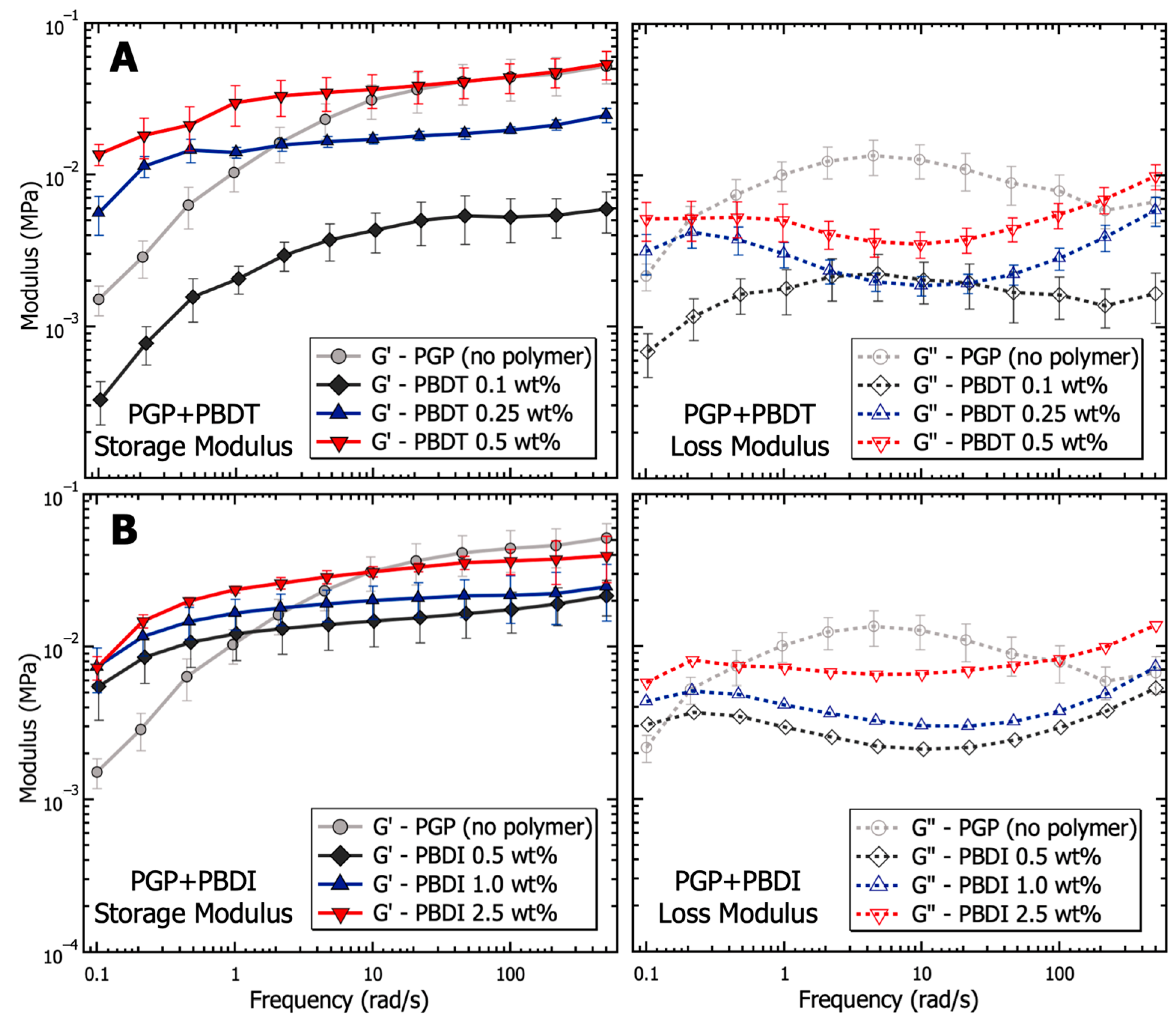
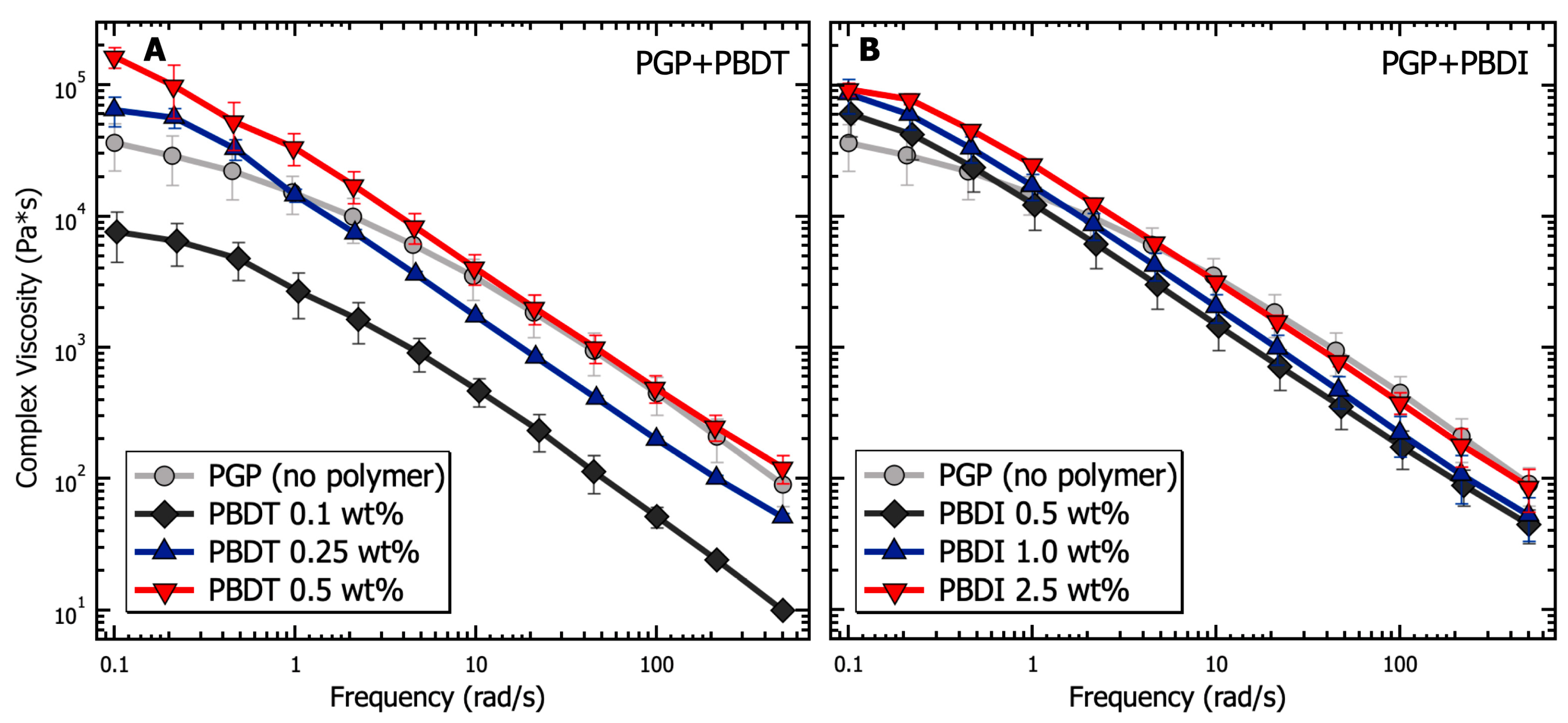
2.3.1. Rheology
2.3.2. Thermal Gravimetric Analysis (TGA)
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Energy Dispersive Spectroscopy (EDS)
2.3.5. Scanning/Transmission Electron Microscopy (S/TEM)
2.3.6. Nitrogen Adsorption (Porosimetry)
2.3.7. X-ray Diffraction (XRD)
2.3.8. Attenuated Total Reflectance Fourier Transform Infrared Radiation Analysis (ATR-FTIR)
2.3.9. Nanoindentation
3. Results and Discussion
3.1. Solution Properties of PGP Sol-Gel Resins
3.2. Composition of PGP Composites
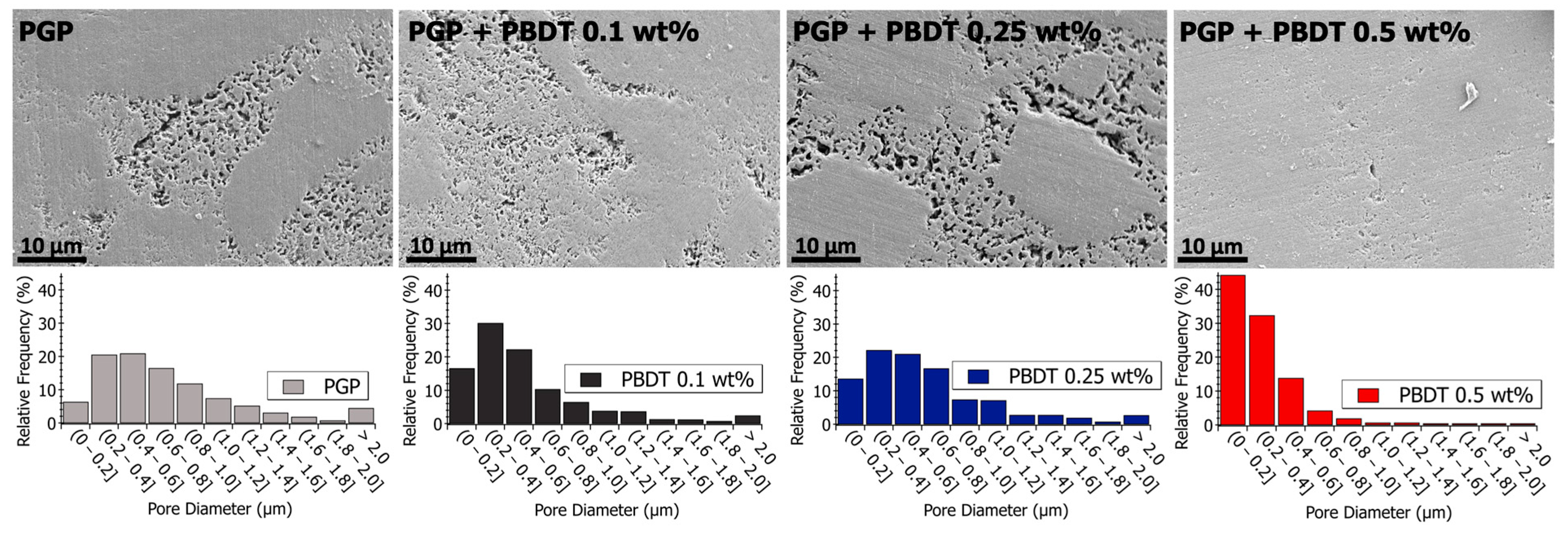
3.3. Porosity of PGP Composites
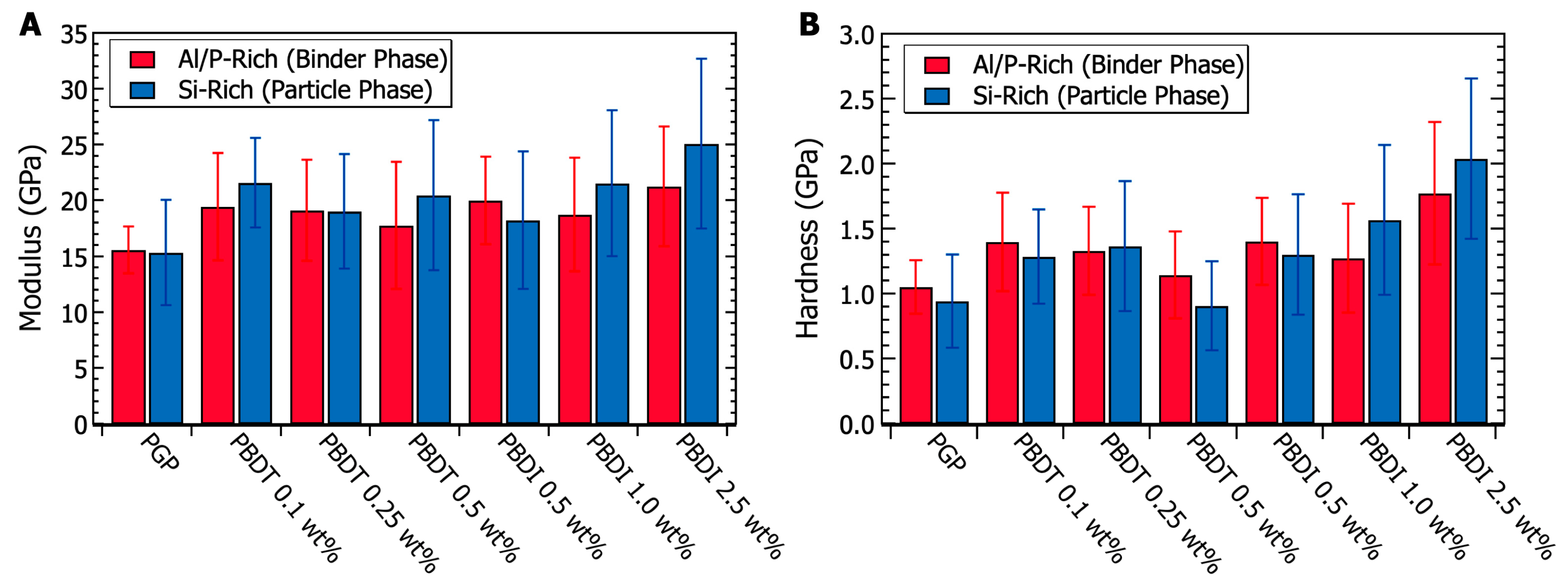
3.4. Micromechanical Properties of PGP Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furtos, G.; Prodan, D.; Sarosi, C.; Moldovan, M.; Korniejenko, K.; Miller, L.; Fiala, L.; Iveta, N. Mechanical Properties of MiniBarsTM Basalt Fiber-Reinforced Geopolymer Composites. Materials 2024, 17, 248. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Niu, Z.; Wang, J.; Zhu, G.R.; Zhou, M. Effect of Sodium Polyacrylate on Mechanical Properties and Microstructure of Metakaolin-Based Geopolymer with Different SiO2/Al2O3 Ratio. Ceram. Int. 2018, 44, 18173–18180. [Google Scholar] [CrossRef]
- Wang, S.; Ma, X.; He, L.; Zhang, Z.; Li, L.; Li, Y. High Strength Inorganic-Organic Polymer Composites (IOPC) Manufactured by Mold Pressing of Geopolymers. Constr. Build. Mater. 2019, 198, 501–511. [Google Scholar] [CrossRef]
- Wagh, A.S. Chemically Bonded Phosphate Ceramics—A Novel Class of Geopolymers. In Advances in Ceramic Matrix Composites X; Singh, J.P., Bansal, N.P., Kriven, W.M., Eds.; The American Ceramic Society: Argonne, IL, USA, 2005; pp. 107–116. ISBN 9781118408353. [Google Scholar]
- Haddaji, Y.; Hamdane, H.; Majdoubi, H.; Mansouri, S.; Allaoui, D.; El Bouchti, M.; Tamraoui, Y.; Manoun, B.; Oumam, M.; Hannache, H. Eco-Friendly Geopolymer Composite Based on Non-Heat-Treated Phosphate Sludge Reinforced With Polypropylene Fibers. Silicon 2021, 13, 2389–2400. [Google Scholar]
- Cekalova, M.; Kovarik, T.; Rieger, D. Rheological Characterization of Geopolymer Binder Modified by Organic Resins. J. Phys. Conf. Ser. 2017, 790. [Google Scholar] [CrossRef]
- Jindal, B.B.; Alomayri, T.; Hasan, A.; Kaze, C.R. Geopolymer Concrete with Metakaolin for Sustainability: A Comprehensive Review on Raw Material’s Properties, Synthesis, Performance, and Potential Application. Environ. Sci. Pollut. Res. 2023, 30, 25299–25324. [Google Scholar] [CrossRef] [PubMed]
- Barbhuiya, S.; Pang, E. Strength and Microstructure of Geopolymer Based on Fly Ash and Metakaolin. Materials 2022, 15, 3732. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Matsimbe, J.; Dinka, M.; Olukanni, D.; Musonda, I. Geopolymer: A Systematic Review of Methodologies. Materials 2022, 15, 6852. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in Geopolymer Materials: A Comprehensive Review. J. Traffic Transp. Eng. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers—Inorganic Polymeric New Materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Davidovits, J. High-Alkali Cements for 21st Century Concretes. In Proceedings of the American Concrete Institute; American Concrete Institute: Farmington Hills, MI, USA, 1994; Volume 144, pp. 383–397. [Google Scholar]
- Lyon, R.E.; Balaguru, P.N.; Foden, A.; Sorathia, U.; Davidovits, J.; Davidovics, M. Fire-Resistant Aluminosilicate Composites. Fire Mater. 1997, 21, 67–73. [Google Scholar] [CrossRef]
- Gilliard Hensel, N.; Franz, G.; Riedl, M.; Gottschalk, M.; Wunder, B.; Galbert, F.; Nissen, J. Polymorphism and Solid Solution in the System SiO2-AlPO4(-H2O): A Review and New Synthesis Experiments up to 3.5 GPa and 1573 K. Neues Jahrb. Mineral. Abhandlungen 2007, 184, 131–149. [Google Scholar] [CrossRef]
- Robinson, P.; McCartney, E.R. Subsolidus Relations in the System SiO2-Al2O3-P2O5. J. Am. Ceram. Soc. 1964, 47, 587–592. [Google Scholar] [CrossRef]
- Alexander, G.B.; Heston, W.M.; Iler, R.K. The Solubility of Amorphous Silica in Water. J. Phys. Chem. 1954, 58, 453–455. [Google Scholar] [CrossRef]
- Boigelot, R.; Graz, Y.; Bourgel, C.; Defoort, F.; Poirier, J. The SiO2-P2O5 Binary System: New Data Concerning the Temperature of Liquidus and the Volatilization of Phosphorus. Ceram. Int. 2015, 41, 2353–2360. [Google Scholar] [CrossRef]
- Hussain, M.; Varely, R.; Cheng, Y.B.; Mathys, Z.; Simon, G.P. Synthesis and Thermal Behavior of Inorganic-Organic Hybrid Geopolymer Composites. J. Appl. Polym. Sci. 2005, 96, 112–121. [Google Scholar] [CrossRef]
- Castillo, H.; Collado, H.; Droguett, T.; Vesely, M.; Garrido, P.; Palma, S. State of the Art of Geopolymers: A Review. E-Polymers 2022, 22, 108–124. [Google Scholar] [CrossRef]
- Celerier, H.; Jouin, J.; Mathivet, V.; Tessier-Doyen, N.; Rossignol, S. Composition and Properties of Phosphoric Acid-Based Geopolymers. J. Non-Cryst. Solids 2018, 493, 94–98. [Google Scholar] [CrossRef]
- Muñiz-Villarreal, M.S.; Manzano-Ramírez, A.; Sampieri-Bulbarela, S.; Ramón Gasca-Tirado, J.; Reyes-Araiza, J.L.; Rubio-Ávalos, J.C.; Pérez-Bueno, J.J.; Apatiga, L.M.; Zaldivar-Cadena, A.; Amigó-Borrás, V. The Effect of Temperature on the Geopolymerization Process of a Metakaolin-Based Geopolymer. Mater. Lett. 2011, 65, 995–998. [Google Scholar] [CrossRef]
- Zribi, M.; Baklouti, S. Investigation of Phosphate Based Geopolymers Formation Mechanism. J. Non-Cryst. Solids 2021, 562, 120777. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Sol-Gel Derived Organic-Inorganic Hybrid Materials: Synthesis, Characterizations and Applications. J. Sol-Gel Sci. Technol. 2011, 59, 73–94. [Google Scholar] [CrossRef]
- Malucelli, G. Hybrid Organic/Inorganic Coatings Through Dual-Cure Processes: State of the Art and Perspectives. Coatings 2016, 6, 10. [Google Scholar] [CrossRef]
- Morris, J.H.; Perkins, P.G.; Rose, A.E.A.; Smith, W.E. The Chemistry and Binding Properties of Aluminium Phosphates. Chem. Soc. Rev. 1977, 6, 173–194. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S. Understanding the Relationship between Geopolymer Composition, Microstructure and Mechanical Properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Wang, W.; Fan, C.; Wang, B.; Zhang, X.; Liu, Z. Workability, Rheology, and Geopolymerization of Fly Ash Geopolymer: Role of Alkali Content, Modulus, and Water-Binder Ratio. Constr. Build. Mater. 2023, 367, 130357. [Google Scholar] [CrossRef]
- Favier, A.; Hot, J.; Habert, G.; Roussel, N.; d’Espinose De Lacaillerie, J.-B. Flow Properties of MK-Based Geopolymer Pastes. A Comparative Study with Standard Portland Cement Pastes. Soft Matter 2014, 10, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Lemougna, P.N.; Wang, K.; Tang, Q.; Melo, U.C.; Cui, X. Recent Developments on Inorganic Polymers Synthesis and Applications. Ceram. Int. 2016, 42, 15142–15159. [Google Scholar] [CrossRef]
- Goryunova, K.; Gahramanli, Y.; Muradkhanli, V.; Nadirov, P. Phosphate-Activated Geopolymers: Advantages and Application. RSC Adv. 2023, 13, 30329–30345. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Z.; Liu, X. Comprehensive Understanding of Aluminosilicate Phosphate Geopolymers: A Critical Review. Materials 2022, 15, 5961. [Google Scholar] [CrossRef]
- Perera, D.S.; Hanna, J.V.; Davis, J.; Blackford, M.G.; Latella, B.A.; Sasaki, Y.; Vance, E.R. Relative Strengths of Phosphoric Acid-Reacted and Alkali-Reacted Metakaolin Materials. J. Mater. Sci. 2008, 43, 6562–6566. [Google Scholar] [CrossRef]
- Tchakoute, H.K.; Ruscher, C.H.; Kamseu, E.; Andreola, F.; Leonelli, C. Influence of the Molar Concentration of Phosphoric Acid Solution on the Properties of Metakaolin-Phosphate-Based Geopolymer Cements. Appl. Clay Sci. 2017, 147, 184–194. [Google Scholar] [CrossRef]
- Cui, X.-M.; Liu, L.-P.; He, Y.; Chen, J.-Y.; Zhou, J. A Novel Aluminosilicate Geopolymer Material with Low Dielectric Loss. Mater. Chem. Phys. 2011, 130, 1–4. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Elimbi, A.; Stephan, D. Phase and Dimensional Stability of Volcanic Ash-Based Phosphate Inorganic Polymers at Elevated Temperatures. SN Appl. Sci. 2020, 2, 828. [Google Scholar] [CrossRef]
- Mackenzie, K.J.D.; Welter, M. Geopolymer (Aluminosilicate) Composites: Synthesis, Properties and Applications; Woodhead Publishing: Sawston, UK, 2014; ISBN 9780857091208. [Google Scholar]
- Xu, H.; Van Deventer, J.S.J. The Geopolymerisation of Alumino-Silicate Minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Alrefaei, Y.; Dai, J.-G. Silico-Aluminophosphate and Alkali-Aluminosilicate Geopolymers: A Comparative Review. Front. Mater. 2019, 6, 106. [Google Scholar] [CrossRef]
- Katsiki, A. Aluminosilicate Phosphate Cements—A Critical Review. Adv. Appl. Ceram. 2019, 118, 274–286. [Google Scholar] [CrossRef]
- Li, J.; Mailhiot, S.; Sreenivasan, H.; Kantola, A.M.; Illikainen, M.; Adesanya, E.; Kriskova, L.; Telkki, V.-V.; Kinnunen, P. Curing Process and Pore Structure of Metakaolin-Based Geopolymers: Liquid-State 1 H NMR Investigation. Cem. Concr. Res. 2021, 143, 106394. [Google Scholar] [CrossRef]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Messina, F.; Ferone, C.; Asprone, D.; Cioffi, R. Lightweight Geopolymer-Based Hybrid Materials. Compos. Part B Eng. 2017, 128, 225–237. [Google Scholar] [CrossRef]
- Ferone, C.; Roviello, G.; Colangelo, F.; Cioffi, R.; Tarallo, O. Novel Hybrid Organic-Geopolymer Materials. Appl. Clay Sci. 2013, 73, 42–50. [Google Scholar] [CrossRef]
- Onutai, S.; Kobayashi, T.; Thavorniti, P.; Jiemsirilers, S. Porous Fly Ash-Based Geopolymer Composite Fiber as an Adsorbent for Removal of Heavy Metal Ions from Wastewater. Mater. Lett. 2019, 236, 30–33. [Google Scholar] [CrossRef]
- Yan, S.; Saeed, A.; Najm, H.M.; Hassan, A.; Muayad, M.; Sabri, S.; Qaidi, S.; Mashaan, N.S.; Ansari, K. Properties and Applications of Geopolymer Composites: A Review Study of Mechanical and Microstructural Properties. Materials 2022, 15, 8250. [Google Scholar] [CrossRef] [PubMed]
- Colorado, H.A.; Hahn, H.T.; Hiel, C. Pultruded Glass Fiber- and Pultruded Carbon Fiber-Reinforced Chemically Bonded Phosphate Ceramics. J. Compos. Mater. 2011, 45, 2391–2399. [Google Scholar] [CrossRef]
- Růžek, V.; Dostayeva, A.M.; Walter, J.; Grab, T.; Korniejenko, K. Carbon Fiber-Reinforced Geopolymer Composites: A Review. Fibers 2023, 11, 17. [Google Scholar] [CrossRef]
- Ali, M.F.; Vijayalakshmi Natrajan, M.M. A Review of Geopolymer Composite Thermal Properties. IOP Conf. Ser. Earth Environ. Sci. 2021, 822, 012051. [Google Scholar] [CrossRef]
- Bounor-Legaré, V.; Cassagnau, P. In Situ Synthesis of Organic-Inorganic Hybrids or Nanocomposites from Sol-Gel Chemistry in Molten Polymers. Prog. Polym. Sci. 2014, 39, 1473–1497. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Xu, D.L.; Wang, B.Q.; Xu, G.M.; Yang, D.F.; Wang, N.; Liu, H.C.; Wang, Y.C. A Novel Method for Preparation of Organic Resins Reinforced Geopolymer Composites. J. Mater. Sci. 2010, 45, 1189–1192. [Google Scholar] [CrossRef]
- Glad, B.E.; Kriven, W.M. Geopolymer with Hydrogel Characteristics via Silane Coupling Agent Additives. J. Am. Ceram. Soc. 2014, 97, 295–302. [Google Scholar] [CrossRef]
- Chiranjeevi, K.; Abraham, M.; Rath, B.; Praveenkumar, T.R. Enhancing the Properties of Geopolymer Concrete Using Nano-Silica and Microstructure Assessment: A Sustainable Approach. Sci. Rep. 2023, 13, 17302. [Google Scholar] [CrossRef]
- Luo, Z.; Li, W.; Gan, Y.; He, X.; Castel, A.; Sheng, D. Nanoindentation on Micromechanical Properties and Microstructure of Geopolymer with Nano-SiO2 and Nano-TiO2. Cem. Concr. Compos. 2021, 117, 103883. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, K.; Lu, J. Novel Modification Method for Inorganic Geopolymer by Using Water Soluble Organic Polymers. Mater. Lett. 2004, 58, 1292–1296. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Mechanical Properties and Prediction of Fracture Parameters of Geopolymer/Alkali-Activated Mortar Modified with PVA Fiber and Nano-SiO2. Ceram. Int. 2020, 46, 20027–20037. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.; Zhang, Z.; Li, N.; Shi, D. Mechanical and Fracture Properties of Ultra-High Performance Geopolymer Concrete: Effects of Steel Fiber and Silica Fume. Cem. Concr. Compos. 2020, 112, 103665. [Google Scholar] [CrossRef]
- Bahranifard, Z.; Farshchi Tabrizi, F.; Vosoughi, A.R. An Investigation on the Effect of Styrene-Butyl Acrylate Copolymer Latex to Improve the Properties of Polymer Modified Concrete. Constr. Build. Mater. 2019, 205, 175–185. [Google Scholar] [CrossRef]
- Rossignolo, J.A.; Agnesini, M.V.C. Durability of Polymer-Modified Lightweight Aggregate Concrete. Cem. Concr. Compos. 2004, 26, 375–380. [Google Scholar] [CrossRef]
- Pei, M.; Kim, W.; Hyung, W.; Ango, A.J.; Soh, Y. Effects of Emulsifiers on Properties of Poly(Styrene-Butyl Acrylate) Latex-Modified Mortars. Cem. Concr. Res. 2002, 32, 837–841. [Google Scholar] [CrossRef]
- Muthadhi, A.; Kothandaraman, S. Experimental Investigations on Polymer-Modified Concrete Subjected to Elevated Temperatures. Mater. Struct. 2014, 47, 977–986. [Google Scholar] [CrossRef]
- Pourchez, J.; Peschard, A.; Grosseau, P.; Guyonnet, R.; Guilhot, B.; Vallée, F. HPMC and HEMC Influence on Cement Hydration. Cem. Concr. Res. 2006, 36, 288–294. [Google Scholar] [CrossRef]
- Jo, Y.K.; Do, J. Hardening Properties of Epoxy-Modified Cement Composites without Hardener. Polym. Polym. Compos. 2016, 24, 195–204. [Google Scholar] [CrossRef]
- Fowler, D.W. Polymers in Concrete: A Vision for the 21st Century. Cem. Concr. Compos. 1999, 21, 449–452. [Google Scholar] [CrossRef]
- Hsu, K.C.; Lee, Y.F. Water-soluble Sulfonated Phenolic Resins. I. Synthesis. J. Appl. Polym. Sci. 1995, 57, 1501–1509. [Google Scholar] [CrossRef]
- Xie, Z.; Yuan, Q.; Yao, H.; Liu, Y.; Zhang, S.; Tian, Y. Understanding the Impact of Polyacrylamide Molecular Weight on the Workability of Cement Paste. Cem. Concr. Compos. 2023, 142, 105171. [Google Scholar] [CrossRef]
- Rocha, D.L.; Júnior, L.U.D.T.; Marvila, M.T.; Pereira, E.C.; Souza, D.; de Azevedo, A.R.G. A Review of the Use of Natural Fibers in Cement Composites: Concepts, Applications and Brazilian History. Polymers 2022, 14, 2043. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; Volpini, V. Mechanical Performance of Fiber Reinforced Cement Composites Including Fully-Recycled Plastic Fibers. Fibers 2021, 9, 16. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Molino, A.J.; Menna, C.; Ferone, C.; Cioffi, R.; Tarallo, O. Hybrid Geopolymers from Fly Ash and Polysiloxanes. Molecules 2019, 24, 3510. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Z.; Zhu, H.; Wang, X.; Gao, J. Effects of Silane on Reaction Process and Microstructure of Metakaolin-Based Geopolymer Composites. J. Build. Eng. 2020, 32, 101695. [Google Scholar] [CrossRef]
- Ouyang, G.; Wu, L.; Ye, C.; Wang, J.; Dong, T. Effect of Silane Coupling Agent on the Rheological and Mechanical Properties of Alkali-Activated Ultrafine Metakaolin Based Geopolymers. Constr. Build. Mater. 2021, 290, 123223. [Google Scholar] [CrossRef]
- Glad, B.E.; Han, C.; Kriven, W.M. Polymer Adhesion to Geopolymer via Silane Coupling Agent Additives. J. Am. Ceram. Soc. 2012, 95, 3758–3762. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Tarallo, O. Fire Resistant Melamine Based Organic-Geopolymer Hybrid Composites. Cem. Concr. Compos. 2015, 59, 89–99. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, B.; Yi, Z.; Du, F.; Zhang, Y. The Properties and Latest Application of Geopolymers. IOP Conf. Ser. Mater. Sci. Eng. 2019, 472, 012029. [Google Scholar] [CrossRef]
- Xiao-ling, S.; Xue-min, C.; Kun-sheng, L.; Guang-jian, Z.; Yan, H. Hot-Pressure Forming Process of PVC/Geopolymer Composite Materials. Appl. Clay Sci. 2013, 71, 32–36. [Google Scholar] [CrossRef]
- Strini, A.; Roviello, G.; Ricciotti, L.; Ferone, C.; Messina, F.; Schiavi, L.; Corsaro, D.; Cioffi, R. TiO2-Based Photocatalytic Geopolymers for Nitric Oxide Degradation. Materials 2016, 9, 513. [Google Scholar] [CrossRef]
- Sekkal, W.; Zaoui, A. High Strength Metakaolin-Based Geopolymer Reinforced by Pristine and Covalent Functionalized Carbon Nanotubes. Constr. Build. Mater. 2022, 327, 126910. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, C.; Qiao, Y.; Wang, X.; Jia, D.; Li, H.; Colombo, P. Porous Geopolymer Composites: A Review. Compos. Part A 2021, 150, 106629. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, G.; Zhou, M.; Wang, J.; Chen, Q. Effect of Organic Polymers on the Properties of Slag-Based Geopolymers. Constr. Build. Mater. 2018, 167, 216–224. [Google Scholar] [CrossRef]
- Rondinella, A.; Furlani, E.; Zanocco, M.; De Leitenburg, C.; Scagnetto, F.; Maschio, S. Synthesis, Microhardness, Fracture Toughness and Microstructural Features of Chitosan Containing Alkali Activated Geopolymers. Ceram. Int. 2023, 49, 26726–26733. [Google Scholar] [CrossRef]
- Park, J.H.; Rutledge, G.C. 50th Anniversary Perspective: Advanced Polymer Fibers: High Performance and Ultrafine. Macromolecules 2017, 50, 5627–5642. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; Wei, X.; Wu, D.; Jin, R. Effects of Water on the Preparation, Morphology, and Properties of Polyimide/Silica Nanocomposite Films Prepared by Sol-Gel Process. J. Appl. Polym. Sci. 2007, 104, 1579–1586. [Google Scholar] [CrossRef]
- Chae, H.G.; Kumar, S. Rigid-Rod Polymeric Fibers. J. Appl. Polym. Sci. 2006, 100, 791–802. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Hybrid Polymer Composite Materials: Processing; Thakur, V.K., Thakur, M.K., Gupta, R.K., Eds.; Woodhead Publishing: Sawston, UK, 2017; ISBN 978-0-08-100789-1. [Google Scholar]
- Roberts, A.D.; Kelly, P.; Bain, J.; Morrison, J.J.; Wimpenny, I.; Barrow, M.; Woodward, R.T.; Gresil, M.; Blanford, C.; Hay, S.; et al. Graphene-Aramid Nanocomposite Fibres: Via Superacid Co-Processing. Chem. Commun. 2019, 55, 11703–11706. [Google Scholar] [CrossRef]
- García, J.M.; García, F.C.; Serna, F.; de la Peña, J.L. High-Performance Aromatic Polyamides. Prog. Polym. Sci. 2010, 35, 623–686. [Google Scholar] [CrossRef]
- Nicholls, A.R.; Perez, Y.; Pellisier, M.; Rodde, A.; Lanusse, P.; Stock, J.A.; Kull, K.; Eubank, J.; Harmon, J.P. Thermomechanical Characterization of Thermoplastic Polyimides to Improve the Chain Collaboration via Ureidopyrimidone Endcaps. Polym. Eng. Sci. 2019, 59, 2231–2246. [Google Scholar] [CrossRef]
- Fraser, A.C.; Chew, N.G.P.; Hegde, M.; Liu, F.; Liu, C.W.; Coronell, O.; Dingemans, T.J. Linear versus Nonlinear Aromatic Polyamides: The Role of Backbone Geometry in Thin Film Salt Exclusion Membranes. ACS Appl. Mater. Interfaces 2022, 14, 36143–36156. [Google Scholar] [CrossRef]
- Yu, D.; Zanelotti, C.J.; Fox, R.J.; Dingemans, T.J.; Madsen, L.A. Solvent-Cast Solid Electrolyte Membranes Based on a Charged Rigid-Rod Polymer and Ionic Liquids. ACS Appl. Energy Mater. 2021, 4, 6599–6605. [Google Scholar] [CrossRef]
- Sarkar, N.; Kershner, L.D. Rigid Rod Water-Soluble Polymers. J. Appl. Polym. Sci. 1996, 62, 393–408. [Google Scholar]
- Vannini, M.; Marchese, P.; Celli, A.; Lorenzetti, C. Synthesis and Characterization of Novel Water-Soluble Polyamides with Enhanced Gas Barrier Properties. Ind. Eng. Chem. Res. 2018, 57, 15254–15261. [Google Scholar] [CrossRef]
- Hegde, M.; Yang, L.; Vita, F.; Fox, R.J.; Van De Watering, R.; Norder, B.; Lafont, U.; Francescangeli, O.; Madsen, L.A.; Picken, S.J.; et al. Strong Graphene Oxide Nanocomposites from Aqueous Hybrid Liquid Crystals. Nat. Commun. 2020, 11, 830. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J.; Hegde, M.; Cole, D.P.; Moore, R.B.; Picken, S.J.; Dingemans, T.J. High-Strength Liquid Crystal Polymer–Graphene Oxide Nanocomposites from Water. ACS Appl. Mater. Interfaces 2022, 14, 16592–16600. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Viale, S.; Best, A.S.; Mendes, E.; Jager, W.F.; Picken, S.J. A Supramolecular Nematic Phase in Sulfonated Polyaramides. Chem. Commun. 2004, 14, 1596–1597. [Google Scholar] [CrossRef] [PubMed]
- Viale, S.; Best, A.S.; Mendes, E.; Picken, S.J. Formation of Aqueous Molecular Nematic Liquid Crystal Phase in Poly(p-Sulfophenylene Sulfoterephthalamide). Chem. Commun. 2005, 12, 1528–1530. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J.; Hegde, M.; Zanelotti, C.J.; Kumbhar, A.S.; Samulski, E.T.; Madsen, L.A.; Picken, S.J.; Dingemans, T.J. Irreversible Shear-Activated Gelation of a Liquid Crystalline Polyelectrolyte. ACS Macro Lett. 2020, 9, 957–963. [Google Scholar] [CrossRef]
- Fox, R.J.; Chen, W.-R.; Do, C.; Picken, S.J.; Forest, M.G.; Dingemans, T.J. Fingerprinting the Nonlinear Rheology of a Liquid Crystalline Polyelectrolyte. Rheol. Acta 2020, 59, 727–743. [Google Scholar] [CrossRef]
- Yang, W.; Furukawa, H.; Gong, J.P. Highly Extensible Double-Network Gels with Self-Assembling Anisotropic Structure. Adv. Mater. 2008, 20, 4499–4503. [Google Scholar] [CrossRef]
- Wan, Q.; Zhang, R.; Zhang, Y. Structure and Properties of Phosphate-Based Geopolymer Synthesized with the Spent Fluid Catalytic-Cracking (SFCC) Catalyst. Gels 2022, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Y.; Yu, Z.; Gao, J.; Ten Brinck, S.; Slebodnick, C.; Fahs, G.B.; Zanelotti, C.J.; Hegde, M.; Moore, R.B.; et al. Double Helical Conformation and Extreme Rigidity in a Rodlike Polyelectrolyte. Nat. Commun. 2019, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.C.; Yankey, J.; Coronell, O.; Dingemans, T.J. A Sulfonated All-Aromatic Polyamide for Heavy Metal Capture: A Model Study with Pb(II). ACS Appl. Polym. Mater. 2023, 5, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Imanaka, N.; Kriven, W.M.; Fukushima, M.; Kale, G. Ceramics for Environmental Systems, 257th ed.; Singh, M., Ohji, T., Michaelis, A., Eds.; The American Ceramic Society: Vancouver, BC, USA, 2016; ISBN 9781119234449. [Google Scholar]
- Enfedaque, A.; Alberti, M.G.; Gálvez, J.C.; Mengie, S. Influence of Natural Weather Conditions in the Long-Term Fracture Energy of Glass Fibre Reinforced Cement (GRC) Modified with Chemical Additions. Materials 2021, 14, 3355. [Google Scholar] [CrossRef]
- Christiansen, M.U. An Investigation of Waste Glass-Based Geopolymers Supplemented with Alumina. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2013. [Google Scholar]
- Hart, F. A Hard Sell? Metakaolin in High-Performance Concrete. Ind. Miner. 2017, 36–39. [Google Scholar]
- Davidovits, R.; Pelegris, C.; Davidovits, J. Standardized Method in Testing Commercial Metakaolins for Geopolymer Formulations; Geopolymer Institute Library: Saint-Quentin, France, 2019; pp. 1–8. [Google Scholar] [CrossRef]
- Viale, S.; Jager, W.F.; Picken, S.J. Synthesis and Characterization of a Water-Soluble Rigid-Rod Polymer. Polymer 2003, 44, 7843–7850. [Google Scholar] [CrossRef]
- Sakulich, A.R.; Li, V.C. Nanoscale Characterization of Engineered Cementitious Composites (ECC). Cem. Concr. Res. 2011, 41, 169–175. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Brandvold, A.S.; Al-Chaar, G.K.; Kriven, W.M. Isolating the Effects of Thixotropy in Geopolymer Pastes. J. Am. Ceram. Soc. 2023, 106, 2797–2807. [Google Scholar] [CrossRef]
- Han, A.; Colby, R.H. Rheology of Entangled Polyelectrolyte Solutions. Macromolecules 2021, 54, 1375–1387. [Google Scholar] [CrossRef]
- Wyatt, N.B.; Liberatore, M.W. Rheology and Viscosity Scaling of the Polyelectrolyte Xanthan Gum. J. Appl. Polym. Sci. 2009, 114, 4076–4084. [Google Scholar] [CrossRef]
- Lee, H.C.; Brant, D.A. Rheology of Concentrated Isotropic and Anisotropic Xanthan Solutions. 1. A Rodlike Low Molecular Weight Sample. Macromolecules 2002, 35, 2212–2222. [Google Scholar] [CrossRef]
- Sayko, R.; Tian, Y.; Liang, H.; Dobrynin, A.V. Charged Polymers: From Polyelectrolyte Solutions to Polyelectrolyte Complexes. Macromolecules 2021, 54, 7183–7192. [Google Scholar] [CrossRef]
- Muthukumar, M. Dynamics of Polyelectrolyte Solutions. J. Chem. Phys. 1997, 107, 2619–2635. [Google Scholar] [CrossRef]
- Verrelli, D.I.; Kilcullen, A.R. Normal Stress Differences and Yield Stresses in Attractive Particle Networks. Adv. Condens. Matter Phys. 2016, 2016, 1716598. [Google Scholar] [CrossRef]
- Brandvold, A.S.; Trindade, A.C.C.; Kriven, W.M. Rheological Assessment of Metakaolin-Based Geopolymer Composites through Squeeze Flow. J. Am. Ceram. Soc. 2023, 106, 4038–4051. [Google Scholar] [CrossRef]
- Dvorkin, L.; Zhitkovsky, V.; Makarenko, R.; Ribakov, Y. The Influence of Polymer Superplasticizers on Properties of High-Strength Concrete Based on Low-Clinker Slag Portland Cement. Materials 2023, 16, 2075. [Google Scholar] [CrossRef]
- Macosko, C.W. Rheology: Principles, Measurements and Applications, 1st ed.; Wiley-VCH: New York, NY, USA, 1994; ISBN 1560815795. [Google Scholar]
- White, C.E.; Provis, J.L.; Proffen, T.; Riley, D.P.; Van Deventer, J.S.J. Density Functional Modeling of the Local Structure of Kaolinite Subjected to Thermal Dehydroxylation. J. Phys. Chem. A 2010, 114, 4988–4996. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.K.; Chen, J.J.; Zhang, X.; Huang, Y.X.; Li, W.W.; Yu, H.Q. Self-Induced Synthesis of Phase-Junction TiO2 with a Tailored Rutile to Anatase Ratio below Phase Transition Temperature. Sci. Rep. 2016, 6, 20491. [Google Scholar] [CrossRef]
- Uddin, M.J.; Cesano, F.; Chowdhury, A.R.; Trad, T.; Cravanzola, S.; Martra, G.; Mino, L.; Zecchina, A.; Scarano, D. Surface Structure and Phase Composition of TiO2 P25 Particles After Thermal Treatments and HF Etching. Front. Mater. 2020, 7, 192. [Google Scholar] [CrossRef]
- Sato, M. X-Ray Study of Low Tridymite. Mineral. J. 1963, 4, 131–146. [Google Scholar] [CrossRef]
- Debnath, R.; Chaudhuri, J. Surface-Bound Titania-Induced Selective Growth and Stabilization of Tridymite Aluminum Phosphate. J. Solid State Chem. 1992, 97, 163–168. [Google Scholar] [CrossRef]
- Wagemaker, M.; Kearley, G.J.; Van Well, A.A.; Mutka, H.; Mulder, F.M. Multiple Li Positions inside Oxygen Octahedra in Lithiated TiO2 Anatase. J. Am. Chem. Soc. 2003, 125, 840–848. [Google Scholar] [CrossRef]
- Neder, R.B.; Burghammer, M.; Grasl, T.; Schulz, H.; Bram, A.; Fiedler, S. Refinement of the KaolinitesStructure from Single-Crystal Synchrotron Data. Clays Clay Miner. 1999, 47, 487–494. [Google Scholar] [CrossRef]
- Dabbebi, R.; de Aguiar, J.L.B.; Samet, B.; Baklouti, S. Mineralogical and Chemical Investigation of Tunisian Phosphate Washing Waste during Calcination. J. Therm. Anal. Calorim. 2019, 137, 1827–1840. [Google Scholar] [CrossRef]
- Yang, T.; Han, E.; Wang, X.; Wu, D. Surface Decoration of Polyimide Fiber with Carbon Nanotubes and Its Application for Mechanical Enhancement of Phosphoric Acid-Based Geopolymers. Appl. Surf. Sci. 2017, 416, 200–212. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Ming, L.Y.; Yong, H.C.; Tahir, M.F.M. Clay-Based Materials in Geopolymer Technology. In Cement Based Materials; Saleh, H.M., Ed.; IntechOpen: London, UK, 2018; pp. 239–264. ISBN 978-1-78984-154-1. [Google Scholar]
- Bittnar, Z.; Bartos, P.J.M.; Nemecek, J.; Smilauer, V.; Zeman, J. Nanotechnology in Construction 3; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9783642009792. [Google Scholar]
- Wei, S.; Mandel, J.A.; Said, S. Study of the Interface Strength in Steel Fiber-Reinforced Cement-Based Composites. Am. Concr. Inst. 1986, 83, 597–605. [Google Scholar] [CrossRef]
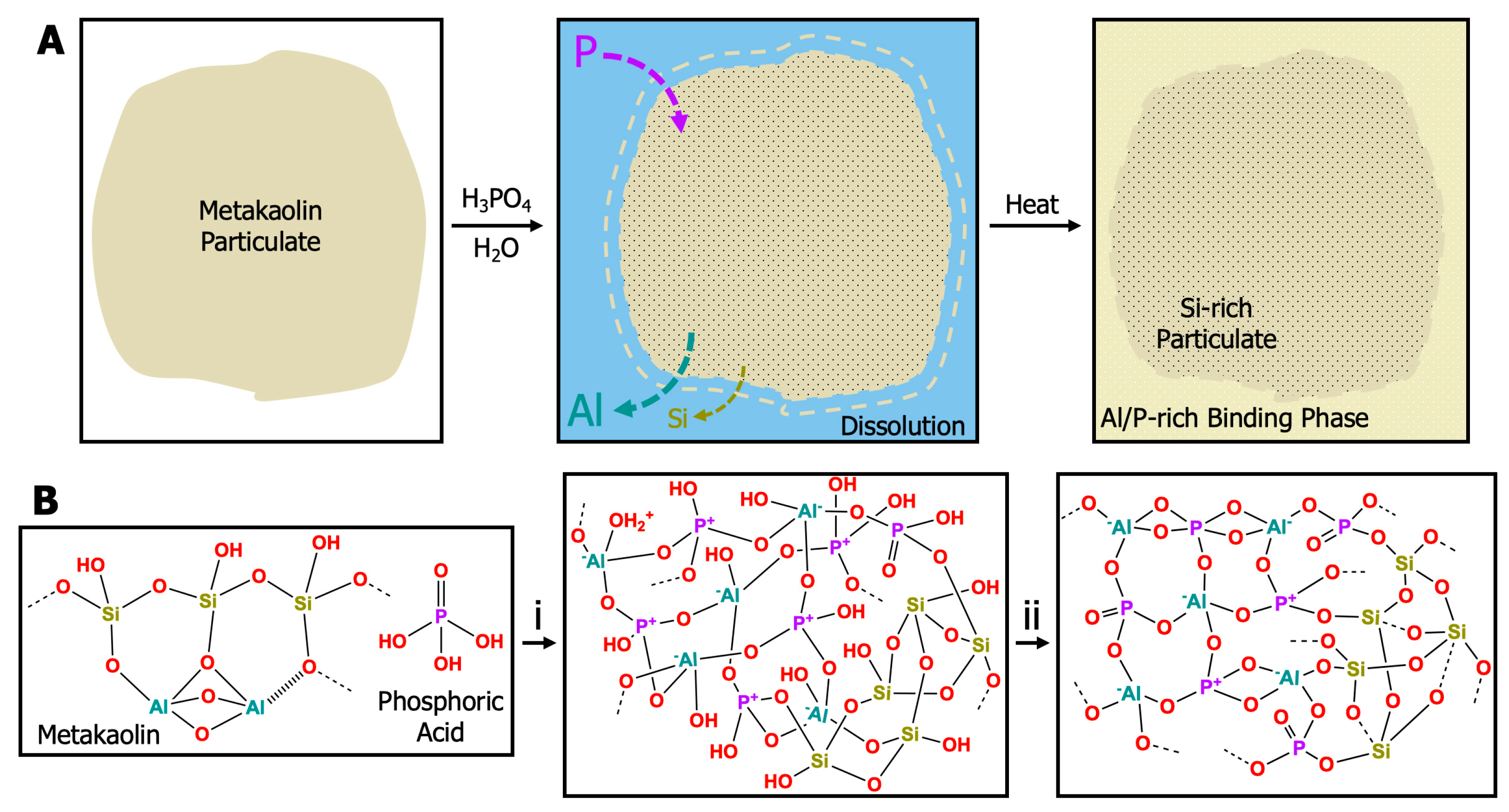
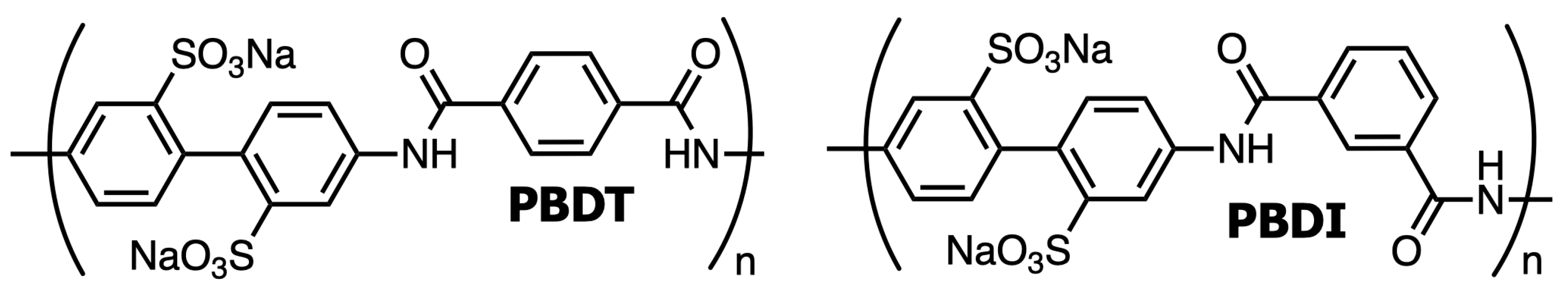
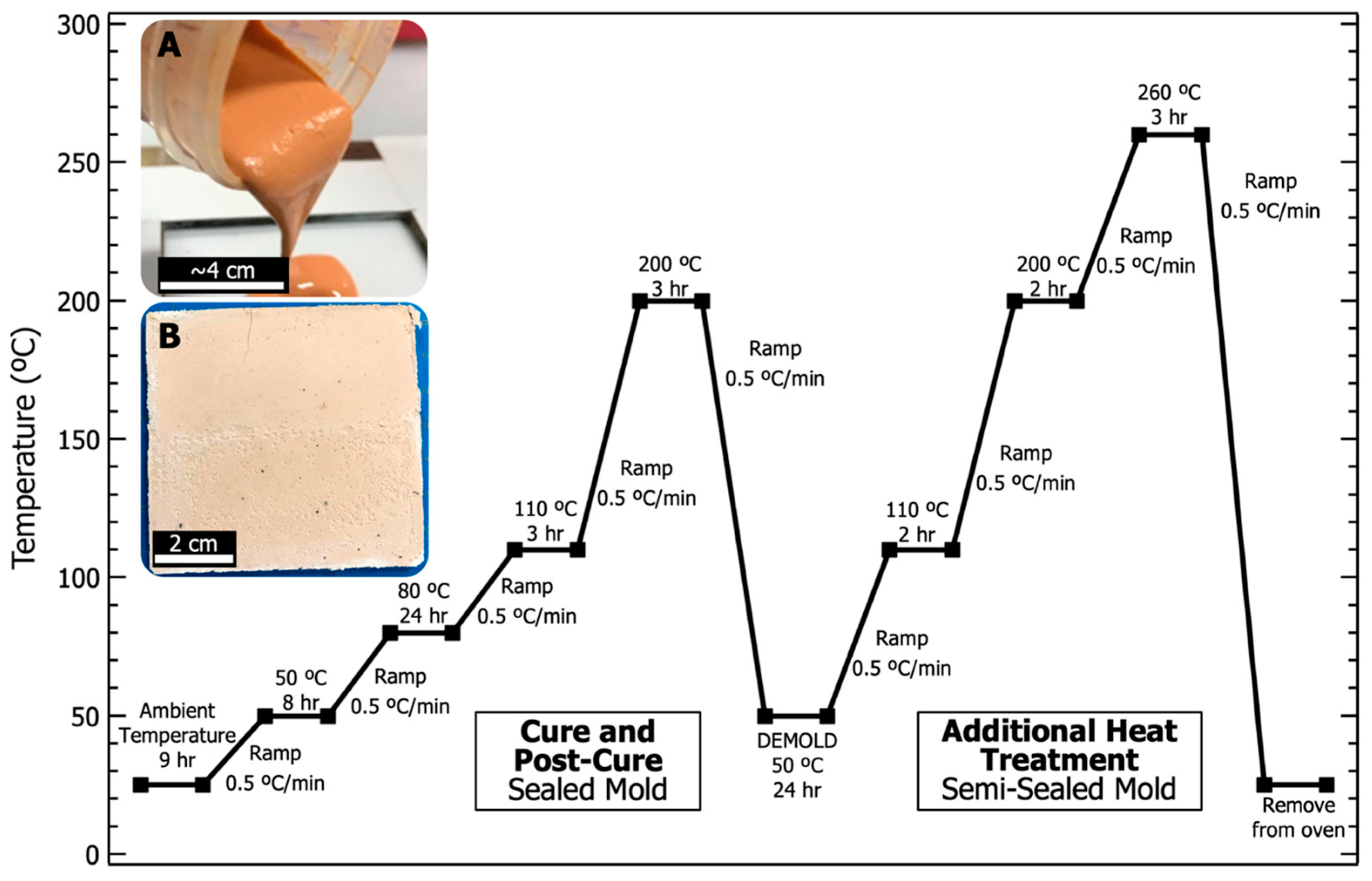
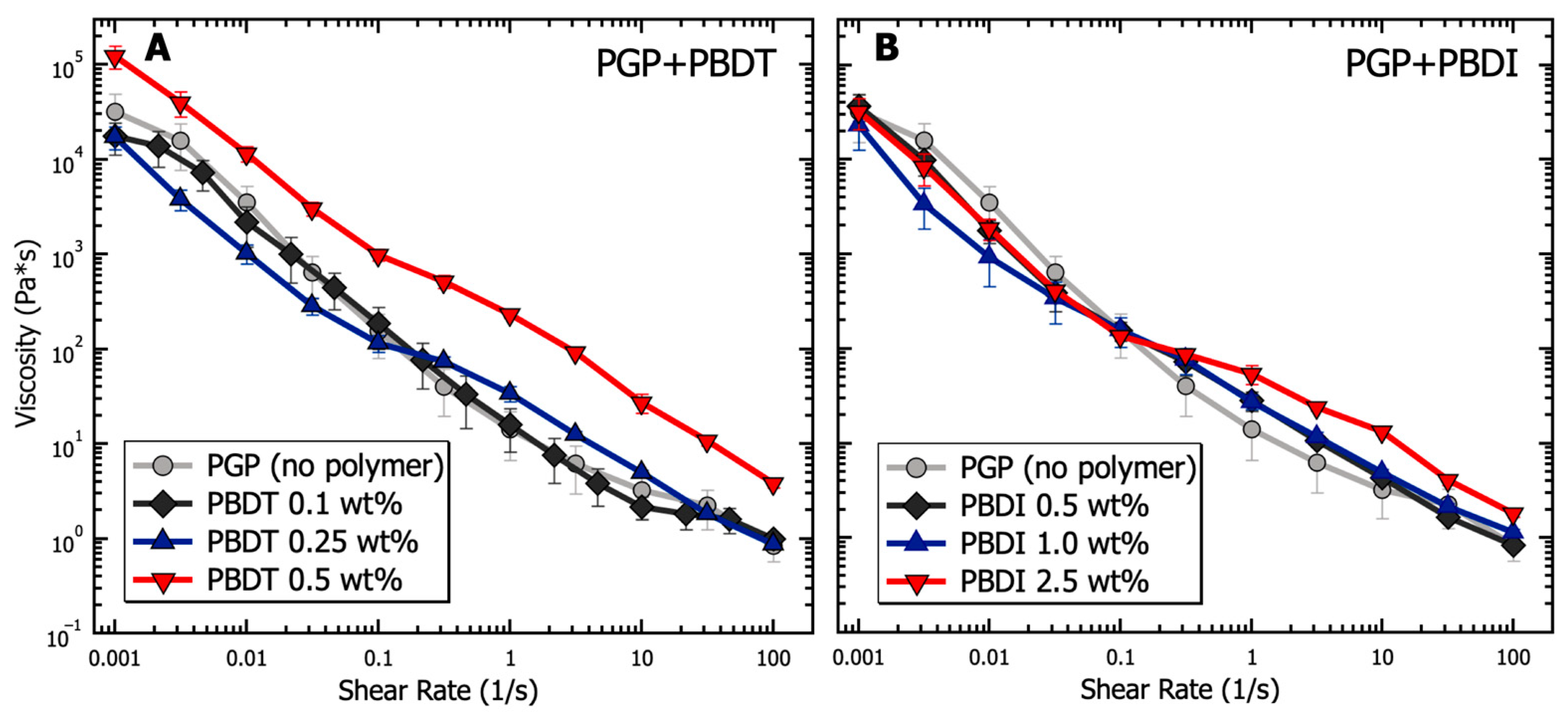


| Atomic Species | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | Na2O | P2O5 | TiO2 | MgO |
|---|---|---|---|---|---|---|---|---|---|
| Composition (wt. %) * | 54.04 | 41.93 | 1.52 | 0.13 | 0.48 | 0.047 | 0.15 | 2.35 | 0.12 |
| Sample | Polymer | Weight Percent of Polymer | Mass of Polymer Added | Mass of Metakaolin Added | Mass of 9M Phosphoric Acid Added | Mass of Total Solids |
|---|---|---|---|---|---|---|
| wt. % | G | g | g | g | ||
| PGP | - | - | - | 20.000 | 26.926 | 31.468 |
| PBDT 0.1 | PBDT | 0.101 | 0.027 | 20.000 | 26.926 | 31.495 |
| PBDT 0.25 | PBDT | 0.254 | 0.068 | 20.000 | 26.926 | 31.538 |
| PBDT 0.5 | PBDT | 0.503 | 0.136 | 20.000 | 26.926 | 31.604 |
| PBDI 0.5 | PBDI | 0.503 | 0.136 | 20.000 | 26.926 | 31.604 |
| PBDI 1.0 | PBDI | 1.003 | 0.273 | 20.000 | 26.926 | 31.741 |
| PBDI 2.5 | PBDI | 2.502 | 0.691 | 20.000 | 26.926 | 32.172 |
| Sample | Td, 5% | Total Water Loss @ 200 °C | Total Mass Loss @ 975 °C | Calculated Mass of Polymer in Composite | Targeted Solids Content of Polymer in Composite |
|---|---|---|---|---|---|
| °C | wt. % | wt. % | wt. % | wt. % | |
| PBDT | 489 | 18.1 | 72.9 | - | - |
| PBDI | 437 | 16.0 | 72.9 | - | - |
| PGP | - | 4.97 | 2.04 | - | - |
| PBDT 0.1 | - | 4.13 | 2.13 | 0.092 | 0.087 |
| PBDT 0.25 | - | 5.24 | 2.25 | 0.216 | 0.222 |
| PBDT 0.5 | - | 5.63 | 2.44 | 0.397 | 0.430 |
| PBDI 0.5 | - | 4.71 | 2.48 | 0.445 | 0.430 |
| PBDI 1.0 | - | 4.13 | 2.67 | 0.632 | 0.860 |
| PBDI 2.5 | - | 4.97 | 3.47 | 1.435 | 2.188 |
| Domains | All Samples | Target Ratio | |||
|---|---|---|---|---|---|
| O | Si | Al | P | O:Si, O:Al, O:P | |
| Atomic % | Atomic % | Atomic % | Atomic % | Atomic % | |
| Si-rich | 65.37 ± 1.39 | 19.77 ± 2.71 | 7.68 ± 2.24 | 7.17 ± 1.38 | 66.7:11.1 |
| Al/P-rich | 64.97 ± 1.53 | 5.63 ± 2.05 | 15.03 ± 0.32 | 14.38 ± 0.37 | 66.7:11.1 |
| Average | 65.20 ± 1.88 | 12.42 ± 5.23 | 11.67 ± 2.79 | 10.71 ± 2.65 | 66.7:11.1 |
| Sample | SEM | SEM | TEM | TEM | N2 Adsorption | N2 Adsorption | N2 Adsorption |
|---|---|---|---|---|---|---|---|
| Average Pore Size | Median Pore Size | Average Pore Size | Median Pore Size | BET Pore Size | BET Surface Area | BJH Pore Volume | |
| nm | nm | nm | Nm | nm | m2/g | cm3/g | |
| PGP | 784 | 627 | 38.1 | 35.9 | 23.0 | 21.4 | 0.129 |
| PBDT 0.1 | 570 | 430 | 30.0 | 27.3 | 18.4 | 12.7 | 0.059 |
| PBDT 0.25 | 658 | 534 | 27.3 | 25.8 | 12.6 | 45.2 | 0.130 |
| PBDT 0.5 | 313 | 234 | 24.5 | 21.7 | 15.0 | 33.2 | 0.112 |
| PBDI 0.5 | 564 | 480 | 25.6 | 27.5 | 10.4 | 42.8 | 0.095 |
| PBDI 1.0 | 820 | 697 | 30.6 | 33.2 | 12.2 | 19.8 | 0.063 |
| PBDI 2.5 | 942 | 696 | 21.0 | 18.5 | 15.4 | 32.2 | 0.116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliore, J.M.; Hewitt, P.; Dingemans, T.J.; Simone, D.L.; Monzel, W.J. Effect of Water-Soluble Polymers on the Rheology and Microstructure of Polymer-Modified Geopolymer Glass-Ceramics. Materials 2024, 17, 2856. https://doi.org/10.3390/ma17122856
Migliore JM, Hewitt P, Dingemans TJ, Simone DL, Monzel WJ. Effect of Water-Soluble Polymers on the Rheology and Microstructure of Polymer-Modified Geopolymer Glass-Ceramics. Materials. 2024; 17(12):2856. https://doi.org/10.3390/ma17122856
Chicago/Turabian StyleMigliore, John M., Patrick Hewitt, Theo J. Dingemans, Davide L. Simone, and William Jacob Monzel. 2024. "Effect of Water-Soluble Polymers on the Rheology and Microstructure of Polymer-Modified Geopolymer Glass-Ceramics" Materials 17, no. 12: 2856. https://doi.org/10.3390/ma17122856






