Corrosion Behavior of CMT Cladding Layer of AZ91 Magnesium Alloy Subjected to Friction Stir Processing
Abstract
1. Introduction
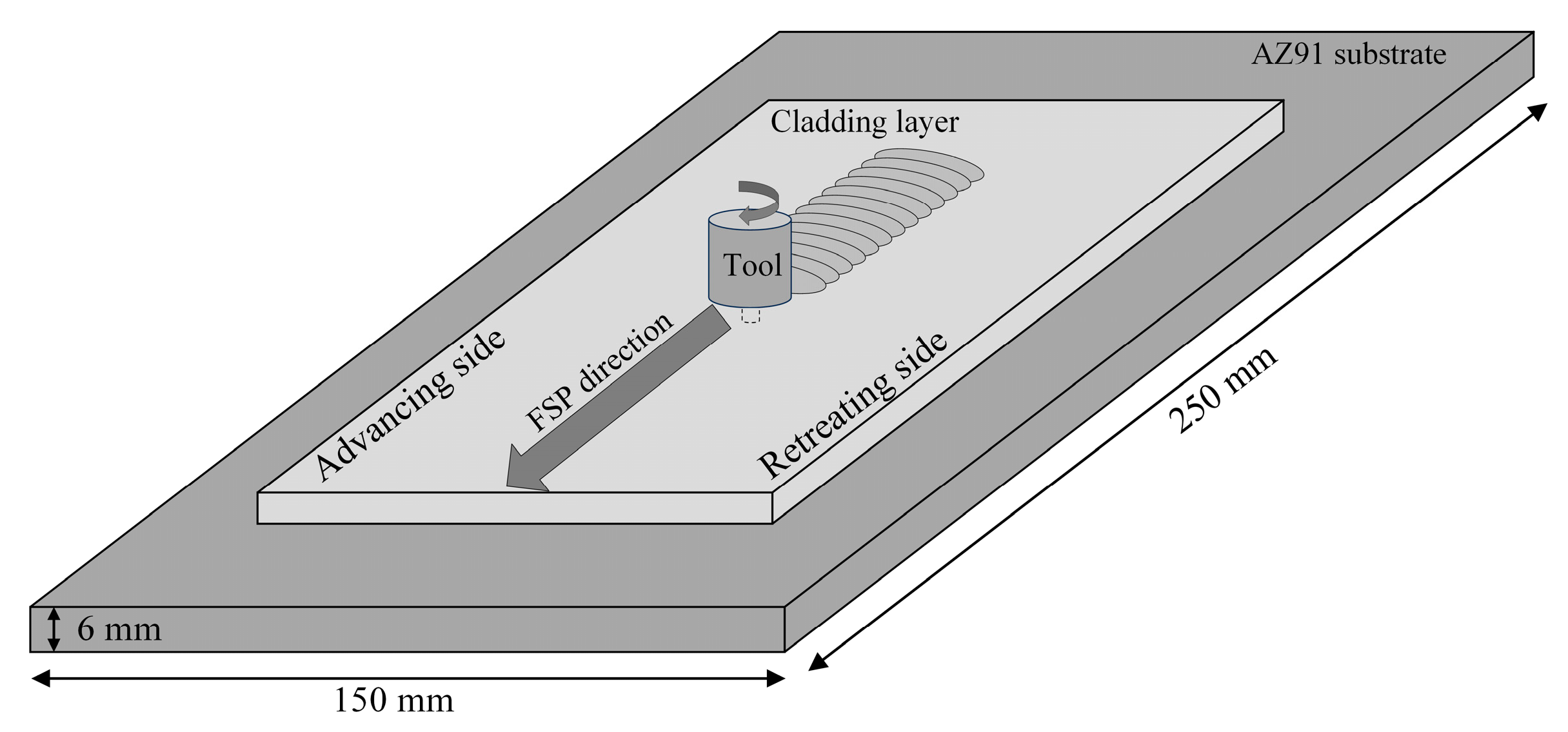
2. Experimental Procedure
3. Results and Discussion
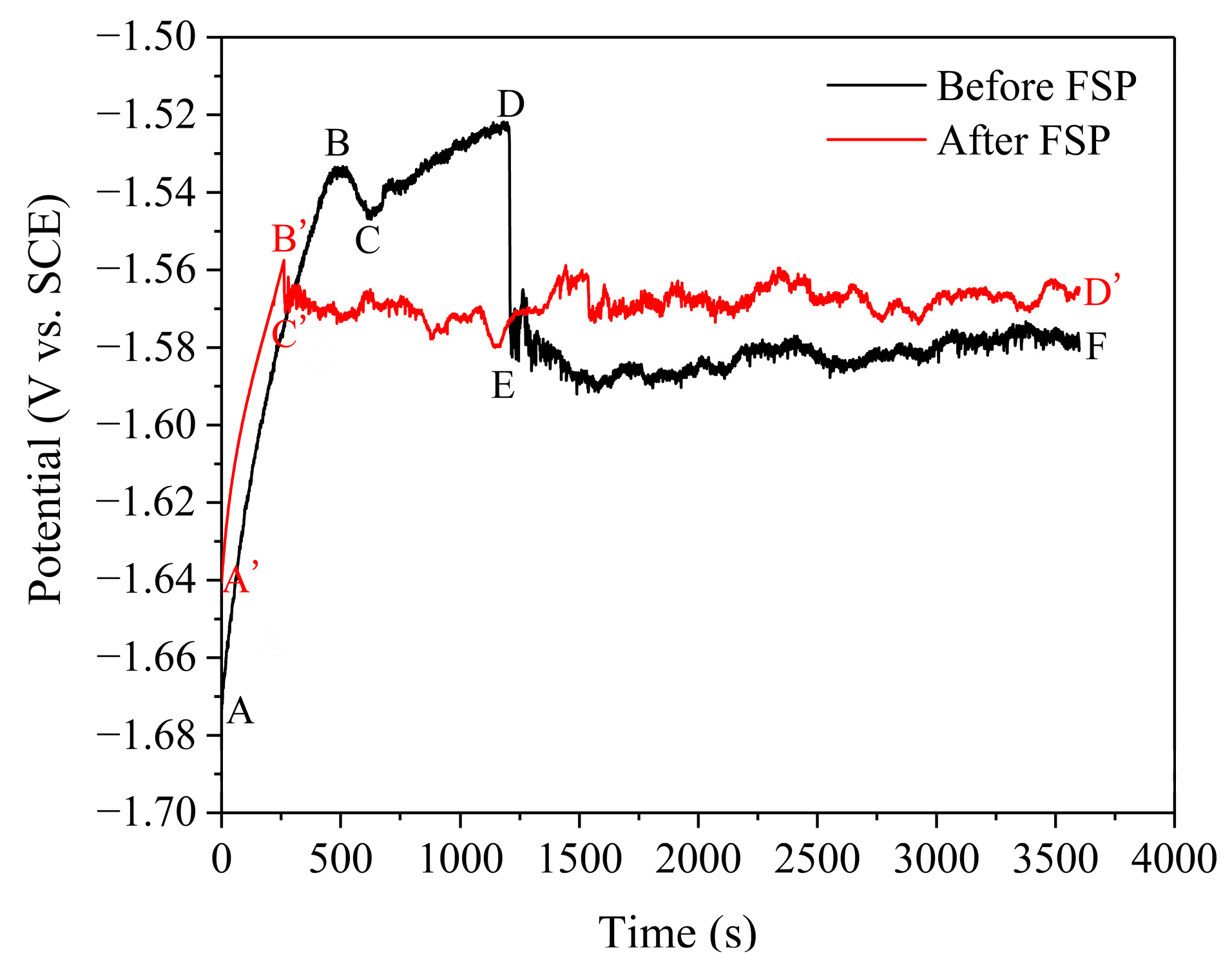
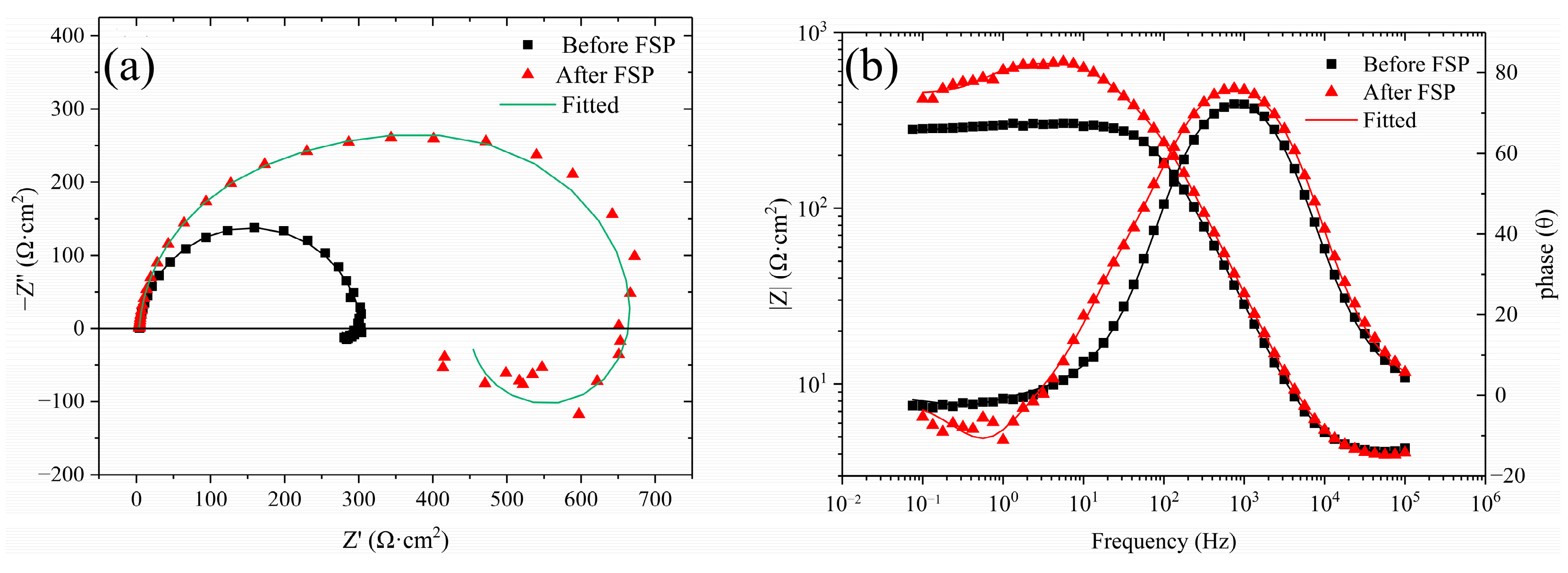
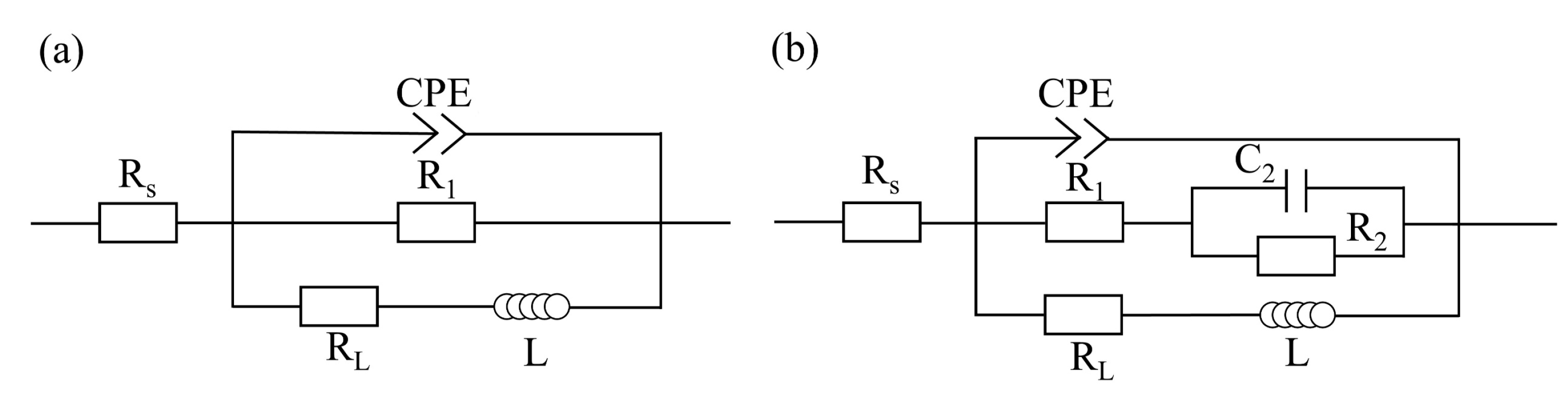
3.1. Electrochemical Measurements
3.2. Immersion Corrosion Behavior
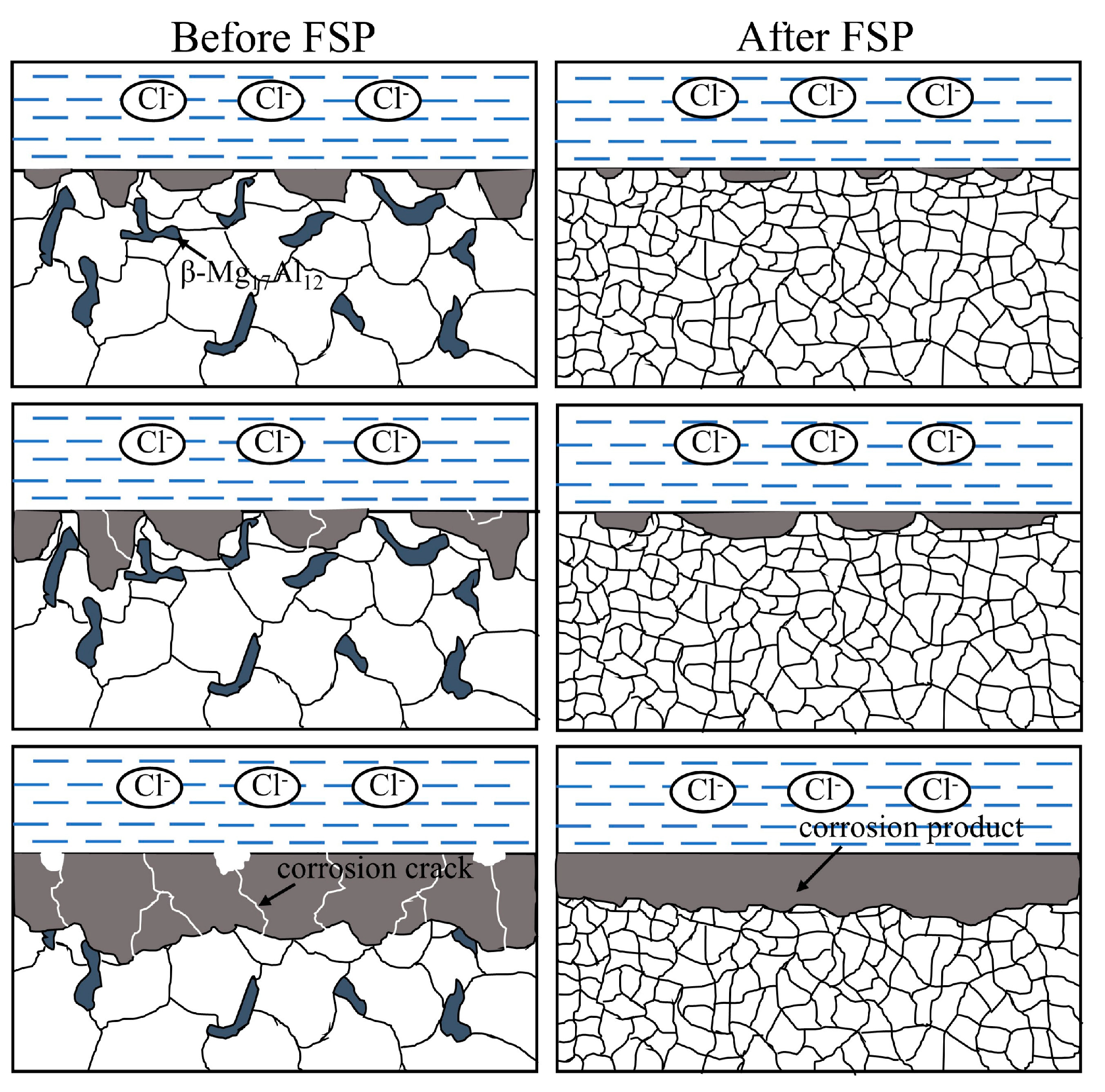
3.3. Corrosion Mechanism
4. Conclusions
- (1)
- Compared with the cladding layer before FSP, the corrosion potential and corrosion current density of the FSPed sample increased from −1.455 V to −1.397 V and decreased from 4.135 μA/cm2 to 1.275 μA/cm2, respectively. The protective corrosion product film formed on the FSPed cladding layer surface was denser, and the breakdown potential of the protective film was significantly increased.
- (2)
- The FSPed cladding layer had a higher charge-transfer resistance, indicating that its dissolution rate in NaCl solution was relatively low. The decrease in Y value and increase in n value of the CPE reflects that the corrosion product film structure of the FSPed cladding layer was denser and more uniform.
- (3)
- The corrosion rate of both the unprocessed and FSPed cladding layers in NaCl solution decreased with the increase in immersion time, and the corrosion rate of the FSPed sample was lower. Severe localized corrosion occurred on the unprocessed cladding layer surface accompanied by the formation of corrosion cracks, while relatively uniform corrosion with shallow corrosion pits occurred on the FSPed cladding layer surface.
- (4)
- An increase in the grain boundary density due to the grain refinement induced by FSP improved the adhesion between the protective film and the cladding layer substrate and facilitated the formation of a denser and more uniform corrosion product film on the cladding layer surface, leading to a decrease in the dissolution rate of the cladding layer. Additionally, due to the uniform distribution of the β phase within the α-Mg matrix in the FSPed cladding layer, the area ratio of cathode to anode decreased during the corrosion process, resulting in a decrease in galvanic corrosion rate.
- (5)
- FSP effectively improved the corrosion resistance of the AZ91 magnesium alloy CMT cladding layer by increasing the Al content in the α-Mg matrix, refining the grains and causing the fragmentation and dispersion of the β phase.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mordike, B.L.; Ebert, T. Magnesium: Properties—Applications—Potential. Mater. Sci. Eng. 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.M.; Chen, J.; Peng, X.D.; Chen, D.L.; Pan, F.S. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloy. 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, M.; Gao, J.C.; Hu, J.Z.; Zhang, Y. Corrosion process of pure magnesium in simulated body fluid. Mater. Lett. 2008, 62, 2181–2184. [Google Scholar] [CrossRef]
- Gan, Y.X.; Solomon, D.; Reinbolt, M. Friction Stir Processing of Particle Reinforced Composite Materials. Materials 2010, 3, 329–350. [Google Scholar] [CrossRef]
- Badkoobeh, F.; Mostaan, H.; Rafiei, M.; Bakhsheshi-Rad, H.R.; Berto, F. Friction Stir Welding/Processing of Mg-Based Alloys: A Critical Review on Advancements and Challenges. Materials 2021, 14, 6726. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Y.; Pilchak, A.L.; Juhas, M.C.; Williams, J.C. Microstructural refinement and property enhancement of cast light alloys via friction stir processing. Scr. Mater. 2008, 58, 361–366. [Google Scholar] [CrossRef]
- Asadi, P.; Mahdavinejad, R.A.; Tutunchilar, S. Simulation and experimental investigation of FSP of AZ91 magnesium alloy. Mater. Sci. Eng. A 2011, 528, 6469–6477. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, W.Q.; Zhang, J.; Yi, J.Z.; Cui, Y.F. Evolution of microstructure of WE43 magnesium alloys fabricated by laser deposition manufacturing with subsequent friction stir processing. Mater. Lett. 2023, 330, 133218. [Google Scholar] [CrossRef]
- Zhen, Y.H.; Shen, J.Q.; Hu, S.S.; Yin, C.X.; Yin, F.L.; Bu, X.Z. Effect of rotation rate on microstructure and mechanical properties of CMT cladding layer of AZ91 magnesium alloy fabricated by friction stir processing. J. Manuf. Process. 2022, 79, 553–561. [Google Scholar] [CrossRef]
- Patel, V.; Li, W.Y.; Andersson, J.; Li, N. Enhancing grain refinement and corrosion behavior in AZ31B magnesium alloy via stationary shoulder friction stir processing. J. Mater. Res. Technol. 2022, 17, 3150–3156. [Google Scholar] [CrossRef]
- Huang, L.; Wang, K.S.; Wang, W.; Yuan, J.; Qiao, K.; Yang, T.; Peng, P.; Li, T.Q. Effects of grain size and texture on stress corrosion cracking of friction stir processed AZ80 magnesium alloy. Eng. Fail. Anal. 2018, 92, 392–404. [Google Scholar] [CrossRef]
- Arora, H.S.; Singh, H.; Dhindaw, B.K. Parametric Study of Friction Stir Processing of Magnesium-Based AE42 Alloy. J. Mater. Eng. Perform. 2012, 21, 2328–2339. [Google Scholar] [CrossRef]
- Qiao, K.; Zhang, T.; Wang, K.S.; Yuan, S.N.; Wang, L.Q.; Chen, S.Y.; Wang, Y.H.; Xue, K.R.; Wang, W. Effect of multi-pass friction stir processing on the microstructure evolution and corrosion behavior of ZrO2/AZ31 magnesium matrix composite. J. Mater. Res. Technol. 2022, 18, 1166–1179. [Google Scholar] [CrossRef]
- Wu, B.; Yusof, F.; Li, F.G.; Razak, B.B.A.; Bin Muhamad, M.R.; Badruddin, I.A.; Hussien, M.; Kamangar, S.; Ibrahim, M.Z. Influence of Friction Stir Processing Parameters on Microstructure, Hardness and Corrosion Resistance of Biocompatible Mg Alloy WE43. Arab. J. Sci. Eng. 2024, 49, 1897–1911. [Google Scholar] [CrossRef]
- Kumar, K.; Das, A.; Prasad, S.B. Effect of multi-pass friction stir processing on mechanical, microstructural, bioactivity, and corrosion properties of novel magnesium-hopeite composite for biomedical applications. Mater. Today Commun. 2023, 35, 105584. [Google Scholar] [CrossRef]
- Baradarani, F.; Mostafapour, A.; Shalvandi, M. Enhanced corrosion behavior and mechanical properties of AZ91 magnesium alloy developed by ultrasonic-assisted friction stir processing. Mater. Corros. 2020, 71, 109–117. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, Q.X.; Chen, G.Q.; Cao, X.; Zhang, S.; Pan, J.L.; Zhang, G.; Shi, Q.Y. Enhanced corrosion resistance of AZ91 magnesium alloy through refinement and homogenization of surface microstructure by friction stir processing. Corros. Sci. 2018, 138, 284–296. [Google Scholar] [CrossRef]
- Arora, H.S.; Singh, H.; Dhindaw, B.K. Corrosion behavior of a Mg alloy AE42 subjected to friction stir processing. Corrosion 2013, 69, 122–135. [Google Scholar] [CrossRef]
- Liao, J.S.; Hotta, M.; Yamamoto, N. Corrosion behavior of fine-grained AZ31B magnesium alloy. Corros. Sci. 2012, 61, 208–214. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.P.; Shinohara, T. Corrosion behavior of AZ91 magnesium alloy in dilute NaCl solutions. Mater. Des. 2010, 31, 857–863. [Google Scholar] [CrossRef]
- Lu, F.M.; Ma, A.B.; Jiang, J.H.; Gou, Y.; Yang, D.H.; Song, D.; Chen, J.Q. Significantly improved corrosion resistance of heat-treated Mg-Al-Gd alloy containing profuse needle-like precipitates within grains. Corros. Sci. 2015, 94, 171–178. [Google Scholar] [CrossRef]
- Miao, H.W.; Huang, H.; Shi, Y.J.; Zhang, H.; Pei, J.; Yuan, G.Y. Effects of solution treatment before extrusion on the microstructure, mechanical properties and corrosion of Mg-Zn-Gd alloy in vitro. Corros. Sci. 2017, 122, 90–99. [Google Scholar] [CrossRef]
- Asmussen, R.M.; Binns, W.J.; Jakupi, P.; Shoesmith, D. Microstructural effects on corrosion of AM50 magnesium alloys. J. Electrochem. Soc. 2014, 161, C501–C508. [Google Scholar] [CrossRef]
- Gao, S.K.; Zhao, H.Y.; Li, G.H.; Sun, G.D.; Zhou, L.; Zhao, Y.B. Strengthening mechanism and corrosion behavior of friction stir welded LAZ933 magnesium-lithium alloy. J. Magnes. Alloy. 2023, in press. [Google Scholar] [CrossRef]
- Banerjee, P.C.; Raman, R.K.S.; Durandet, Y.; McAdam, G. Electrochemical investigation of the influence of laser surface melting on the microstructure and corrosion behaviour of ZE41 magnesium alloy—An EIS based study. Corros. Sci. 2011, 53, 1505–1514. [Google Scholar] [CrossRef]
- Song, G.L.; Bowles, A.L.; Stjohn, D.H. Corrosion resistance of aged die cast magnesium alloy AZ91D. Mater. Sci. Eng. A 2004, 366, 74–86. [Google Scholar] [CrossRef]
- Cui, L.Y.; Hu, Y.; Zeng, R.C.; Yang, Y.X.; Sun, D.D.; Li, S.Q.; Zhang, F.; Han, E.H. New insights into the effect of Tris-HCl and Tris on corrosion of magnesium alloy in presence of bicarbonate, sulfate, hydrogen phosphate and dihydrogen phosphate ions. J. Mater. Sci. Technol. 2017, 33, 971–986. [Google Scholar] [CrossRef]
- Huang, L.Y.; Wang, K.S.; Wang, W.; Peng, P.; Qiao, K.; Liu, Q. Microstructural evolution and corrosion behavior of friction stir processed fine-grained AZ80 Mg alloy. Mater. Corros. 2020, 71, 93–108. [Google Scholar] [CrossRef]
- Saikrishna, N.; Reddy, G.P.K.; Munirathinam, B.; Sunil, B.R. Influence of bimodal grain size distribution on the corrosion behavior of friction stir processed biodegradable AZ31 magnesium alloy. J. Magnes. Alloy. 2016, 4, 68–76. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, G.Q.; Zeng, S.B.; Zhang, S.; Long, F.; Shi, Q.Y. The corrosion behavior of Mg-9Al-xRE magnesium alloys modified by friction stir processing. J. Alloy. Compd. 2021, 851, 156835. [Google Scholar] [CrossRef]
- Song, D.; Ma, A.B.; Jiang, J.H.; Lin, P.H.; Yang, D.H.; Fan, J.F. Corrosion behavior of equal-channel-angular-pressed pure magnesium in NaCl aqueous solution. Corros. Sci. 2010, 52, 481–490. [Google Scholar] [CrossRef]
- Mathieu, S.; Rapin, C.; Hazan, J.; Steinmetz, P. Corrosion behavior of high pressure die-cast and semi-solid cast AZ91D alloys. Corros. Sci. 2002, 44, 2737–2756. [Google Scholar] [CrossRef]
- Zhen, Y.H.; Shen, J.Q.; Hu, S.S.; Bi, J.; Yin, F.L.; Bu, X.Z. Effect of friction stir processing on microstructure and properties of AZ91 magnesium alloy CMT cladding layer. Mater. Lett. 2021, 282, 128701. [Google Scholar] [CrossRef]
- Birbilis, N.; Ralston, K.D.; Virtanen, S.; Fraser, H.L.; Davies, C.H.J. Grain character influences on corrosion of ECAPed pure magnesium. Corros. Eng. Sci. Technol. 2010, 45, 224–230. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 319–324. [Google Scholar] [CrossRef]
- Zhao, M.C.; Liu, M.; Song, G.L.; Atrens, A. Influence of the β-phase morphology on the corrosion of the Mg alloy AZ91. Corros. Sci. 2008, 50, 1939–1953. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A.; Dargusch, M. Influence of microstructure on the corrosion of diecast AZ91D. Corros. Sci. 1999, 41, 249–273. [Google Scholar] [CrossRef]




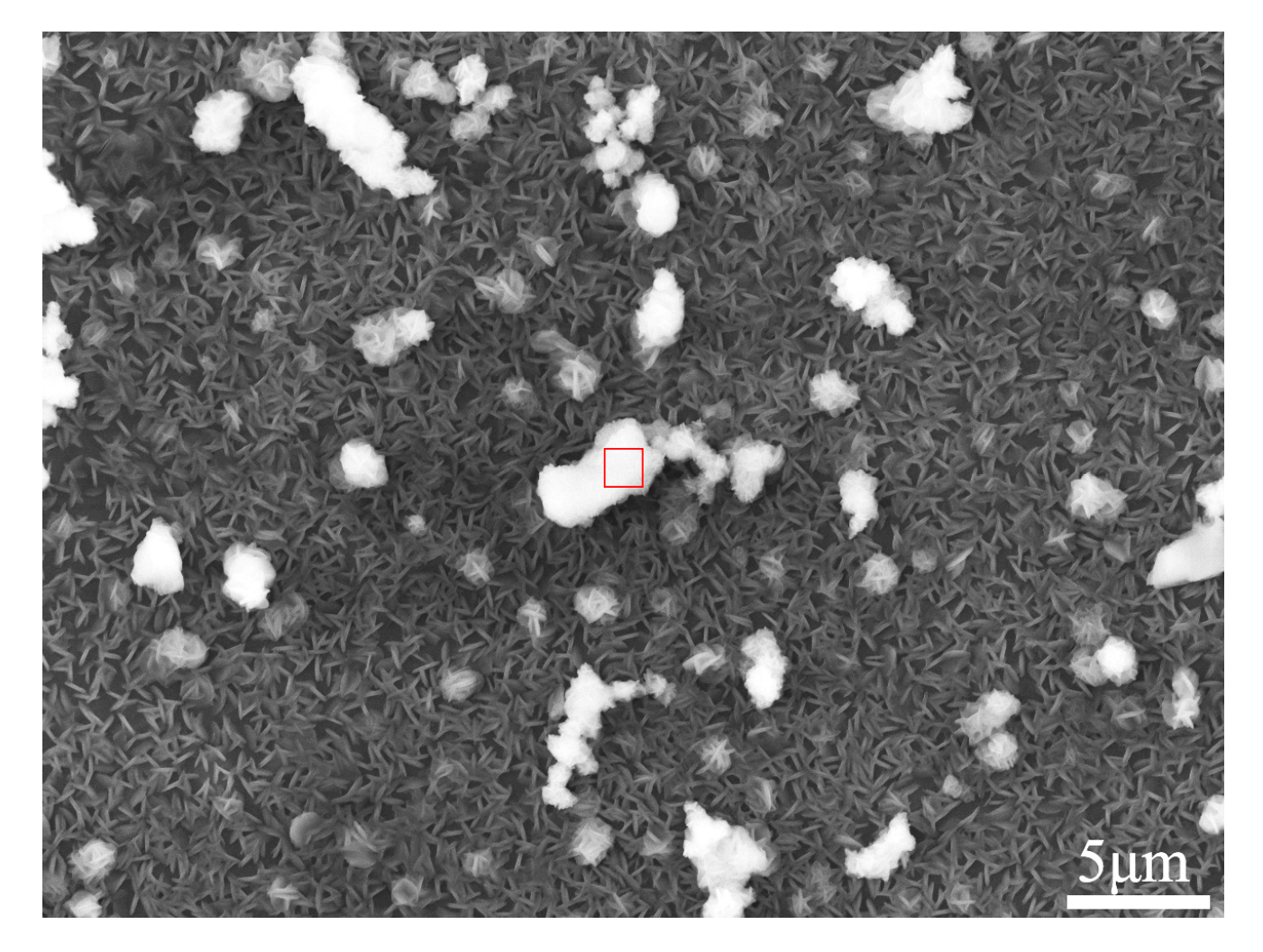
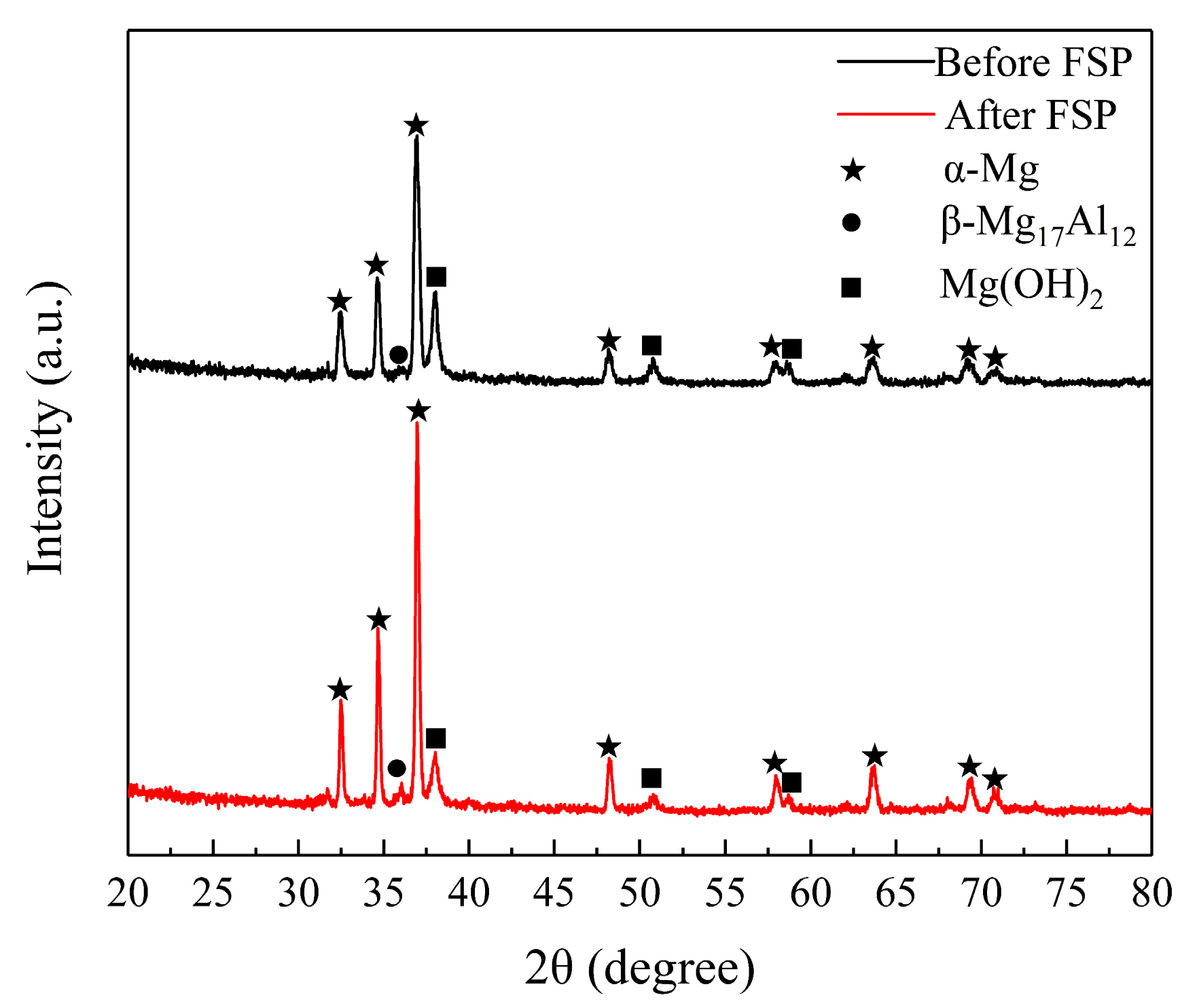

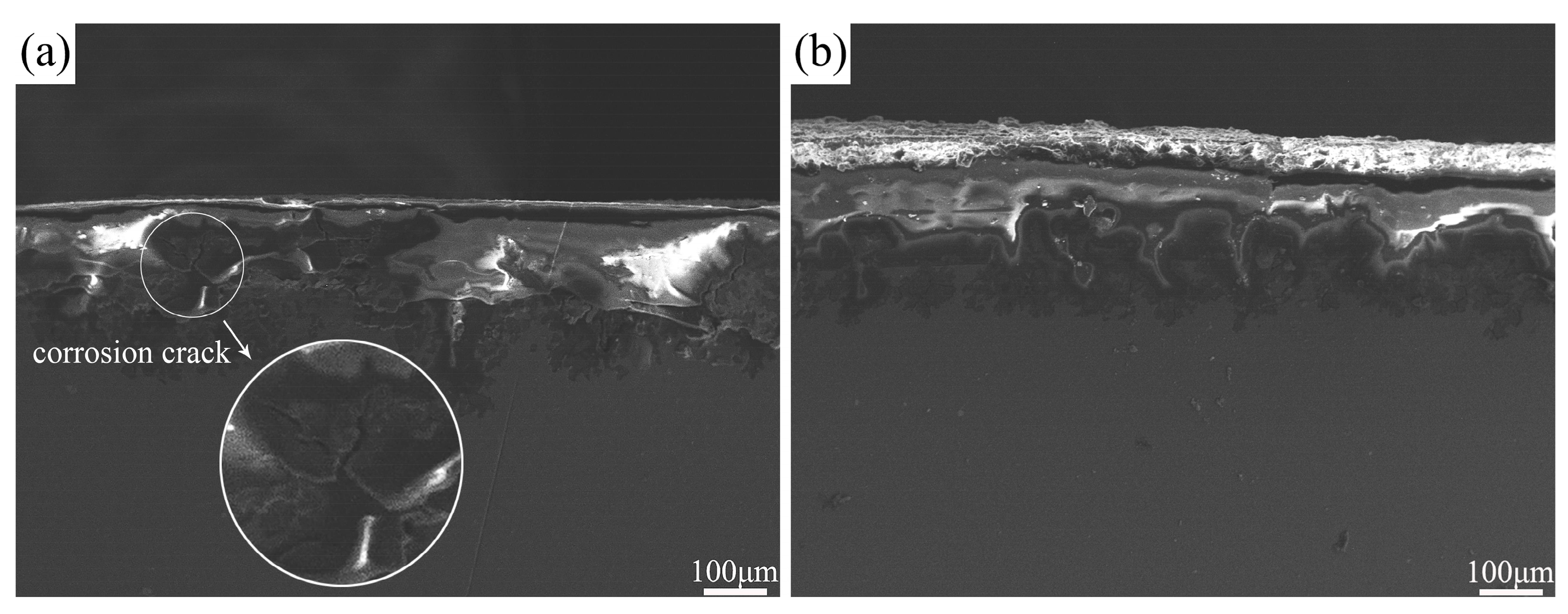

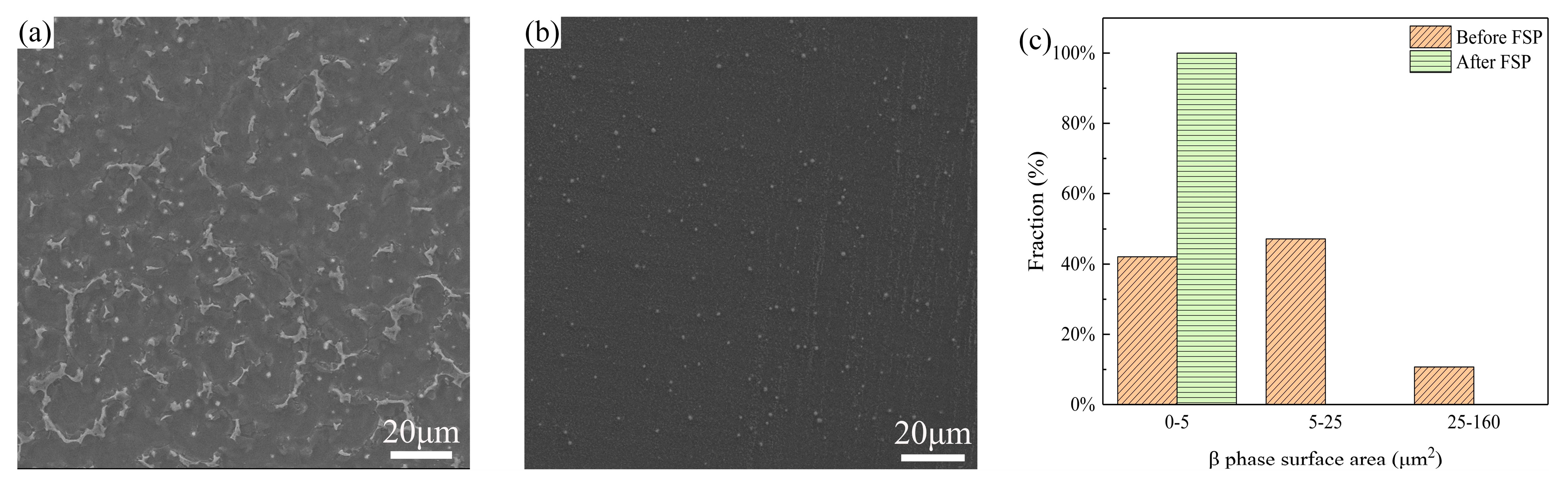
| Material | Al | Zn | Mn | Si | Cu | Fe | Ni | Mg |
|---|---|---|---|---|---|---|---|---|
| Substrate (AZ91) | 8.70 | 0.58 | 0.24 | 0.020 | 0.0050 | 0.0020 | 0.00100 | Bal. |
| Wire (AZ91) | 8.99 | 0.65 | 0.26 | 0.037 | 0.0025 | 0.0018 | 0.00043 | Bal. |
| Parameter | Before FSP | After FSP |
|---|---|---|
| Rs (Ω·cm2) | 4.03 | 3.86 |
| R1 (Ω·cm2) | 300 | 479.4 |
| Y (sn·Ω−1·cm−2) | 9.22 × 10−6 | 7.22 × 10−6 |
| n | 0.95 | 0.96 |
| C2 (F·cm−2) | / | 3.32 × 10−5 |
| R2 (Ω·cm2) | / | 198.2 |
| RL (Ω·cm2) | 2990 | 1311 |
| L (H·cm−2) | 1452 | 415.8 |
| Element | O | Mg | Al | Cl |
|---|---|---|---|---|
| wt.% | 44.57 | 41.63 | 3.16 | 10.63 |
| at.% | 56.68 | 34.84 | 2.38 | 6.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Shen, J.; Hu, S.; Zhen, Y.; Zhao, H. Corrosion Behavior of CMT Cladding Layer of AZ91 Magnesium Alloy Subjected to Friction Stir Processing. Materials 2024, 17, 2875. https://doi.org/10.3390/ma17122875
Chen Y, Shen J, Hu S, Zhen Y, Zhao H. Corrosion Behavior of CMT Cladding Layer of AZ91 Magnesium Alloy Subjected to Friction Stir Processing. Materials. 2024; 17(12):2875. https://doi.org/10.3390/ma17122875
Chicago/Turabian StyleChen, Yang, Junqi Shen, Shengsun Hu, Yahui Zhen, and Huichao Zhao. 2024. "Corrosion Behavior of CMT Cladding Layer of AZ91 Magnesium Alloy Subjected to Friction Stir Processing" Materials 17, no. 12: 2875. https://doi.org/10.3390/ma17122875
APA StyleChen, Y., Shen, J., Hu, S., Zhen, Y., & Zhao, H. (2024). Corrosion Behavior of CMT Cladding Layer of AZ91 Magnesium Alloy Subjected to Friction Stir Processing. Materials, 17(12), 2875. https://doi.org/10.3390/ma17122875







