Silver Decoration of Vertically Aligned MoS2-MoOx Nanosheets: A Comprehensive XPS Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Vertically Aligned MoS2
2.3. Silver Deposition and Functionalization of the Samples
2.4. Characterization of the Samples
3. Results and Discussion
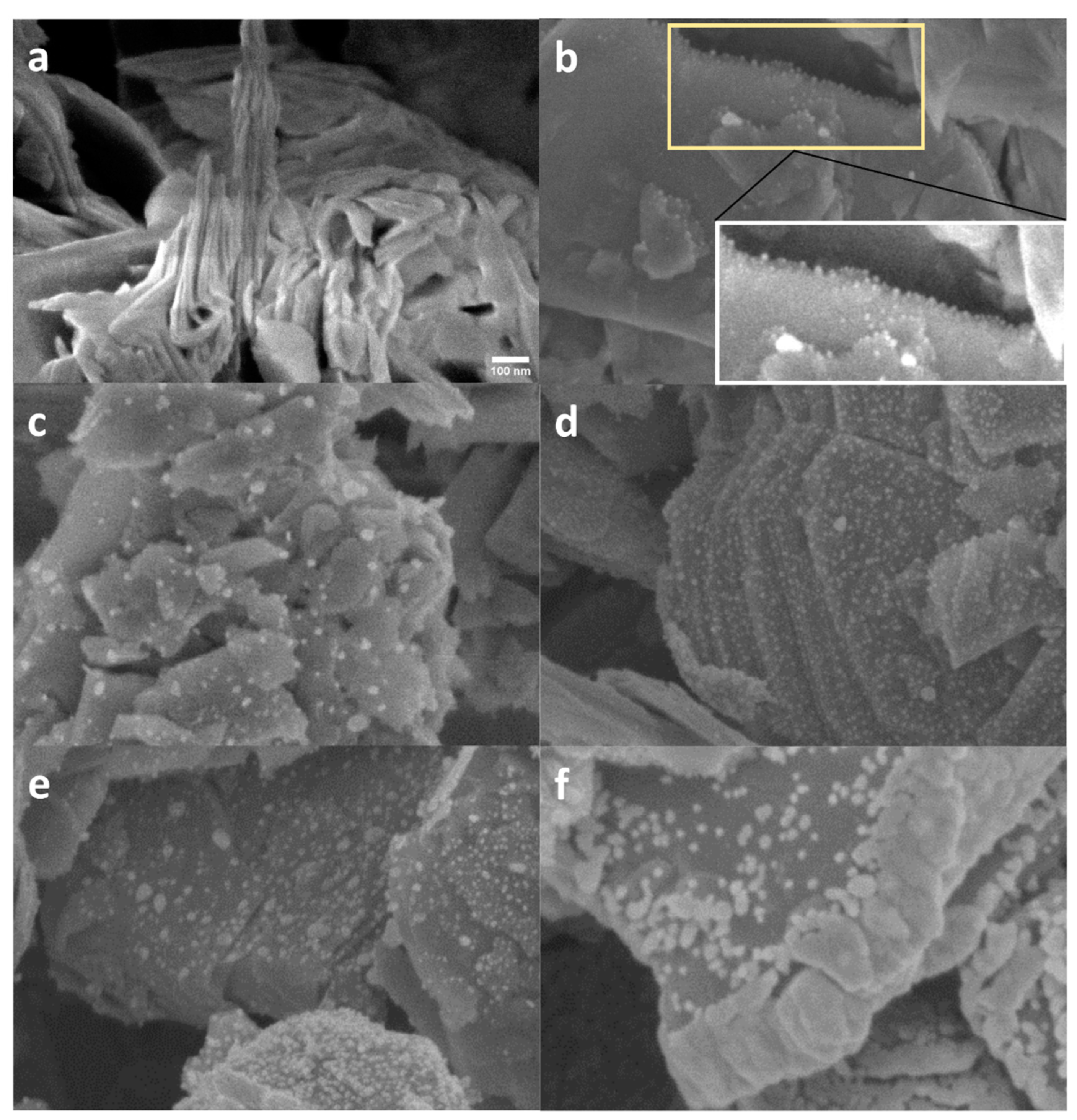
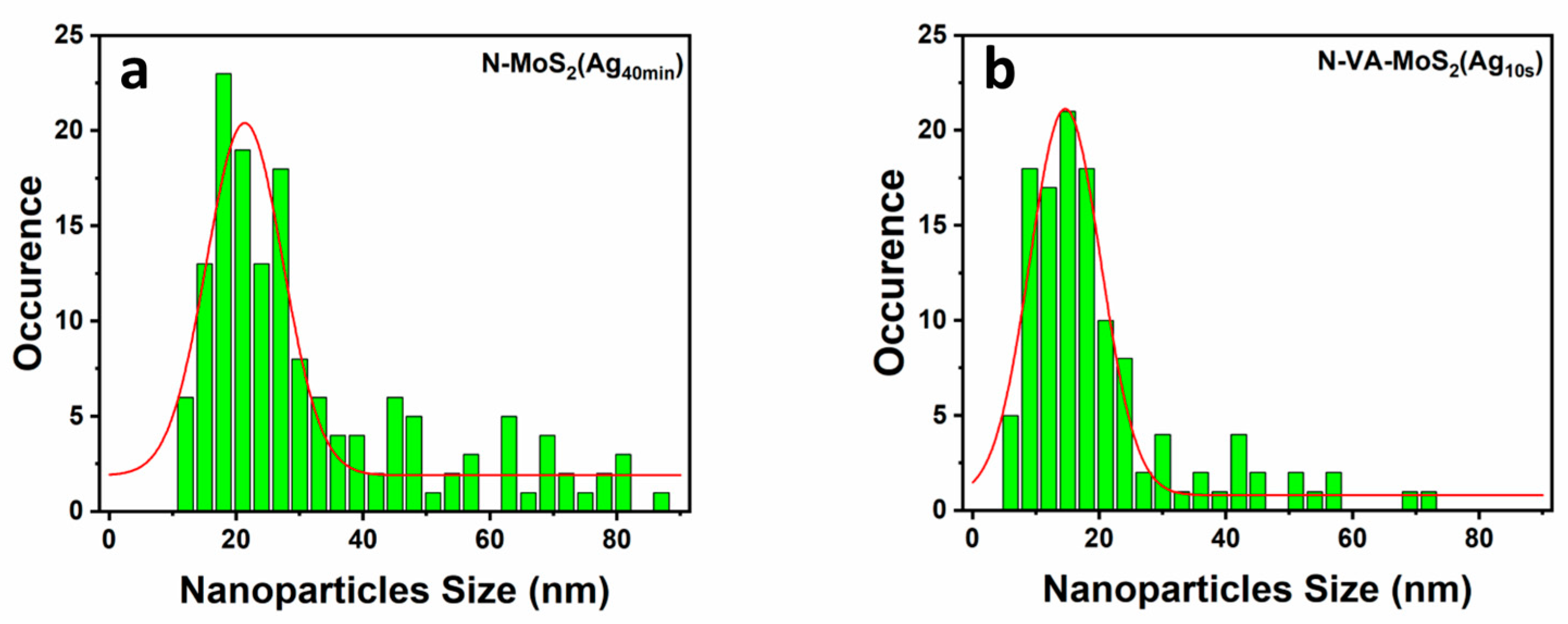
3.1. Scanning Electron Microscopy (SEM)
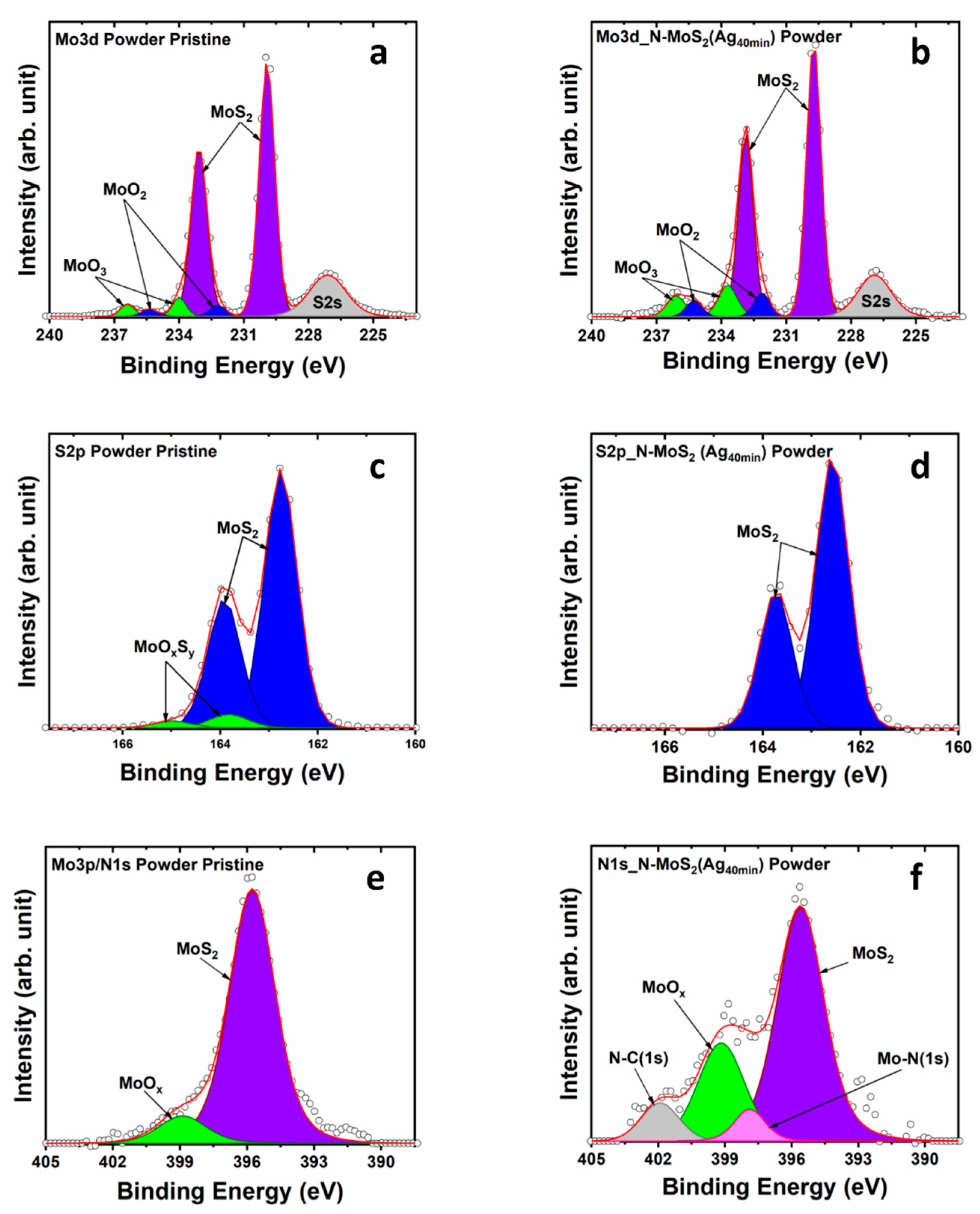
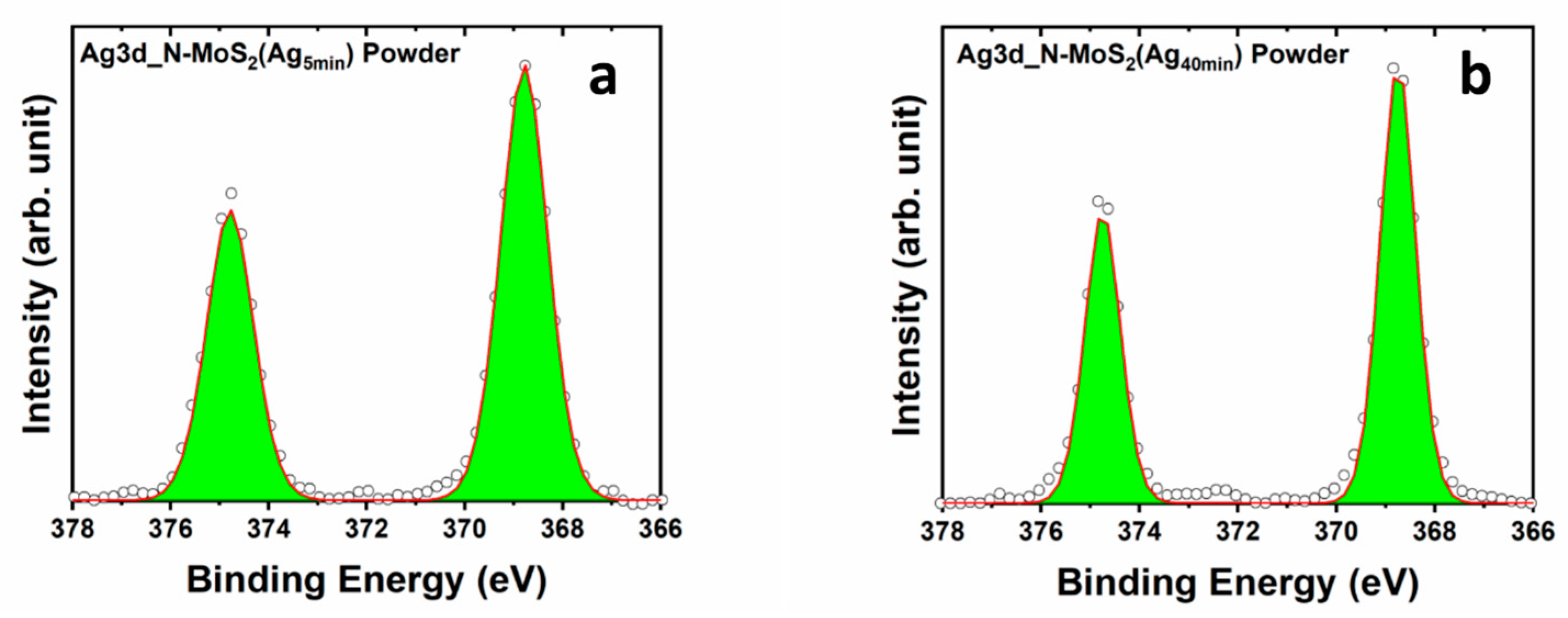
3.2. X-ray Photoelectron Spectroscopy
3.3. Valence Band Offset (VBO)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, H.; Xu, T.; Wang, Z.; Ren, W.; Nan, H.; Ni, Z.; Chen, Q.; Yuan, S.; Miao, F.; Song, F.; et al. Hopping transport through defect-induced localized states in molybdenum disulphide. Nat. Commun. 2013, 4, 2642. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Nagashio, K.; Toriumi, A. Experimental detection of active defects in few layers MoS2 through random telegraphic signals analysis observed in its FET characteristics. 2D Mater. 2016, 4, 015035. [Google Scholar] [CrossRef]
- Yang, L.; Majumdar, K.; Liu, H.; Du, Y.; Wu, H.; Hatzistergos, M.; Hung, P.Y.; Tieckelmann, R.; Tsai, W.; Hobbs, C.; et al. Chloride Molecular Doping Technique on 2D Materials: WS2 and MoS2. Nano Lett. 2014, 14, 6275–6280. [Google Scholar] [CrossRef] [PubMed]
- Khazaeizhad, R.; Kassani, S.H.; Jeong, H.; Yeom, D.-I.; Oh, K. Mode-locking of Er-doped fiber laser using a multilayer MoS2 thin film as a saturable absorber in both anomalous and normal dispersion regimes. Opt. Express 2014, 22, 23732. [Google Scholar] [CrossRef] [PubMed]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Kim, H.; Cho, J. MoS2 Nanoplates Consisting of Disordered Graphene-like Layers for High Rate Lithium Battery Anode Materials. Nano Lett. 2011, 11, 4826–4830. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 Films with Vertically Aligned Layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kim, M.; Cho, S.H.; Jung, Y.S. Vertically aligned nanostructures for a reliable and ultrasensitive SERS-active platform: Fabrication and engineering strategies. Nano Today 2021, 37, 101063. [Google Scholar] [CrossRef]
- Bruix, A.; Füchtbauer, H.G.; Tuxen, A.K.; Walton, A.S.; Andersen, M.; Porsgaard, S.; Besenbacher, F.; Hammer, B.; Lauritsen, J.V. In Situ Detection of Active Edge Sites in Single-Layer MoS2 Catalysts. ACS Nano 2015, 9, 9322–9330. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Zapien, J.A.; Afrose, R.; Anam, T.K.; Rahman, M.; Liton, M.N.H.; Helal, M.A.; Khan, M.K.R.; Ayotunde Emmanuel, A. A comparative study of Ag doping effects on the electronic, optical, carrier conversion, photocatalytic and electrical properties of MoS2. Mater. Sci. Eng. B 2021, 273, 115442. [Google Scholar] [CrossRef]
- Sierra-Castillo, A.; Haye, E.; Acosta, S.; Arenal, R.; Bittencourt, C.; Colomer, J.-F. Atmospheric pressure chemical vapor deposition growth of vertically aligned SnS2 and SnSe2 nanosheets. RSC Adv. 2021, 11, 36483–36493. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.; Grinvald, S.; Kirshner, M.; Sinai, O.; Oksman, M.; Alon, H.; Meiron, O.E.; Bar-Sadan, M.; Houben, L.; Naveh, D. Growth Mechanisms and Electronic Properties of Vertically Aligned MoS2. Sci. Rep. 2018, 8, 16480. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, S.; Li, H.; Li, M.; Chen, T.; Xiao, X.; Wang, Y. Recent advances in MoS2-based nanomaterial sensors for room-temperature gas detection: A review. Sens. Diagn. 2023, 2, 361–381. [Google Scholar] [CrossRef]
- Radziuk, D.; Moehwald, H. Prospects for plasmonic hot spots in single molecule SERS towards the chemical imaging of live cells. Phys. Chem. Chem. Phys. 2015, 17, 21072–21093. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-M.; Oh, J.-W.; Lee, H.; Suh, Y.D. Plasmonic Nanogap-Enhanced Raman Scattering with Nanoparticles. Acc. Chem. Res. 2016, 49, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Rogach, A.L.; Jäckel, F.; Klar, T.A.; Feldmann, J. Properties and Applications of Colloidal Nonspherical Noble Metal Nanoparticles. Adv. Mater. 2010, 22, 1805–1825. [Google Scholar] [CrossRef] [PubMed]
- Fiameni, S.; Battiston, S.; Castellani, V.; Barison, S.; Armelao, L. Implementing sustainability in laboratory activities: A case study on aluminum titanium nitride based thin film magnetron sputtering deposition onto commercial laminated steel. J. Clean. Prod. 2021, 285, 124869. [Google Scholar] [CrossRef]
- Hassan, M.M. Antimicrobial Coatings for Textiles. In Handbook of Antimicrobial Coatings; Elsevier: Amsterdam, The Netherlands, 2013; pp. 321–355. [Google Scholar]
- Yin, Y.; Li, C.; Yan, Y.; Xiong, W.; Ren, J.; Luo, W. MoS2-Based Substrates for Surface-Enhanced Raman Scattering: Fundamentals, Progress and Perspective. Coatings 2022, 12, 360. [Google Scholar] [CrossRef]
- Dolui, K.; Rungger, I.; Das Pemmaraju, C.; Sanvito, S. Possible doping strategies for MoS2 monolayers: An ab initio study. Phys. Rev. B 2013, 88, 075420. [Google Scholar] [CrossRef]
- Yue, Q.; Chang, S.; Qin, S.; Li, J. Functionalization of monolayer MoS2 by substitutional doping: A first-principles study. Phys. Lett. A 2013, 377, 1362–1367. [Google Scholar] [CrossRef]
- Deokar, G.; Rajput, N.S.; Vancsó, P.; Ravaux, F.; Jouiad, M.; Vignaud, D.; Cecchet, F.; Colomer, J.-F. Large area growth of vertically aligned luminescent MoS2 nanosheets. Nanoscale 2017, 9, 277–287. [Google Scholar] [CrossRef]
- Michiels, M.; Hemberg, A.; Godfroid, T.; Douheret, O.; Colaux, J.L.; Moskovkin, P.; Lucas, S.; Caillard, A.; Thomann, A.-L.; Laha, P.; et al. On the relationship between the plasma characteristics, the microstructure and the optical properties of reactively sputtered TiO2 thin films. J. Phys. D. Appl. Phys. 2021, 54, 415202. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Zhao, Y.; Ouyang, G. Thickness-dependent photoelectric properties of MoS2/Si heterostructure solar cells. Sci. Rep. 2019, 9, 17381. [Google Scholar] [CrossRef]
- Rycenga, M.; Camargo, P.H.C.; Li, W.; Moran, C.H.; Xia, Y. Understanding the SERS Effects of Single Silver Nanoparticles and Their Dimers, One at a Time. J. Phys. Chem. Lett. 2010, 1, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Li, X. Optimal Size of Gold Nanoparticles for Surface-Enhanced Raman Spectroscopy under Different Conditions. J. Nanomater. 2013, 2013, 790323. [Google Scholar] [CrossRef]
- Stamplecoskie, K.G.; Scaiano, J.C.; Tiwari, V.S.; Anis, H. Optimal Size of Silver Nanoparticles for Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2011, 115, 1403–1409. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced Raman spectroscopy: A brief retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Njoki, P.N.; Lim, I.-I.S.; Mott, D.; Park, H.-Y.; Khan, B.; Mishra, S.; Sujakumar, R.; Luo, J.; Zhong, C.-J. Size Correlation of Optical and Spectroscopic Properties for Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 14664–14669. [Google Scholar] [CrossRef]
- Sigmund, P. Theory of Sputtering. I. Sputtering Yield of Amorphous and Polycrystalline Targets. Phys. Rev. 1969, 184, 383–416. [Google Scholar] [CrossRef]
- Farmanbar, M.; Brocks, G. First-principles study of van der Waals interactions and lattice mismatch at MoS2/metal interfaces. Phys. Rev. B 2016, 93, 085304. [Google Scholar] [CrossRef]
- Zhou, W.; Zou, X.; Najmaei, S.; Liu, Z.; Shi, Y.; Kong, J.; Lou, J.; Ajayan, P.M.; Yakobson, B.I.; Idrobo, J.-C. Intrinsic Structural Defects in Monolayer Molybdenum Disulfide. Nano Lett. 2013, 13, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; He, K.; Yuan, Y.; Sharifi-Asl, S.; Cheng, M.; Lu, J.; Saidi, W.A.; Shahbazian-Yassar, R. In situ study of nucleation and growth dynamics of Au nanoparticles on MoS2 nanoflakes. Nanoscale 2018, 10, 15809–15818. [Google Scholar] [CrossRef] [PubMed]
- He, R.X.; Liang, R.; Peng, P.; Norman Zhou, Y. Effect of the size of silver nanoparticles on SERS signal enhancement. J. Nanoparticle Res. 2017, 19, 267. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Xie, J.; Xue, L. Calibration of Binding Energy Positions with C1s for XPS Results. J. Wuhan Univ. Technol. Sci. Ed. 2020, 35, 711–718. [Google Scholar] [CrossRef]
- Ganta, D.; Sinha, S.; Haasch, R.T. 2-D Material Molybdenum Disulfide Analyzed by XPS. Surf. Sci. Spectra 2014, 21, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ristova, M.; Ristov, M. XPS profile analysis on CdS thin film modified with Ag by an ion exchange. Appl. Surf. Sci. 2001, 181, 68–77. [Google Scholar] [CrossRef]
- Yao, Y.; Ao, K.; Lv, P.; Wei, Q. MoS2 Coexisting in 1T and 2H Phases Synthesized by Common Hydrothermal Method for Hydrogen Evolution Reaction. Nanomaterials 2019, 9, 844. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Watson, G.W.; Payne, D.J.; Atkinson, G.R.; Egdell, R.G.; Law, D.S.L. Theoretical and Experimental Study of the Electronic Structures of MoO3 and MoO2. J. Phys. Chem. C 2010, 114, 4636–4645. [Google Scholar] [CrossRef]
- Qin, S.; Lei, W.; Liu, D.; Chen, Y. In-situ and tunable nitrogen-doping of MoS2 nanosheets. Sci. Rep. 2014, 4, 7582. [Google Scholar] [CrossRef]
- Kim, G.-T.; Park, T.-K.; Chung, H.; Kim, Y.-T.; Kwon, M.-H.; Choi, J.-G. Growth and characterization of chloronitroaniline crystals for optical parametric oscillators. Appl. Surf. Sci. 1999, 152, 35–43. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Z.; Dong, L.; Zhao, W.; Jin, Y.; Fang, L.; Hu, B.; Dong, M. Activating MoS2 with Super-High Nitrogen-Doping Concentration as Efficient Catalyst for Hydrogen Evolution Reaction. J. Phys. Chem. C 2019, 123, 10917–10925. [Google Scholar] [CrossRef]
- Titantah, J.T.; Lamoen, D. Carbon and nitrogen 1s energy levels in amorphous carbon nitride systems: XPS interpretation using first-principles. Diam. Relat. Mater. 2007, 16, 581–588. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, L.; Xiong, H.; Shao, M.; Xu, L.; Xie, A.; Zhuang, S.; Tang, Y.; Yang, X.; Chen, Y.; et al. 3D self-supported Ni nanoparticle@N-doped carbon nanotubes anchored on NiMoN pillars for the hydrogen evolution reaction with high activity and anti-oxidation ability. J. Mater. Chem. A 2019, 7, 13671–13678. [Google Scholar] [CrossRef]
- Boyen, H.-G.; Ethirajan, A.; Kästle, G.; Weigl, F.; Ziemann, P.; Schmid, G.; Garnier, M.G.; Büttner, M.; Oelhafen, P. Alloy Formation of Supported Gold Nanoparticles at Their Transition from Clusters to Solids: Does Size Matter? Phys. Rev. Lett. 2005, 94, 016804. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, P. Photoemission and the electronic structure of surfaces. In Surface Science and Synchrotron Radiation; IOP Publishing: Bristol, UK, 2023; pp. 3-1–3-67. [Google Scholar]
- Lambaré, C.; Tessier, P.-Y.; Poncin-Epaillard, F.; Debarnot, D. Plasma functionalization and etching for enhancing metal adhesion onto polymeric substrates. RSC Adv. 2015, 5, 62348–62357. [Google Scholar] [CrossRef]
- Braun, M. Magnetron Sputtering Technique. In Handbook of Manufacturing Engineering and Technology; Springer: London, UK, 2015; pp. 2929–2957. [Google Scholar]
- Kraut, E.A. Heterojunction band off-sets: Variation with ionization potential compared to experiment. J. Vac. Sci. Technol. B Microelectron. Process. Phenom. 1984, 2, 486–490. [Google Scholar] [CrossRef]
- Tuan, A.C.; Kaspar, T.C.; Droubay, T.; Rogers, J.W.; Chambers, S.A. Band offsets for the epitaxial TiO2/SrTiO3/Si(001) system. Appl. Phys. Lett. 2003, 83, 3734–3736. [Google Scholar] [CrossRef]
- Yeh, J.J.; Lindau, I. Atomic subshell photoionization cross sections and asymmetry parameters: 1 ≤ Z ≤ 103. At. Data Nucl. Data Tables 1985, 32, 1–155. [Google Scholar] [CrossRef]
- Cox, P.A. The Electronic Structure and Chemistry of Solids; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Andriotis, A.N.; Nicolaides, C.A. Variations of the surface dipole moment due to anisotropy and chemisorption. Surf. Sci. 1982, 116, 513–521. [Google Scholar] [CrossRef]
- Chiu, M.-H.; Zhang, C.; Shiu, H.-W.; Chuu, C.-P.; Chen, C.-H.; Chang, C.-Y.S.; Chen, C.-H.; Chou, M.-Y.; Shih, C.-K.; Li, L.-J. Determination of band alignment in the single-layer MoS2/WSe2 heterojunction. Nat. Commun. 2015, 6, 7666. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, C.; Li, K.; Wang, W.; Li, Z.; Ao, J.; Wu, J.; He, W.; Mao, W.; Liu, Q.; et al. Investigation of the Energy Band at the Molybdenum Disulfide and ZrO2 Heterojunctions. Nanoscale Res. Lett. 2018, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Chai, J.W.; Zhang, Z.; Pan, J.S.; Wang, S.J. The energy-band alignment at molybdenum disulphide and high-k dielectrics interfaces. Appl. Phys. Lett. 2014, 104, 232110. [Google Scholar] [CrossRef]
- Afanas’ev, V.V.; Chiappe, D.; Huyghebaert, C.; Radu, I.; De Gendt, S.; Houssa, M.; Stesmans, A. Band alignment at interfaces of few-monolayer MoS2 with SiO2 and HfO2. Microelectron. Eng. 2015, 147, 294–297. [Google Scholar] [CrossRef]






| Powder | Ag(NPs) Size Average (nm) | VA-MoS2 | Ag(NPs) Size Average (nm) |
|---|---|---|---|
| N-MoS2 (Ag5min) | 8.2 ± 1.0 | N-VA-MoS2 (Ag5s) | 6.7 ± 0.2 |
| N-MoS2 (Ag10min) | 13.4 ± 0.8 | N-VA-MoS2 (Ag10s) | 26.4 ± 2.3 |
| N-MoS2 (Ag15min) | 16.6 ± 1.0 | N-VA-MoS2 (Ag15s) | * |
| N-MoS2 (Ag20min) | 18.0 ± 0.9 | ||
| N-MoS2 (Ag40min) | 36.4 ± 2.7 |
| Materials | Ag (%) | N (%) |
|---|---|---|
| N-MoS2 (Ag5min) | 1.3 ± 0.06 | 2.1 |
| N-MoS2 (Ag10min) | 4.1 ± 0.04 | 2.2 |
| N-MoS2 (Ag15min) | 4.1 ± 0.04 | 2.3 |
| N-MoS2 (Ag20min) | 7.0 ± 0.07 | 3.2 |
| N-MoS2 (Ag40min) | 10.4 ± 0.10 | 4.0 |
| Materials | Ag (%) | N (%) |
|---|---|---|
| N-VA-MoS2 (Ag5s) | 13.8 | 13.8 |
| N-VA-MoS2 (Ag10s) | 23.3 | 10.3 |
| N-VA-MoS2 (Ag15s) | 31.3 | 7.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, K.A.; Das, A.; Colomer, J.-F.; Hemberg, A.; Noirfalise, X.; Bittencourt, C. Silver Decoration of Vertically Aligned MoS2-MoOx Nanosheets: A Comprehensive XPS Investigation. Materials 2024, 17, 2882. https://doi.org/10.3390/ma17122882
Youssef KA, Das A, Colomer J-F, Hemberg A, Noirfalise X, Bittencourt C. Silver Decoration of Vertically Aligned MoS2-MoOx Nanosheets: A Comprehensive XPS Investigation. Materials. 2024; 17(12):2882. https://doi.org/10.3390/ma17122882
Chicago/Turabian StyleYoussef, Khaled Al, Arkaprava Das, Jean-François Colomer, Axel Hemberg, Xavier Noirfalise, and Carla Bittencourt. 2024. "Silver Decoration of Vertically Aligned MoS2-MoOx Nanosheets: A Comprehensive XPS Investigation" Materials 17, no. 12: 2882. https://doi.org/10.3390/ma17122882






