Novel Saccharomyces cerevisiae-Loaded Polyvinylpyrrolidone/SiO2 Nanofiber for Wound Dressing Prepared Using Electrospinning Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SC
2.3. Preparation of Electrospun Composite SD/PVP Nanofibers
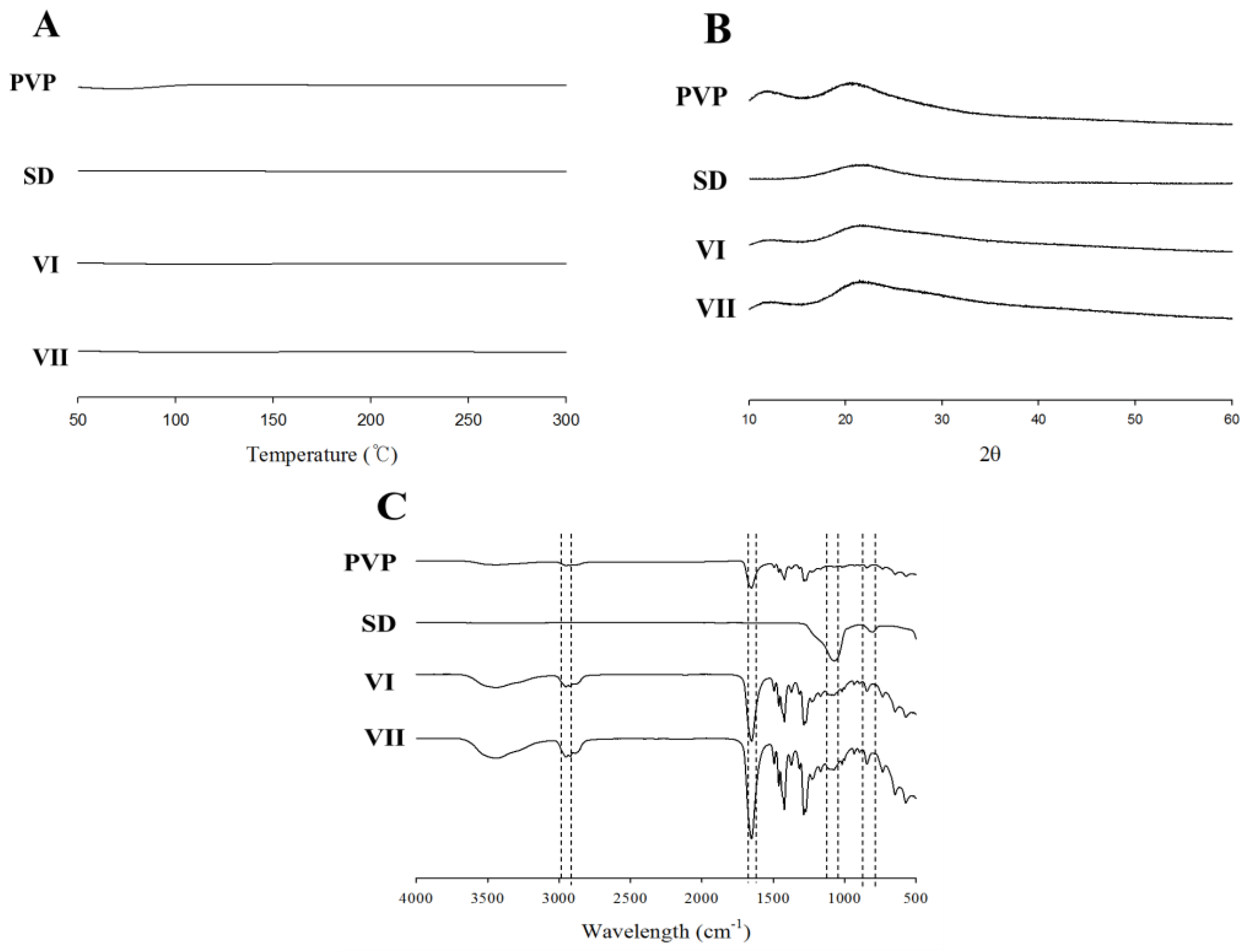
2.4. Physicochemical Properties of Electrospun Composite SD/PVP Nanofibers
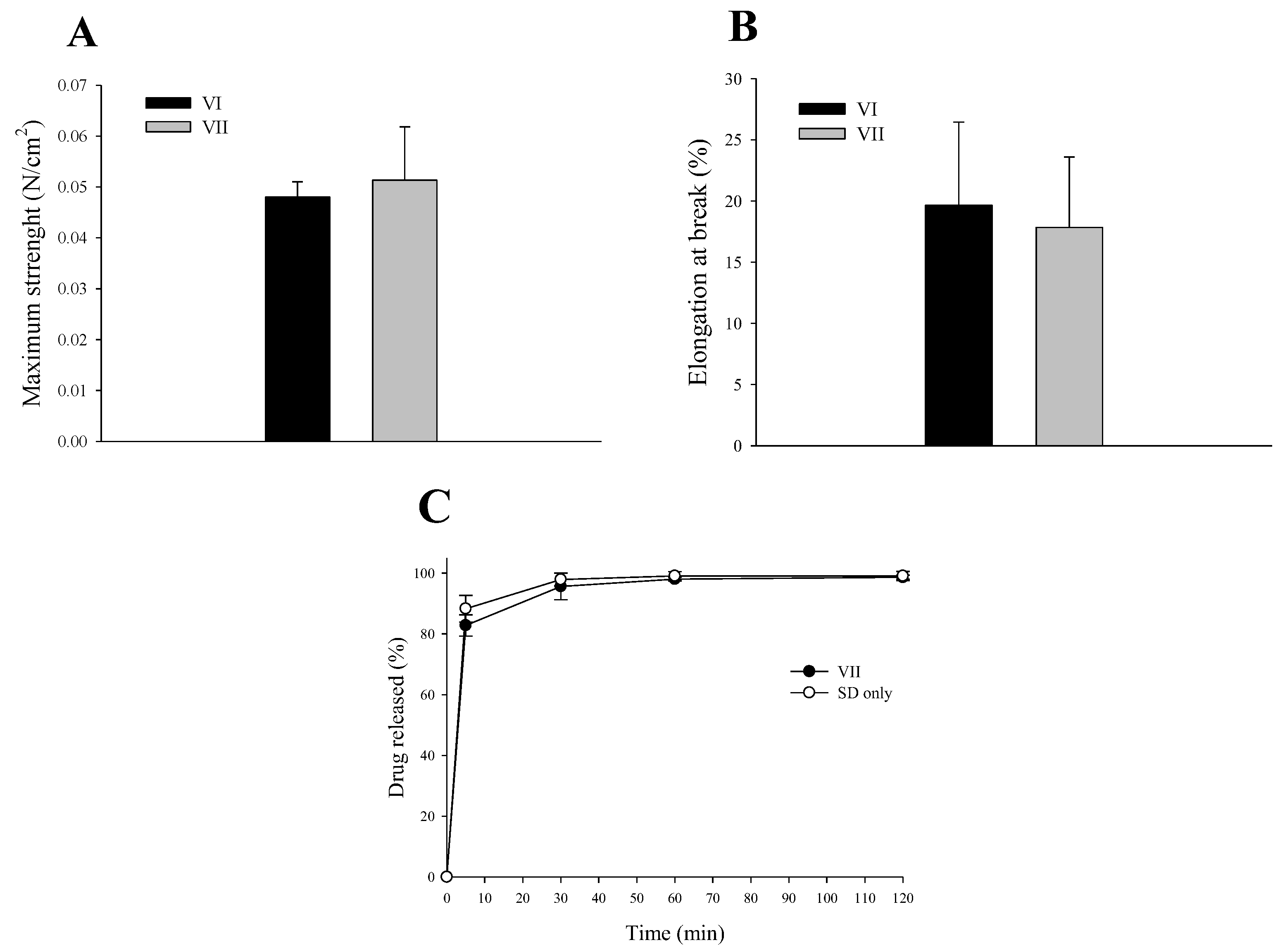
2.5. Mechanical Properties of Electrospun Composite SD/PVP Nanofibers
2.6. In Vitro Release Study of SC-Loaded Electrospun Composite SD/PVP Nanofibers
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of PVP and SD Concentration
3.2. SC-Loaded Electrospun Nanofiber
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.S.; Kim, J.; Lee, S.M.; Woo, M.R.; Kim, D.W.; Kim, J.O.; Choi, H.G.; Jin, S.G. Development of guar gum-based dual-layer wound dressing containing Lactobacillus plantarum: Rapid recovery and mechanically flexibility. Int. J. Biol. Macromol. 2022, 221, 1572–1579. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kim, J.O.; Kim, J. Antimicrobial strategies for topical biofilm-based wound infections: Past, present, and future. J. Pharma. Investig. 2023, 53, 627–641. [Google Scholar] [CrossRef]
- Kim, J.S.; Yu, H.; Woo, M.R.; Kim, D.W.; Kim, J.O.; Ku, S.K.; Jin, S.G.; Choi, H.G. Influence of hydrophilic polymers on mechanical property and wound recovery of hybrid bilayer wound dressing system for delivering thermally unstable probiotic. Biomater. Adv. 2022, 135, 112696. [Google Scholar] [CrossRef]
- Sofi, H.S.; Rashid, R.; Amna, T.; Hamid, R.; Sheikh, F.A. Recent advances in formulating electrospun nanofiber membranes: Delivering active phytoconstituents. J. Drug Deliv. Sci. Technol. 2020, 60, 102038. [Google Scholar] [CrossRef]
- Keshavarz, R.; Olsen, S.; Almeida, B. Using biomaterials to improve mesenchymal stem cell therapies for chronic, nonhealing wounds. Bioeng. Transl. Med. 2024, 9, e10598. [Google Scholar] [CrossRef] [PubMed]
- Elsadek, N.E.; Nagah, A.; Ibrahim, T.M.; Chopra, H.; Ghonaim, G.A.; Emam, S.E.; Cavalu, S.; Attia, M.S. Electrospun nanofibers revisited: An update on the emerging applications in nanomedicine. Materials 2022, 15, 1934. [Google Scholar] [CrossRef] [PubMed]
- Shehab-ElDin, A.N.; Sobh, R.A.; Rabie, A.M.; Mohamed, W.S.; Nasr, H.E. Polyamide 6/tallow modified clay nanofibrous mat coupled with hydrogels for potential topical/transdermal delivery of doxycycline hydrochloride. J. Pharm. Investig. 2023, 53, 307–321. [Google Scholar] [CrossRef]
- Tan, S.M.; Teoh, X.Y.; Le Hwang, J.; Khong, Z.P.; Sejare, R.; Almashhadani, A.Q.; Assi, R.A.; Chan, S.Y. Electrospinning and its potential in fabricating pharmaceutical dosage form. J. Drug Deliv. Sci. Technol. 2022, 76, 103761. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Ignacyk, M.; Miklaszewski, A.; Plech, T.; Karpiński, T.M.; Kwiatek, J.; Swora-Cwynar, E.; Walendowski, M.; Cielecka-Piontek, J. Electrospun Nanofibers with Pomegranate Peel Extract as a New Concept for Treating Oral Infections. Materials 2024, 17, 2558. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L. Electrospun Nanofiber Membranes with Various Structures for Wound Dressing. Materials 2023, 16, 6021. [Google Scholar] [CrossRef]
- Arif, M.M.; Khan, S.M.; Gull, N.; Tabish, T.A.; Zia, S.; Khan, R.U.; Awais, S.M.; Butt, M.A. Polymer-based biomaterials for chronic wound management: Promises and challenges. Int. J. Pharm. 2021, 598, 120270. [Google Scholar]
- Croitoru, A.M.; Ficai, D.; Ficai, A.; Mihailescu, N.; Andronescu, E.; Turculet, S.C. Nanostructured fibers containing natural or synthetic bioactive compounds in wound dressing applications. Materials 2020, 13, 2407. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kokil, G.R.; He, Y.; Lowe, B.; Salam, A.; Altalhi, T.A.; Ye, Q.; Kumeria, T. Inorganic/organic combination: Inorganic particles/polymer composites for tissue engineering applications. Bioact. Mater. 2023, 24, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, K.; Dang, T.K.; Vu, L.T.D.; Ha, N.T.; Truong, H.D.; Tran, T.H. Orodispersible film incorporating nanoparticulate loratadine for an enhanced oral bioavailability. J. Pharma. Investig. 2023, 53, 417–426. [Google Scholar] [CrossRef]
- Passaro, J.; Imparato, C.; Parida, D.; Bifulco, A.; Branda, F.; Aronne, A. Electrospinning of PVP-based ternary composites containing SiO2 nanoparticles and hybrid TiO2 microparticles with adsorbed superoxide radicals. Compos. B Eng. 2022, 238, 109874. [Google Scholar] [CrossRef]
- Kandasamy, S.; Narayanan, V.; Sumathi, S. Zinc and manganese substituted hydroxyapatite/CMC/PVP electrospun composite for bone repair applications. Int. J. Biol. Macromol. 2020, 145, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Forysenkova, A.A.; Konovalova, M.V.; Fadeeva, I.V.; Antonova, O.S.; Kotsareva, O.D.; Slonskaya, T.K.; Julietta, V.R.; Svirshchevskaya, E.V. Polyvinylpyrrolidone–Alginate Film Barriers for Abdominal Surgery: Anti-Adhesion Effect in Murine Model. Materials 2023, 16, 5532. [Google Scholar] [CrossRef] [PubMed]
- Derakhshankhah, H.; Malekshah, R.E.; Izadi, Z.; Samari, M.; Rezaee, M.; Samadian, H. Fabrication, characterization, and application of wound dressing based on PVP/PVA nanofibers containing β-cyclodextrin/curcumin complex: From in silico to in vitro studies. J. Mol. Liq. 2023, 386, 122500. [Google Scholar] [CrossRef]
- Cazorla-Luna, R.; Ruiz-Caro, R.; Veiga, M.D.; Malcolm, R.K.; Lamprou, D.A. Recent advances in electrospun nanofiber vaginal formulations for women’s sexual and reproductive health. Int. J. Pharm. 2021, 607, 121040. [Google Scholar] [CrossRef]
- Yu, H.; Kim, J.S.; Kim, D.W.; Park, E.S.; Youn, Y.S.; Ud Din, F.; Kim, J.O.; Ku, S.K.; Jin, S.G.; Choi, H.G. Novel composite double-layered dressing with improved mechanical properties and wound recovery for thermosensitive drug, Lactobacillus brevis. Compos. B Eng. 2021, 225, 109276. [Google Scholar] [CrossRef]
- Çanga, E.M.; Dudak, F.C. Improved digestive stability of probiotics encapsulated within poly (vinyl alcohol)/cellulose acetate hybrid fibers. Carbohydr. Polym. 2021, 264, 117990. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Spent brewer’s yeast (Saccharomyces cerevisiae) as a potential source of bioactive peptides: An overview. Int. J. Biol. Macromol. 2022, 208, 1116–1126. [Google Scholar] [CrossRef]
- Branco, P.; Maurício, E.M.; Costa, A.; Ventura, D.; Roma-Rodrigues, C.; Duarte, M.P.; Fernandes, A.R.; Prista, C. Exploring the Multifaceted Potential of a Peptide Fraction Derived from Saccharomyces cerevisiae Metabolism: Antimicrobial, Antioxidant, Antidiabetic, and Anti-Inflammatory Properties. Antibiotics 2023, 12, 1332. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Yu, D.G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater. Sci. Eng. C 2020, 111, 110805. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Naqvi, S.A.; Jaskani, M.J.; Waseem, M.; Ali, E.; Khan, I.A.; Manzoor, M.F.; Siddeeg, A.; Aadil, R.M. Efficient utilization of date palm waste for the bioethanol production through Saccharomyces cerevisiae strain. Food Sci. Nutr. 2021, 9, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, L.; Satpathy, M.; Duan, Y. Effect of electrospinning parameters on the fiber diameter and morphology of PLGA nanofibers. Dent. Oral Biol. Craniofacial Res. 2021, 4, 1. [Google Scholar]
- Singh, Y.P.; Dasgupta, S.; Nayar, S.; Bhaskar, R. Optimization of electrospinning process & parameters for producing defect-free chitosan/polyethylene oxide nanofibers for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2020, 31, 781–803. [Google Scholar] [PubMed]
- Kim, J.S.; Choi, Y.J.; Woo, M.R.; Cheon, S.; Ji, S.H.; Im, D.; Un Din, F.; Kim, J.O.; Youn, Y.S.; Oh, K.T.; et al. New potential application of hydroxypropyl-β-cyclodextrin in solid self-nanoemulsifying drug delivery system and solid dispersion. Carbohydr. Polym. 2021, 271, 118433. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Kim, J.S.; Yu, H.; Woo, M.R.; Choi, J.E.; Baek, K.; Kim, J.O.; Choi, Y.S.; Choi, H.G.; Jin, S.G. Comparison of the physicochemical properties, aqueous solubility, and oral bioavailability of rivaroxaban-loaded high-pressure homogenised and Shirasu porous glass membrane emulsified solid self-nanoemulsifying drug delivery systems. J. Mol. Liq. 2021, 346, 117057. [Google Scholar] [CrossRef]
- Emami, F.; Keihan Shokooh, M.; Mostafavi Yazdi, S.J. Recent progress in drying technologies for improving the stability and delivery efficiency of biopharmaceuticals. J. Pharma. Investig. 2023, 53, 35–57. [Google Scholar] [CrossRef]
- Choi, M.J.; Woo, M.R.; Baek, K.; Park, J.H.; Joung, S.; Choi, Y.S.; Choi, H.G.; Jin, S.G. Enhanced oral bioavailability of Rivaroxaban-Loaded microspheres by optimizing the polymer and surfactant based on molecular interaction mechanisms. Mol. Pharmaceutics 2023, 20, 4153–4164. [Google Scholar] [CrossRef] [PubMed]
- Jaberifard, F.; Ramezani, S.; Ghorbani, M.; Arsalani, N.; Moghadam, F.M. Investigation of wound healing efficiency of multifunctional eudragit/soy protein isolate electrospun nanofiber incorporated with ZnO loaded halloysite nanotubes and allantoin. Int. J. Pharm. 2023, 630, 122434. [Google Scholar] [CrossRef] [PubMed]
- Gençtürk, A.; Kahraman, E.; Güngör, S.; Özsoy, Y.; Sarac, A.S. Effects of polyvinylpyrrolidone and ethyl cellulose in polyurethane electrospun nanofibers on morphology and drug release characteristics. Turk. J. Pharma. Sci. 2020, 17, 638. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N. Apparent diameter and cell density of yeast strains with different ploidy. Sci. Rep. 2023, 13, 1513. [Google Scholar] [CrossRef] [PubMed]
- Kalva, S.N.; Augustine, R.; Al Mamun, A.; Dalvi, Y.B.; Vijay, N.; Hasan, A. Active agents loaded extracellular matrix mimetic electrospun membranes for wound healing applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102500. [Google Scholar] [CrossRef]
- Zhang, M.; Ahmed, A.; Xu, L. Electrospun nanofibers for functional food packaging application. Materials 2023, 16, 5937. [Google Scholar] [CrossRef]
- Djayanti, K.; Maharjan, P.; Cho, K.H.; Jeong, S.; Kim, M.S.; Shin, M.C.; Min, K.A. Mesoporous Silica Nanoparticles as a Potential Nanoplatform: Therapeutic Applications and Considerations. Int. J. Mol. Sci. 2023, 24, 6349. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Yu, M.; Wang, W.; Wang, Y.; Zhang, Z.; Zhou, H.; Dai, Y.; Wang, C.; Xu, Y. Enhanced microwave absorption performance of Fe/C nanofibers by adjusting the magnetic particle size using different electrospinning solvents. Ceram. Int. 2020, 46, 28603–28612. [Google Scholar] [CrossRef]
- Kim, W.T.; Park, D.C.; Yang, W.H.; Cho, C.H.; Choi, W.Y. Effects of electrospinning parameters on the microstructure of pvp/tio2 nanofibers. Nanomater. 2021, 11, 1616. [Google Scholar] [CrossRef]
- Rasouli, M.; Pirsalami, S.; Zebarjad, S.M. Study on the formation and structural evolution of bead-on-string in electrospun polysulfone mats. Polym. Int. 2020, 69, 822–832. [Google Scholar] [CrossRef]
- Nuamcharoen, P.; Kobayashi, T.; Potiyaraj, P. Influence of volatile solvents and mixing ratios of binary solvent systems on morphology and performance of electrospun poly (vinylidene fluoride) nanofibers. Polym. Int. 2021, 70, 1465–1477. [Google Scholar] [CrossRef]
- Wei, L.; Zhou, D.; Kang, X. Electrospinning as a novel strategy for the encapsulation of living probiotics in polyvinyl alcohol/silk fibroin. Innov. Food Sci. Emerg. Technol. 2021, 71, 102726. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, W.; Li, Y.; Zhang, S.; Liang, H.; Ji, C.; Lin, X. High-speed electrospinning of phycocyanin and probiotics complex nanofibrous with higher probiotic activity and antioxidation. Food Res. Int. 2023, 167, 112715. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Zhu, Y.; Shen, J.; Yang, X.; Chen, C.; Cao, H.; Li, C. Preparation of silica-sustained electrospun polyvinylpyrrolidone fibers with uniform mesopores via oxidative removal of template molecules by H2O2 treatment. Mater. Res. Bull. 2010, 45, 830–837. [Google Scholar] [CrossRef]

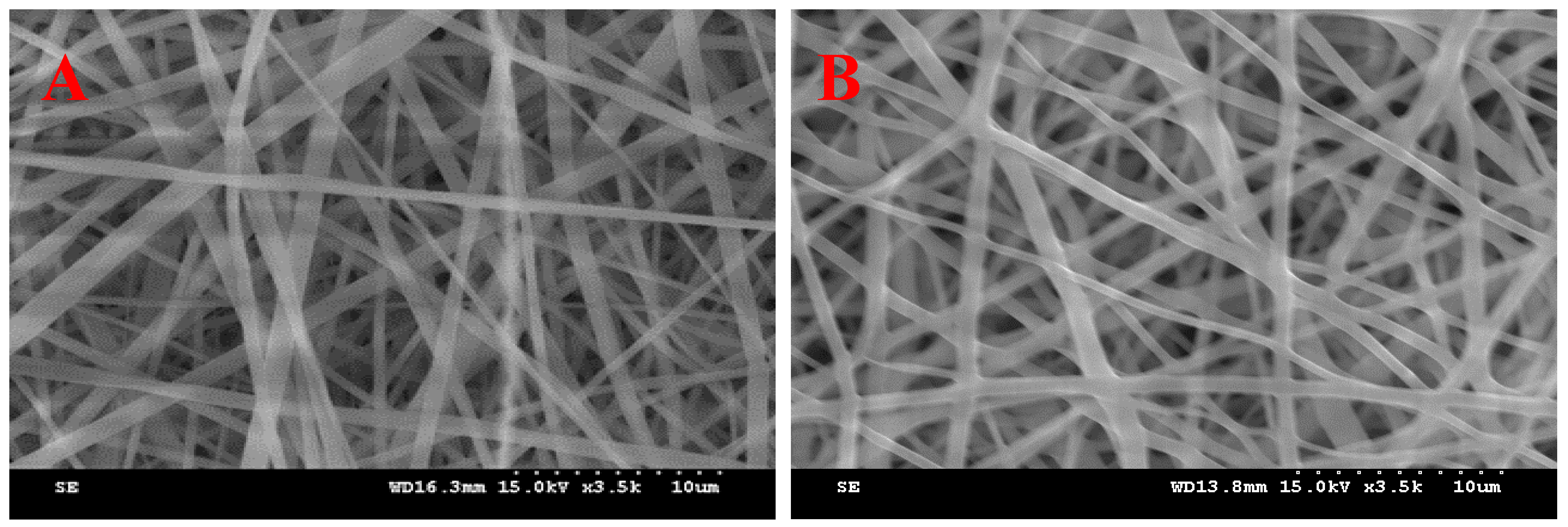
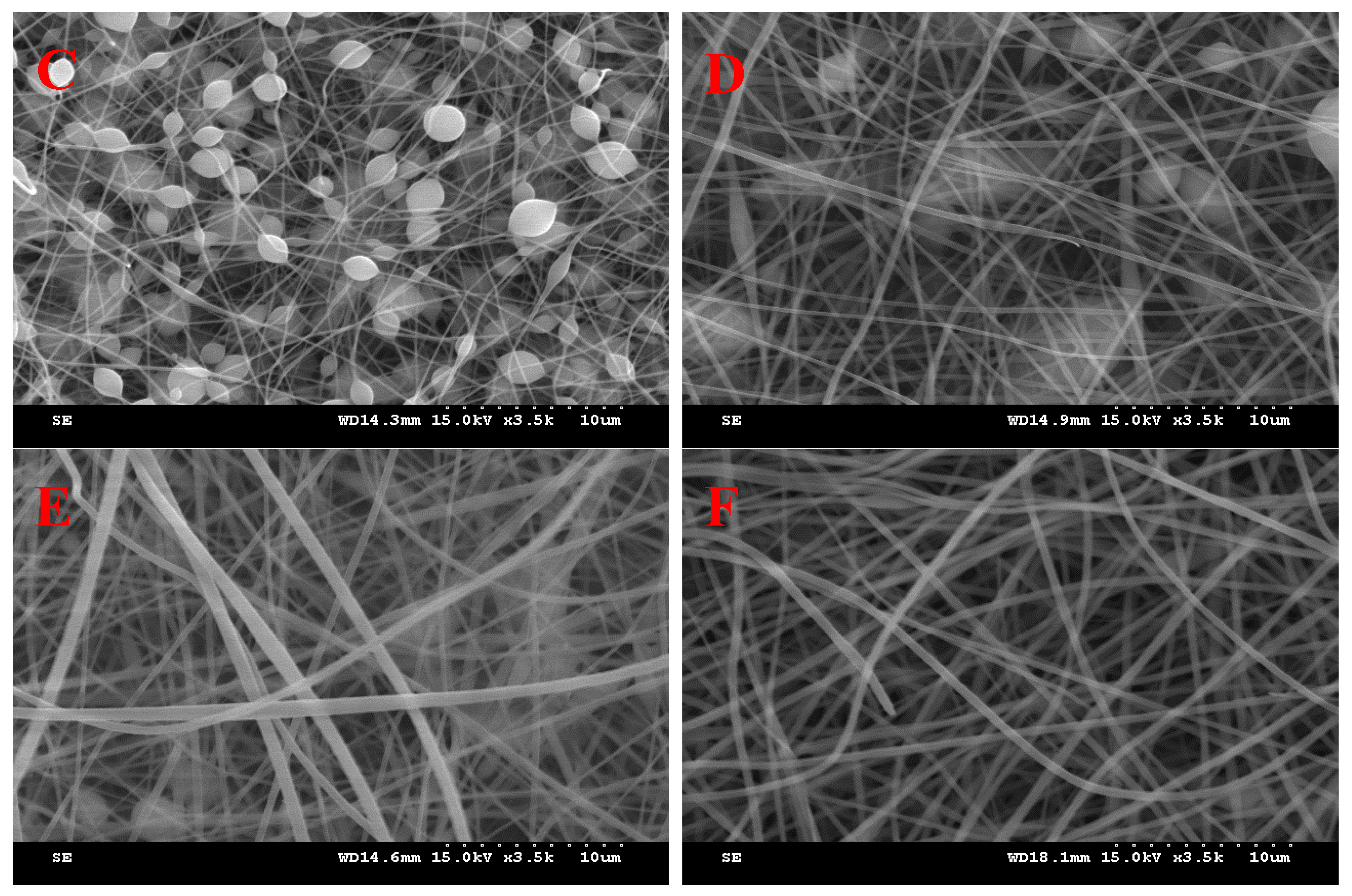
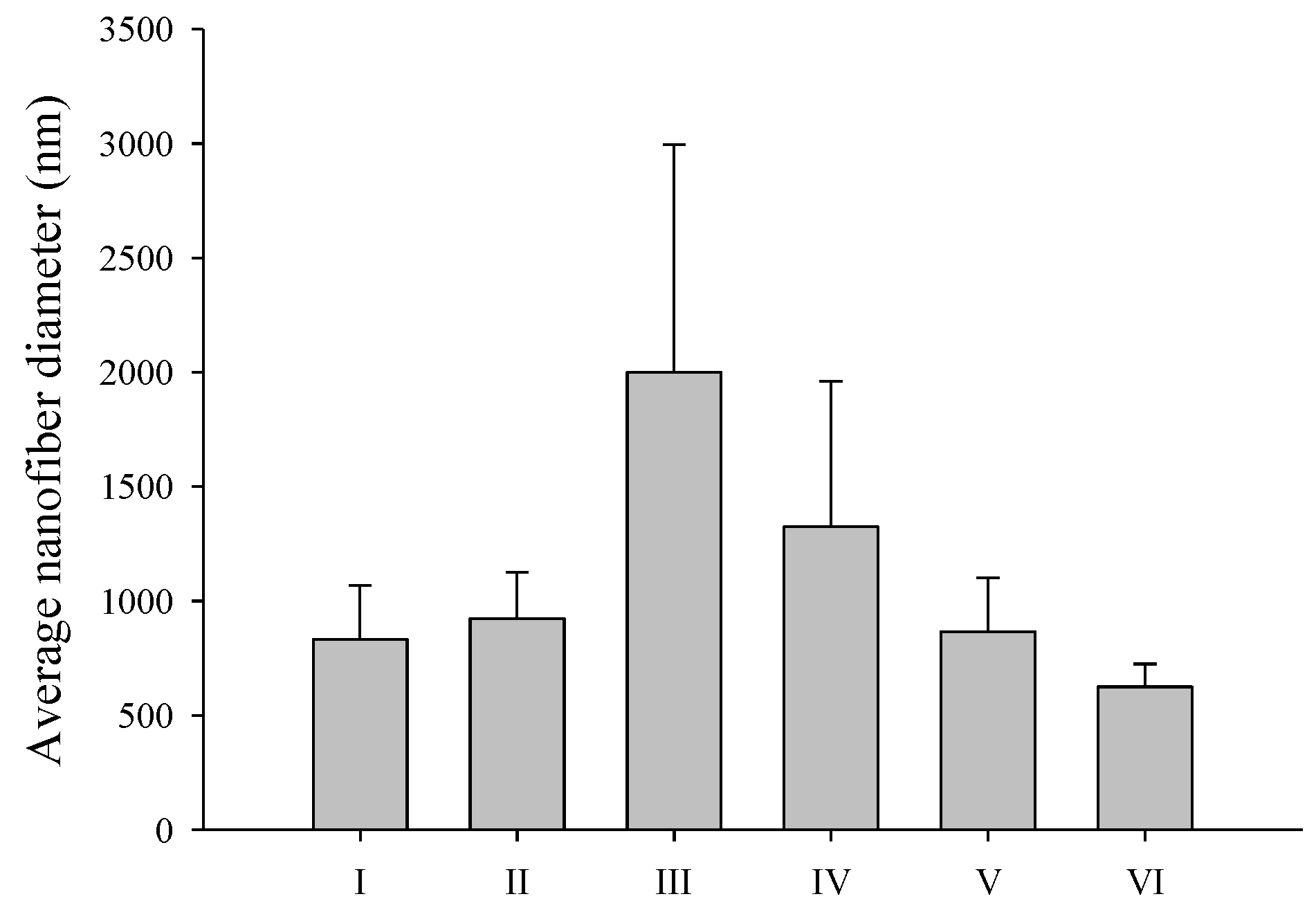
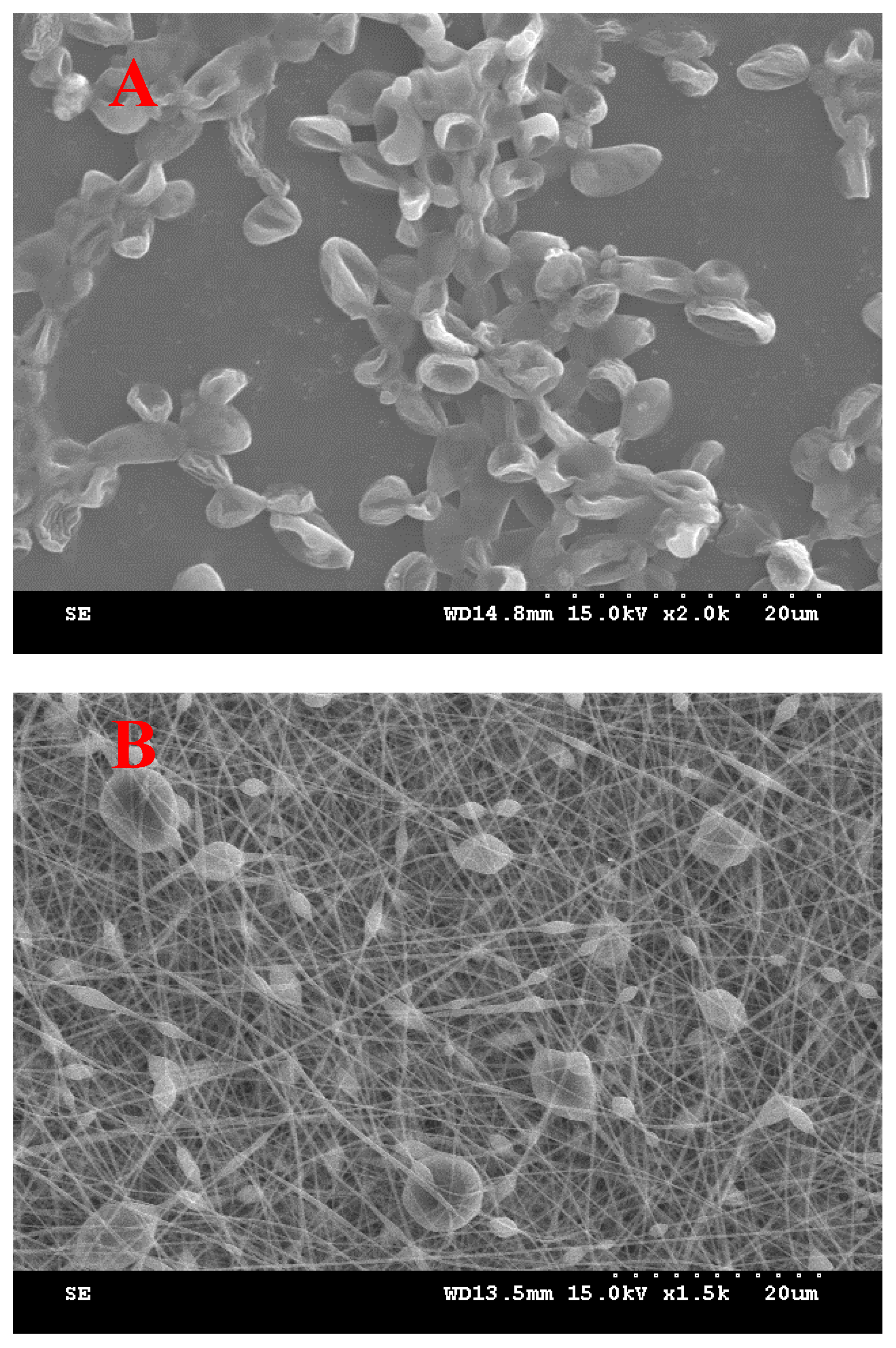
| Ingredients (g) | I | II | III | IV | V | VI | VII |
|---|---|---|---|---|---|---|---|
| SC | - | - | - | - | - | - | 3.0 |
| PVP | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| SD | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Water (Solvent) | - | - | 5.7 | 5.4 | 5.1 | 4.8 | 4.8 |
| Ethanol (Solvent) | 6.0 | 6.0 | 0.3 | 0.6 | 0.9 | 1.2 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.S.; Yoon, H.; Jin, S.G. Novel Saccharomyces cerevisiae-Loaded Polyvinylpyrrolidone/SiO2 Nanofiber for Wound Dressing Prepared Using Electrospinning Method. Materials 2024, 17, 2903. https://doi.org/10.3390/ma17122903
Cho YS, Yoon H, Jin SG. Novel Saccharomyces cerevisiae-Loaded Polyvinylpyrrolidone/SiO2 Nanofiber for Wound Dressing Prepared Using Electrospinning Method. Materials. 2024; 17(12):2903. https://doi.org/10.3390/ma17122903
Chicago/Turabian StyleCho, Yeon Seo, Hongjun Yoon, and Sung Giu Jin. 2024. "Novel Saccharomyces cerevisiae-Loaded Polyvinylpyrrolidone/SiO2 Nanofiber for Wound Dressing Prepared Using Electrospinning Method" Materials 17, no. 12: 2903. https://doi.org/10.3390/ma17122903






