New Insights into the Application of Biocompatible (Un)Modified TiO2 and TiO2-ZrO2 Oxide Fillers in Light-Curing Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
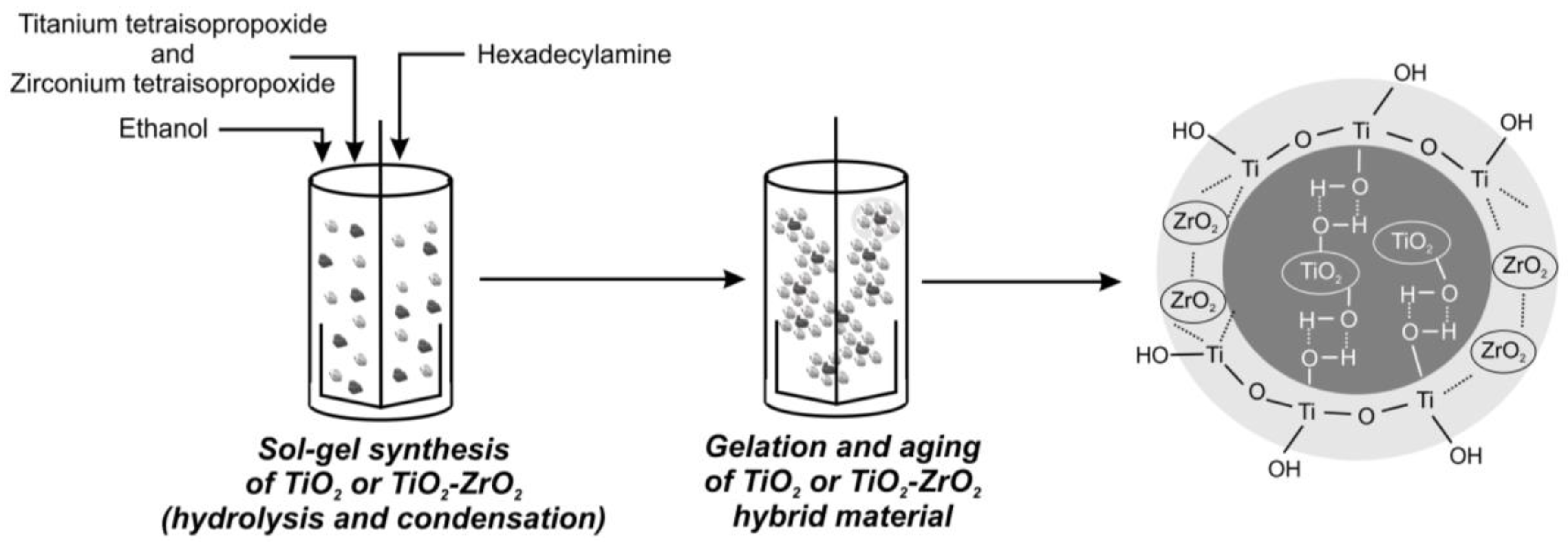
2.2. Fabrication of Inorganic Oxide Fillers
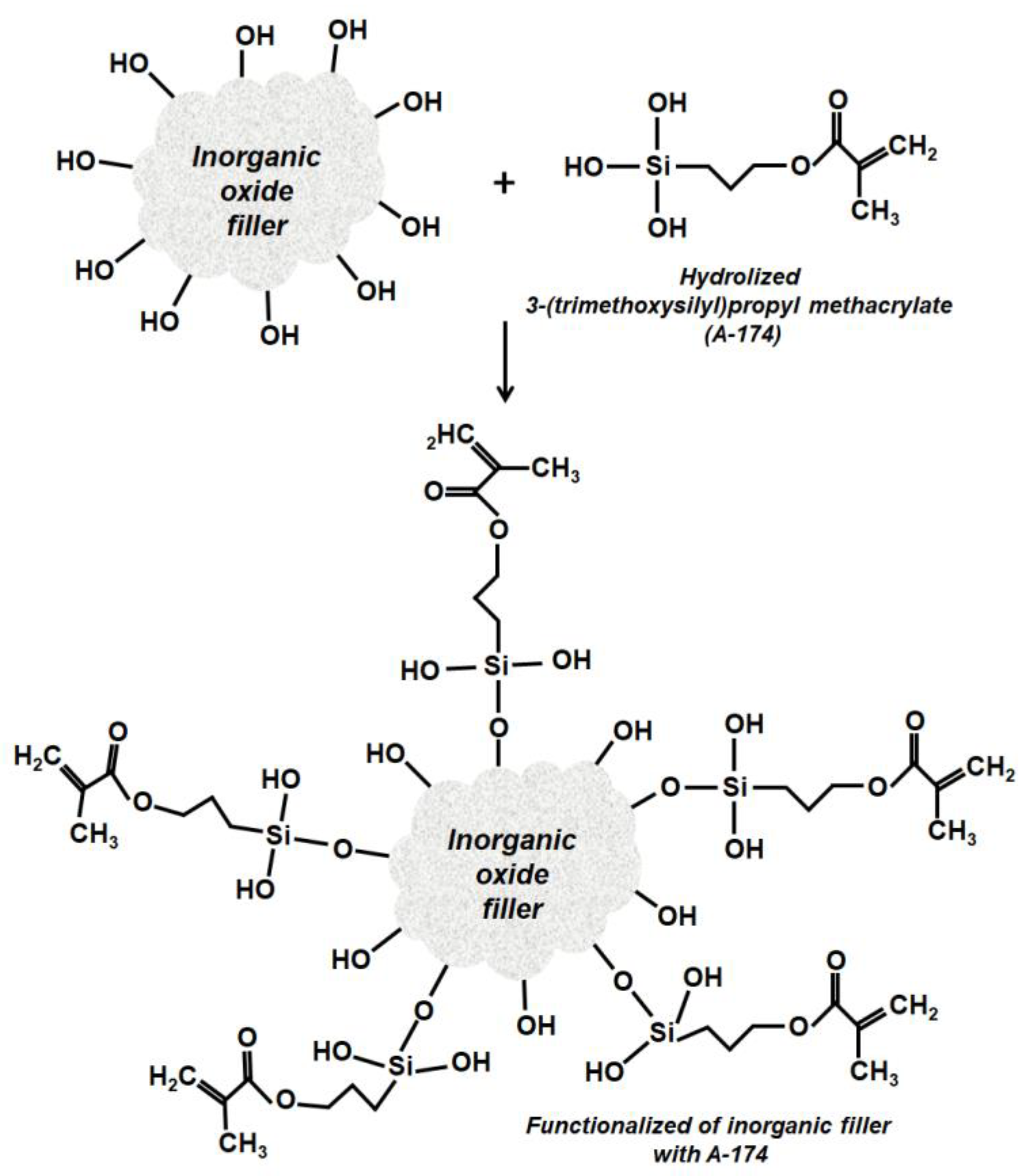
2.3. Surface Modification of Inorganic Oxide Systems
2.4. Preparation of Light-Curing Compositions
2.5. Characterization of Obtained Inorganic Oxide Fillers
2.6. Characterization of Light-Curing Compositions and Polymer Composites
3. Results and Discussion
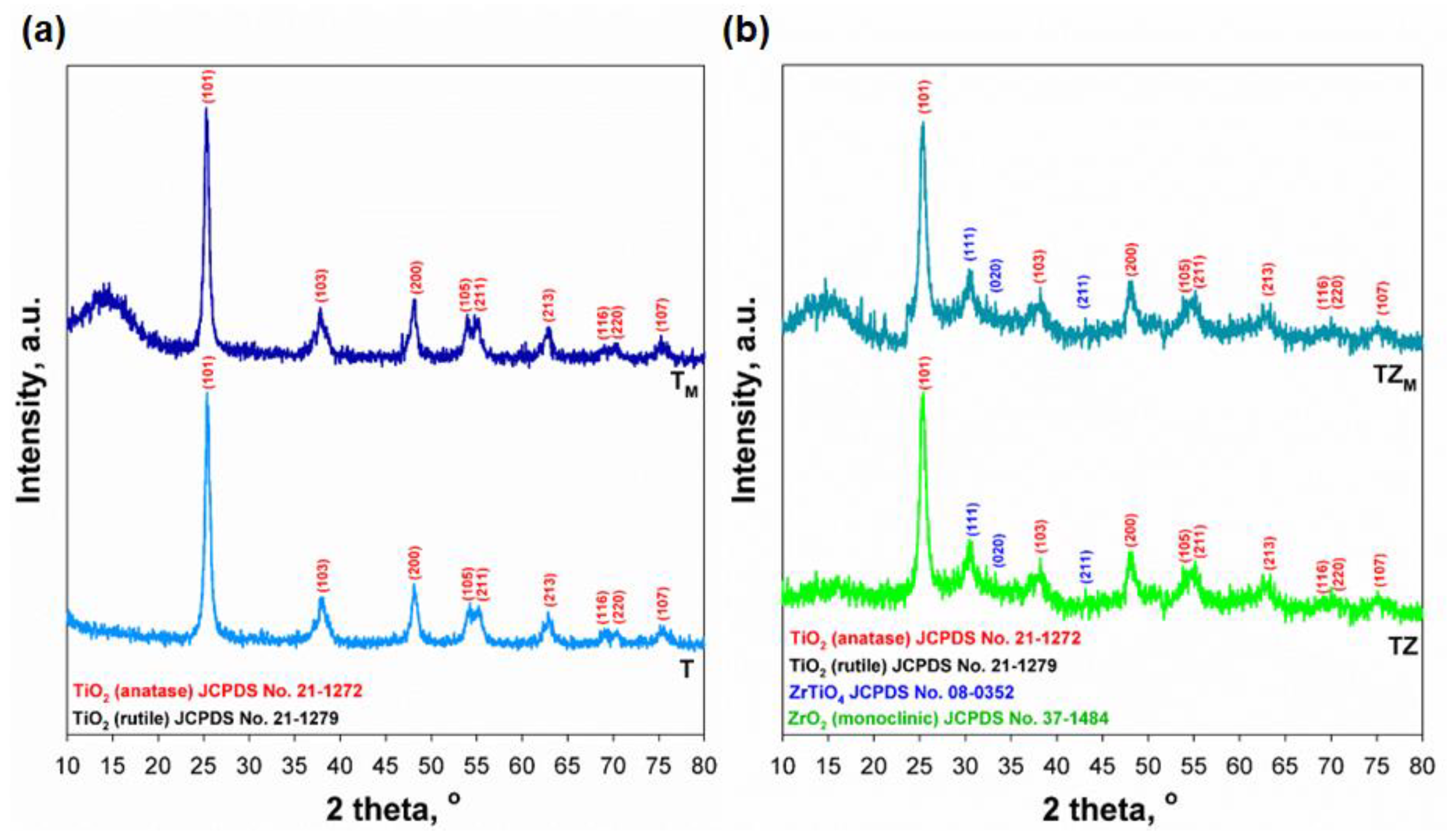
3.1. Physicochemical Characterization of Inorganic Oxide Fillers
3.2. Characterization of Monomer/Inorganic Oxide Filler Compositions
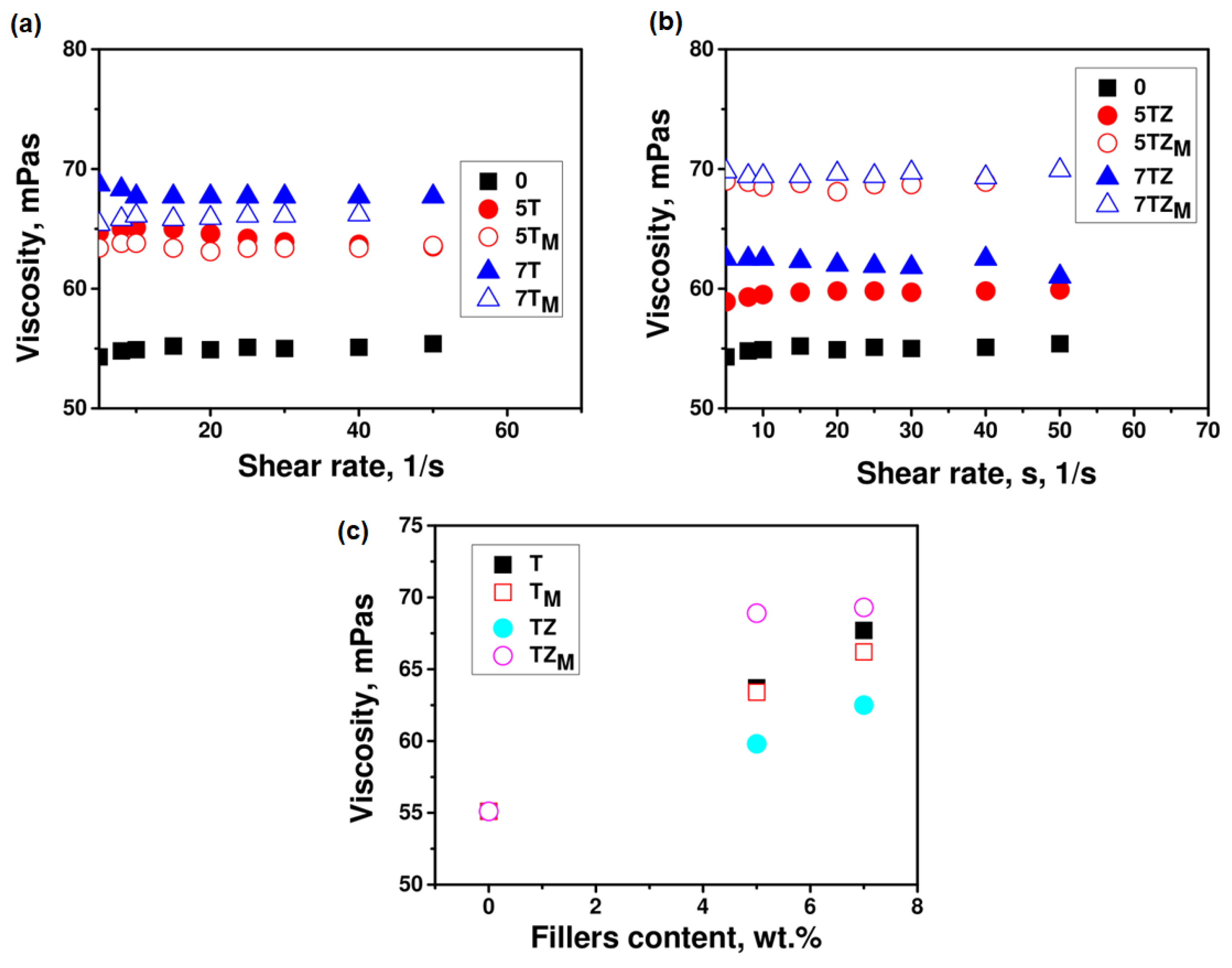
3.2.1. Viscosity
3.2.2. Photocuring
3.2.3. Spectral Analysis
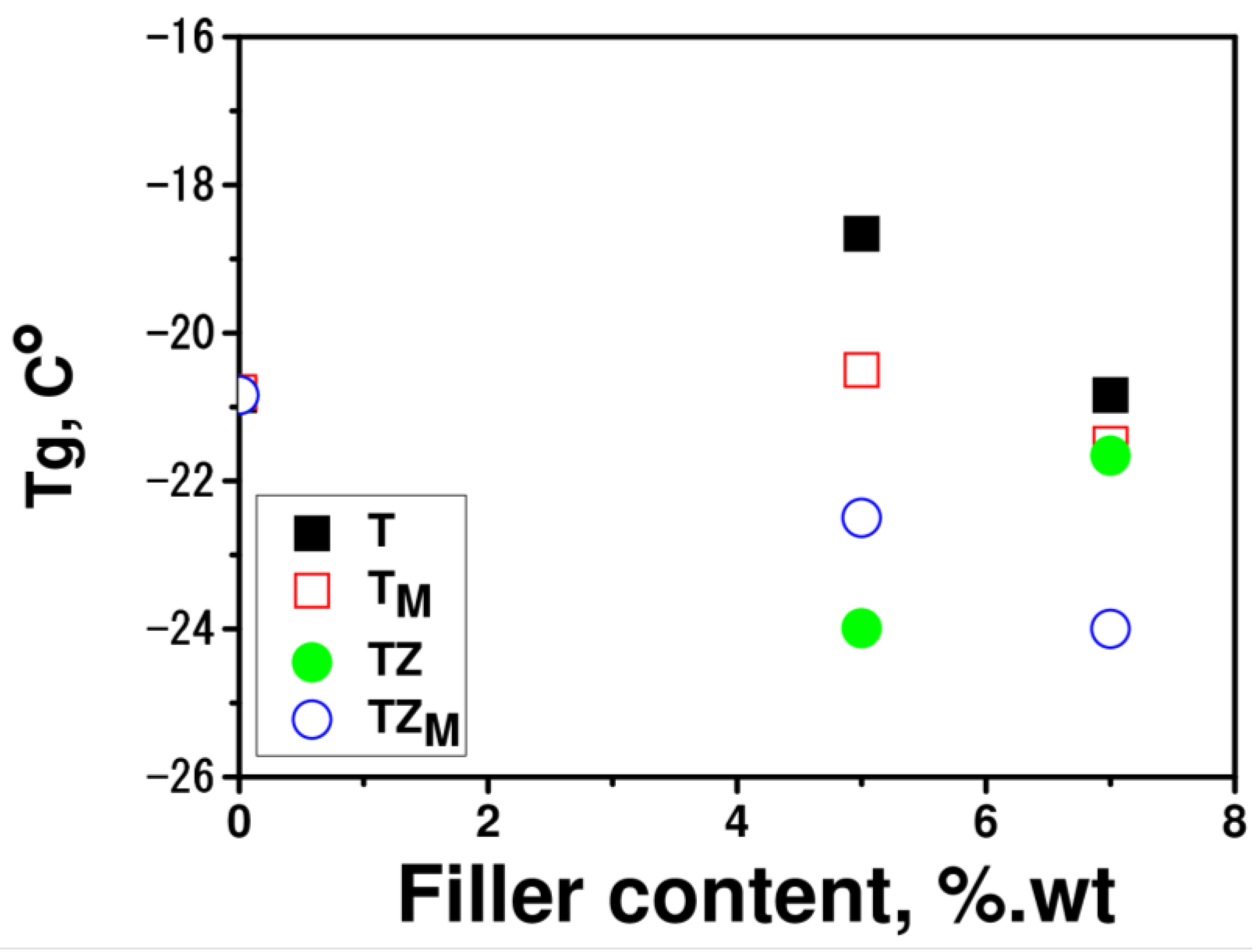
3.2.4. Thermal Properties
3.2.5. Morphology of Fabricated Composites
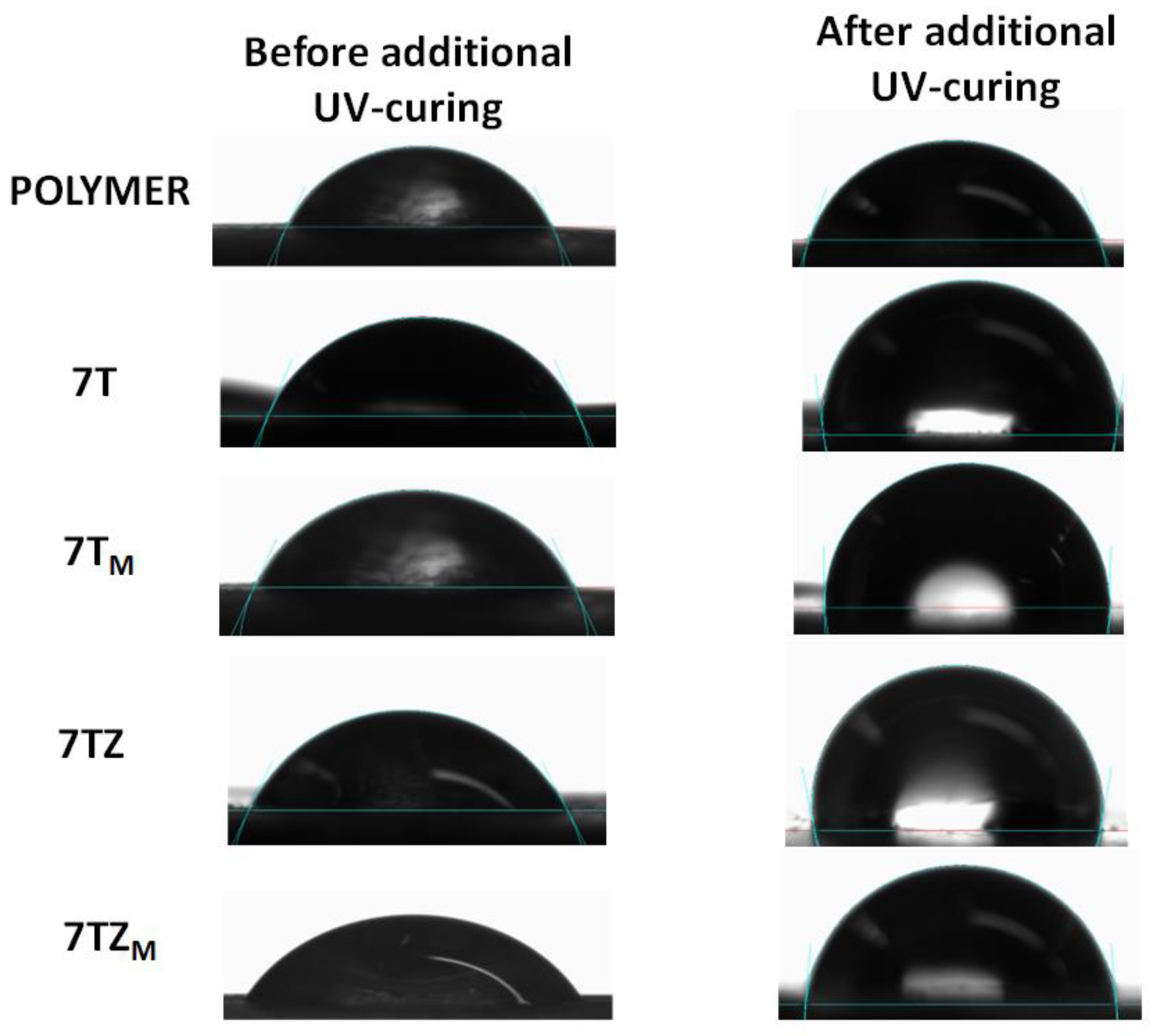
3.2.6. Contact Angle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brett, C.J.; Montani, S.; Schwartzkopf, M.; van Benthem, R.A.T.M.; Jansen, J.F.G.A.; Griffini, G.; Roth, S.V.; Johansson, M.K.G. Revealing structural evolution occurring from photo-initiated polymer network formation. Commun. Chem. 2020, 3, 88. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Granskog, V.; García-Gallego, S.; von Kieseritzky, J.; Rosendahl, J.; Stenlund, P.; Zhang, Y.; Petronis, S.; Lyvén, B.; Arner, M.; Håkansson, J.; et al. Bone Repair: High-performance thiol-ene composites unveil a new era of adhesives suited for bone repair. Adv. Funct. Mater. 2018, 28, 1870180. [Google Scholar] [CrossRef]
- Shirai, M. Photoinitiated Polymerization. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S.E., Mullen, K.E., Eds.; 3.V; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1579–1585. [Google Scholar]
- Sadej, M.; Gojzewski, H.; Andrzejewska, E. Photocurable polymethacrylate-silica nanocomposites: Correlation between dispersion stability, curing kinetics, morphology and properties. J. Polym. Res. 2016, 23, 116. [Google Scholar] [CrossRef]
- Sadej-Bajerlain, M.; Gojzewski, H.; Andrzejewska, E. Monomer/modified nanosilica systems: Photopolymerization kinetics and composite characterization. Polymer 2011, 52, 1495–1503. [Google Scholar] [CrossRef]
- Medellin, A.; Du, W.; Miao, G.; Zou, J.; Pei, Z.; Ma, C. Vat photopolymerization 3D printing of nanocomposites: A literature review. J. Micro Nano-Manuf. 2019, 7, 031006. [Google Scholar] [CrossRef]
- Robakowska, M.; Gierz, Ł.; Gojzewski, H. Sialon and alumina modified UV-curable coatings with improved mechanical properties and hydrophobicity. Coatings 2021, 11, 1424. [Google Scholar] [CrossRef]
- Robakowska, M.; Gierz, Ł.; Mayer, P.; Szcześniak, K.; Marcinkowska, A.; Lewandowska, A.; Gajewski, P. Influence of the addition of sialon and aluminum nitride fillers on the photocuring process of polymer coatings. Coatings 2022, 12, 1389. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabó, D.V. Polymer-nanoparticle composites: From synthesis to modern applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Song, S.W.; Jeong, Y.; Kwon, S. Photocurable polymer nanocomposites for magnetic, optical, and biological applications. IEEE J. Select. Top. Quantum Electron. 2015, 21, 324–335. [Google Scholar] [CrossRef]
- Liu, F.; Liu, A.; Tao, W.; Yang, Y. Preparation of UV curable organic/inorganic hybrid coatings—A review. Prog. Org. Coat. 2020, 145, 105685. [Google Scholar] [CrossRef]
- Cazan, C.; Enesca, A.; Andronic, L. Synergic effect of TiO2 filler on the mechanical properties of polymer nanocomposites. Polymers 2021, 13, 2017. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, B.; Chen, W.; Yang, J. Preparation and photocatalytic activities of TiO2-based composite catalysts. Catalysts 2022, 12, 1263. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Q.; Wang, X.; Joo, J.; Dahl, M.; Liu, B.; Gao, C.; Yin, Y. Photocatalytic surface-initiated polymerization on TiO2 toward well-defined composite nanostructures. ACS Appl. Mater. Interfaces 2016, 8, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Akinci, A.; Sen, S.; Sen, U. Friction and wear behavior of zirconium oxide reinforced PMMA composites. Compos. B Eng. 2014, 56, 42–47. [Google Scholar] [CrossRef]
- Halder, S.; Ahemad, S.; Das, S.; Wang, J. Epoxy/Glass Fiber Laminated Composites Integrated with Amino Functionalized ZrO2 for Advanced Structural Applications. ACS Appl. Mater. Interfaces 2016, 8, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.; Haupert, F.; Schlarb, A.K. Improvement of tensile properties and toughness of an epoxy resin by nanozirconium-dioxide reinforcement. J. Mater. Sci. 2008, 43, 3245–3252. [Google Scholar] [CrossRef]
- Naik, J.; Bhajantri, R.F.; Hebbar, V.; Rathod, S.G. Influence of ZrO2 filler on physico-chemical properties of PVA/NaClO4 polymer composite electrolytes. Adv. Compos. Hybrid Mater. 2018, 1, 518–529. [Google Scholar] [CrossRef]
- Yalagala, B.P.; Dahiya, A.S.; Dahiya, R. ZnO nanowires based degradable high-performance photodetectors for eco-friendly green electronics. Opto-Electron. Adv. 2023, 6, 220020. [Google Scholar] [CrossRef]
- Liang, S.; Xu, F.; Li, W.; Yang, W.; Cheng, S.; Yang, H.; Chen, J.; Yi, Z.; Jiang, P. Tunable smart mid infrared thermal control emitter based on phase change material VO2 thin film. Appl. Therm. Eng. 2023, 232, 121074. [Google Scholar] [CrossRef]
- Becker-Willinger, C.; Schmitz-Stöwe, S.; Bentz, D.; Veith, M. Kinetic investigations on TiO2 nanoparticles as photo initiators for UV-polymerization in acrylic matrix. In Micromachining and Microfabrication Process Technology XV; Maher, M.A., Chiao, J.-C., Resnick, P.J., Eds.; SPIE: Bellingham, WA, USA, 2010; p. 75900I. [Google Scholar]
- Ingrosso, C.; Corcione, C.E.; Striani, R.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Frigione, M. UV-curable nanocomposite based on methacrylic-siloxane resin and surface-modified TiO2 nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 15494–15505. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhou, S.; You, B.; Wu, L. Kinetic study on the UV-induced photopolymerization of epoxy acrylate/TiO2 nanocomposites by FTIR spectroscopy. J. Appl. Polym. Sci. 2006, 99, 3281–3287. [Google Scholar] [CrossRef]
- Damm, C.; Völtzke, D.; Abicht, H.-P.; Israel, G. Influence of the properties of TiO2 particles on a photocatalytic acrylate polymerisation. J. Photochem. Photobiol. A 2005, 174, 171–179. [Google Scholar] [CrossRef]
- Nakayama, N.; Hayashi, T. Preparation and characterization of TiO2 and polymer nanocomposite films with high refractive index. J. Appl. Polym. Sci. 2007, 105, 3662–3672. [Google Scholar] [CrossRef]
- Ghorbanhossaini, A.; Rafiee, R.; Pligovka, A.; Salerno, M. Dental composites with strength after aging improved by using anodic nanoporous fillers: Experimental results, modeling, and simulations. Eng. Comput. 2023, 39, 387–398. [Google Scholar] [CrossRef]
- Liu, K.; Cao, M.; Fujishima, A.; Jiang, L. Bio-inspired titanium dioxide materials with special wettability and their applications. Chem. Rev. 2014, 114, 10044–10094. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Wang, R.; Sakai, N.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Studies of surface wettability conversion on TiO2 single-crystal surfaces. J. Phys. Chem. B 1999, 103, 2188–2194. [Google Scholar] [CrossRef]
- Kong, X.; Hu, Y.; Wang, X.; Pan, W. Effect of surface morphology on wettability conversion. J. Adv. Ceram. 2016, 5, 284–290. [Google Scholar] [CrossRef]
- Hamid, N.; Telgi, R.L.; Tirth, A.; Tandon, V.; Chandra, S.; Chaturvedi, R.K. Titanium Dioxide Nanoparticles and Cetylpyridinium Chloride Enriched Glass-Ionomer Restorative Cement: A Comparative Study Assessing Compressive Strength and Antibacterial Activity. J. Clin. Pediatr. Dent. 2019, 43, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Khudyakov, I.V.; Legg, J.C.; Purvis, M.B.; Overton, B.J. Kinetics of photopolymerization of acrylates with functionality of 1−6. Ind. Eng. Chem. Res. 1999, 38, 3353–3359. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Das, S.; Balan, R.; Lekshmi, I.C. TiO2-ZrO2 nanocomposite with tetragonal zirconia phase and photocatalytic degradation of Alizarin Yellow GG azo dye under natural sunlight. Mater. Today Proc. 2021, 47, 4641–4646. [Google Scholar] [CrossRef]
- Siwińska-Ciesielczyk, K.; Andrzejczak, A.; Paukszta, D.; Piasecki, A.; Moszyński, D.; Zgoła-Grześkowiak, A.; Jesionowski, T. Synthesis of selected mixed oxide materials with tailored photocatalytic activity in the degradation of tetracycline. Materials 2021, 14, 5361. [Google Scholar] [CrossRef] [PubMed]
- Aydınoğlu, A.; Yoruç, A.B.H. Effects of silane-modified fillers on properties of dental composite resin. Mater. Sci. Eng. C 2017, 79, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Ciesielczyk, F.; Nowacka, M.; Jesionowski, T. Influence of selected alkoxysilanes on dispersive properties and surface chemistry of titanium dioxide and TiO2-SiO2 composite material. J. Nanomater. 2012, 2012, 1–19. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Ciesielczyk, F.; Jesionowski, T.; Sójka-Ledakowicz, J.; Lota, W.; Walawska, A. Evaluation of the photocatalytic properties of textile fabrics modifed with titanium dioxide of anatase structute. Fibres Text. East. Eur. 2011, 19, 76–83. [Google Scholar]
- Ambrożewicz, D.; Ciesielczyk, F.; Nowacka, M.; Karasiewicz, J.; Piasecki, A.; Maciejewski, H.; Jesionowski, T. Fluoroalkylsilane versus alkylsilane as hydrophobic agents for silica and silicates. J. Nanomater. 2013, 2013, 631938. [Google Scholar] [CrossRef]
- Berendsen, G.E.; de Galan, L. Preparation and chromatographic properties of some chemically bonded phases for reversed-phase liquid chromatography. J. Liq. Chromatogr. 1978, 1, 561–586. [Google Scholar] [CrossRef]
- Pujiarti, H.; Bahar, H.; Hidayat, R. Poly(ionic-liquid) from imidazoline-functionalized siloxane prepared by simple sol-gel route for efficient quasi-solid-state DSSC. Mater. Res. Express 2019, 6, 75507. [Google Scholar] [CrossRef]
- Boussehel, H. Influence of 3-(trimethoxysilyl) propyl methacrylate coupling agent treatment of olive pomace flour reinforced polystyrene composites. J. Compos. Adv. Mater. Rev. Des Compos. Matériaux Avancés 2019, 29, 375–380. [Google Scholar] [CrossRef]
- Gojzewski, H.; Sadej, M.; Andrzejewska, E.; Kokowska, M. Dataset for acrylate/silica nanoparticles formulations and photocured composites: Viscosity, filler dispersion and bulk Poisson’s ratio. Data Brief 2017, 12, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Bendaoued, A.; Messaoud, M.; Harzallah, O.; Bistac, S.; Salhi, R. Impact of spherical anatase TiO2 nanoparticles on thermal properties of polypropylene resin. J. Nanosci. Curr. Res. 2021, 6, 135. [Google Scholar]
- Suzuki, T.; Hori, N.; Att, W.; Kubo, K.; Iwasa, F.; Ueno, T.; Maeda, H.; Ogawa, T. Ultraviolet Treatment Overcomes Time-Related Degrading Bioactivity of Titanium. Tissue Eng. Part A 2009, 15, 3679–3688. [Google Scholar] [CrossRef]
- Kuroiwa, A.; Nomura, Y.; Ochiai, T.; Sudo, T.; Nomoto, R.; Hayakawa, T.; Kanzaki, H.; Nakamura, Y.; Hanada, N. Antibacterial, Hydrophilic Effect and Mechanical Properties of Orthodontic Resin Coated with UV-Responsive Photocatalyst. Materials 2018, 11, 889. [Google Scholar] [CrossRef]




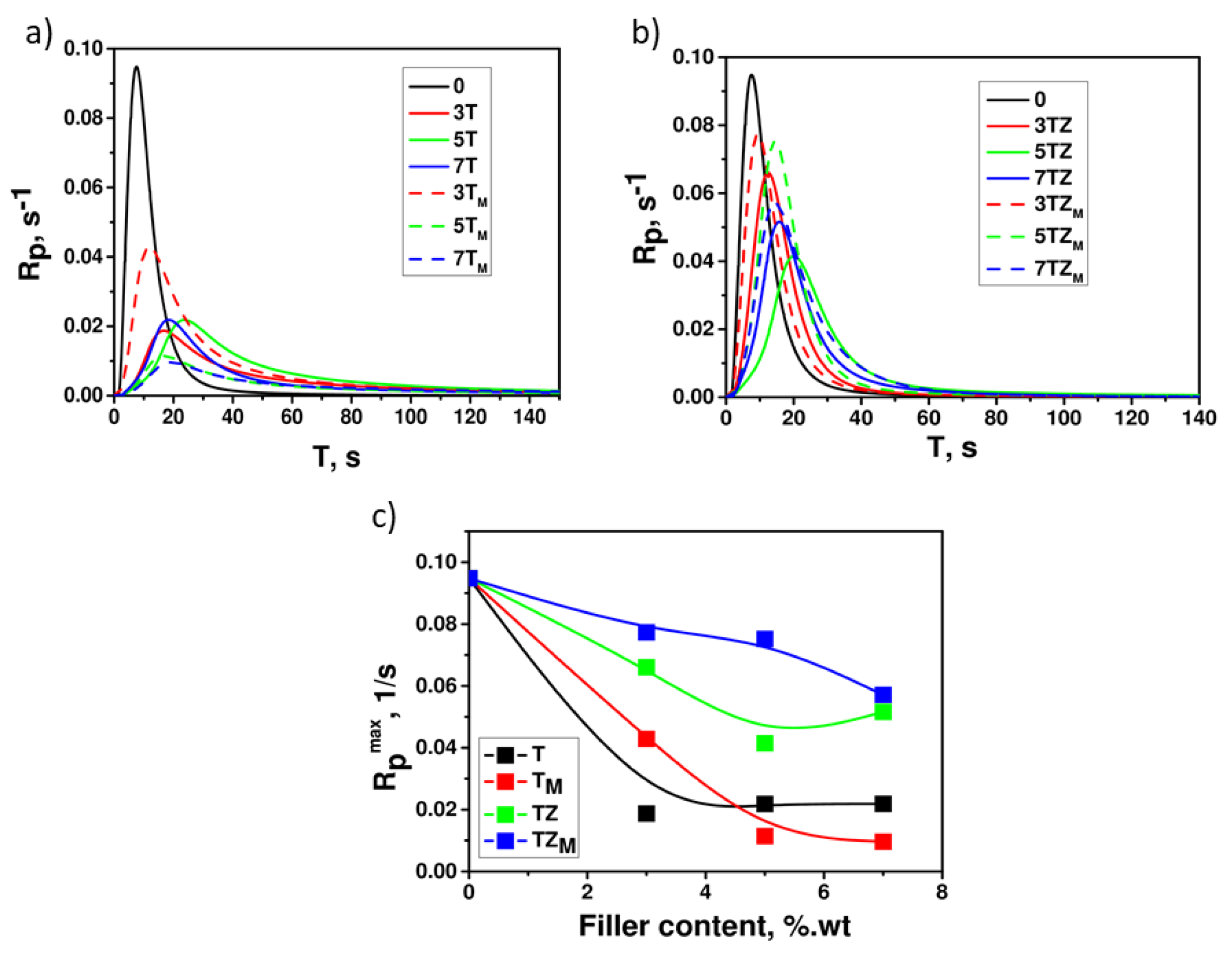

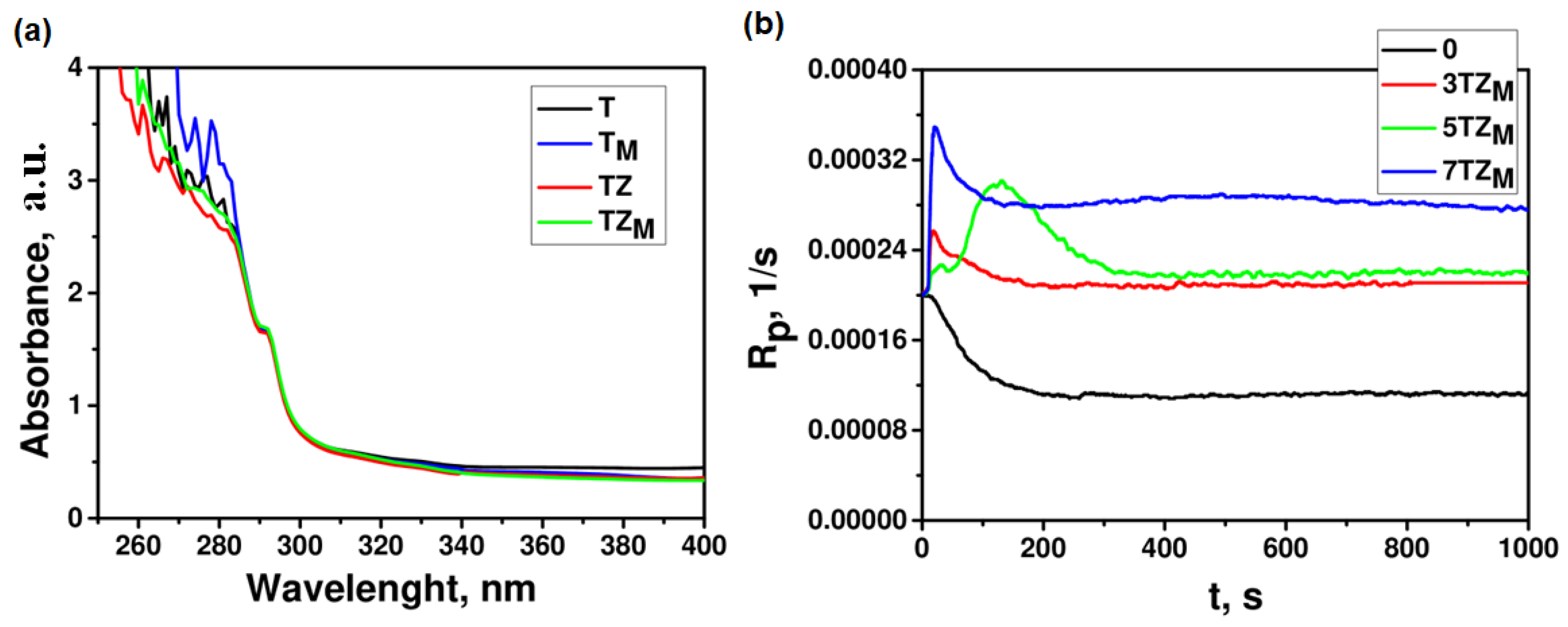



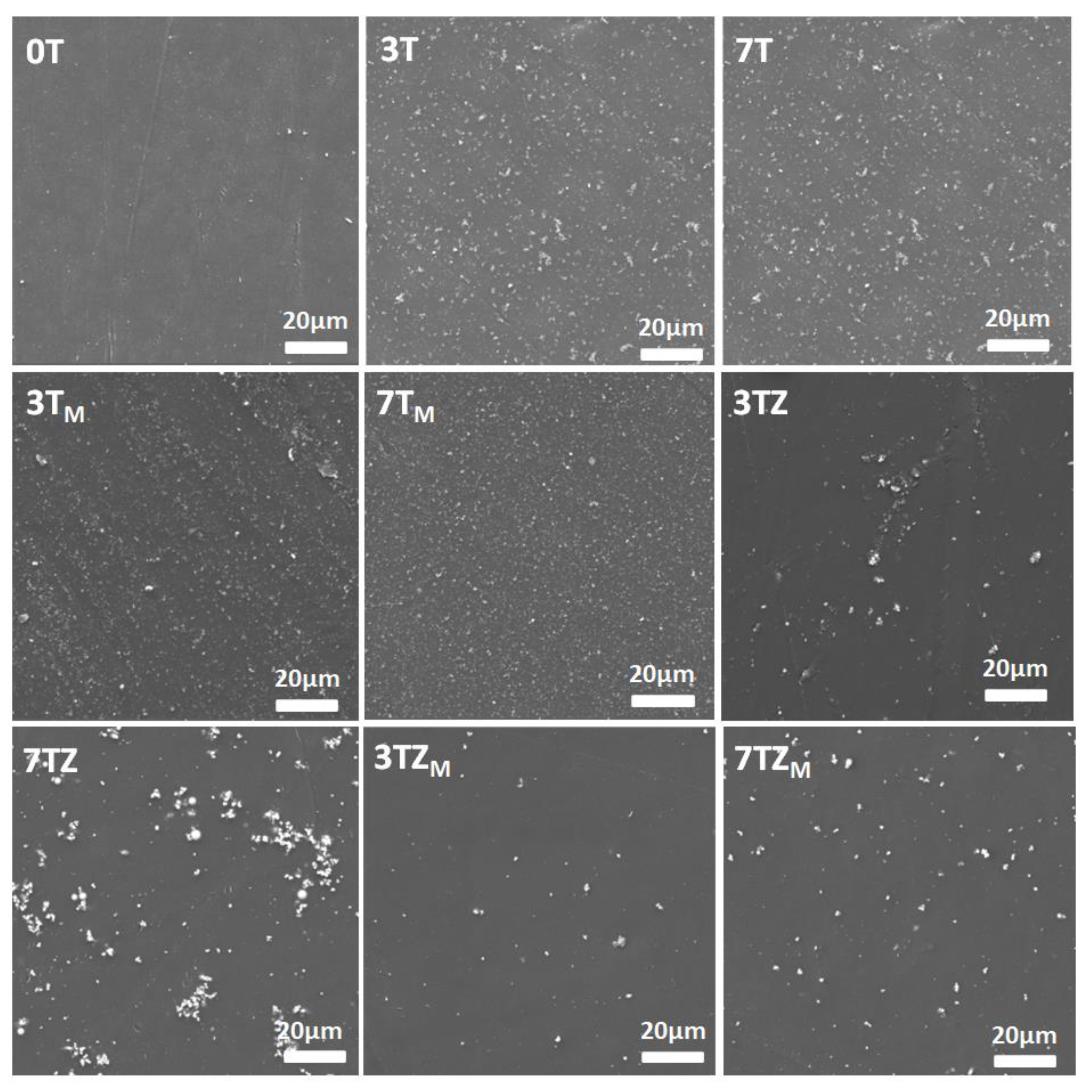
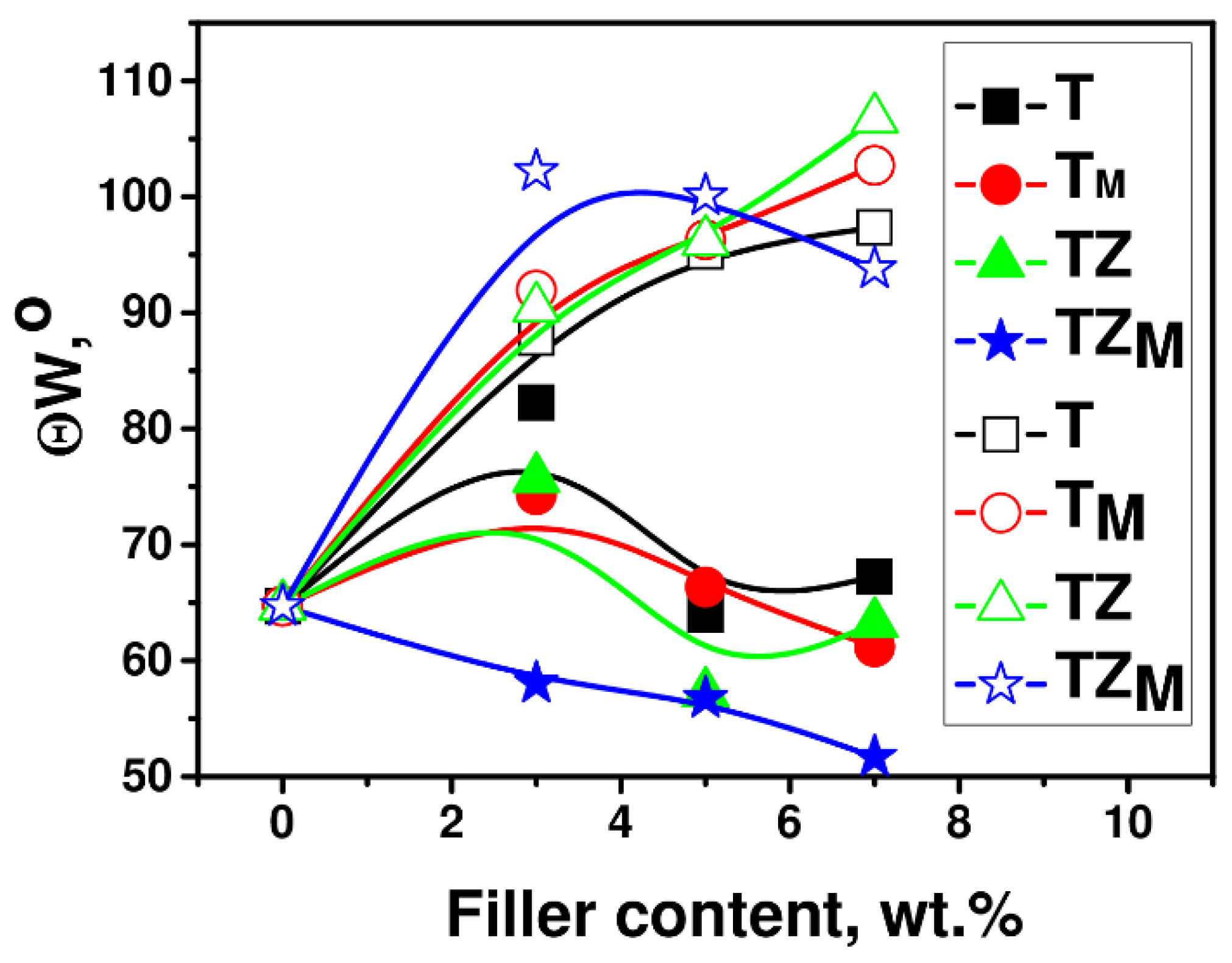
| Sample | T | TM | TZ | TZM |
|---|---|---|---|---|
| Particle size distribution by volume, nm | 164–531 | 459–1110 | 342–615 | 459–825 |
| Maximum volume contribution, % | 295 nm–22.7 | 712 nm–28.2 | 459 nm–43.0 | 615 nm–38.8 |
| Polydispersity index, PdI | 0.382 | 0.225 | 1.000 | 0.209 |
| Sample | T | TM | TZ | TZM | |
|---|---|---|---|---|---|
| Phase composition (%) | Anatase | 100 | 100 | 79.2 | 79.2 |
| Rutile | - | - | - | - | |
| ZrTiO4 | - | - | 20.8 | 20.8 | |
| Monoclinic ZrO2 | - | - | - | - | |
| Sample | T | TM | TZ | TZM | |
|---|---|---|---|---|---|
| Elemental analysis (%) | N | 0.0254 | 0.0212 | 0.0185 | 0.0228 |
| C | 0.1075 | 2.5447 | 0.0933 | 2.4837 | |
| H | 0.0390 | 0.3469 | 0.3418 | 0.4233 | |
| P (μmol/m2) | - | 5.5 | - | 1.8 | |
| Wavenumber, cm−1 | Functional Group | Sample | ||||
|---|---|---|---|---|---|---|
| A-174 | T | TM | TZ | TZM | ||
| 640 | Ti-O-Ti | − | + | + | + | + |
| 714 | − | + | + | + | + | |
| 721 | Ti-O-Zr | − | − | − | + | + |
| 742 | Zr-O | − | − | − | + | + |
| 830 | Si-CH2 (asymmetric) | + | − | + | − | + |
| 910–890 | Si-OH | + | − | + | − | + |
| 1080 | Si-O | + | − | + | − | + |
| 1087 | Si-O-C | + | − | + | − | + |
| 1160 | Si-O-CH3 | + | − | + | − | + |
| 1410 | C-H | + | − | + | − | + |
| 1170–1080 | Si-O-Ti | − | − | + | − | + |
| 1640 | O-H | − | + | + | + | + |
| 1710 | C=O (ester) | + | − | + | − | + |
| 2830 | -CH2- (asymmetric) | + | − | + | − | + |
| 2930 | C-H | − | + | + | + | + |
| 2950 | -CH2- (symmetric) | + | − | + | − | + |
| 2973 | -CH3 | + | − | + | − | + |
| 3380 | O-H | − | + | + | + | + |
| Sample | R (%) | T5 (°C) | T50 (°C) | Tmax (°C) |
|---|---|---|---|---|
| 0 | 4.9 | 327 | 395 | 392 |
| 3T | 10.5 | 330 | 400 | 397 |
| 7T | 13.0 | 337 | 404 | 397 |
| 3TM | 5.5 | 337 | 397 | 394 |
| 7TM | 11.2 | 330 | 402 | 397 |
| 3TZ | 8.87 | 344 | 397 | 394 |
| 7TZ | 12.7 | 342 | 402 | 394 |
| 3TZM | 11.3 | 327 | 402 | 399 |
| 7TZM | 9.84 | 337 | 393 | 400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siwińska-Ciesielczyk, K.; Andrzejczak, A.; Jesionowski, T.; Gierz, Ł.; Marcinkowska, A.; Robakowska, M. New Insights into the Application of Biocompatible (Un)Modified TiO2 and TiO2-ZrO2 Oxide Fillers in Light-Curing Materials. Materials 2024, 17, 2908. https://doi.org/10.3390/ma17122908
Siwińska-Ciesielczyk K, Andrzejczak A, Jesionowski T, Gierz Ł, Marcinkowska A, Robakowska M. New Insights into the Application of Biocompatible (Un)Modified TiO2 and TiO2-ZrO2 Oxide Fillers in Light-Curing Materials. Materials. 2024; 17(12):2908. https://doi.org/10.3390/ma17122908
Chicago/Turabian StyleSiwińska-Ciesielczyk, Katarzyna, Angelika Andrzejczak, Teofil Jesionowski, Łukasz Gierz, Agnieszka Marcinkowska, and Mariola Robakowska. 2024. "New Insights into the Application of Biocompatible (Un)Modified TiO2 and TiO2-ZrO2 Oxide Fillers in Light-Curing Materials" Materials 17, no. 12: 2908. https://doi.org/10.3390/ma17122908
APA StyleSiwińska-Ciesielczyk, K., Andrzejczak, A., Jesionowski, T., Gierz, Ł., Marcinkowska, A., & Robakowska, M. (2024). New Insights into the Application of Biocompatible (Un)Modified TiO2 and TiO2-ZrO2 Oxide Fillers in Light-Curing Materials. Materials, 17(12), 2908. https://doi.org/10.3390/ma17122908












