Cobalt–Imidazole Complexes: Effect of Anion Nature on Thermochemical Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Study of the Cobalt Complexes
2.2. Study of Thermal Decomposition of the Cobalt Complexes
2.3. Evaluation of Activation Energy for Thermal Decomposition of Cobalt Complexes
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Complex Compounds [Co(C3H4N2)6](NO3)2 and [Co(C3H4N2)6](ClO4)2
3.2.1. Synthesis of Co(ClO4)2·6H2O
3.2.2. Solvent-Free Synthesis of [Co(C3H4N2)6](NO3)2 and [Co(C3H4N2)6](ClO4)2
3.3. Characterization of Complex Compounds and Solid Products of Combustion
3.4. Study of the Thermal Properties of [Co(C3H4N2)6](NO3)2 and [Co(C3H4N2)6](ClO4)2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muhammed, A.; Sreelakshmi, P.; Praseeda, P.N.; Jishnu; Shan, S. A review on gas-generators for application in exploding fire extinguisher balls. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1114, 012079. [Google Scholar] [CrossRef]
- Barsukov, V.; Goldaev, S.; Basalaev, S. Applying of open gas generators in solid fuel for rising underwater objects. AIP Conf. Proc. 2019, 2103, 020002. [Google Scholar]
- Neutz, J.; König, A.; Knauss, H.; Jordan, S.; Rödiger, T.; Smorodsky, B.; Blümcke, E.W. Mass Flow Discharge and Total Temperature Characterization of a Pyrotechnic Gas Generator Formulation for Airbag Systems. Propellants Explos. Pyrotech. 2009, 34, 267–273. [Google Scholar] [CrossRef]
- Varisova, R.R. Technologies for the development of high-viscosity oil fields. J. Phys. Conf. Ser. 2022, 2388, 012071. [Google Scholar] [CrossRef]
- Netskina, O.V.; Dmitruk, K.A.; Mazina, O.I.; Paletsky, A.A.; Mukha, S.A.; Prosvirin, I.P.; Pochtar, A.A.; Bulavchenko, O.A.; Shmakov, A.G.; Veselovskaya, J.V.; et al. CO2 methanation: Solvent-Free Synthesis of Nickel-Containing Catalysts from Complexes with Ethylenediamine. Materials 2023, 16, 2616. [Google Scholar] [CrossRef] [PubMed]
- Tella, A.C.; Eke, U.B.; Owalude, S.O. Solvent-free mechanochemical synthesis and X-ray studies of Cu(II) and Ni(II) complexes of 5-(3,4,5-Trimethoxybenzyl)pyrimidine-2,4-diamine (Trimethoprim) in a ball-mill. J. Saudi Chem. Soc. 2016, 20, S376–S381. [Google Scholar] [CrossRef]
- Netskina, O.; Mukha, S.; Veselovskaya, J.; Bolotov, V.; Komova, O.; Ishchenko, A.; Bulavchenko, O.; Prosvirin, I.; Pochtar, A.; Rogov, V. CO2 Methanation: Nickel-Alumina Catalyst Prepared by Solid-State Combustion. Materials 2021, 14, 6789. [Google Scholar] [CrossRef]
- Netskina, O.V.; Mukha, S.A.; Dmitruk, K.A.; Ishchenko, A.V.; Bulavchenko, O.A.; Pochtar, A.A.; Suknev, A.P.; Komova, O.V. Solvent-Free Method for Nanoparticles Synthesis by Solid-State Combustion Using Tetra(Imidazole)Copper(II) Nitrate. Inorganics 2022, 10, 15. [Google Scholar] [CrossRef]
- Netskina, O.V.; Dmitruk, K.A.; Paletsky, A.A.; Mukha, S.A.; Pochtar, A.A.; Bulavchenko, O.A.; Prosvirin, I.P.; Shmakov, A.G.; Ozerova, A.M.; Veselovskaya, J.V.; et al. Solvent-Free Synthesis of Nickel Nanoparticles as Catalysts for CO2 hydrogenation to Methane. Catalysts 2022, 12, 1274. [Google Scholar] [CrossRef]
- Chai, L.-Q.; Zhou, L.; Zhang, K.-Y.; Zhang, H.-S. Structural characterizations, spectroscopic, electrochemical properties, and antibacterial activities of copper (II) and cobalt (II) complexes containing imidazole ring. Appl. Organomet. Chem. 2018, 32, e4576. [Google Scholar] [CrossRef]
- Roncaroli, F.; Dal Molin, E.S.; Viva, F.A.; Bruno, M.M.; Halac, E.B. Cobalt and Iron Complexes with N-heterocyclic Ligands as Pyrolysis Precursors for Oxygen Reduction Catalysts. Electrochim. Acta 2015, 174, 66–77. [Google Scholar] [CrossRef]
- Barreca, D.; Massignan, C.; Daolio, S.; Fabrizio, M.; Piccirillo, C.; Armelao, L.; Tondello, E. Composition and microstructure of cobalt oxide thin films obtained from a novel cobalt (II) precursor by chemical vapor deposition. Chem. Mater. 2001, 13, 588–593. [Google Scholar] [CrossRef]
- Pająk, M.; Woźniczka, M.; Vogt, A.; Kufelnicki, A. Reversible uptake of molecular oxygen by heteroligand Co(II)–l-α-amino acid–imidazole systems: Equilibrium models at full mass balance. Chem. Cent. J. 2017, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Norkus, E.; Vaškelis, A.; Grigucevičienė, A.; Rozovskis, G.; Reklaitis, J.; Norkus, P. Oxidation of cobalt(II) with air oxygen in aqueous ethylenediamine solutions. Transit. Met. Chem. 2001, 26, 465–472. [Google Scholar] [CrossRef]
- Peral, F.; Gallego, E. Self-association of imidazole and its methyl derivatives in aqueous solution. A study by ultraviolet spectroscopy. J. Mol. Struct. 1997, 415, 187–196. [Google Scholar] [CrossRef]
- Reimann, C.W. Electron Absorption Spectrum of Cobalt (II)–Doped Trisphenanthrolinezinc Nitrate Dihydrate. J. Res. Natl. Bur. Standards. Sect. A Phys. Chem. 1966, 70A, 417–419. [Google Scholar] [CrossRef]
- Battistuzzi, R. Cobalt(II) perchlorate, tetrafluoroborate, nitrate and sulphate complexes with 4,6-dimethylpyrimidine-2(1H)-thione. Polyhedron 1985, 4, 933–941. [Google Scholar] [CrossRef]
- Solomon, E.; Lever, I.A.B.P. (Eds.) Inorganic Electronic Structure and Spectroscopy. Volume II. Applications and Case Studies; Wiley-Interscience: New York, NY, USA, 1999; pp. 1–658. [Google Scholar]
- Hodgson, J.B.; Percy, G.C.; Thornton, D.A. The IR spectra of imidazole complexes of 1st transition series metal(II) nitrates and perchlorates. J. Mol. Struct. 1980, 66, 81–92. [Google Scholar] [CrossRef]
- Madanagopal, A.; Periandy, S.; Gayathri, P.; Ramalingam, S.; Xavier, S.; Ivanov, V.K. Spectroscopic and computational investigation of the structure and pharmacological activity of 1-benzylimidazole. J. Taibah Univ. Sci. 2017, 11, 975–996. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.J.; Ahn, A.; Kim, Y.; Min, A.; Choi, M.Y.; Miller, R.E. Infrared Spectroscopy of Imidazole Trimer in Helium Nanodroplets: Free NH Stretch Mode. Bull. Korean Chem. Soc. 2011, 32, 885–888. [Google Scholar] [CrossRef]
- Tikhonov, D.S.; Scutelnic, V.; Sharapa, D.I.; Krotova, A.A.; Dmitrieva, A.V.; Obenchain, D.A.; Schnell, M. Structures of the (Imidazole)nH+ … Ar (n = 1, 2, 3) complexes determined from IR spectroscopy and quantum chemical calculations. Struct. Chem. 2023, 34, 203–213. [Google Scholar] [CrossRef]
- CCDC. Advancing Structural Science. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 15 April 2024).
- Grimmett, M.R. Advances in Imidazole Chemistry. Adv. Heterocycl. Chem. 1970, 12, 103–183. [Google Scholar] [CrossRef]
- Małecka, B.; Łącz, A.; Drożdż, E.; Małecki, A. Thermal decomposition of d-metal nitrates supported on alumina. J. Therm. Anal. Calorim. 2015, 119, 1053–1061. [Google Scholar] [CrossRef]
- Xue, L.; Zhao, F.Q.; Hu, R.Z.; Gao, H.X. A Simple Method to Estimate the Critical Temperature of Thermal Explosion for Energetic Materials Using Nonisothermal DSC. J. Energ. Mater. 2010, 28, 17–34. [Google Scholar] [CrossRef]
- Stern, K.H. High temperature properties and decomposition of inorganic salts. Part 4. Oxy-Salts of the halogens. J. Phys. Chem. Ref. Data 1974, 3, 481–526. [Google Scholar] [CrossRef]
- Małecki, A.; Gajerski, R.; Łabuś, S.; Prochowska-Klisch, B.; Wojciechowski, K.T. Mechanism of Thermal Decomposition of d-metals Nitrates Hydrates. J. Therm. Anal. Calorim. 2000, 60, 17–23. [Google Scholar] [CrossRef]
- Manouchehri, I.; Kameli, P.; Salamati, H. Facile Synthesis of Co3O4/CoO Nanoparticles by thermal treatment of ball-milled precursors. J. Supercond. Nov. Magn. 2011, 24, 1907–1910. [Google Scholar] [CrossRef]
- Rodil, S.E. Infrared spectra of amorphous carbon based materials. Diam. Relat. Mater. 2005, 14, 1262–1269. [Google Scholar] [CrossRef]
- Das, S.C.; Majumdar, A.; Hippler, R. Electronic and chemical property of amorphous carbon, hydrocarbon, hydrogenated/hydrogen free carbon nitride: Spectroscopic. Int. J. Innov. Sci. Res. 2014, 12, 148–156. [Google Scholar]
- Gowda, N.M.N.; Naikar, S.B.; Reddy, G.K.N. Perchlorate ion complex. Adv. Inorg. Chem. 1984, 28, 255–299. [Google Scholar] [CrossRef]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Gómez-Serrano, V. FT-IR analysis of pyrone and chromene structures in activated carbon. Energy Fuels 2014, 28, 4096–4103. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 22 April 2024).

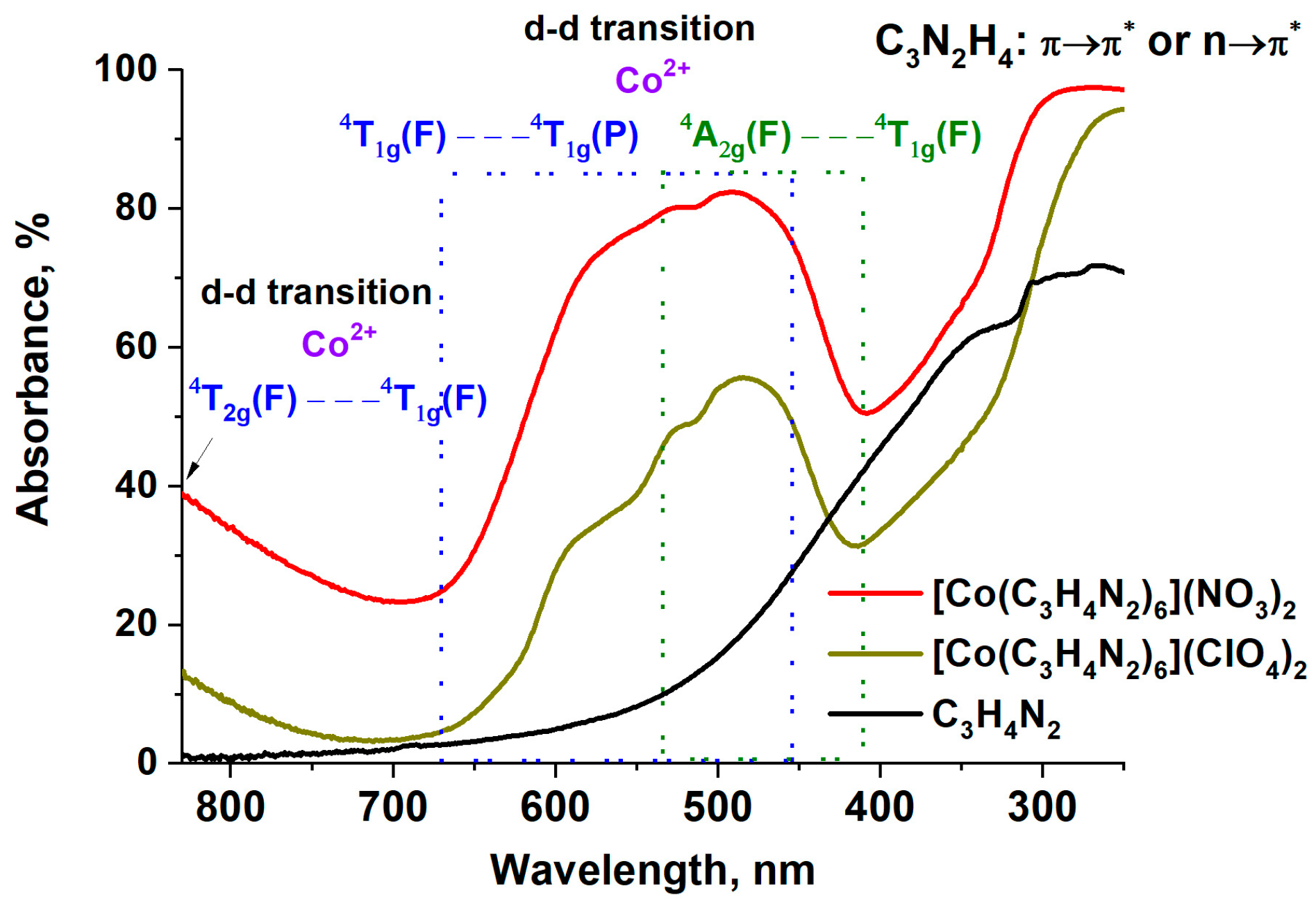

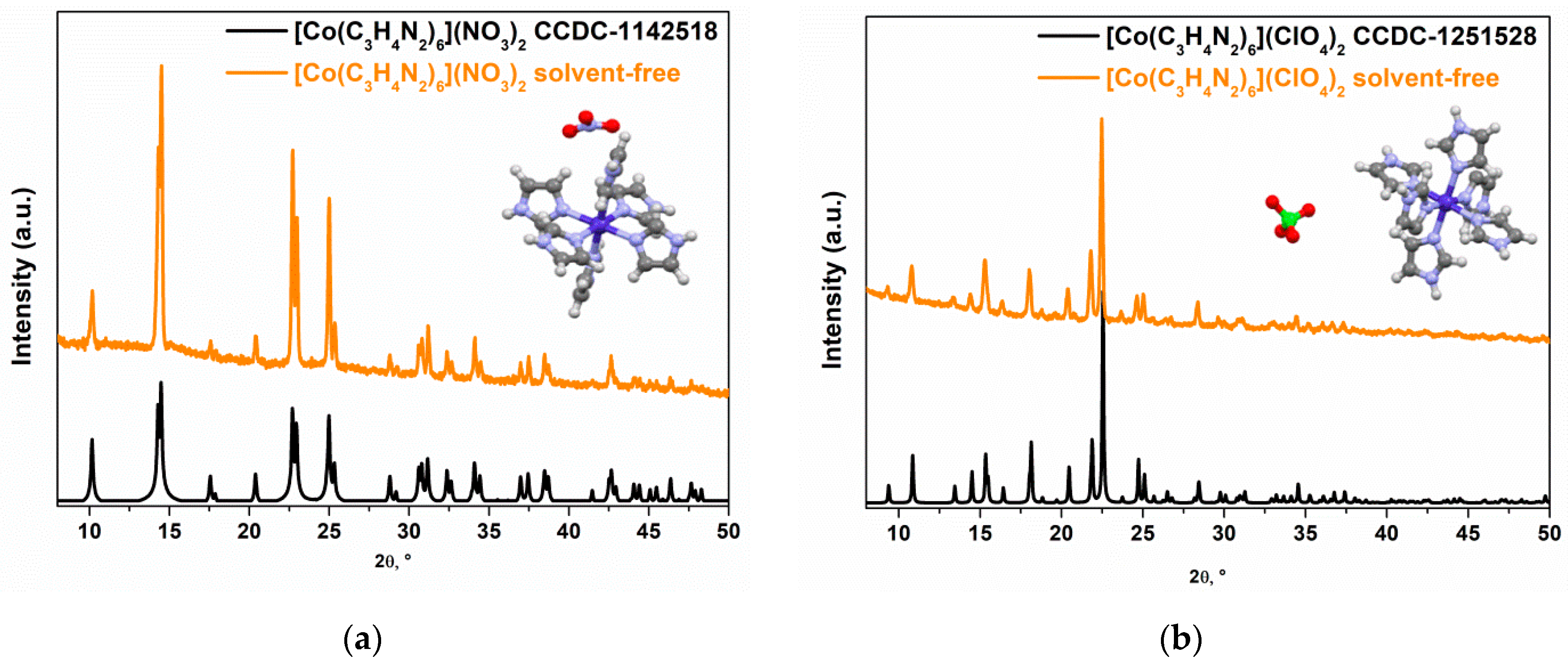
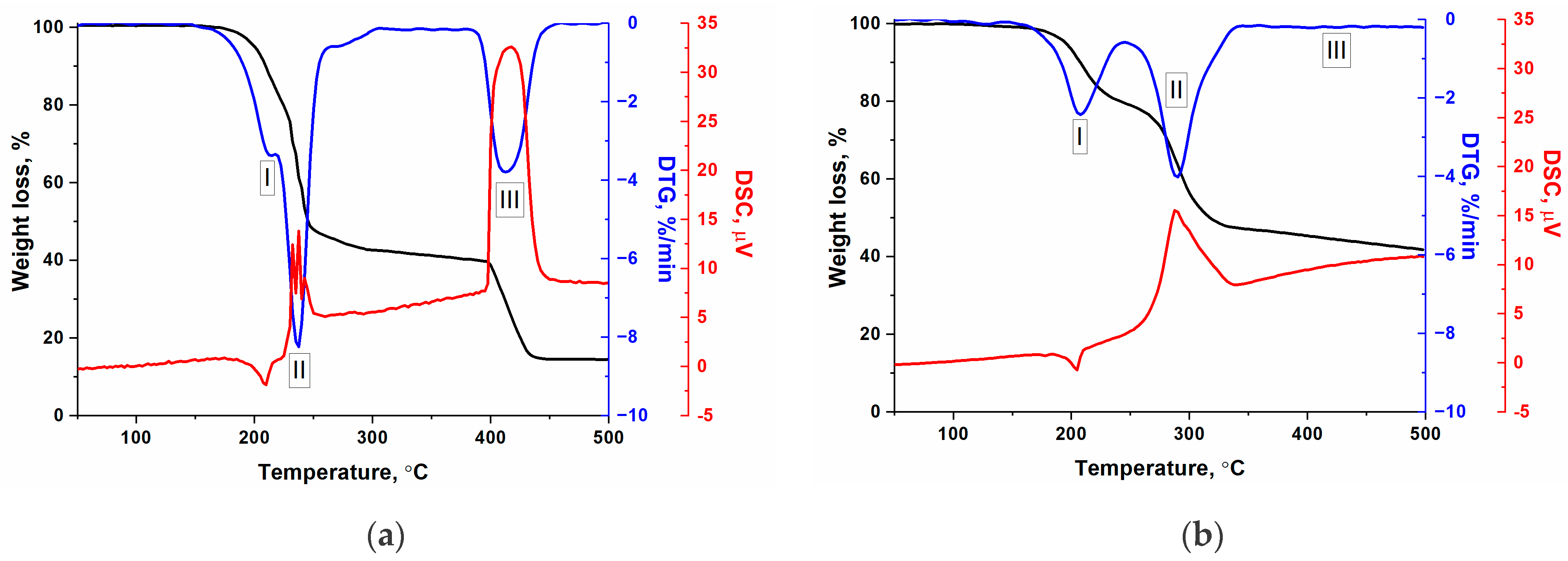
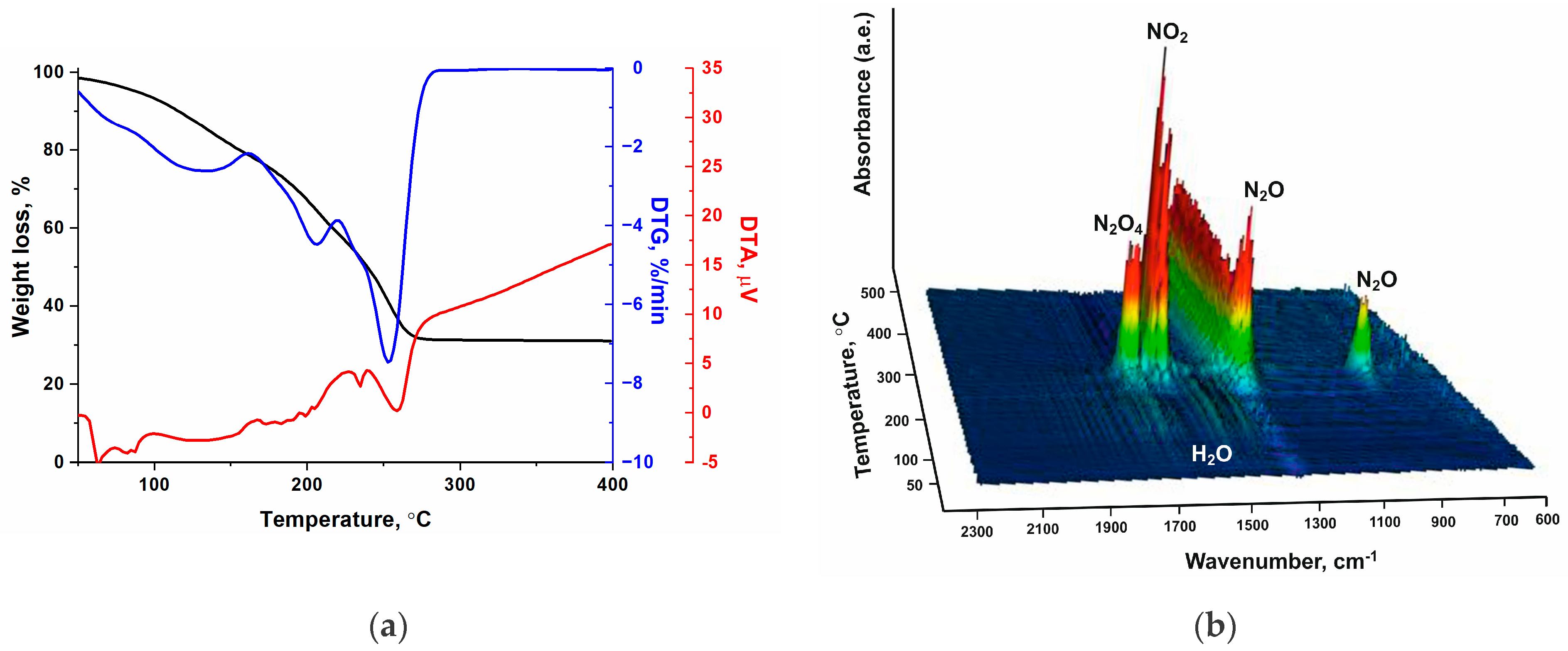



| Complex, Molar Mass | Composition, % | Gross Formula | Oxygen Balance | Heat of Combustion | |
|---|---|---|---|---|---|
| Calculated | Found | ||||
| [Co(C3H4N2)6](NO3)2 CoC18H24N14O6 591 g/mol | Co—10.0 C—36.6 H—4.1 N—33.2 O—16.2 | Co—10.5 C—36.1 H—3.9 N—32.7 Other («O») 1—16.8 | CoC16.9H21.9N13.1«O»5.9 | −117% | 17.6 |
| [Co(C3H4N2)6](ClO4)2 CoC18H24Cl2N12O8 666 g/mol | Co—8.8 C—32.4 H—3.6 N—25.2 Cl—10.6 O—19.2 | Co—8.9 C—32.5 H—3.6 N—25.2 Cl—10.6 Other («O») 1—19.2 | CoC17.9H23.8N11.9«O»7.9Cl2 | −112% | 14.7 |
| Complex | Stage 1 (Endothermic) | Stage 2 (Exothermic) | ||||
|---|---|---|---|---|---|---|
| Heat Rate, °C/min | Temperature, °C | Activation Energy, kJ/mol | Heat Rate, °C/min | Temperature, °C | Activation Energy, kJ/mol | |
| [Co(C3H4N2)6](NO3)2 | 2.5 | 203 | 275 ± 44 | 2.5 | 220 | 100 ± 13 |
| 5 | 210 | 5 | 228 | |||
| 10 | 213 | 10 | 243 | |||
| 15 | 215 | 15 | 255 | |||
| [Co(C3H4N2)6](ClO4)2 | 2.5 | 200 | 283 ± 17 | 2.5 | 276 | 136 ± 5 |
| 5 | 204 | 5 | 289 | |||
| 10 | 209 | 10 | 301 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netskina, O.V.; Sukhorukov, D.A.; Dmitruk, K.A.; Mukha, S.A.; Prosvirin, I.P.; Pochtar, A.A.; Bulavchenko, O.A.; Paletsky, A.A.; Shmakov, A.G.; Suknev, A.P.; et al. Cobalt–Imidazole Complexes: Effect of Anion Nature on Thermochemical Properties. Materials 2024, 17, 2911. https://doi.org/10.3390/ma17122911
Netskina OV, Sukhorukov DA, Dmitruk KA, Mukha SA, Prosvirin IP, Pochtar AA, Bulavchenko OA, Paletsky AA, Shmakov AG, Suknev AP, et al. Cobalt–Imidazole Complexes: Effect of Anion Nature on Thermochemical Properties. Materials. 2024; 17(12):2911. https://doi.org/10.3390/ma17122911
Chicago/Turabian StyleNetskina, Olga V., Dmitry A. Sukhorukov, Kirill A. Dmitruk, Svetlana A. Mukha, Igor P. Prosvirin, Alena A. Pochtar, Olga A. Bulavchenko, Alexander A. Paletsky, Andrey G. Shmakov, Alexey P. Suknev, and et al. 2024. "Cobalt–Imidazole Complexes: Effect of Anion Nature on Thermochemical Properties" Materials 17, no. 12: 2911. https://doi.org/10.3390/ma17122911
APA StyleNetskina, O. V., Sukhorukov, D. A., Dmitruk, K. A., Mukha, S. A., Prosvirin, I. P., Pochtar, A. A., Bulavchenko, O. A., Paletsky, A. A., Shmakov, A. G., Suknev, A. P., & Komova, O. V. (2024). Cobalt–Imidazole Complexes: Effect of Anion Nature on Thermochemical Properties. Materials, 17(12), 2911. https://doi.org/10.3390/ma17122911






