CO2 Capture with Polyethylenimine Supported on 3D-Printed Porous SiO2 Structures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Feedstock Preparation
2.3. Printing
2.4. Filament Extrusion
2.5. Debinding/Sintering
2.6. Characterization (Microscopy, SEM, BET, Compression Strength)
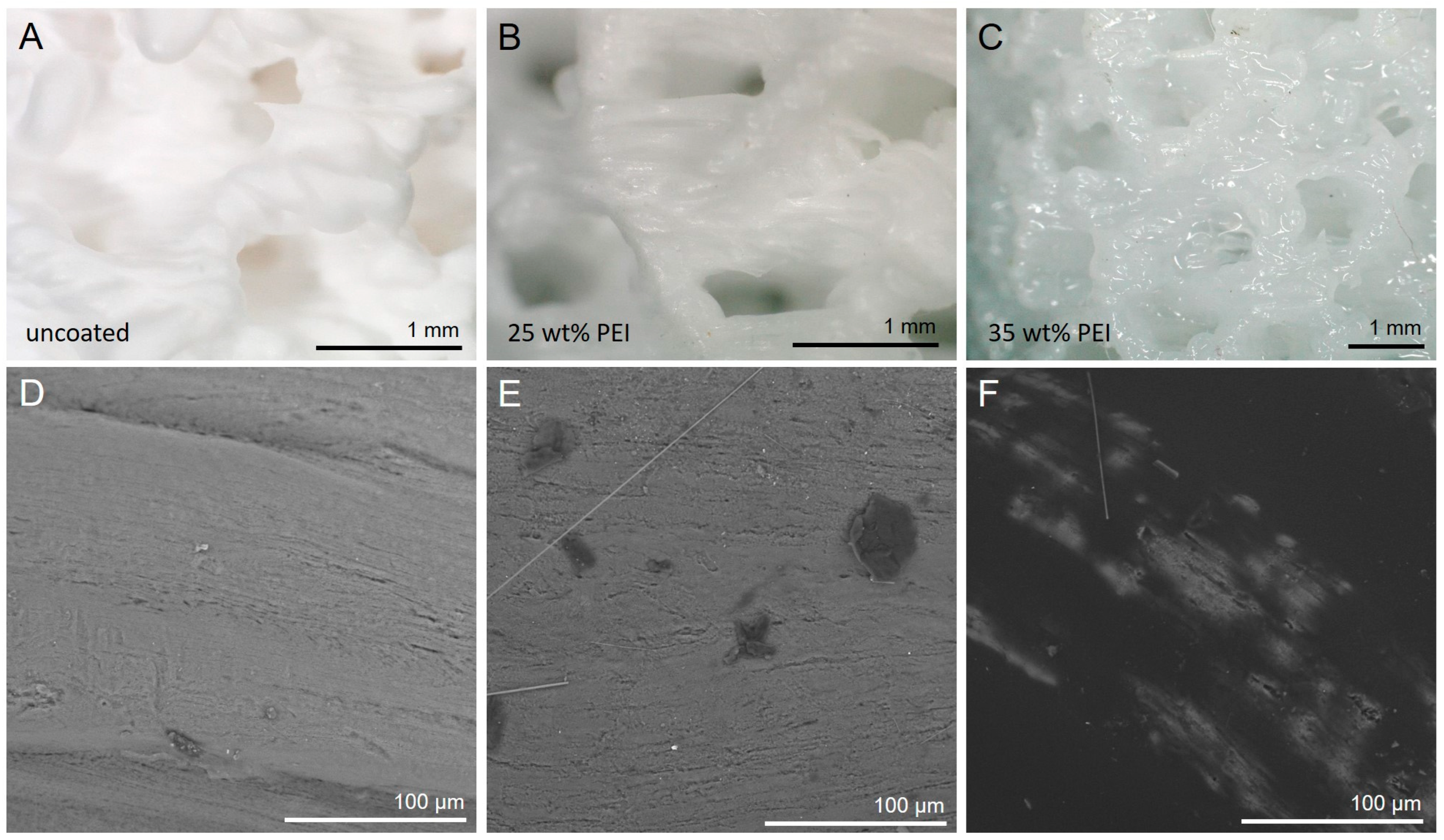
2.7. PEI Surface Modification
2.8. CO2 Adsorption
3. Results and Discussion
3.1. Feedstock
3.2. Three-Dimensional Printing
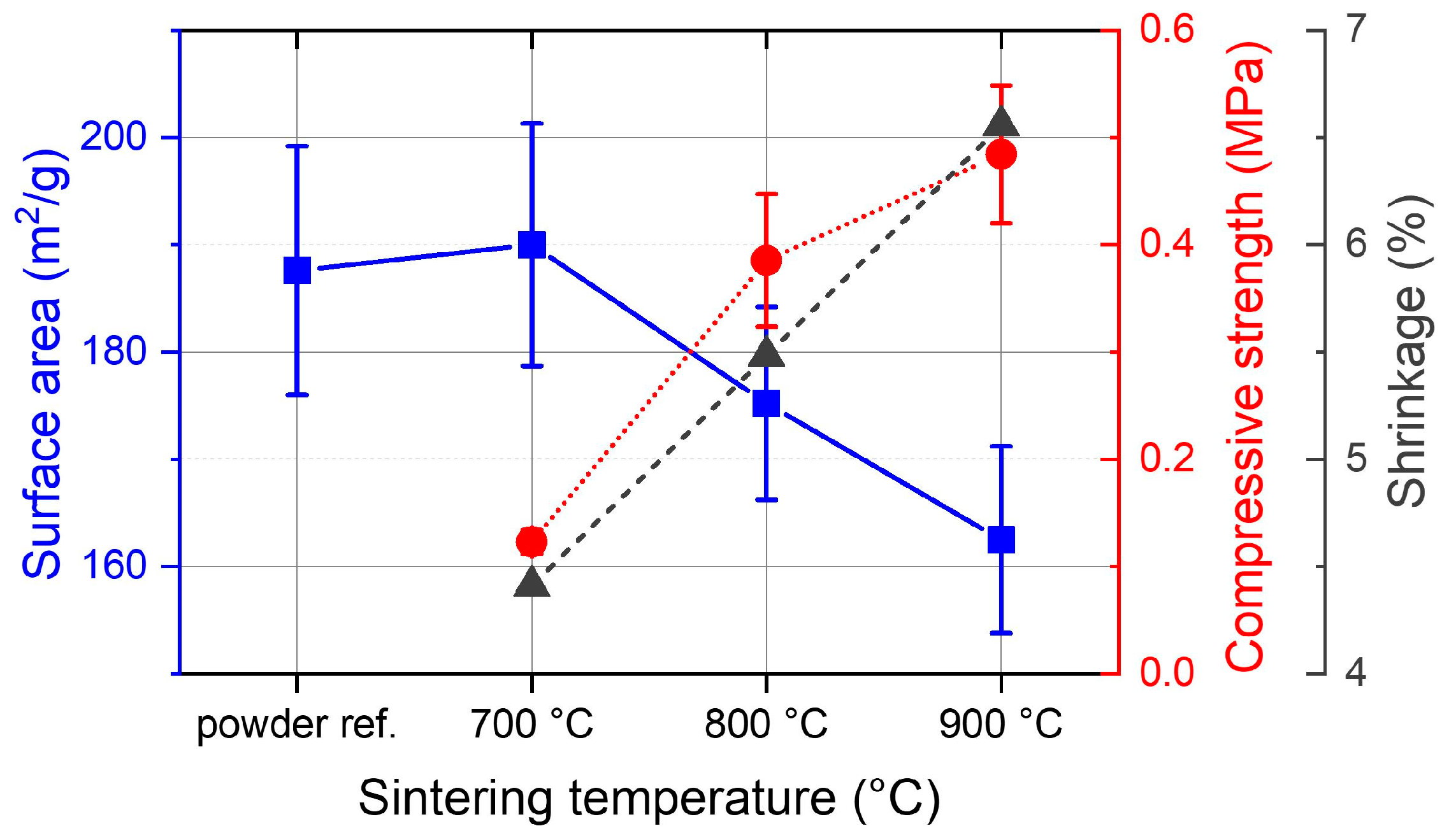
3.3. Sintering
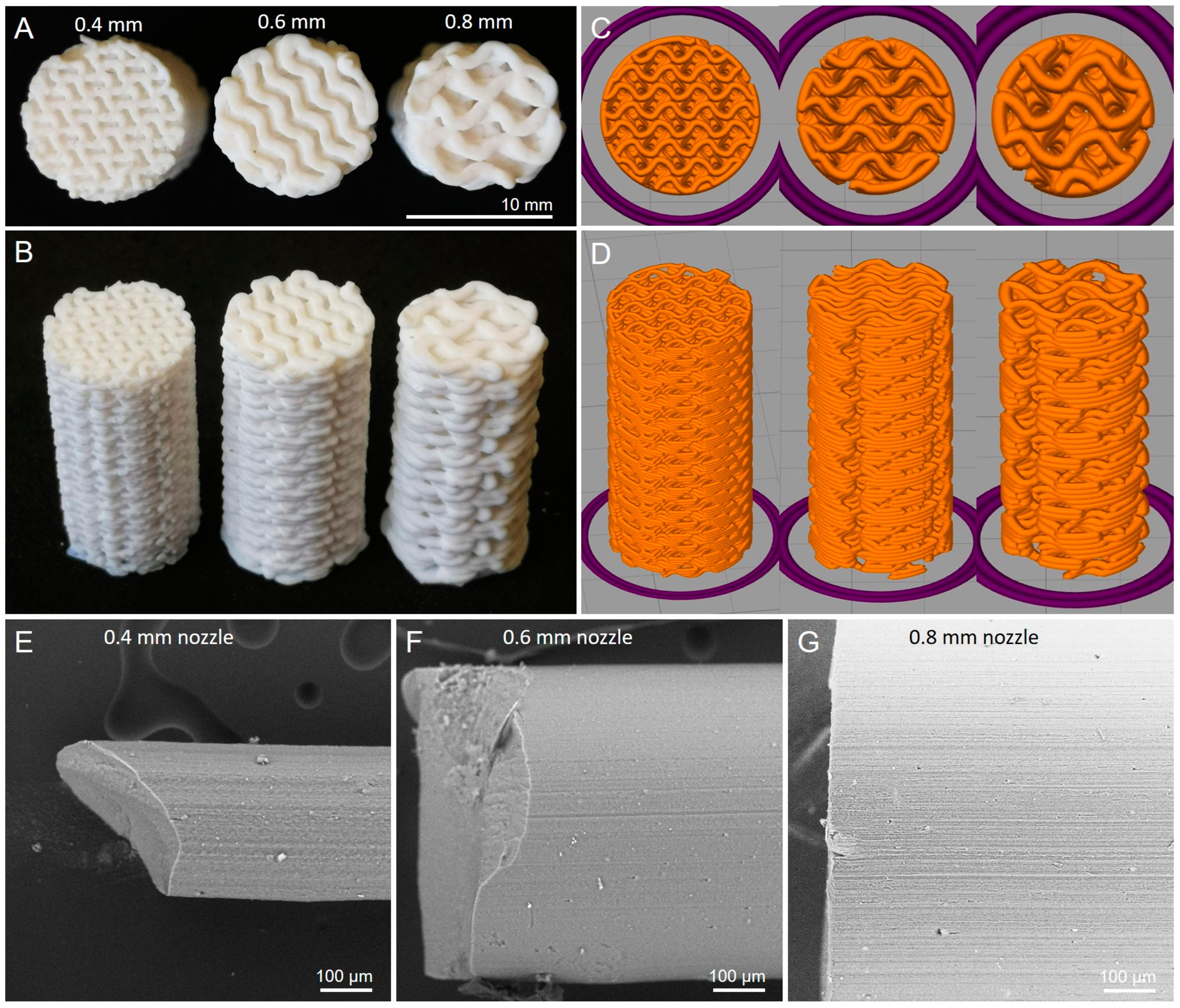
3.4. Samples with Different Nozzle Sizes
3.5. Filament Granulates
3.6. CO2 Capture
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hack, J.; Maeda, N.; Meier, D.M. Review on CO2 Capture Using Amine-Functionalized Materials. ACS Omega 2022, 7, 39520–39530. [Google Scholar] [CrossRef]
- Fisher, J.C.; Tanthana, J.; Chuang, S.S.C. Oxide-supported Tetraethylenepentamine for CO2 Capture. Environ. Prog. Sustain. Energy 2009, 28, 589–598. [Google Scholar] [CrossRef]
- Guo, L.; Hu, X.; Hu, G.; Chen, J.; Li, Z.; Dai, W.; Dacosta, H.F.M.; Fan, M. Tetraethylenepentamine Modified Protonated Titanate Nanotubes for CO2 Capture. Fuel Process. Technol. 2015, 138, 663–669. [Google Scholar] [CrossRef]
- Irani, M.; Jacobson, A.T.; Gasem, K.A.M.; Fan, M. Modified Carbon Nanotubes/Tetraethylenepentamine for CO2 Capture. Fuel 2017, 206, 10–18. [Google Scholar] [CrossRef]
- Rosu, C.; Pang, S.H.; Sujan, A.R.; Sakwa-Novak, M.A.; Ping, E.W.; Jones, C.W. Effect of Extended Aging and Oxidation on Linear Poly(Propylenimine)-Mesoporous Silica Composites for CO2 Capture from Simulated Air and Flue Gas Streams. ACS Appl. Mater. Interfaces 2020, 12, 38085–38097. [Google Scholar] [CrossRef]
- Zhang, H.; Goeppert, A.; Olah, G.A.; Prakash, G.K.S. Remarkable Effect of Moisture on the CO2 Adsorption of Nano-Silica Supported Linear and Branched Polyethylenimine. J. CO2 Util. 2017, 19, 91–99. [Google Scholar] [CrossRef]
- Hack, J.; Frazzetto, S.; Evers, L.; Maeda, N.; Meier, D.M. Branched Versus Linear Structure: Lowering the CO2 Desorption Temperature of Polyethylenimine-Functionalized Silica Adsorbents. Energies 2022, 15, 1075. [Google Scholar] [CrossRef]
- Weisshar, F.; Gau, A.; Hack, J.; Maeda, N.; Meier, D.M. Toward Carbon Dioxide Capture from the Atmosphere: Lowering the Regeneration Temperature of Polyethylenimine-Based Adsorbents by Ionic Liquid. Energy Fuels 2021, 35, 9059–9062. [Google Scholar] [CrossRef]
- Wang, Y.; Rim, G.; Song, M.; Holmes, H.E.; Jones, C.W.; Lively, R.P. Cold Temperature Direct Air CO2 Capture with Amine-Loaded Metal–Organic Framework Monoliths. ACS Appl. Mater. Interfaces 2023, 16, 1404–1415. [Google Scholar] [CrossRef]
- Kearns, E.R.; Gillespie, R.; D’Alessandro, D.M. 3D Printing of Metal-Organic Framework Composite Materials for Clean Energy and Environmental Applications. J. Mater. Chem. A 2021, 9, 27252–27270. [Google Scholar] [CrossRef]
- Lawson, S.; Snarzyk, M.; Hanify, D.; Rownaghi, A.A.; Rezaei, F. Development of 3D-Printed Polymer-MOF Monoliths for CO2 Adsorption. Ind. Eng. Chem. Res. 2020, 59, 7151–7160. [Google Scholar] [CrossRef]
- Soliman, A.; Alamoodi, N.; Karanikolos, G.N.; Doumanidis, C.C.; Polychronopoulou, K. A Review on New 3-d Printed Materials’ Geometries for Catalysis and Adsorption: Paradigms from Reforming Reactions and CO2 Capture. Nanomaterials 2020, 10, 2198. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, H.; Eastman, S.; Al-Mamoori, A.; Hajari, A.; Rownaghi, A.A.; Rezaei, F. Formulation of Aminosilica Adsorbents into 3D-Printed Monoliths and Evaluation of Their CO2 Capture Performance. ACS Appl. Mater. Interfaces 2017, 9, 7489–7498. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, S.N.; Boon, J.; James, J.; Krishnamurthy, S.; Lind, A.; Blom, R.; Andreassen, K.A.; Cormos, A.M.; Sandu, V.C.; de Boer, R. 3D-Printing of Adsorbents for Increased Productivity in Carbon Capture Applications (3D-CAPS). Int. J. Greenh. Gas Control 2021, 112, 103512. [Google Scholar] [CrossRef]
- Krishnamurthy, S. Vacuum Swing Adsorption Process for Post-Combustion Carbon Capture with 3D Printed Sorbents: Quantifying the Improvement in Productivity and Specific Energy over a Packed Bed System through Process Simulation and Optimization. Chem. Eng. Sci. 2022, 253, 117585. [Google Scholar] [CrossRef]
- Lengauer, W.; Duretek, I.; Fürst, M.; Schwarz, V.; Gonzalez-Gutierrez, J.; Schuschnigg, S.; Kukla, C.; Kitzmantel, M.; Neubauer, E.; Lieberwirth, C.; et al. Fabrication and Properties of Extrusion-Based 3D-Printed Hardmetal and Cermet Components. Int. J. Refract. Met. Hard Mater. 2019, 82, 141–149. [Google Scholar] [CrossRef]
- Cano, S.; Gonzalez-Gutierrez, J.; Sapkota, J.; Spoerk, M.; Arbeiter, F.; Schuschnigg, S.; Holzer, C.; Kukla, C. Additive Manufacturing of Zirconia Parts by Fused Filament Fabrication and Solvent Debinding: Selection of Binder Formulation. Addit. Manuf. 2019, 26, 117–128. [Google Scholar] [CrossRef]
- Orlovská, M.; Chlup, Z.; Bača, Ľ.; Janek, M.; Kitzmantel, M. Fracture and Mechanical Properties of Lightweight Alumina Ceramics Prepared by Fused Filament Fabrication. J. Eur. Ceram. Soc. 2020, 40, 4837–4843. [Google Scholar] [CrossRef]
- Shen, T.; Xiong, H.; Li, Z.; Zhang, L.; Zhou, K. Fused Deposition Fabrication of High-Quality Zirconia Ceramics Using Granular Feedstock. Ceram. Int. 2021, 47, 34352–34360. [Google Scholar] [CrossRef]
- Hadian, A.; Koch, L.; Koberg, P.; Sarraf, F.; Liersch, A.; Sebastian, T.; Clemens, F. Material Extrusion Based Additive Manufacturing of Large Zirconia Structures Using Filaments with Ethylene Vinyl Acetate Based Binder Composition. Addit. Manuf. 2021, 47, 102227. [Google Scholar] [CrossRef]
- Wick-Joliat, R.; Penner, D. Flexible Interconnected Ceramic Parts 3D Printed by Two-Component Material Extrusion with Water-Soluble Support Structures. J. Eur. Ceram. Soc. 2023, 43, 4877–4884. [Google Scholar] [CrossRef]
- Wick-Joliat, R.; Penner, D. Metal Casting into NaCl Molds Fabricated by Material Extrusion 3D Printing. Prog. Addit. Manuf. 2023. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Abu Al-Rub, R.K. Multifunctional Mechanical Metamaterials Based on Triply Periodic Minimal Surface Lattices. Adv. Eng. Mater. 2019, 21, 1900524. [Google Scholar] [CrossRef]
- Kelly, J.P.; Finkenauer, L.R.; Roy, P.; Stolaroff, J.K.; Nguyen, D.T.; Ross, M.S.; Hoff, A.T.; Haslam, J.J. Binder Jet Additive Manufacturing of Ceramic Heat Exchangers for Concentrating Solar Power Applications with Thermal Energy Storage in Molten Chlorides. Addit. Manuf. 2022, 56, 102937. [Google Scholar] [CrossRef]
- Dolamore, F.; Houlton, B.; Fee, C.J.; Watson, M.J.; Holland, D.J. A Numerical Investigation of the Hydrodynamic Dispersion in Triply Periodic Chromatographic Stationary Phases. J. Chromatogr. A 2022, 1685, 463637. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yao, L.; Wang, J.; Zhang, Z.; Qiao, W.; Long, D.; Ling, L. Adsorption and Regeneration Study of Polyethylenimine-Impregnated Millimeter-Sized Mesoporous Carbon Spheres for Post-Combustion CO2 Capture. Appl. Energy 2016, 168, 282–290. [Google Scholar] [CrossRef]
- Meng, Y.; Jiang, J.; Gao, Y.; Yan, F.; Liu, N.; Aihemaiti, A. Comprehensive Study of CO2 Capture Performance under a Wide Temperature Range Using Polyethyleneimine-Modified Adsorbents. J. CO2 Util. 2018, 27, 89–98. [Google Scholar] [CrossRef]
- Wick-Joliat, R.; Schroffenegger, M.; Penner, D. Multi-Material Ceramic Material Extrusion 3D Printing with Granulated Injection Molding Feedstocks. Ceram. Int. 2023, 49, 6361–6367. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Pelanconi, M.; Ortona, A.; Abu Al-Rub, R.K. Additive Manufacturing of Architected Catalytic Ceramic Substrates Based on Triply Periodic Minimal Surfaces. J. Am. Ceram. Soc. 2019, 102, 6176–6193. [Google Scholar] [CrossRef]
- Seeber, B.S.M.; Gonzenbach, U.T.; Gauckler, L.J. Mechanical Properties of Highly Porous Alumina Foams. J. Mater. Res. 2013, 28, 2281–2287. [Google Scholar] [CrossRef]



| Nozzle temperature | 150 °C |
| Pre-heating zone temperature | 70 °C |
| Print bed temperature | 70 °C |
| Nozzle Diameter | 0.4, 0.6, 0.8 mm |
| Layer height | Half of the nozzle diameter |
| Print speed | 15 m/s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wick-Joliat, R.; Weisshar, F.B.; Gorbar, M.; Meier, D.M.; Penner, D. CO2 Capture with Polyethylenimine Supported on 3D-Printed Porous SiO2 Structures. Materials 2024, 17, 2913. https://doi.org/10.3390/ma17122913
Wick-Joliat R, Weisshar FB, Gorbar M, Meier DM, Penner D. CO2 Capture with Polyethylenimine Supported on 3D-Printed Porous SiO2 Structures. Materials. 2024; 17(12):2913. https://doi.org/10.3390/ma17122913
Chicago/Turabian StyleWick-Joliat, René, Florian B. Weisshar, Michal Gorbar, Daniel M. Meier, and Dirk Penner. 2024. "CO2 Capture with Polyethylenimine Supported on 3D-Printed Porous SiO2 Structures" Materials 17, no. 12: 2913. https://doi.org/10.3390/ma17122913
APA StyleWick-Joliat, R., Weisshar, F. B., Gorbar, M., Meier, D. M., & Penner, D. (2024). CO2 Capture with Polyethylenimine Supported on 3D-Printed Porous SiO2 Structures. Materials, 17(12), 2913. https://doi.org/10.3390/ma17122913







