Round-the-Clock Adsorption–Degradation of Tetracycline Hydrochloride by Ag/Ni-TiO2
Abstract
:1. Introduction
2. Materials and Methods
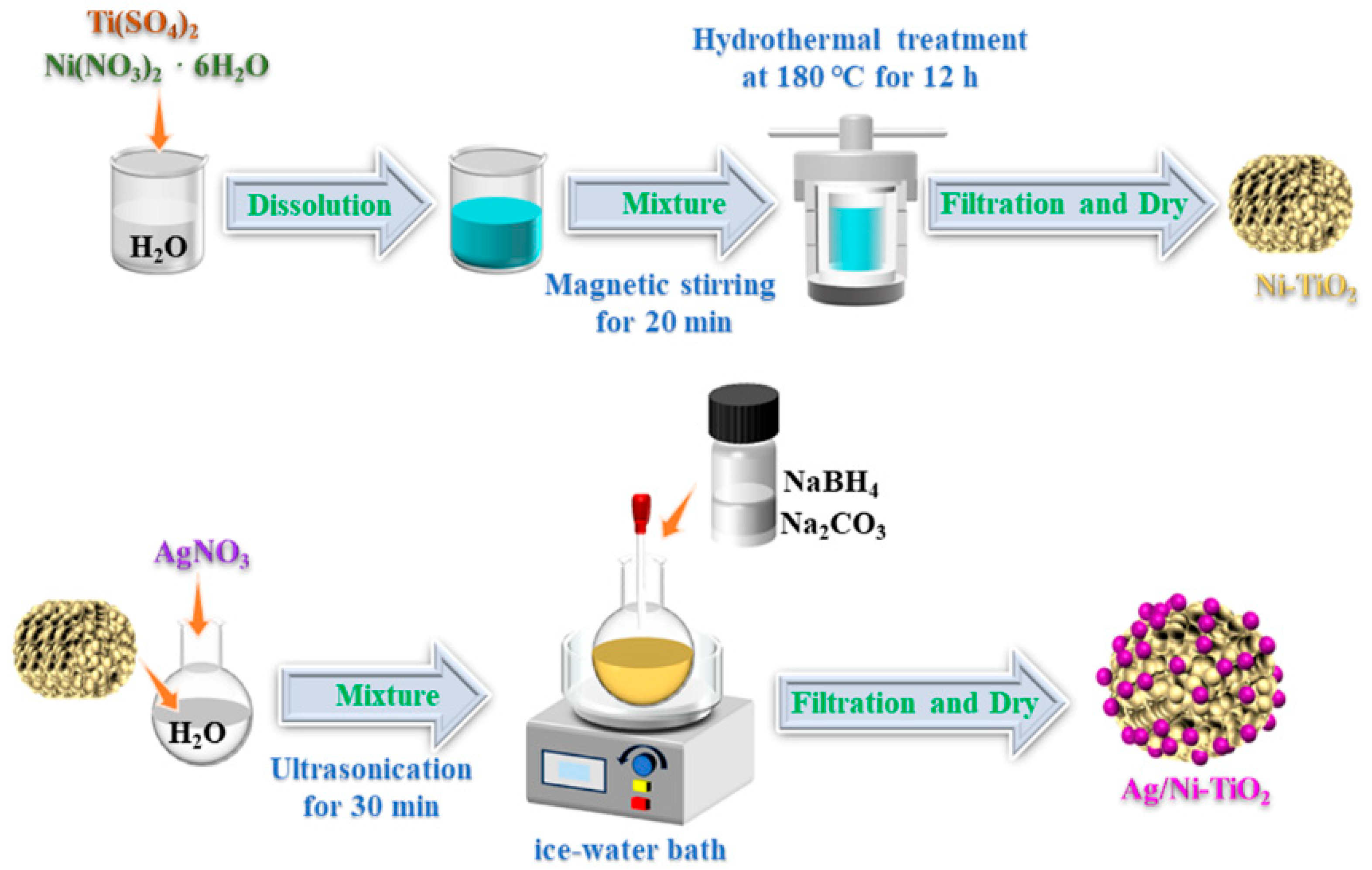
2.1. Preparation of Materials
2.2. Characterization
2.3. Adsorption and Degradation Experiment
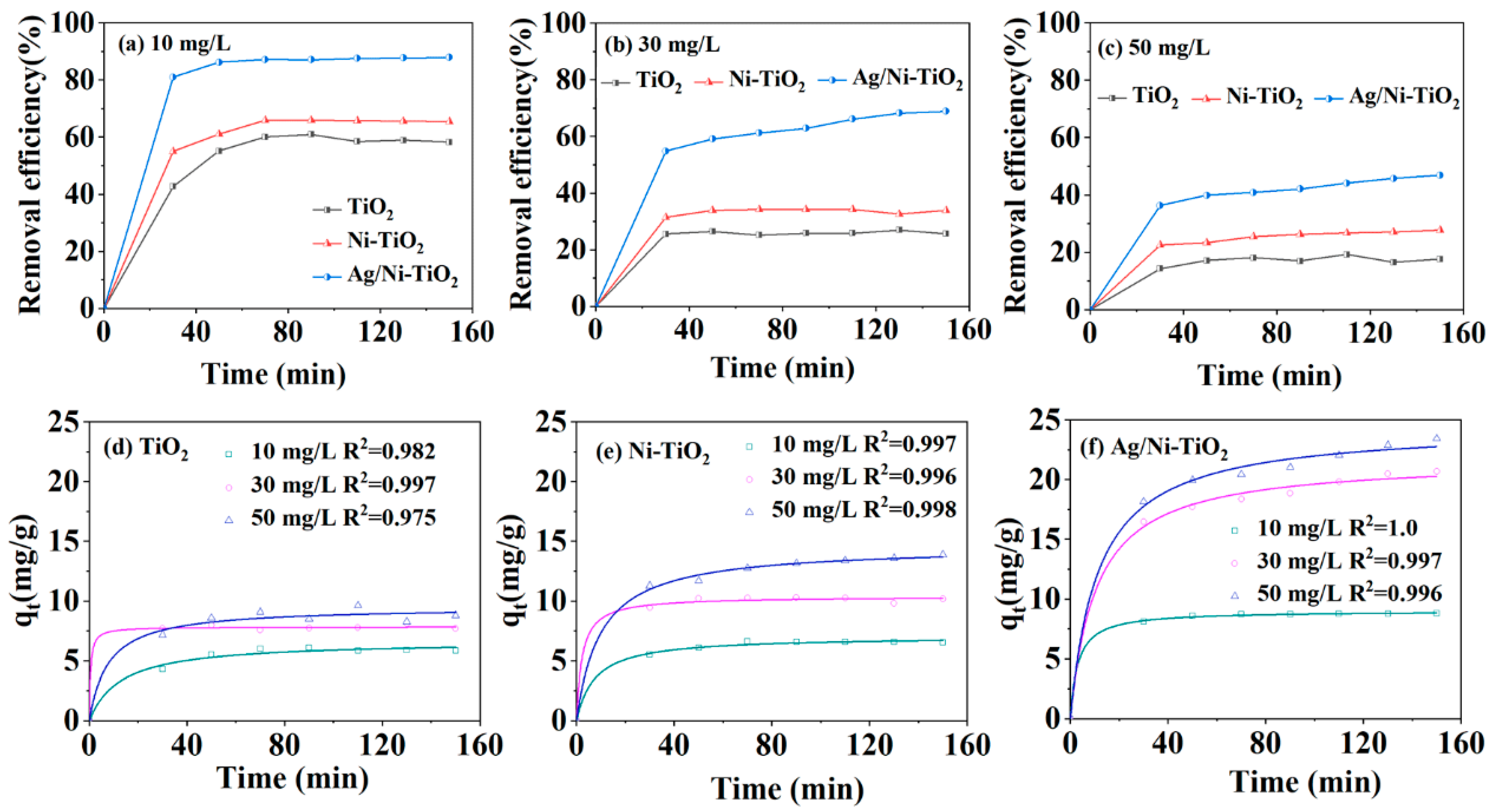
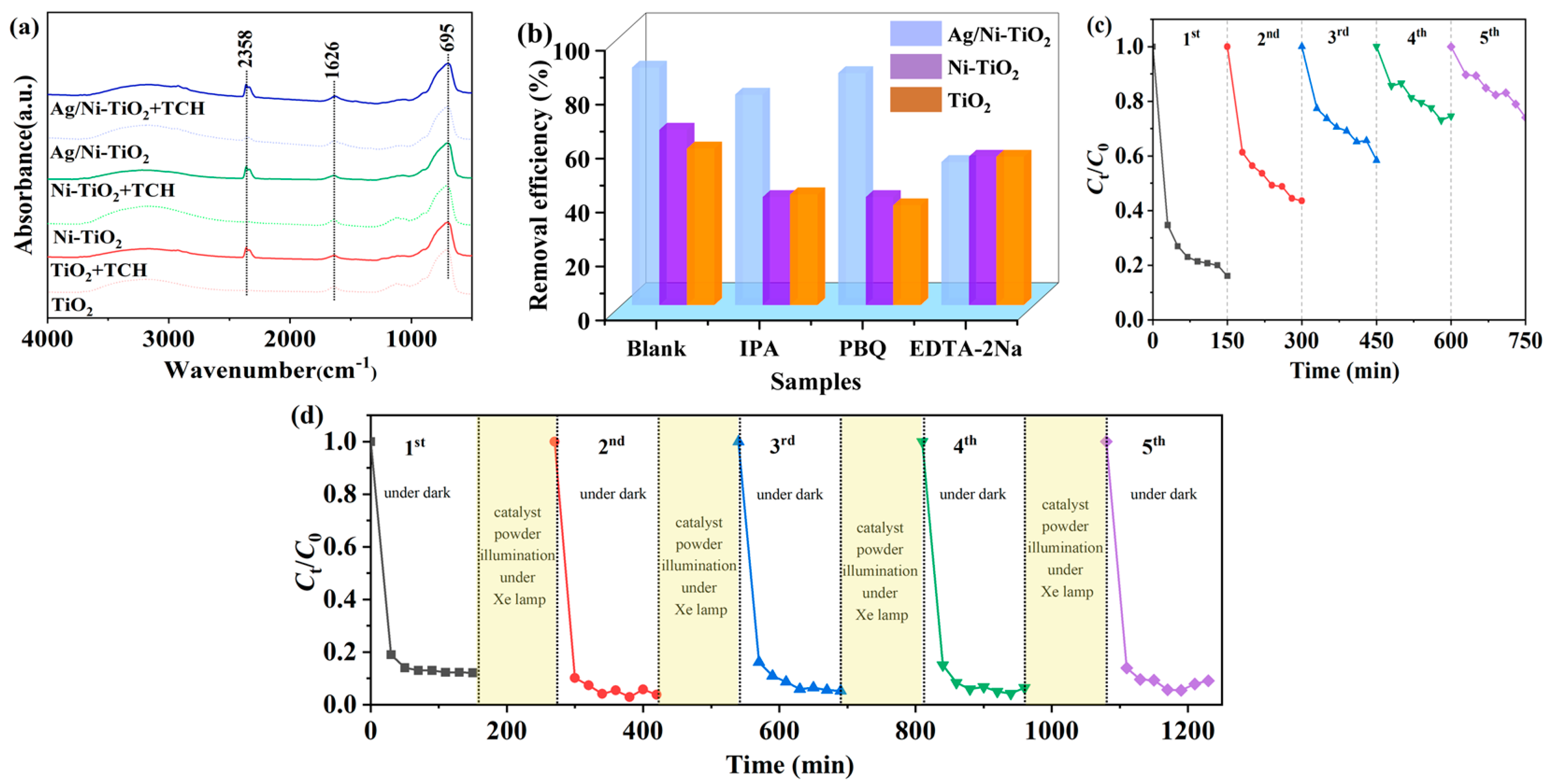
2.3.1. Adsorption–Degradation Performance of TCH
2.3.2. Adsorption–Degradation Cycles of TCH over Ag/Ni-TiO2
2.3.3. Adsorption–Degradation Cycles of TCH over Illuminated Materials
2.4. Determination of TCH
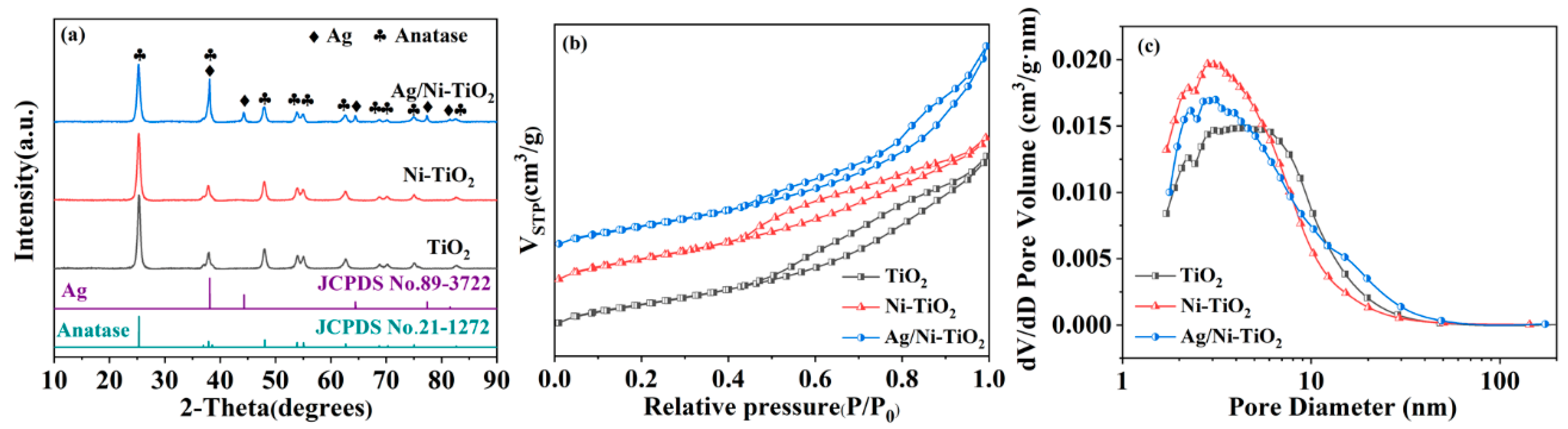
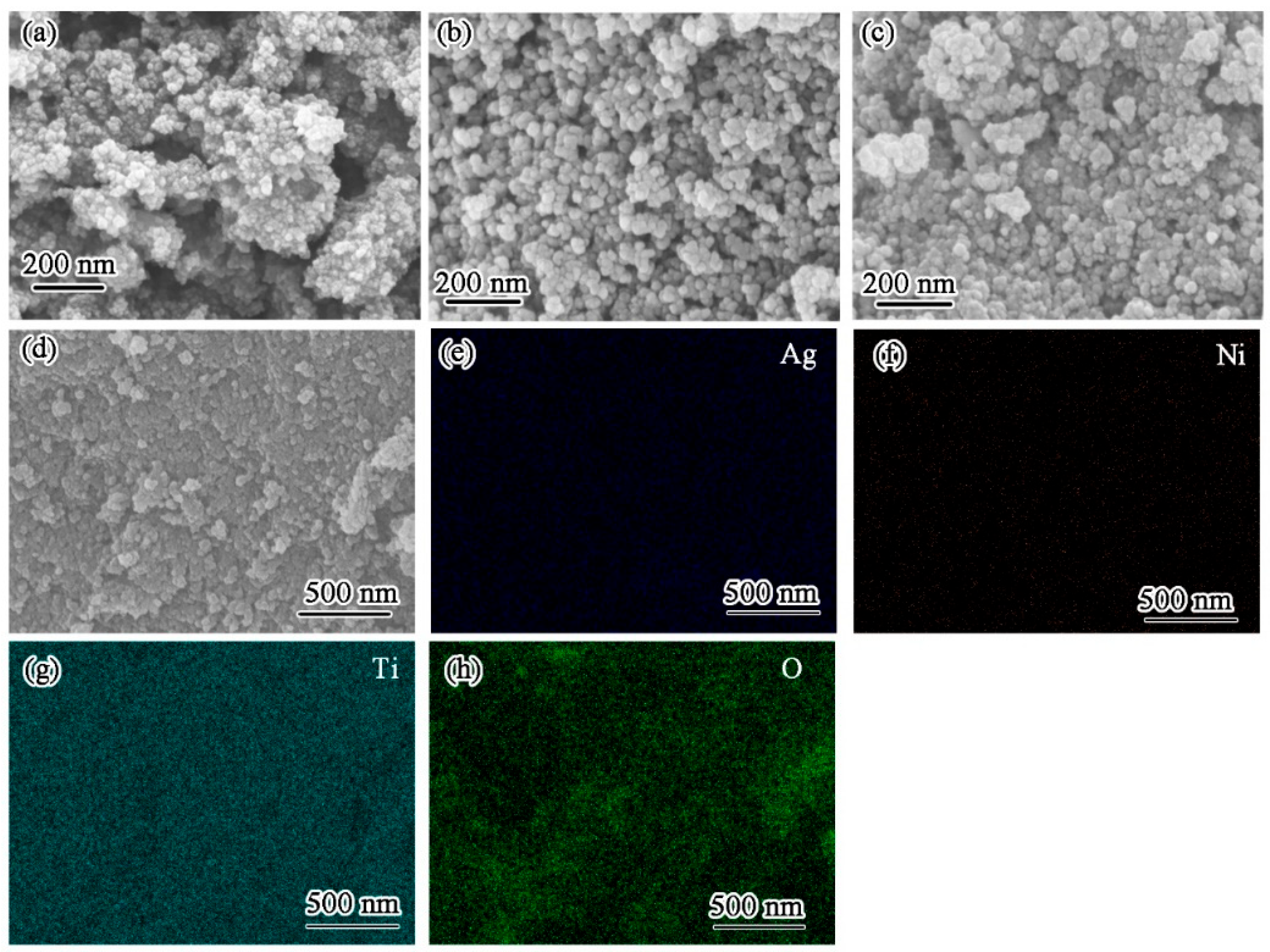
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nie, Y.; Zhao, C.; Zhou, Z.; Kong, Y.; Ma, J. Hydrochloric acid-modified fungi-microalgae biochar for adsorption of tetracycline hydrochloride: Performance and mechanism. Bioresour. Technol. 2023, 383, 129224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bian, Z.; Peng, Y.; Tang, H.; Wang, H. Dual-function oxygen vacancy of BiOBr intensifies pollutant adsorption and molecular oxygen activation to remove tetracycline hydrochloride. Chem. Eng. J. 2023, 451, 138731. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Dai, X.; Zhang, J.; Li, J.; Ma, Y.; Han, Q.; Dong, Y. Research on the adsorption-photocatalytic synergistic degradation of tetracycline by Au nanoparticles/TiO2 nanorods/biochar. J. Alloys Compd. 2024, 976, 172985. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Rao, Y.; Li, X.; Yuan, D.; Tang, S.; Zhao, Q. Enhanced photocatalytic activity of TiO2 with acetylene black and persulfate for degradation of tetracycline hydrochloride under visible light. Chem. Eng. J. 2020, 384, 123350. [Google Scholar] [CrossRef]
- Kong, Y.; Lu, H.; Wang, R.; Yang, Q.; Huang, B.; Zhou, Q.; Hu, W.; Zou, J.; Chen, Q. Adsorption characteristics of tetracycline hydrochloride and oxytetracycline by a MOF-525(Co) metal organic framework. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132443. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; Dong, X.; Zhao, Y.; Li, F.; Cen, Q. Efficient adsorption of tetracycline hydrochloride on biochar-ceramsite composite: Optimization of response surface methodology and investigation of adsorption mechanism. Mater. Today Sustain. 2023, 24, 100525. [Google Scholar] [CrossRef]
- Wang, C.; Yan, R.; Cai, M.; Liu, Y.; Li, S. A novel organic/inorganic S-scheme heterostructure of TCPP/Bi12O17Cl2 for boosting photodegradation of tetracycline hydrochloride: Kinetic, degradation mechanism, and toxic assessment. Appl. Surf. Sci. 2023, 610, 155346. [Google Scholar] [CrossRef]
- Zhang, C.; Xiong, W.; Li, Y.; Lin, L.; Zhou, X.; Xiong, X. Continuous inactivation of human adenoviruses in water by a novel g-C3N4/WO3/biochar memory photocatalyst under light-dark cycles. J. Hazard. Mater. 2023, 442, 130013. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Yang, C.; Chang, M.; Liu, D. Structural design of core-shell ZIF-8@NH2-MIL-125 photocatalyst for synergistic adsorption-photocatalytic degradation of tetracycline hydrochloride. J. Alloys Compd. 2024, 973, 172850. [Google Scholar] [CrossRef]
- Cheng, X.; Guan, R.; Chen, Y.; Qian, Y.; Shang, Q.; Sun, Y. Adsorption and photocatalytic degradation process of oxytetracycline using mesoporous Fe-TiO2 based on high-resolution mass spectrometry. Chem. Eng. J. 2023, 460, 141618. [Google Scholar] [CrossRef]
- Peng, H.; Wang, L.; Zheng, X. Efficient adsorption-photodegradation activity of MoS2 coupling with S,N-codoped porous biochar derived from chitosan. J. Water Process Eng. 2023, 51, 103426. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, S.; Yang, Y.; Wang, Y.; Huang, H.; Hu, Y.; Rodriguez, R.; Chen, J. Efficient adsorption and photocatalytic degradation of organic contaminants using TiO2/(Bi2O3/Bi2O2.33) nanotubes. Mater. Today Chem. 2023, 32, 101641. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Li, M.; Shuai, D.; Zhou, X.; Xiong, X.; Wang, C.; Hu, Q. Continuous photocatalysis via photo-charging and dark-discharging for sustainable environmental remediation: Performance, mechanism, and influencing factors. J. Hazard. Mater. 2021, 420, 126607. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Kong, Y.; Wang, M.; Cai, T.; Zeng, Q. MXene derived Ti3C2/TiO2/Ag persistent photocatalyst with enhanced electron storage capacity for round-the-clock degradation of organic pollutant. J. Colloid Interface Sci. 2024, 656, 233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-M.; Chen, L.-J.; Zhao, X.; Yan, L.-X.; Yan, X.-P. Persistent production of multiple active species with copper doped zinc gallate nanoparticles for light-independent photocatalytic degradation of organic pollutants. J. Colloid Interface Sci. 2024, 668, 540. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.Y.Y.; Sharma, G.; Kherani, N.P.; Ozin, G.A. Post-illumination photoconductivity enables extension of photo-catalysis after sunset. Adv. Energy Mater. 2021, 11, 2101566. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Fan, M.; Yu, X.; Ding, J.; Yan, L.; Qin, G.; Yang, J.; Zhang, Y. Controllable growth of silver nanoparticles on titanium dioxide/nitrogen-doped carbon nanofiber/molybdenum disulfide: Toward enhanced photocatalytic-activating peroxymonosulfate performance and “memory catalysis”. Chem. Eng. J. 2024, 479, 147752. [Google Scholar] [CrossRef]
- Liang, C.; Li, C.; Zhu, Y.; Du, X.; Zeng, Y.; Zhou, Y.; Zhao, J.; Li, S.; Liu, X.; Yu, Q.; et al. Light-driven photothermal catalysis for degradation of toluene on CuO/TiO2 Composite: Dominating photocatalysis and auxiliary thermalcatalysis. Appl. Surf. Sci. 2022, 601, 154144. [Google Scholar] [CrossRef]
- Shaban, M.; Ahmed, A.M.; Shehata, N.; Betiha, M.A.; Rabie, A.M. Ni-doped and Ni/Cr co-doped TiO2 nanotubes for enhancement of photocatalytic degradation of methylene blue. J. Colloid Interface Sci. 2019, 555, 31. [Google Scholar] [CrossRef]
- Kaur, N.; Verma, A.; Thakur, I.; Basu, S. In-situ dual effect of Ag-Fe-TiO2 composite for the photocatalytic degradation of Ciprofloxacin in aqueous solution. Chemosphere 2021, 276, 130180. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Liu, L.; Zhao, G. Role of Ag Loading on the concentration of surface-reaching photoexcited holes in TiO2 nanoparticles. J. Phys. Chem. C 2024, 128, 810. [Google Scholar] [CrossRef]
- Septiningrum, F.; Yuwono, A.H.; Maulana, F.A.; Nurhidayah, E.; Dhaneswara, D.; Sofyan, N.; Hermansyah, H.; Purwanto, W.W. Mangosteen pericarp extract mediated synthesis of Ag/TiO2 nanocomposite and its application on organic pollutant degradation by adsorption-photocatalytic activity. Curr. Res. Green Sustain. Chem. 2024, 8, 100394. [Google Scholar] [CrossRef]
- Li, X.; Shen, R.; Ma, S.; Chen, X.; Xie, J. Graphene-based heterojunction photocatalysts. Appl. Surf. Sci. 2018, 430, 53. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.; Zhu, E.; Liao, L.; Lee, S.-J.; Ding, M.; Shakir, I.; Gambin, V.; Huang, Y.; Duan, X. Approaching the Schottky–Mott limit in van der Waals metal–semiconductor junctions. Nature 2018, 557, 696. [Google Scholar] [CrossRef]
- Monteblanco, E.; Donatini, F.; Hehn, M.; Lacour, D.; Lassailly, Y.; Peretti, J.; Rougemaille, N. Transmission of high-energy electrons through metal-semiconductor Schottky junctions. Phys. Rev. B 2019, 100, 205301. [Google Scholar] [CrossRef]
- Liu, S.; Liu, C.; Wang, W.; Cheng, B.; Yu, J. Unique photocatalytic oxidation reactivity and selectivity of TiO2–graphene nanocomposites. Nanoscale 2012, 4, 3193. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Cheng, Y.; Li, J.; Zhang, Q.; Cao, Y.; Wang, X. Synergistic effect of surfactant hexadecyltrimethylammonium supporting and hydrous titania impregnation onto zeolite in enhanced removal of anionic X-3B dye from aqueous solution. Environ. Prog. Sustain. Energy 2022, 41, e13843. [Google Scholar] [CrossRef]
- Paul, T.C.; Podder, J. Synthesis and characterization of Zn-incorporated TiO2 thin films: Impact of crystallite size on X-ray line broadening and bandgap tuning. Appl. Phys. A 2019, 125, 818. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Xu, M.; Yuan, H.; Chen, Y.; Zhang, J.; Luo, K.; Zhang, J.; You, B. Novel and efficient synthesis of Ag-ZnO nanoparticles for the sunlight-induced photocatalytic degradation. Appl. Surf. Sci. 2019, 476, 632. [Google Scholar] [CrossRef]
- Sun, Q.M.; Xu, J.J.; Tao, F.F.; Ye, W.; Zhou, C.; He, J.H.; Lu, J.M. Boosted inner surface charge transfer in perovskite nanodots@mesoporous titania frameworks for efficient and selective photocatalytic CO2 reduction to methane. Angew. Chem. Int. Ed. 2022, 61, e202200872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Ni, D.; Chen, Z.; Wang, X.; Bu, Y.; Ao, J.P. Improvement of BiVO4 Photoanode Performance during Water Photo-Oxidation Using Rh-Doped SrTiO3 Perovskite as a Co-Catalyst. Adv. Funct. Mater. 2019, 29, 1902101. [Google Scholar] [CrossRef]
- Lei, Q.; Miao, R.; Li, X.; Liu, X.; Li, Y.; He, Z.; Xie, H.; Song, F.; Liu, X.; Liu, H. Efficient hydrogen production from formic acid over Ag@AgPd nanotriangulars at room temperature. Fuel 2024, 355, 129539. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, M.; Wang, L.; Wang, F.; Yang, L.; Li, X.; Wang, C. Plasmonic Ag-TiO2−x nanocomposites for the photocatalytic removal of NO under visible light with high selectivity: The role of oxygen vacancies. Appl. Catal. B Environ. 2017, 204, 67. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, P.; Shi, L.; Yue, X.; Qiu, Q.; Xie, T.; Wang, D.; Lin, Y.; Mu, Z. Insights into the interface effect in Pt@BiOI/ZnO ternary hybrid composite for efficient photodegradation of phenol and photogenerated charge transfer properties. J. Colloid Interface Sci. 2018, 518, 102. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, G.; Xiong, Z.; Hu, Z.; Liao, Y.; Wang, J.; Li, J. Enhanced photocatalytic activity under visible light by the synergistic effects of plasmonics and Ti3+-doping at the Ag/TiO2-heterojunction. Ceram. Int. 2020, 46, 10667. [Google Scholar] [CrossRef]
- Chen, P.; Du, T.; Jia, H.; Zhou, L.; Yue, Q.; Wang, H.; Wang, Y. A novel Bi2WO6/Si heterostructure photocatalyst with Fermi level shift in valence band realizes efficient reduction of CO2 under visible light. Appl. Surf. Sci. 2022, 585, 152665. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Kim, H.-G.; Choi, K.; Lee, K.; Lee, S.; Jung, K.-W.; Choi, J.-W. Controlling the structural robustness of zirconium-based metal organic frameworks for efficient adsorption on tetracycline antibiotics. Water 2021, 13, 1869. [Google Scholar] [CrossRef]
- Sui, G.; Li, J.; Du, L.; Zhuang, Y.; Zhang, Y.; Zou, Y.; Li, B. Preparation and characterization of g-C3N4/Ag–TiO2 ternary hollowsphere nanoheterojunction catalyst with high visible light photocatalytic performance. J. Alloys Compd. 2020, 823, 153851. [Google Scholar] [CrossRef]
- Coenen, K.; Gallucci, F.; Mezari, B.; Hensen, E.; van Sint Annaland, M. An in-situ IR study on the adsorption of CO2 and H2O on hydrotalcites. J. CO2 Util. 2018, 24, 228. [Google Scholar] [CrossRef]
- Ni, J.; Wang, W.; Liu, D.; Zhu, Q.; Jia, J.; Tian, J.; Li, Z.; Wang, X.; Xing, Z. Oxygen vacancy-mediated sandwich-structural TiO2−x /ultrathin g-C3N4/TiO2−x direct Z-scheme heterojunction visible-light-driven photocatalyst for efficient removal of high toxic tetracycline antibiotics. J. Hazard. Mater. 2021, 408, 124432. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Lu, Y.; Zhang, Y.; Qian, A.; Zhang, P.; Tong, M.; Yuan, S. Significant contribution of solid organic matter for hydroxyl radical production during oxygenation. Environ. Sci. Technol. 2022, 56, 11878. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, J.; Fu, Q.; Yang, H.; Tong, Q.; Hao, Z.; Ouyang, G. Unprecedented nonphotomediated hole (h+) oxidation system constructed from defective carbon nanotubes and superoxides. ACS Cent. Sci. 2021, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Baker, L.R.; Somorjai, G.A. Role of hot electrons and metal–oxide interfaces in surface chemistry and catalytic reactions. Chem. Rev. 2015, 115, 2781. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Qin, Y.; Sun, K.; Ahmed, J.; Tian, W.; Ma, Z. Round-the-Clock Adsorption–Degradation of Tetracycline Hydrochloride by Ag/Ni-TiO2. Materials 2024, 17, 2930. https://doi.org/10.3390/ma17122930
Ma S, Qin Y, Sun K, Ahmed J, Tian W, Ma Z. Round-the-Clock Adsorption–Degradation of Tetracycline Hydrochloride by Ag/Ni-TiO2. Materials. 2024; 17(12):2930. https://doi.org/10.3390/ma17122930
Chicago/Turabian StyleMa, Siyu, Yiying Qin, Kongyuan Sun, Jahangeer Ahmed, Wei Tian, and Zhaoxia Ma. 2024. "Round-the-Clock Adsorption–Degradation of Tetracycline Hydrochloride by Ag/Ni-TiO2" Materials 17, no. 12: 2930. https://doi.org/10.3390/ma17122930





