Green and Abrasion-Resistant Superhydrophobic Coatings Constructed with Tung Oil/Carnauba Wax/Silica for Wood Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
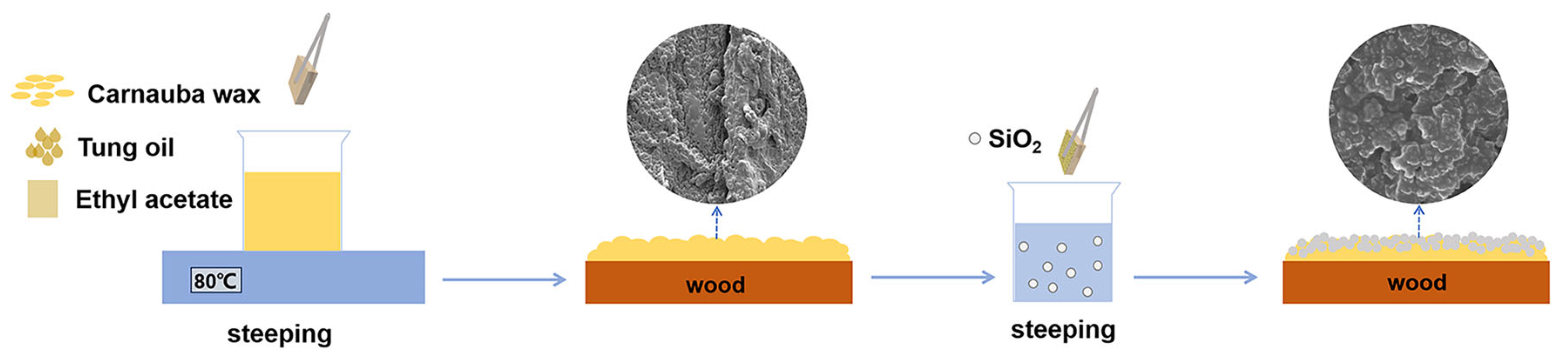
2.2. Preparation of Superhydrophobic Composite Coatings
2.3. Characterization
3. Results
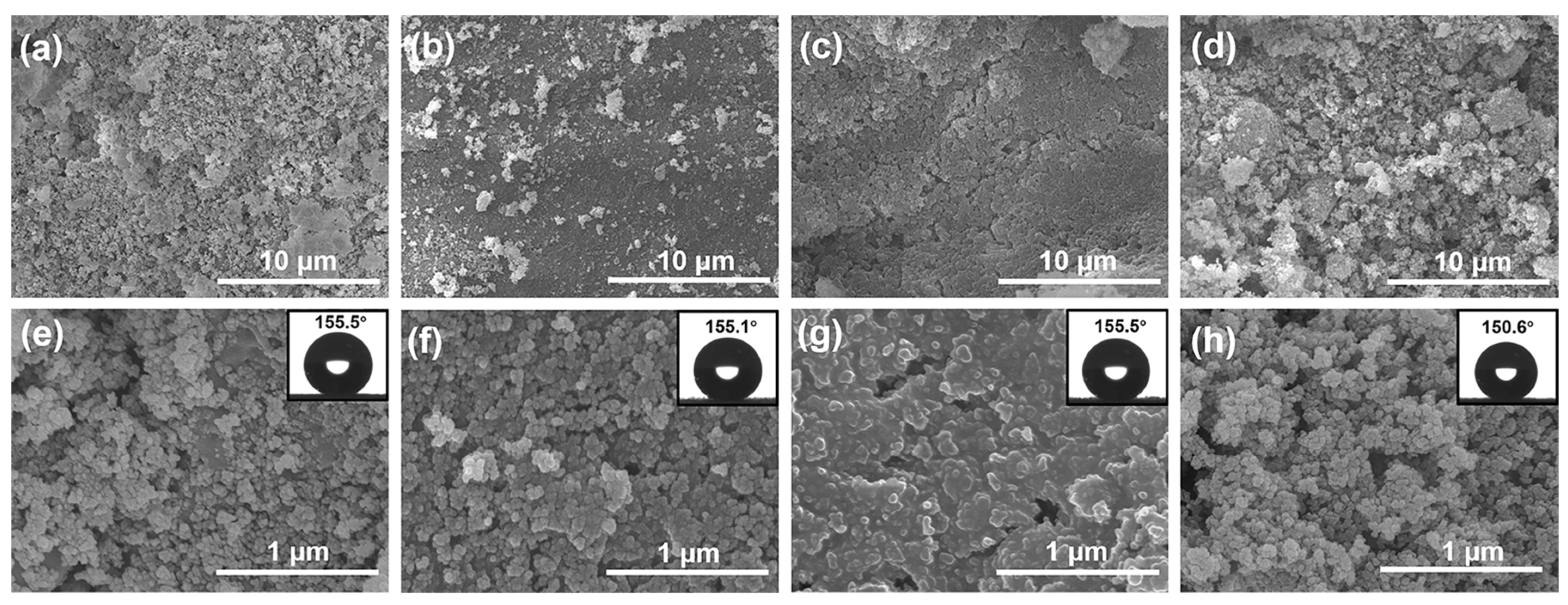
3.1. Morphology and Wettability
3.2. Abrasion Resistance Analysis
3.3. Chemical Stability Analysis
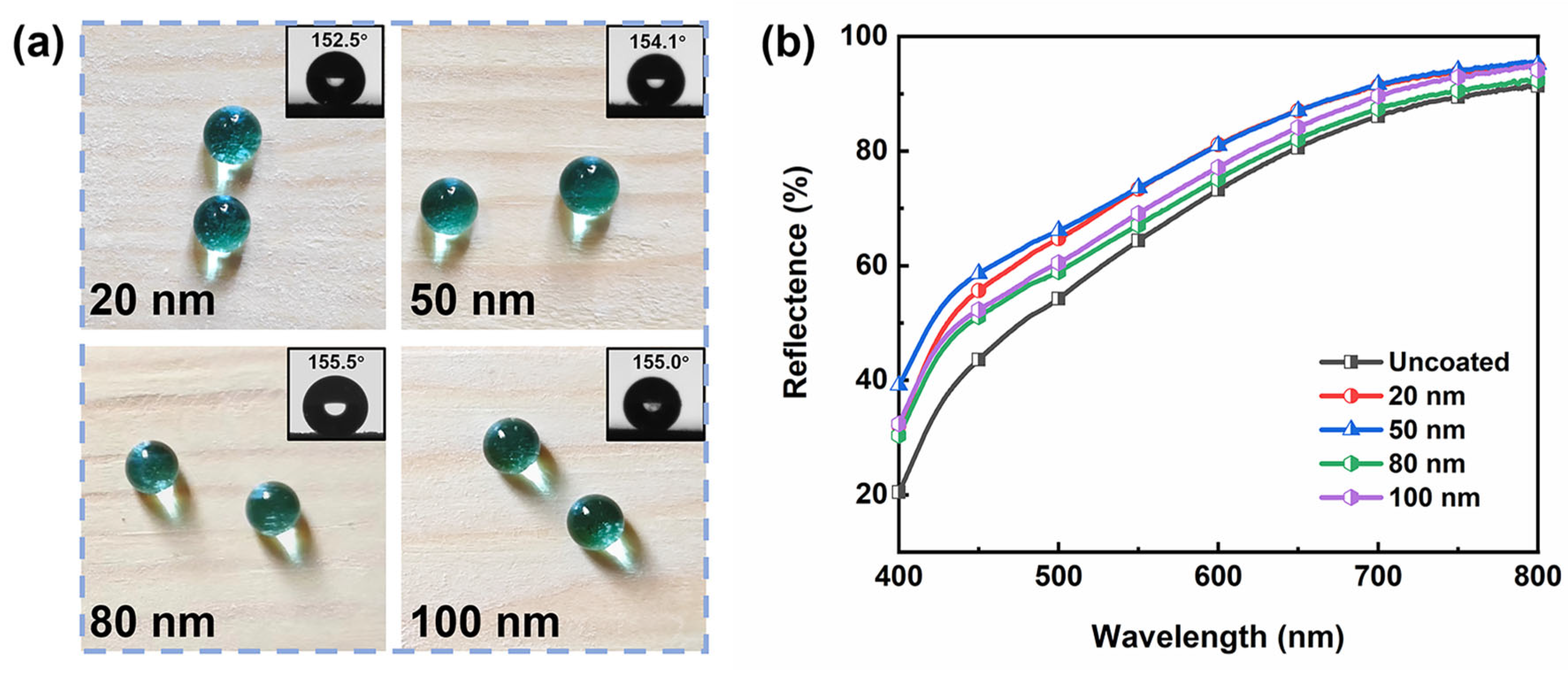
3.4. Transparency Analysis
3.5. Self-Cleaning Properties
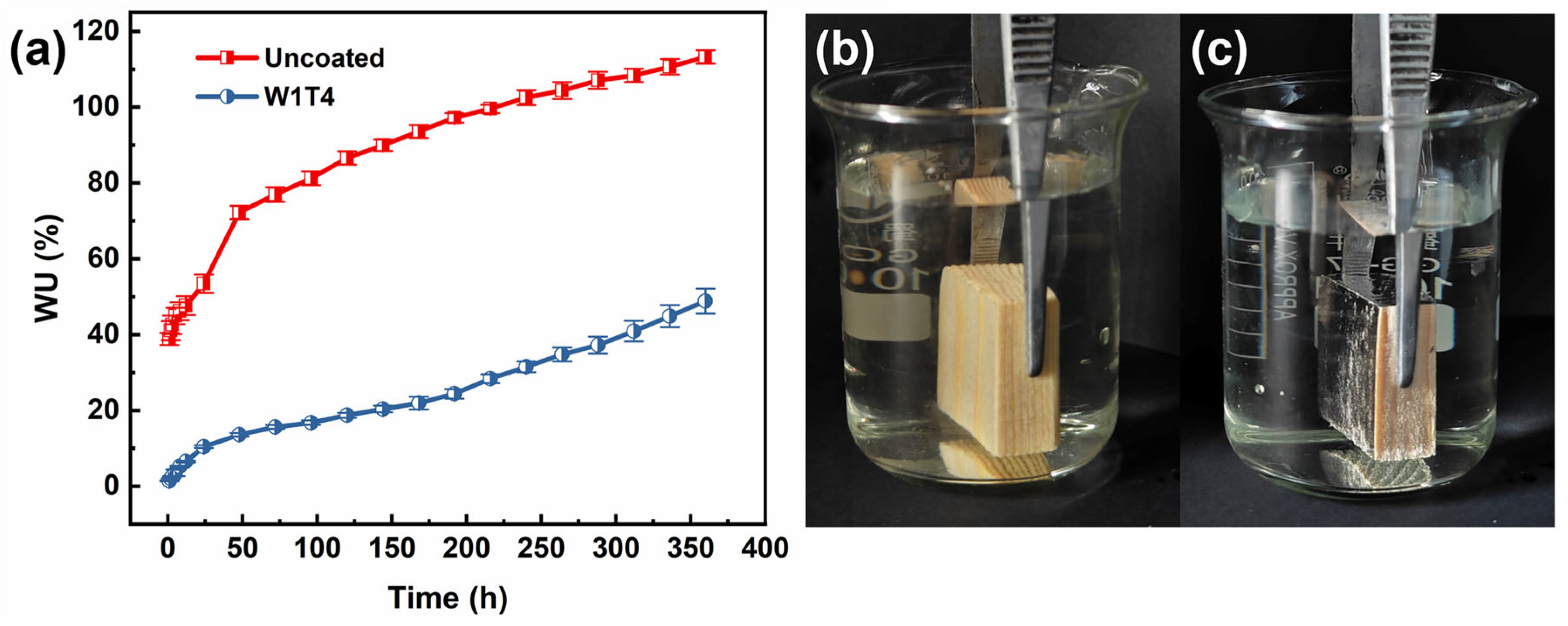
3.6. Water Absorptions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, W.; Jian, Y.; Shao, M.; Cheng, Y.; Liu, J.; Liu, Y.; Hess, D.W.; Wan, H.; Xie, L. A novel two-step strategy to construct multifunctional superhydrophobic wood by liquid-vapor phase deposition of methyltrimethoxysilane for improving moisture resistance, anti-corrosion and mechanical strength. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 13134. [Google Scholar] [CrossRef]
- Pandit, S.K.; Tudu, B.K.; Mishra, I.M.; Kumar, A. Development of stain resistant, superhydrophobic and self-cleaning coating on wood surface. Prog. Org. Coat. 2020, 139, 105453. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Song, J.; Yang, C.; Kuang, Y.; Vellore, A.; Hitz, E.; Zhu, M.; Jiang, F.; Yao, Y.; et al. Strong and Superhydrophobic Wood with Aligned Cellulose Nanofibers as a Waterproof Structural Material. Chin. J. Chem. 2020, 38, 823–829. [Google Scholar] [CrossRef]
- Lin, W.; Huang, Y.; Li, J.; Liu, Z.; Yang, W.; Li, R.; Chen, H.; Zhang, X. Preparation of highly hydrophobic and anti-fouling wood using poly(methylhydrogen)siloxane. Cellulose 2018, 25, 7341–7353. [Google Scholar] [CrossRef]
- Ou, J.; Zhao, G.; Wang, F.; Li, W.; Lei, S.; Fang, X.; Siddiqui, A.R.; Xia, Y.; Amirfazli, A. Durable Superhydrophobic Wood via One-Step Immersion in Composite Silane Solution. ACS Omega 2021, 6, 7266–7274. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Cheng, S.; Wang, P.; Ge, S.; Cai, L.; Cai, J. Construction and characterization of superhydrophobic wood coatings using one-step technique. Colloid Interface Sci. Commun. 2023, 57, 100757. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic Surfaces: Insights from Theory and Experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef]
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic materials and coatings: A review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.K.; Kumar, A.M.; Kumar Sadasivuni, K.; Xing, R.; Liu, S. Self—cleaning superhydrophobic coatings: Potential industrial applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Zhou, C. Anti-corrosive superhydrophobic coatings fabricated by a simple dipping method. Surf. Eng. 2021, 37, 1404–1413. [Google Scholar] [CrossRef]
- Huang, W.; Huang, J.; Guo, Z.; Liu, W. Icephobic/anti-icing properties of superhydrophobic surfaces. Adv. Colloid Interface Sci. 2022, 304, 102658. [Google Scholar] [CrossRef]
- Xiao, F.; Zhang, H.; Wu, T.; Liu, J.; Liu, J.; Zhang, J.; Liu, W.; Liang, T.; Hu, J. Superhydrophobic/superlipophilic interface layer for oil-water separation. Process Saf. Environ. Prot. 2022, 161, 13–21. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Liu, G.; Zhang, M.; Li, J.; Wang, C. Fabrication of superhydrophobic wood surface by a sol–gel process. Appl. Surf. Sci. 2011, 258, 806–810. [Google Scholar] [CrossRef]
- Yue, D.; Lin, S.; Cao, M.; Lin, W.; Zhang, X. Fabrication of transparent and durable superhydrophobic polysiloxane/SiO2 coating on the wood surface. Cellulose 2021, 28, 3745–3758. [Google Scholar] [CrossRef]
- Wan, J.; Xu, J.; Zhu, S.; Li, J.; Wang, B.; Zeng, J.; Li, J.; Chen, K. Eco-Friendly Superhydrophobic Composites with Thermostability, UV Resistance, and Coating Transparency. ACS Appl. Mater. Interfaces 2021, 13, 61681–61692. [Google Scholar] [CrossRef]
- Xie, Y.; Tu, P.; Xiao, Y.; Li, X.; Ren, M.; Cai, Z.; Xu, B. Designing Non-Fluorinated Superhydrophobic Fabrics with Durable Stability and Photocatalytic Functionality. ACS Appl. Mater. Interfaces 2023, 15, 40011–40021. [Google Scholar] [CrossRef]
- Bayer, I.S. Superhydrophobic Coatings from Ecofriendly Materials and Processes: A Review. Adv. Mater. Interfaces 2020, 7, 2000095. [Google Scholar] [CrossRef]
- Saji, V.S. Wax-based artificial superhydrophobic surfaces and coatings. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125132. [Google Scholar] [CrossRef]
- Gupta, S.; Ivvala, J.; Grewal, H.S. Development of natural wax based durable superhydrophobic coatings. Ind. Crops Prod. 2021, 171, 113871. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, P.; Qian, L.; Xiao, H. Fabrication of superhydrophobic paper surface via wax mixture coating. Chem. Eng. J. 2014, 250, 431–436. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, F.; Zhang, W.; Liu, H.; Ge, M.; Zhang, Y.; Dai, J.; Wang, H.; Xing, G.; Lai, Y.; et al. Constructing Mechanochemical Durable and Self-Healing Superhydrophobic Surfaces. ACS Omega 2020, 5, 986–994. [Google Scholar] [CrossRef]
- Milionis, A.; Loth, E.; Bayer, I.S. Recent advances in the mechanical durability of superhydrophobic materials. Adv. Colloid Interface Sci. 2016, 229, 57–79. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Xue, F.; Jing, X. A robust and versatile superhydrophobic coating: Wear-resistance study upon sandpaper abrasion. Appl. Surf. Sci. 2019, 480, 738–748. [Google Scholar] [CrossRef]
- Arbatan, T.; Zhang, L.; Fang, X.-Y.; Shen, W. Cellulose nanofibers as binder for fabrication of superhydrophobic paper. Chem. Eng. J. 2012, 210, 74–79. [Google Scholar] [CrossRef]
- Xue, C.-H.; Zhao, L.-L.; Guo, X.-J.; Ji, Z.-Y.; Wu, Y.; Jia, S.-T.; An, Q.-F. Mechanically durable superhydrophobic surfaces by binding polystyene nanoparticles on fibers with aluminum phosphate followed by hydrophobization. Chem. Eng. J. 2020, 396, 125231. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Zhang, M.; Ma, M.; Wang, C.; Li, J. Improvement of mechanical robustness of the superhydrophobic wood surface by coating PVA/SiO2 composite polymer. Appl. Surf. Sci. 2013, 280, 686–692. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Bai, W.; Li, J.E.; Zhu, S.; Li, Y.; Ding, J.; Liu, Y.; Feng, L. Robust superhydrophobic materials with outstanding durability fabricated by epoxy adhesive-assisted facile spray method. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131109. [Google Scholar] [CrossRef]
- Mastouri, A.; Efhamisisi, D.; Tarmian, A.; Boukherroub, R.; Lexa, M.; Karami, E.; Panek, M.; Frigione, M. Sustainable superhydrophobic and self-cleaning wood via wax within Epoxy/PDMS nano-composite coatings: Durability related to surface morphology. Prog. Org. Coat. 2024, 186, 107951. [Google Scholar] [CrossRef]
- Meiorin, C.; Aranguren, M.I.; Mosiewicki, M.A. Polymeric networks based on tung oil: Reaction and modification with green oil monomers. Eur. Polym. J. 2015, 67, 551–560. [Google Scholar] [CrossRef]
- Honzíček, J. Curing of Air-Drying Paints: A Critical Review. Ind. Eng. Chem. Res. 2019, 58, 12485–12505. [Google Scholar] [CrossRef]
- He, Z.; Qian, J.; Qu, L.; Yan, N.; Yi, S. Effects of Tung oil treatment on wood hygroscopicity, dimensional stability and thermostability. Ind. Crops Prod. 2019, 140, 111647. [Google Scholar] [CrossRef]
- Tamburini, D.; Sardi, D.; Spepi, A.; Duce, C.; Tinè, M.R.; Colombini, M.P.; Bonaduce, I. An investigation into the curing of urushi and tung oil films by thermoanalytical and mass spectrometric techniques. Polym. Degrad. Stab. 2016, 134, 251–264. [Google Scholar] [CrossRef]
- Li, X.; Gao, L.; Wang, M.; Lv, D.; He, P.; Xie, Y.; Zhan, X.; Li, J.; Lin, Z. Recent development and emerging applications of robust biomimetic superhydrophobic wood. J. Mater. Chem. A 2023, 11, 6772–6795. [Google Scholar] [CrossRef]
- Martini, S.; Tan, C.Y.; Jana, S. Physical Characterization of Wax/Oil Crystalline Networks. J. Food Sci. 2015, 80, C989–C997. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, T. Importance of moderate size of pillars and dual-scale structures for stable superhydrophobic surfaces: A molecular dynamics simulation study. Comput. Mater. Sci. 2020, 175, 109613. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, X.; Yan, Y.; Qian, T.; Wang, J.; Sun, Q.; Jin, C. Facile fabrication of a PDMS @ stearic acid-Al(OH)3 coating on lignocellulose composite with superhydrophobicity and flame retardancy. Appl. Surf. Sci. 2018, 450, 387–395. [Google Scholar] [CrossRef]
- Tu, K.; Wang, X.; Kong, L.; Chang, H.; Liu, J. Fabrication of robust, damage-tolerant superhydrophobic coatings on naturally micro-grooved wood surfaces. RSC Adv. 2016, 6, 701–707. [Google Scholar] [CrossRef]
- Huang, J.; Cai, P.; Li, M.; Wu, Q.; Li, Q.; Wang, S. Preparation of CNF/PDMS Superhydrophobic Coatings with Good Abrasion Resistance Using a One-Step Spray Method. Materials 2020, 13, 5380. [Google Scholar] [CrossRef]
- Turnbull, S.; Bradshaw, L.; Yildiz, Y. Determination of Saponification Value in Carnuba Wax by USP/NF Residual Titration Method. Am. J. Anal. Chem. 2019, 10, 423–427. [Google Scholar] [CrossRef]
- Benkovičová, M.; Kisová, Z.; Bučková, M.; Majková, E.; Šiffalovič, P.; Pangallo, D. The Antifungal Properties of Super-Hydrophobic Nanoparticles and Essential Oils on Different Material Surfaces. Coatings 2019, 9, 176. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Britto Joseph, G.; Durairaj, R.B.; Mageshwaran, G. Superhydrophobic surfaces: A review on fundamentals, applications, and challenges. J. Coat. Technol. Res. 2018, 15, 231–250. [Google Scholar] [CrossRef]
- Xue, C.-H.; Li, Y.-R.; Zhang, P.; Ma, J.-Z.; Jia, S.-T. Washable and Wear-Resistant Superhydrophobic Surfaces with Self-Cleaning Property by Chemical Etching of Fibers and Hydrophobization. ACS Appl. Mater. Interfaces 2014, 6, 10153–10161. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, S. A robust superhydrophobic surface and origins of its self-cleaning properties. Appl. Surf. Sci. 2017, 420, 336–345. [Google Scholar] [CrossRef]
- Extrand, C.W. Origins of Wetting. Langmuir 2016, 32, 7697–7706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, F.; Feng, Q.; Zheng, J.; Chen, C.; Chen, H.; Yang, W. Preparation of SiO2 nanoparticles with adjustable size for fabrication of SiO2/PMHS ORMOSIL superhydrophobic surface on cellulose-based substrates. Prog. Org. Coat. 2020, 138, 105384. [Google Scholar] [CrossRef]
- Lu, Q.; Cheng, R.; Jiang, H.; Xia, S.; Zhan, K.; Yi, T.; Morrell, J.J.; Yang, L.; Wan, H.; Du, G.; et al. Superhydrophobic wood fabricated by epoxy/Cu2(OH)3Cl NPs/stearic acid with performance of desirable self-cleaning, anti-mold, dimensional stability, mechanical and chemical durability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129162. [Google Scholar] [CrossRef]
- Yue, D.; Feng, Q.; Huang, X.; Zhang, X.; Chen, H. In Situ Fabrication of a Superhydrophobic ORMOSIL Coating on Wood by an Ammonia–HMDS Vapor Treatment. Coatings 2019, 9, 556. [Google Scholar] [CrossRef]




| Sample | Carnauba Wax (g) | Tung Oil (g) |
|---|---|---|
| W1T0 | 5 | 0 |
| W1T1 | 2.5 | 2.5 |
| W1T4 | 1 | 4 |
| W0T1 | 0 | 5 |
| Coating | Fabrication Technique | Contact Angle (°) | Weight (g) | Sandpaper (mesh) | Abrasion Length/Cycles | Ref. |
|---|---|---|---|---|---|---|
| PDMS @ stearic acid-Al(OH)3 | Dip and deposition coating | 156.0 | 200 | 1500 | 240 cm abrasion length | [36] |
| ZIF-8/HDTMS | Brush coating | 153.0 | 50 | 1000 | 10 abrasion cycles (100 cm) | [6] |
| Silica/epoxy resin/FAS | Dip coating | 152.0 | 200 | 1500 | 250 cm abrasion length | [37] |
| CNF/PDMS | Spray coating | 158.0 | 100 | 800 | 19 abrasion cycles (380 cm) | [38] |
| Tung oil/carnauba wax/silica | Dip coating | 155.5 | 100 | 800 | 600 cm abrasion length | This work |
| Solution | Time (h) | Contact Angle (°) |
|---|---|---|
| Sodium chloride solution (3 wt%) | 24 | 152.7 |
| Sodium hydroxide solution (pH = 13) | 1 | 140.9 |
| Sodium hydroxide solution (pH = 13) | 4 | 79.3 |
| Hydrochloric acid solution (pH = 1) | 12 | 150.2 |
| Base Material | Coating | Fabrication Technique | Time (h) | Uncoated (WU%) | Coated (WU%) | Ref. |
|---|---|---|---|---|---|---|
| Cunninghamia lanceolata | SiO2/PMHS ORMOSIL | Dip coating | 24 | 70.30 | 24.10 | [45] |
| Pinus wood | Epoxy/Cu2(OH)3Cl NPs/stearic acid | In situ growth and Dip coating | 120 | 80.00 | 36.00 | [46] |
| Cunninghamia lanceolata | Ammonia–HMDS | Vapor treatment coating | 24 | 72.30 | 31.90 | [47] |
| Pinus wood | Tung oil/carnauba wax/silica | Dip coating | 120 | 86.61 | 18.72 | This work |
| Pinus wood | Tung oil/carnauba wax/silica | Dip coating | 360 | 113.25 | 48.84 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Zhang, H.; Zhu, M.; Cai, J.; Xu, B. Green and Abrasion-Resistant Superhydrophobic Coatings Constructed with Tung Oil/Carnauba Wax/Silica for Wood Surface. Materials 2024, 17, 3000. https://doi.org/10.3390/ma17123000
Su J, Zhang H, Zhu M, Cai J, Xu B. Green and Abrasion-Resistant Superhydrophobic Coatings Constructed with Tung Oil/Carnauba Wax/Silica for Wood Surface. Materials. 2024; 17(12):3000. https://doi.org/10.3390/ma17123000
Chicago/Turabian StyleSu, Jieying, Haitao Zhang, Meiting Zhu, Jiajie Cai, and Bin Xu. 2024. "Green and Abrasion-Resistant Superhydrophobic Coatings Constructed with Tung Oil/Carnauba Wax/Silica for Wood Surface" Materials 17, no. 12: 3000. https://doi.org/10.3390/ma17123000
APA StyleSu, J., Zhang, H., Zhu, M., Cai, J., & Xu, B. (2024). Green and Abrasion-Resistant Superhydrophobic Coatings Constructed with Tung Oil/Carnauba Wax/Silica for Wood Surface. Materials, 17(12), 3000. https://doi.org/10.3390/ma17123000





